Abstract
The objective of this study was to design 1, 3, and 6 month sustained-release poly (lactide-co-glycolide) (PLGA) microspheres of SAR 1118, a lymphocyte function-associated antigen-1 antagonist, using Design of Experiments. A full-factorial design was used to identify the polymers suitable for degradation in 1, 3, and 6 months and the Box-Behnken design was used to study the influence of the polymer type, polymer concentration, and drug to polymer ratio on drug loading, burst release, and particle size. From the full-factorial design, PLGA (50:50), PLGA (75:25), and PLGA (85:15) with an inherent viscosity of 0.3–0.5 dL/g were identified as polymers suitable for degradation in 1, 3, and 6 months, respectively. From the Box-Behnken design, the optimized polymer concentration (12% w/v) and drug to polymer ratio (0.15) were identified and used to prepare the SAR 1118-encapsulated microspheres with the above 3 polymers and evaluated for drug loading, burst release, and sustained drug release. The burst release in these 3 batches was less than 20% and the drug loading ranged from 15%–18%. More than 90% of SAR 1118 release from PLGA (50:50), PLGA (75:25), and PLGA (85:15) microspheres occurred in 1, 3, and 6 months, respectively. Thus, the in vitro cumulative release data are remarkably close to the predicted values. The results demonstrated the potential of the Design of Experiments in designing the SAR 1118 microspheres with a high loading efficiency, low burst release, and sustained release for a desired duration.
Introduction
Sustained drug delivery to the back of the eye is beneficial in treating chronic ocular diseases, such as age-related macular degeneration, diabetic macular edema, and proliferative diabetic retinopathy.1,2 To treat chronic eye diseases, the development of sustained release microspheres based on biodegradable polymers has been of considerable interest.3,4 Poly (lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers, poly(lactic-co-glycolic) acid (PLGA), are biocompatible, biodegradable, and have a long history of clinical use.5 In recent years, PLGA-based implants (Ozurdex®) were approved by the US FDA for sustained delivery of dexamethasone in the vitreous humor of the eye. Injectable microspheres based on these polymers are also of potential value in treating eye diseases.6 Microspheres based on PLGA polymers slowly hydrolyze to lactic acid and glycolic acid, which can be cleared rapidly through further metabolism or excretion.5 From such microspheres, drug release can occur via diffusion or polymer degradation.7 Due to their biodegradable nature, microspheres prepared using these polymers do not require removal from the site of administration after drug release, unlike surgically sutured nondegradable implants.
SAR 1118 is an investigational small molecule lymphocyte function-associated antigen-1 (LFA-1) antagonist. SAR 1118 is a white to off-white solid crystallized from methylethylketone, with a molecular weight of 618.5 Da. Sodium salt of SAR 1118 is freely soluble in water and it is under clinical investigation for the treatment of dry eye as an ophthalmic solution.8 SAR 1118 binds to I-domain of the CD11a subunit of LFA-1 and serves as a competitive antagonist for LFA-1 binding to ICAM-1.9 Our earlier studies indicated that topically administered SAR 1118 inhibits leukostasis and retinal vascular leakage in diabetic rats.10 The purpose of this study was to design and develop slow release microsphere formulations of SAR 1118.
In the development of controlled release microsphere formulations, various factors, such as the polymer type, polymer concentration, and method of preparation, influence the properties of prepared particles, including particle size, encapsulation efficiency, and drug release. Polymer and process parameters can be potentially optimized to control the SAR 1118 microsphere size, while minimizing burst release and enhancing drug loading. The Design of Experiments (DoE) is a software-guided experimental design approach for studying the influence of several factors simultaneously. DoE provides information on the interaction of factors with a limited set of experiments. In addition, DoE fits the response data to mathematical equations and these equations serve as models to predict responses at desired parameter (factor) values. This approach is particularly relevant for identifying the parameter space relevant for a product with specific features.11–14 DoE helps in deriving maximum information from a minimal number of experiments. DoE can be implemented using software programs, such as Statgraphics Plus (Statpoint Technologies, Inc.). A variety of statistical design algorithms, such as factorial designs and Box-Behnken designs, can be employed for DoE. Full-factorial designs support linear responses and are particularly useful when the number of factors is as few as 2. However, the Box-Behnken design is an independent, rotatable or nearly rotatable quadratic design based on a 3-level incomplete factorial design, wherein treatment combinations are at the midpoints of edges of the process space and at the center.15,16 The Box-Behnken design requires at least 3 factors and does not contain combinations for which all factors are simultaneously at their highest or lowest levels. Thus, this design excludes the experimentation at the extreme level. The Box-Behnken designs are widely used in the response surface optimization of drug delivery systems.15,17–19
The present study focused on the development of sustained release formulations with a high drug loading, low burst release, and micron size particles for the delivery of SAR 1118 for 1, 3, and 6 months using the experimental design. First, we identified polymers suitable for 1, 3, and 6 months of slow release using a 2-factor, 3-level, full-factorial design incorporating polymer type (% glycolide content) and polymer viscosity as factors (independent variables) and time for complete polymer degradation as the response (dependent variable). Secondly, a 3-factorial, 3-level Box-Behnken DoE was employed using the glycolide ratio (%), polymer concentration, and drug-to-polymer ratio as factors, and drug loading, burst release (% release in 24 h), and mean particle size as the responses, to identify optimum process parameters. Finally, SAR 1118-encapsulated microspheres suitable for 1, 3, and 6 months were prepared using selected polymers and the optimized process parameters, and characterized for drug loading, particle size, drug form, burst release, and sustained release.
