Abstract
Rationale
The ability of tobacco harm reduction strategies to produce significant reductions in toxin exposure is limited by compensatory increases in smoking behavior. Characterizing factors contributing to the marked individual variability in compensation may be useful for understanding this phenomenon and assessing the feasibility of harm reduction interventions.
Objective
To use an animal model of human compensatory smoking that involves a decrease in unit dose supporting nicotine self-administration (NSA) to examine potential contributors to individual differences in compensation.
Methods
Rats were trained for NSA during daily 23 hr sessions at a unit dose of 0.06 mg/kg/inf until responding was stable. The unit dose was then reduced to 0.03 mg/kg/inf for at least 10 sessions. Following reacquisition of NSA at the training dose and extinction, single-dose nicotine pharmacokinetic parameters were determined.
Results
Decreases in nicotine intake following dose reduction were proportionally less than the decrease in unit dose, indicating partial compensation. Compensatory increases in infusion rates were observed across the course of the 23 hr sessions. The magnitude of compensation differed considerably between rats. Rats exhibiting the highest baseline infusion rates exhibited the lowest levels of compensation. Nicotine pharmacokinetic parameters were not significantly correlated with compensation. Infusion rates immediately returned to pre-reduction levels when baseline conditions were restored.
Conclusions
These findings provide initial insights into correlates of individual differences in compensation following a reduction in nicotine unit dose. The present assay may be useful for characterizing mechanisms and potential consequences of the marked individual differences in compensatory smoking observed in humans.
Keywords: Nicotine, Self-administration, Rat, Harm Reduction, Compensation
Introduction
Tobacco harm reduction, or the attempt to reduce disease associated with tobacco use by reducing exposure to tobacco toxins, is being considered as an alternative or complementary strategy to cessation for smokers who are unable or unwilling to quit (Stratton et al. 2001; Hatsukami et al. 2002; Shiffman et al. 2002; Stead and Lancaster 2007). Approaches to harm reduction include smoking reduction (e.g., decreasing cigarettes per day) and the use of potential reduced exposure products (PREPs) containing reduced levels of toxins or nicotine (Hatsukami et al. 2004; Stead and Lancaster 2007), the primary addictive component of tobacco (e.g., Benowitz 1996; U.S. Department of Health and Human Services 1999; Benowitz 2008). While the use of harm reduction strategies can result in decreased levels of tobacco exposure biomarkers (Hecht et al. 2004; Hatsukami et al. 2005) and improvements in certain health outcomes (e.g. diminished risk of lung cancer; Godtfredsen et al. 2005), the decrease in biomarkers is often substantially less than would be predicted based on decreases in cigarettes per day or nicotine yield per cigarette (Hatsukami et al. 2007; Pisinger and Godtfredsen 2007). This disproportionate relationship has been attributed to compensatory changes in smoking topography (e.g., increasing puff depth or frequency) that occur in an attempt to titrate nicotine intake and compensate for lower smoke delivery (Scherer 1999; Benowitz et al. 2005; Hatsukami et al. 2006; Joseph et al. 2008). Compensatory smoking represents a significant obstacle to achieving a meaningful reduction in exposure to harmful tobacco constituents.
Marked individual differences in compensation have been reported in smokers undergoing harm reduction interventions. For example, compensation in individuals switched from their usual brand to cigarettes with reduced nicotine yield ranged from none (i.e., nicotine intake decreased proportionally to decreases in cigarette nicotine yield) to complete (i.e., nicotine intake was maintained despite decreases in nicotine yield) (Benowitz et al. 2006). The substantial individual variability in compensation suggests that harm reduction strategies may be useful for decreasing toxin exposure in at least some smokers. Characterizing determinants of this variability may be useful for selecting candidates for reduction strategies, and could also help in developing treatments for minimizing compensation and improving the efficacy of harm reduction as a potential approach to reducing the health burden of tobacco dependence. Given that smokers not undergoing harm reduction interventions may still limit their smoking during certain times of day (e.g., Frederiksen and Frazier 1977; Morgan et al. 1985), identifying factors contributing to compensation could also be useful for understanding normal smoking behavior.
The determinants of compensation have not been well-established (Scherer 1999; Harris et al. 2008). Factors already known to be related to smoking behavior or to smoking cessation represent logical initial areas of focus. Baseline smoking levels and diurnal patterns of nicotine intake are strongly associated with the ability to stop smoking. Individuals who smoke heavily or who smoke first thing in the morning or at night are less likely to successfully quit (Farkas et al. 1996; Abrams et al. 2000). Another factor thought to contribute to tobacco dependence is nicotine metabolism, with faster nicotine elimination being associated with greater nicotine intake (Benowitz and Jacob 1985; Benowitz et al. 2003; Sellers et al. 2003) and greater difficulty in quitting (Patterson et al. 2008). Each of these factors could potentially contribute to compensation.
Animal models of nicotine self-administration (NSA) may be useful for elucidating variables involved in compensation, as they allow control over nicotine intake history and isolation of the effects of nicotine from those of other smoke constituents or social factors. A number of findings predict that compensatory increases in NSA should occur in rats following nicotine exposure reduction. Infusion rates are higher in rats with shorter access to NSA compared to other rats with longer access (e.g., 1 hr/day versus 23 hr/day), or in animals responding for moderate nicotine unit doses compared to others responding for higher nicotine unit doses (Shoaib et al. 1997; Valentine et al. 1997; LeSage et al. 2002). Although these findings are reminiscent of compensation, a true demonstration of this phenomenon requires a change in nicotine-seeking behavior within the same subjects (Scherer 1999).
We have previously demonstrated that rats exhibit compensatory increases in NSA when duration of daily access to nicotine is progressively reduced within-subjects from 23 hr/day to 2 hr/day (Harris et al. 2008). In addition, numerous studies manipulating nicotine dose within-subjects have reported greater infusion rates for moderate compared to high NSA doses (e.g., Corrigall and Coen 1989; Donny et al. 1995; Bardo et al. 1999; Watkins et al. 1999; DeNoble and Mele 2006). However, none of these studies sought to broadly characterize individual variability in compensation when the unit dose was reduced, the sequence of nicotine exposure conditions that most accurately simulates a harm reduction strategy (e.g., switching to cigarettes with reduced nicotine yield).
The goal of the current study was to develop an animal NSA assay in which the nicotine unit dose is reduced within-subjects in order to evoke a compensatory increase in NSA, and to examine whether baseline behavioral and pharmacokinetic factors are associated with individual differences in compensation. This animal model somewhat resembles the use of a currently marketed PREP (Quest; Vector Tobacco Inc., Durham, NC) consisting of “low”, “extra-low,” and “nicotine-free” cigarettes that is being examined as a potential aid to smoking cessation (Donny et al. 2007; Strasser et al. 2007; Becker et al. 2008). The current model may also be relevant to the proposed population strategy of decreasing the nicotine content of cigarettes to a non-addictive level to promote smoking cessation and prevent initiation of smoking in adolescents (Benowitz and Henningfield 1994; Henningfield et al. 1998). Compensation represents an adverse consequence of this approach, which may be implemented if current legislation allowing the Food and Drug Administration (FDA) regulatory authority over tobacco products is passed (Zeller et al. in press). Following reduction testing, animals in the present study were allowed to reacquire NSA at the training dose to determine whether the higher rate of NSA associated with compensation would persist when baseline conditions were restored, a potential adverse consequence of tobacco exposure reduction (Shiffman et al. 1998; Hatsukami et al. 2004; Harris et al. 2008). Rats were then tested under conditions of saline extinction to examine the relationship between compensation and increases in responding during the first extinction session (i.e., an “extinction burst”) (Harris et al. 2007; Harris et al. 2008), as both phenomena reflect increased drug-seeking behavior following reductions in nicotine availability.
Materials and methods
Animals
Experimentally-naïve male Holtzman rats (Harlan, Indianapolis, IN) weighing 300–400 g were maintained under a restricted feeding regimen (18 g/day rat chow). Each rat was individually housed in an operant-conditioning chamber in a temperature-and humidity-controlled colony room with unlimited access to water under a reversed 12 h light/dark cycle (lights off at 11:00 hr). Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Apparatus
Each operant chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, an aluminum ceiling, and a stainless steel grid floor. Two response levers were located on the front wall 10 cm above the chamber floor on either side of a food aperture located 2 cm above the floor. Stimulus lights were located 2 cm above each response lever. Water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside each cubicle delivered infusions through PE 90 tubing connected to a fluid swivel mounted above the chamber, and from the swivel through a spring leash connected to a guide cannula mounted in a harness assembly on the back of the rat. MED-PC IV software (Med Associates, Inc., St. Albans, VT) was used for operating the apparatus and recording data.
Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline containing 25 units/ml heparin. The pH of the solution was adjusted to 7.4 with dilute NaOH. Nicotine doses are expressed as the base.
Procedure
Catheter Implantation
Each rat was implanted with a chronic indwelling jugular catheter under droperidol/fentanyl anesthesia as described previously (LeSage et al. 2002; Harris et al. 2008). The catheter was externalized between the scapulae and attached to a harness assembly that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least four days after surgery, during which time they received daily intravenous (i.v.) infusions of heparinized saline (30 units/ml) and antibiotic (rocephin, 5.25 mg) into the jugular catheter.
Nicotine Self-Administration Training
Rats were trained to self-administer nicotine in 23-h/day sessions conducted seven days a week. This access schedule results in patterns of nicotine intake and serum nicotine levels more similar to those of smokers than NSA sessions of shorter (e.g., 1 hr) duration (see LeSage et al. 2002). Nicotine availability was signaled by illumination of the stimulus light above the active response lever (always the right lever). Following completion of the response requirement, the stimulus light was extinguished and nicotine (0.06 mg/kg/inf) was infused over the course of 1 sec in a volume of 50 μl heparinized saline. This training dose was chosen because it lies on the descending arm of the “inverted U” NSA dose-response curve (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006), allowing for measurement of compensatory increases in NSA following unit dose reduction. Following a 7-sec time-out, during which time additional responses had no programmed consequences, the stimulus light turned on and the next nicotine infusion was available. Responses on the other (inactive) lever were recorded but had no programmed consequences. Sessions were suspended from 10:00 – 11:00 hr each day to allow for cage maintenance, feeding of animals, and evaluation of general health. The response requirement was initially a fixed ratio 1 (FR 1). After robust responding developed, the FR was gradually increased to FR 3 across several sessions. The criteria for acquisition were a minimum of 10 infusions per day under the FR 3 schedule and a ratio of active to inactive lever presses of at least 2:1 for five consecutive sessions. These acquisition criteria are typical for NSA under unlimited access conditions (e.g., Valentine et al. 1997; Brower et al. 2002; LeSage et al. 2003).
Dose Reduction Group
Figure 1 shows a timeline for the experimental procedure for rats in the dose reduction group (n = 25). Rats were trained for NSA until acquisition criteria were met and NSA was stable (i.e., no trend in infusion rate across five consecutive sessions and a coefficient of variation < 15%). The nicotine unit dose was then reduced to 0.03 mg/kg/inf for at least 10 sessions and until stable. This unit dose was used because it is near the peak of the NSA dose-response curve (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006). Rats were then allowed to reacquire NSA at the 0.06 mg/kg/inf unit dose for at least 10 sessions and until stable. A period of extinction was subsequently arranged for seven consecutive sessions. Extinction sessions were identical to previous sessions with the exception that saline was substituted for the nicotine unit dose (i.e., cues previously paired with nicotine infusions were presented).
Fig 1.
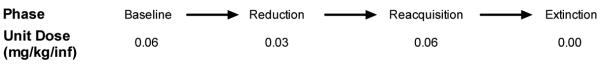
Timeline for experimental procedure for the reduction group. Also shown is the nicotine unit dose (mg/kg/inf) available at each test phase.
Control Group
An additional group of 10 rats was used to examine whether NSA (0.06 mg/kg/inf) remains stable over time in the absence of a reduction in nicotine unit dose. Rats were trained for NSA until acquisition criteria were met and NSA deemed stable as for rats in the reduction group. Animals were then allowed to continue to self-administer the 0.06 mg/kg/inf unit dose for an additional 27 sessions (i.e, the average number of sessions that the reduction group required to complete reduction and reacquisition testing). A period of saline extinction was subsequently arranged as for the reduction group.
Single Dose Nicotine Pharmacokinetics
This protocol was conducted in rats in the reduction group following the final extinction session (i.e., from ≈ 10:00 – 11:00 hr). Each rat was anesthetized as described above and a sampling catheter (PE 50 tubing) was placed in the left femoral vein. A single bolus dose of 0.1 mg/kg nicotine was then administered via the existing jugular catheter. This dose was used because it is well-tolerated in anesthetized rats and produces readily measured serum nicotine concentrations (e.g., Satoskar et al. 2003). Blood sampling occurred 15, 30, 60, 120, 180, and 240 min after the nicotine dose via the femoral catheter for measurement of serum nicotine concentrations. These time points encompass ≈ 4 nicotine elimination half-lives and are suitable for calculating nicotine pharmacokinetic parameters (Keyler et al. 1999; Keyler et al. 2005; Harris et al. 2008).
Nicotine Analysis and Estimation of Nicotine Pharmacokinetic Parameters
Serum nicotine concentrations were measured by gas chromatography with nitrogen-phosphorous detection (Jacob et al. 1981; Hieda et al. 1999). Pharmacokinetic parameters were estimated using noncompartmental methods from individual concentration-time data using WinNonlin version 4.1 (Pharsight, Mountain View, CA) as described in Harris et al. (2008).
Data Analysis
Effects of Dose Reduction on Daily Rates of NSA and Total Daily Nicotine Intake (Primary Outcomes)
Daily infusion rates in the reduction group during baseline (mean of the final five sessions prior to reduction) and each of the first and final five sessions of reduction, as well as infusion rates during a comparable period of testing in the control group (see below), were analyzed using a two-way analysis of variance (ANOVA) with group and session as factors. Bonferroni's post hoc tests were used for between-group comparisons at each session. Infusion rates across sessions for each group were also compared to baseline using a one-way repeated measures ANOVA followed by Dunnett's post hoc tests. Data used for the control group were the average daily infusion rate during the final five days prior to achievement of stability (i.e., “baseline”), as well as each of sessions 1– 5 and 13–17 following achievement of stability. These sessions were used because they coincide with the first and last 5 sessions of the reduction schedule, the average length of which was 17 days.
Total daily nicotine intake (mg/kg/day) in the reduction group during baseline and the mean of the final five days of reduction, as well as total daily nicotine intake during comparable periods in the control group, were analyzed using two-way ANOVA with group and test phase (i.e., baseline or reduction) as factors. Bonferroni's post hoc tests were used for between group comparisons at each test phase. Total daily nicotine intake in the reduction group during baseline and reduction was compared using a paired samples t-test. A single-sample t-test was also used to compare total daily nicotine intake during reduction to a theoretical mean of 0.85 mg/kg/day, which represents the predicted total daily nicotine intake if intake decreased proportionally to unit dose (i.e., no compensation had occurred).
Effects of Dose Reduction on Within-Session Rates of NSA (Secondary Outcome)
To examine compensation during different times of day, within-session infusion rates in the reduction group were first separated into 2-hr blocks during baseline and the mean of the final five days of reduction. A 2-hr block interval was used because it best reflects the circadian patterns of behavior evident in cumulative response records of 23 h/day NSA (see Harris et al. 2007). These data were analyzed using a repeated measures ANOVA with day and 2-hr block as factors, followed by Bonferroni post hoc tests comparing infusion rates at each 2-hr block during baseline and reduction.
Compensation Index and Correlates
Compensation is typically measured in humans using a formula that compares decreases in nicotine exposure (intake) with decreases in cigarettes smoked per day or cigarette nicotine yield (Scherer 1999; Hughes and Carpenter 2005). To simulate this approach in the current model, a Compensation Index (CI) for each rat in the reduction group was calculated using the formula: 1 – (% decrease in total daily nicotine intake following reduction / % decrease in nicotine unit dose following reduction). A CI of 0 indicates no compensation (i.e., total daily nicotine intake decreased proportionally to the reduction in unit dose), while a CI of 1.0 indicates full compensation (i.e., total daily nicotine intake was unchanged following reduction in unit dose). To confirm whether the degree of compensation using this measure was statistically significant, the mean CI was compared to a theoretical mean of 0 using a single-sample t-test.
Linear regression (for normally distributed data) or Spearman's correlation coefficient (for non-normally distributed data) was used to examine the relationship between each rat's CI and baseline behavioral and pharmacokinetic variables. Baseline behavioral correlates of interest were the daily infusion rate and Diurnal Index (i.e., proportion of total infusions taken during the light (inactive) phase of the session). A Diurnal Index less than 0.5 indicates that the majority of infusions were taken during the dark (active) phase of baseline sessions. Pharmacokinetic correlates of interest were the nicotine volume of distribution, clearance, and half-life determined at the end of the experiment. Pharmacokinetic data were not available for one rat. However, all behavioral data for this animal have been included in the current analyses.
Relationship Between Nicotine Pharmacokinetic Parameters and Baseline Infusion Rates
As a secondary analysis, the relationship between nicotine pharmacokinetic parameters and both daily and within-session baseline infusion rates were analyzed using linear regression or Spearman's nonparametric correlation coefficient, where appropriate.
Reacquisition of NSA at the 0.06 mg/kg/inf unit dose
Daily infusion rates in the reduction group during each of the first 10 sessions of reacquisition of NSA at the training dose, as well as infusion rates during a comparable period in the control group (i.e., sessions 18–27 following achievement of stability), were computed as percent of baseline and compared using a two-way ANOVA with group and session as factors.
Extinction
Daily infusion rates in the reduction group during the final five days of reacquisition at the 0.06 mg/kg/inf unit dose (referred to as “pre-extinction” in this analysis) and each day of extinction, as well as infusion rates in the control group during a comparable period (i.e. sessions 28–37), were analyzed using a two-way ANOVA with group and session as factors. “Extinction burst“ magnitude was calculated as both the change in overall (i.e, 23 hr) infusion rate (“between-session extinction burst”) and the change in infusion rate during the first 2 hr of the session (“within-session extinction burst”) between pre-extinction and extinction day 1. The number of days to “early extinction” (defined as >50% decrease in infusion rate compared to pre-extinction for two consecutive sessions; see Harris et al. 2007; Harris et al. 2008), was also computed for each rat in the reduction group. The relationship between these measures and CI was examined using linear regression. To examine whether initial rates of responding during extinction were related to initial rates of responding during reduction, linear regression was used to examine the relationship between change in infusion rate (compared to pre-extinction) during the first 2 hrs of testing on extinction day 1 (i.e., the within-session extinction burst, see above) and change in infusion rate (compared to baseline) during the first 2 hrs of testing on the first day of reduction.
Results
Effects of Dose Reduction on Daily Rates of NSA and Total Daily Nicotine Intake (Primary Outcomes)
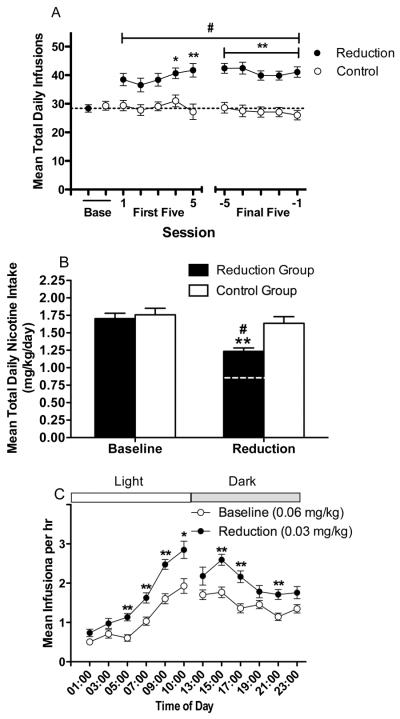
Analysis of daily infusion rates during baseline and reduction indicated significant effects of group (F (1, 330) = 15.5, p < .0001) and session (F (10, 330) = 3.9, p < .0001), and a significant interaction (F (10, 330) = 5.8, p < .0001). Infusion rates were significantly higher in the reduction group than in the control group beginning on the fourth day of reduction (Fig 2A, p < .05 or .01). There was no effect of session in the control group, but a significant effect in the reduction group (F (10, 240) = 12.7, p < .0001), with infusion rates significantly increased compared to baseline on all days of reduction testing (Fig 2A; p < .01).
Fig 2.
(A) Mean (±SEM) total number of infusions earned per daily session during baseline (0.06 mg/kg/inf) and the first and final five days of reduction (0.03 mg/kg/inf) in the reduction group. Daily infusion rate during a comparable period for the control group (0.06 mg/kg/inf throughout) is also shown. *,** Significantly different from control group, p < 0.05, 0.01. #Significantly different from baseline, p < 0.01. (B) Mean (±SEM) total daily nicotine intake (mg/kg/day) during baseline and the final five days of reduction in the reduction group and a comparable period in the control group. Dotted line represents predicted total daily nicotine intake for the reduction group if intake had decreased proportionally to reduction in nicotine unit dose (i.e., no compensation had occurred). ** Significantly different from baseline and control group, p < 0.01. #Significantly different from predicted total daily nicotine intake if no compensation had occurred, p < 0.01. (C) Mean (±SEM) hourly infusion rate per 2 hr block during baseline and the final five days of reduction in the reduction group. Light = light phase of the light/dark cycle. Dark = dark phase of the light/dark cycle. The break in the graph represents the 1 hr cage maintenance period from 10:00 – 11:00 hr (see text). *,** Significantly different from baseline at that 2 hr block, p < 0.05, 0.01.
There were significant effects of group (F (1, 33) = 4.1, p < .05) and test phase (i.e. baseline or reduction) (F (1, 33) = 53.1, p < .0001) on total daily nicotine intake and a group by test phase interaction (F (1, 33) = 18.2, p < .0001). During reduction, nicotine intake in the reduction group was lower than in the control group (Fig 2B; p < .01) and was also lower than during baseline (t (24) = 10.7, p < .01). However, decreases in nicotine intake following reduction were proportionally less than the decrease in unit dose (Fig 2B; t(24) = 7.9, p < .01), indicating partial compensation.
Effects of Dose Reduction on Within-Session Rates of NSA (Secondary Outcome)
There were significant effects of test phase (i.e. baseline or reduction) (F (1, 24) = 239.1, p < .0001) and 2 hr block (F (11, 264) = 30.3, p < .0001) on within-session infusion rates, and a significant interaction (F (11, 264) = 2.8, p < .01). Within-session infusion rates during reduction were significantly increased compared to baseline at numerous 2 hr blocks across the session (Fig 2C: p < .01 or 0.05).
Compensation Index and Correlates
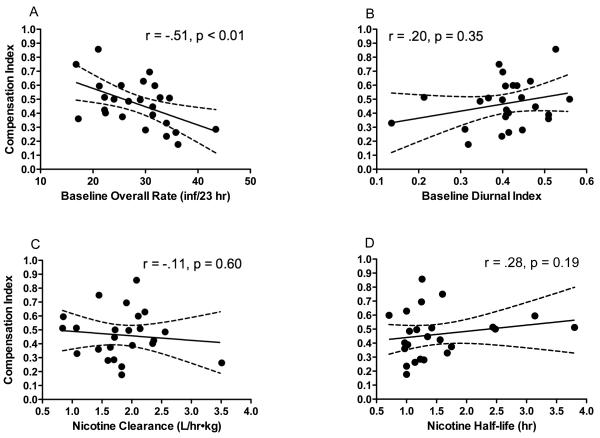
The average Compensation Index (CI) for rats in the reduction group (± SEM) was 0.47 (± 0.03) (range = 0.18 to 0.86). This value was significantly greater than 0 (a CI representing no compensation; t(24) = 14.0, p < .0001), yet was less than 1.0 (a CI representing complete compensation). Thus, partial compensation was generally observed.
There was a significant negative correlation between baseline daily infusion rate and CI (r=−0.51, p < 0.01) (Table 1, Figure 3A). Rats with the highest baseline infusion rates exhibited the lowest levels of compensation during reduction. Baseline Diurnal Index and nicotine pharmacokinetic parameters were not significantly correlated with CI (Table 1, Fig 3B – 3D).
Table 1.
Baseline behavioral and pharmacokinetic measures for rats in the reduction group. Correlation coefficients between these measures and the Compensation Index are also shown.
| Measure | Mean ± SEM (Range) | Compensation Index |
|---|---|---|
| Baseline behavior | ||
| Daily Infusion Rate | 28.4 ± 1.3 (16.8 to 43.4) | r = −0.51** |
| Diurnal Index | 0.41 ± 0.02 (0.14 to 0.56) | r = 0.20, p = 0.35 |
|
| ||
| Pharmacokinetics | ||
| Volume of Distribution (l/kg) | 3.4 ± 0.2 (1.9 to 5.4) | r = 0.23, p = 0.28 |
| Clearance (l/h•kg) | 1.8 ± 0.1 (0.8 to 3.5) | r = −0.11, p = 0.60 |
| Half-Life (h) | 1.5 ± 0.2 (0.7 to 3.8) | r = 0.28, p = 0.19 |
Significantly correlated, p < 0.01.
Fig 3.
Correlation between each rat's Compensation Index (CI) and baseline daily infusion rate (A), baseline Diurnal Index (B), nicotine clearance (C), and nicotine half-life (D). Figures 3A – 3D also illustrate the considerable degree of between-subject variability associated with compensation in this model.
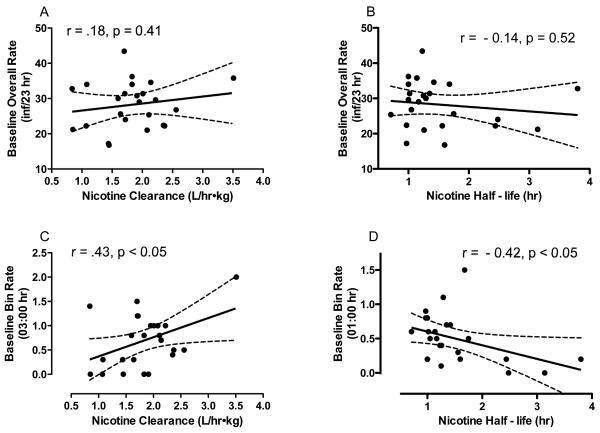
Relationship Between Nicotine Pharmacokinetic Parameters and Baseline Infusion Rates
Daily baseline infusion rates were not significantly correlated with nicotine clearance (Fig 4A), half-life (Fig 4B), or volume of distribution. Clearance was positively correlated with baseline within-session infusion rates during the 2 hr block ending at 03:00 (r = .43, p <.05; Fig 4C) and half-life was negatively correlated with baseline rates during the 2 hr block ending at 01:00 (r = −.42, p <.05; Fig 4D). Thus, faster nicotine elimination was associated with higher infusion rates during a period of the inactive phase when infusion rates were lowest (see Fig 2C). There were no other significant relationships between pharmacokinetic parameters and baseline within-session infusion rates.
Fig 4.
Correlation between each rat's daily baseline infusion rate and nicotine clearance (A) and half-life (B). Also shown is the correlation between each rat's baseline infusion rate at the 2 hr block ending at 03:00 hr and nicotine clearance (C), as well as the relationship between each rat's baseline infusion rate at the 2 hr block ending at 01:00 hr and nicotine half-life (D).
Reacquisition of NSA at the 0.06 mg/kg/inf unit dose
There was no significant effect of group, session, or group by session interaction on infusion rates (expressed as percent of baseline) in the reduction and control groups during baseline and reacquisition (Fig 5A). Nevertheless, infusion rates in several rats in the reduction group were above baseline range on the first day of reacquisition testing.
Fig 5.
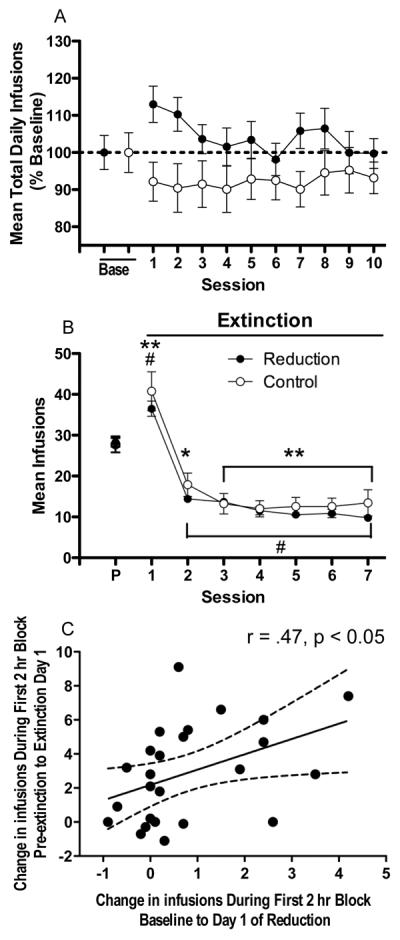
(A) Mean (±SEM) number of infusions earned per session during baseline and reacquisition of NSA (0.06 mg/kg/inf) in the reduction group and a comparable period in the control group, expressed as percent of baseline. Data from the intervening sessions are not shown. (B) Mean (±SEM) number of infusions per 23 hr session during pre-extinction (P) and extinction in the reduction and control groups. *,** Significantly different from pre-extinction (control group), p < 0.05, 0.01. # Significantly different from pre-extinction (reduction group), p <0.01. (C) Correlation between change in infusion rate (compared to pre-extinction) during the first 2 hr block on extinction day 1 (i.e., “within-session extinction burst”, see text) and change in infusion rate (compared to baseline) during the first 2 hr block on the first day of reduction.
Extinction
There was a significant effect of session (F(7, 224) = 91.0, p < .0001) but no effect of group or group by session interaction on daily infusion rates in the reduction and control groups (Fig 5B). There were significant effects of session in both the reduction group (F (7, 168) = 104.7, p < .0001) and the control group (F (7, 56) = 5.0, p < .0001), with infusion rates in both groups significantly increased compared to baseline on extinction day 1 (Fig 5B; p < .01) and decreased compared to baseline on subsequent days (p < .05 or 0.01). Stable levels of compensation were not significantly correlated with the between-session extinction burst (mean ± SEM = 8.2 ± 1.8 infusions; range = −15.0 to 29.0 infusions), within-session extinction burst (mean ± SEM = 2.8 ± 0.6 infusions; range = −1.1 to 9.1 infusions), or days to early extinction (mean ± SEM = 5.0 ± 0.3 days; range = 3 – 7 days). There was a significant positive correlation between change in infusion rate during the first 2 hr on the first day of extinction (i.e., the within-session extinction burst) and change in infusion rate during the first 2 hr on the first day of reduction (r = .47, p <.05; Fig 5C). Animals exhibiting greater initial rates of responding during extinction also exhibited greater initial rates of responding during reduction.
Discussion
Reduction in nicotine unit dose resulted in compensatory increases in NSA that occurred across the course of the 23 hr sessions. Compensation was associated with a considerable degree of individual variability (mean Compensation Index ± SEM = 0.47 ± 0.03, range = 0.18 to 0.86). Rats exhibiting the highest baseline infusion rates exhibited the lowest levels of compensation. Nicotine pharmacokinetic parameters were not correlated with compensation. Infusion rates immediately returned to pre-reduction levels when baseline conditions were restored. This study is the first to explicitly and broadly characterize individual differences in compensation following reduction in nicotine unit dose in either humans or animals, and provides some initial insights into potential correlates and consequences of compensation.
The present findings are consistent with previous within-subject studies demonstrating higher infusion rates for moderate rather than high NSA unit doses (e.g., Corrigall and Coen 1989; Donny et al. 1995; Bardo et al. 1999; Watkins et al. 1999; DeNoble and Mele 2006). In both the current and prior studies, increases in infusion rates did not maintain constant levels of nicotine intake across high and moderate unit doses (i.e., compensation was only partial). Compensation is also incomplete when rats are switched from moderate to low NSA unit doses (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006). In contrast, rats self-administering other drugs of abuse (e.g. cocaine) typically maintain similar levels of intake across a wide range of unit doses (e.g, Pickens and Thompson 1968; Yokel and Pickens 1974), consistent with other reports that nicotine intake is less precisely regulated than intake of other drugs (see Lynch and Carroll 1999; 2001).
The considerable degree of between-subject variability in compensation in the current study was exploited in a large sample of rats to examine whether individual differences in compensation were related to certain aspects of baseline NSA and nicotine pharmacokinetics. Higher daily infusion rates during baseline sessions were associated with lower levels of compensation. We have also previously reported that higher baseline infusion rates were associated with less compensation following a reduction in duration of daily access to NSA (Harris et al. 2008). These findings are somewhat consistent with a report that smokers with the highest levels of nicotine intake per cigarette exhibited the lowest levels of compensation (Benowitz et al. 1986), and suggest that baseline nicotine intake may serve as a readily determined predictor of compensation.
Individual differences in nicotine potency may account for the inverse relationship between baseline infusion rates and compensation in this study. Assuming equal nicotine efficacy between rats (i.e., a similar peak in the dose-response curve), those in which nicotine potency is lower (i.e., their dose-response curve is shifted to the right compared to other rats) would exhibit higher infusion rates at the training dose. As such, the training dose would lie closer to the peak of the curve for those rats, resulting in less “potential” to compensate due to a ceiling effect. It is also possible that individual differences in nicotine efficacy (i.e., peak of the dose-response curve) may have played a role. Future studies involving a within-subject assessment of NSA during unit dose reduction across a wide range of doses are necessary to clarify the relative contribution of individual differences in nicotine reinforcing potency versus efficacy in the observed relationship between baseline rates and compensation.
Compensation was not correlated with baseline Diurnal Index or nicotine pharmacokinetic parameters. However, a secondary analysis indicated that faster nicotine elimination was associated with slightly higher within-session infusion rates during the inactive phase of baseline sessions. A potential explanation for this finding is that the greater decline of brain nicotine levels in rats with faster nicotine metabolism may have resulted in a greater degree of nicotine deprivation during this time of day, disrupting sleep and evoking NSA. Such a phenomenon would be consistent with a report that humans with faster nicotine metabolism are more likely to wake up at night to smoke (Mooney et al. 2008). Although preliminary, these data suggest a relationship between individual differences in nicotine pharmacokinetics and NSA in rats, and complement a report that faster nicotine metabolism is associated with greater oral NSA in male mice (Siu et al. 2006).
A potential adverse consequence of tobacco exposure reduction is that the more intensive smoking associated with reduction could continue if unrestricted smoking is resumed, resulting in greater toxin exposure (Shiffman et al. 1998; Hatsukami et al. 2004; Harris et al. 2008). Although some rats in the current study exhibited infusion rates above baseline range on the first day of reacquisition, infusion rates were not significantly increased compared to baseline at any point during reacquisition for rats as a group. This suggests that relapse to smoking regular cigarettes following the use of cigarettes with reduced nicotine yield should not be associated with increased smoke intake in most smokers. Consistent with this prediction, compensatory increases in smoking in individuals switched to reduced nicotine yield cigarettes did not persist when they were switched back to their usual brand (Benowitz et al. 2005).
Rats exhibited a temporary increase in infusion rates on the first day of extinction testing (i.e., an “extinction burst”), which is consistent with previous studies using a lower NSA unit dose (Harris et al. 2007; Harris et al. 2008). The relationship between the extinction burst and compensation was of interest because both phenomena reflect increased drug-seeking behavior following reductions in nicotine availability. Stable levels of compensation were not correlated with rate of extinction or magnitude of the extinction burst measured using either a between- or within-session analysis. However, animals exhibiting greater initial increases in infusion rates on the first day of extinction (i.e., a higher within-session extinction burst) also exhibited greater initial increases in infusion rates on the first day of reduction. Similar mechanisms may therefore mediate drug-seeking during the earliest stages of extinction and reduction.
The fact that the reduction and control groups exhibited similar infusion rates throughout extinction suggests that extinction behavior is not influenced by a history of reduced nicotine exposure. To the extent that response rate during extinction is indicative of the motivation to engage in drug-seeking and relapse during drug abstinence (Markou et al. 1993; Epstein et al. 2006), these findings suggest that exposure to harm reduction interventions should not affect subsequent smoking cessation success. Consistent with this interpretation, studies have shown that smoking cessation success is not compromised by a history of tobacco exposure reduction (Hughes et al. 1999; Carpenter et al. 2004; Hughes and Carpenter 2006).
Certain features of compensation in the current study were similar to features of compensation in humans. The magnitude of compensation in the current model (CI = 0.47) is similar to the magnitude of an analogous measure of compensation in humans smoking cigarettes with reduced nicotine yield (CI = 0.51 averaged across 11 brand-switching studies; Scherer 1999). That is, despite differences in species and procedures, nicotine intake following reduction in both humans and rats was ≈ 50% higher than what would be expected if nicotine intake decreased proportionally to decreases in nicotine yield/unit dose (i.e., no compensation occurred). Compensation developed rapidly in the current study, as infusion rates reached near maximal levels on the first day of reduction (see Fig 2A). Similarly, compensation in smokers switching to cigarettes with reduced nicotine yield reaches maximum levels within days (Russell et al. 1982). Finally, the substantial degree of individual variability associated with compensation in the current study simulates the substantial between-subject differences in compensation seen in smokers (e.g,. Benowitz et al. 1986; Hecht et al. 2004). Similarities between compensation in the current study and compensatory smoking lend support for the validity of the present assay.
The current study provides an initial assessment of a limited number of factors in compensation. Several other variables implicated in tobacco dependence could also play a role including severity of nicotine withdrawal (i.e., level of dependence) (Kenny and Markou 2001; Hughes 2007), individual differences in the ability of nicotine to enhance the reinforcing effects of non-drug reinforcers (Donny et al. 2003; Liu et al. 2007), or individual differences in reactivity to environmental cues associated with nicotine delivery (e.g., Caggiula et al., 2001, 2002; Chaudhri et al., 2005). Certain non-nicotine constituents of tobacco (e.g., acetaldehyde, MAO inhibitors) may also contribute to nicotine reinforcement (e.g., Belluzzi et al. 2005; Guillem et al. 2005; Villegier et al. 2007) and compensation.
The Compensation Index (CI) used in the present study is directly analogous to a measure of compensation in smokers (Scherer 1999; Hughes and Carpenter 2005) and was used to facilitate comparison of the present findings with human studies. Although the CI is not intended to be an index of nicotine reinforcement efficacy, the fact that the current CI indicated partial compensation is consistent with previous studies of the relationship between nicotine dose and measures of nicotine reinforcement. For example, using a behavioral economics approach in a meta-analysis of studies examining the effects of changes in nicotine yield, DeGrandpre et al. (1992) demonstrated that the relationship between nicotine intake and nicotine yield is well described by a non-linear demand curve (r2 typically greater than 0.9). That is, a given change in nicotine yield did not generally result in a proportional change in nicotine self-administration. Therefore, although a simple measure, the current CI provided useful information and provided a suitable measure of compensation for this initial model.
A limitation of the current CI is that it is a transformation of a percent change score, a measure that can be inflated if baseline levels of the dependent variable are extremely low. Such a measurement artifact may have contributed to the inverse relationship between baseline nicotine intake and compensation in this study. However, baseline infusion rates were likely high enough to avoid this effect. Adapting this model to include a range of nicotine unit doses will allow for more sophisticated analytical approaches (e.g., behavioral economic analyses) that avoid this limitation.
While the dose-reduction model simulates only one possible approach to harm reduction (i.e., use of cigarettes with reduced nicotine yield), results were similar to those obtained with an access-reduction model analogous to limiting situations in which cigarettes are smoked (Harris et al. 2008). As in the current study, compensation in the access-reduction model was partial and associated with a considerable degree of individual variability. Higher baseline infusion rates were also associated with less compensation following access reduction, and baseline Diurnal Index and nicotine pharmacokinetics were not major contributors to compensation. These similarities support the generalizability of the current findings.
The current model also differed from the access-reduction model in certain respects. In contrast to the current study, rats exhibited a transient increase in infusion rates compared to pre-reduction levels during the reacquisition phase of the access reduction protocol (Harris et al. 2008), potentially indicating differences in the characteristics of relapse from different harm reduction interventions. These models therefore each provide unique information and together provide a more complete characterization of compensatory smoking than either model alone.
Further work with this and other models may be useful for identifying additional predictors and consequences of compensation and for assessing the potential role of tobacco harm reduction as an approach to managing tobacco dependence in individuals. Characterizing correlates of compensation may also inform tobacco policy. Legislation is currently pending that would provide the FDA regulatory authority over tobacco products that may result in an FDA-mandated reduction in the nicotine content of cigarettes to render them non-addictive (Waxman 2007; Zeller et al. in press). Identifying populations of smokers at greatest risk of compensating following nicotine reduction may facilitate development of strategies to avoid this potential adverse consequence of nicotine regulation.
Acknowledgements
Supported by NIH grants T32 DA 07097 (NIDA), F32 DA021935 (NIDA), and P50-DA013333 (NIDA/NCI) and the Minneapolis Medical Research Foundation Translational Addiction Research Program.
Footnotes
No conflicts of interest.
References
- Abrams DB, Herzog TA, Emmons KM, Linnan L. Stages of change versus addiction: a replication and extension. Nicotine Tob Res. 2000;2:223–9. doi: 10.1080/14622200050147484. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–6. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–48. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Nicotine renal excretion rate influences nicotine intake during cigarette smoking. J Pharmacol Exp Ther. 1985;234:153–5. [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Bernert JT, Wilson M, Wang L, Allen F, Dempsey D. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:1376–83. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315:1310–3. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–4. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–30. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72:371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005 doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited- access schedule. Psychopharmacology. 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. III. A reanalysis of the nicotine regulation hypothesis. Psychopharmacology. 1992;108:1–10. doi: 10.1007/BF02245277. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology (Berl) 2006;184:266–72. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–94. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas AJ, Pierce JP, Zhu SH, Rosbrook B, Gilpin EA, Berry C, Kaplan RM. Addiction versus stages of change models in predicting smoking cessation. Addiction. 1996;91:1271–80. discussion 1281–92. [PubMed] [Google Scholar]
- Frederiksen LW, Frazier M. Temporal distribution of smoking. Addict Behav. 1977;2:187–94. doi: 10.1016/0306-4603(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. Jama. 2005;294:1505–10. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16:86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Lesage MG. Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology (Berl) 2007;194:395–402. doi: 10.1007/s00213-007-0848-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Henningfield JE, Kotlyar M. Harm reduction approaches to reducing tobacco-related mortality. Ann Rev Public health. 2004;25:1–19. doi: 10.1146/annurev.publhealth.25.102802.124406. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Joseph AM, Lesage M, Jensen J, Murphy SE, Pentel PR, Kotlyar M, Borgida E, Le C, Hecht SS. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9(Suppl 4):S537–53. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Allen S, Jensen J, Li S, Le C, Murphy S. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128:2528–37. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Le CT, Zhang Y, Joseph AM, Mooney ME, Carmella SG, Hecht SS. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiol Biomarkers Prev. 2006;15:2355–8. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Slade J, Benowitz NL, Giovino GA, Gritz ER, Leischow S, Warner KE. Reducing tobacco harm: research challenges and issues. Nicotine Tob Res. 2002;4(Suppl 2):S89–101. doi: 10.1080/1462220021000032852. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. 1998;7:281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–7. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction. 2005;100:1074–89. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. 2006;8:739–49. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Cummings KM, Hyland A. Ability of smokers to reduce their smoking and its association with future smoking cessation. Addiction. 1999;94:109–14. doi: 10.1046/j.1360-0443.1999.9411097.x. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Lando H, Carmella SG, Gross M, Bliss R, Le CT, Hatsukami DK. Smoking reduction fails to improve clinical and biological markers of cardiac disease: a randomized controlled trial. Nicotine Tob Res. 2008;10:471–81. doi: 10.1080/14622200801901948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Hieda Y, St Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res. 1999;1:241–9. doi: 10.1080/14622299050011361. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, Lesage MG, St Peter JV, Stewart S, Fuller S, Le CT, Pentel PR. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metab Disposition. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–86. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–89. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology (Berl) 2007;194:463–73. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of intravenously self-administered nicotine in rats. Exp Clin Psychopharmacol. 1999;7:198–207. doi: 10.1037//1064-1297.7.3.198. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–82. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1396–400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SF, Gust SW, Pickens RW, Champagne SE, Hughes JR. Temporal patterns of smoking topography in the natural environment. Int J Addict. 1985;20:613–21. doi: 10.3109/10826088509044940. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, Hawk LW, Tyndale RF, Benowitz N, Lerman C. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–9. [PubMed] [Google Scholar]
- Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9:631–46. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- Russell MA, Sutton SR, Iyer R, Feyerabend C, Vesey CJ. Long-term switching to low-tar low-nicotine cigarettes. Br J Addict. 1982;77:145–58. doi: 10.1111/j.1360-0443.1982.tb01416.x. [DOI] [PubMed] [Google Scholar]
- Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol. 2003;3:957–70. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Tyndale RF, Fernandes LC. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov Today. 2003;8:487–93. doi: 10.1016/s1359-6446(03)02704-1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gitchell JG, Warner KE, Slade J, Henningfield JE, Pinney JM. Tobacco harm reduction: conceptual structure and nomenclature for analysis and research. Nicotine Tob Res. 2002;4(Suppl 2):S113–29. doi: 10.1080/1462220021000032717. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Mason KM, Henningfield JE. Tobacco dependence treatments: review and prospectus. Annu Rev Public Health. 1998;19:335–58. doi: 10.1146/annurev.publhealth.19.1.335. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl) 2006;184:401–8. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007:CD005231. doi: 10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10:189–95. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Nicotine addiction: Health consequences of smoking. DHHS; 1999. [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–25. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–51. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Waxman H. Familiy Smoking Prevention and Tobacco Control Act. House of Representatives. 2007 [Google Scholar]
- Yokel RA, Pickens R. Drug level of d- and l-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–64. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D, Backinger C, Benowitz N, Biener L, Burns D, Clark P, Connolly G, Djordjevic MV, Eissenberg T, Giovino GA, Healton C, Hecht SS, Henningfield JE, Husten C, Kobus K, Leischow S, Levy DT, Marcus S, Myers ML, Parascandola M, Ponkshe P, Shields PG, Slovic P, Sweanor D, Warner KE. The strategic dialogue on tobacco harm reduction: A vision and blueprint for action in the United States. Tob Control. doi: 10.1136/tc.2008.027318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]