Methods
Materials
SAR 1118 was provided by SARcode Biosciences. PLA and PLGA copolymers with varying lactide:glyclode ratios (50:50, 75:25, and 85:15) of 0.3–0.5 dL/g inherent viscosity were obtained from Lactel Absorbable Polymers. PVA (cold water soluble) of 30,000–70,000 molecular weight was obtained from Sigma-Aldrich Chemical Co. All solvents and other chemicals were of a high-performance liquid chromatography grade and obtained from Fisher Scientific.
Selection of polymers for 1, 3, and 6 months degradation by full-factorial design
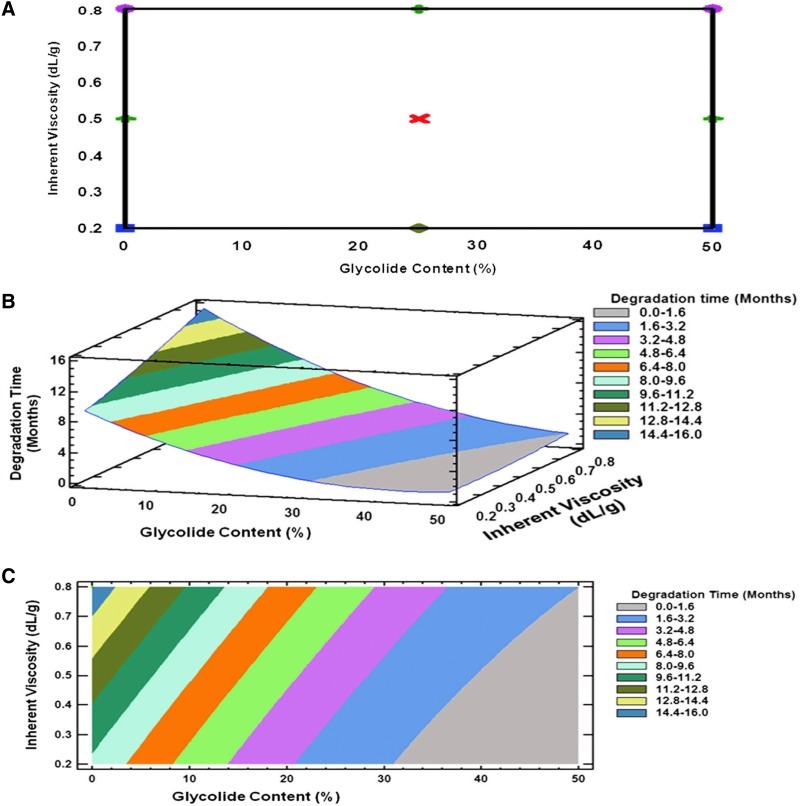
To select polymers with 1, 3, and 6 months degradation time for the development of SAR 1118-encapsulated microspheres, a 3-level, 2 full-factorial design (Statgarphics Centurion XV software, Statpoint Technologies, Inc.) was performed using a glycolide content (%) and polymer inherent viscosity as independent variables and degradation time as the dependent variable. The high and low values of independent variables are given in the Table 1. The degradation times of the various polymers with different glycolide content and inherent viscosity were collected from vendors and the contour plots were constructed (Fig. 1). The contour plots were used to identify the polymers suitable for degradation in 1, 3, and 6 months. The selected polymers were subsequently used for the preparation of SAR 1118-encapsulated microspheres.
Table 1.
The Independent and Dependent Variables Used in the Experimental Designs
| |
|
Levels |
|
|
|---|---|---|---|---|
| Design | Independent variables (Factors) | Low | High | Dependent variables (Responses) |
| Box-Behnken design | Polymer type (% glycolide) | 0 | 50 | Burst release |
| Polymer concentration | 3 | 15 | Drug loading | |
| Drug-to-polymer ratio | 0.1 | 0.2 | Particle size | |
| Full-factorial design | Glycolide content | 0 | 50 | Polymer degradation |
| Inherent viscosity | 0.2 | 0.8 | ||
Full factorial design was used for predicting degradation time based on glycolide content and inherent viscosity poly (lactide-co-glycolide) family of polymers. The Box-Behnken design was used for predicting the influence of % glycolide content, polymer concentration, and drug-to-polymer ratio on drug loading, burst release, and particle size.
FIG. 1.
Experimental design to predict degradation time of poly (lactide-co-glycolide) (PLGA) family of polymers based on percent glycolide content and polymer inherent viscosity. This information was used in the selection of polymers for the preparation of SAR 1118-encapsulated microspheres. Plots show design layout and the response surface and contour plots. (A) 32 full factorial design layout. (B) Response surface plot. (C) Contour plot. Statistical design was generated using the Statgraphics Centurion XV and the relationship between independent and dependent variables was evaluated.
Box-Behnken design to evaluate the effect of process parameters on drug loading, burst release, and particle size
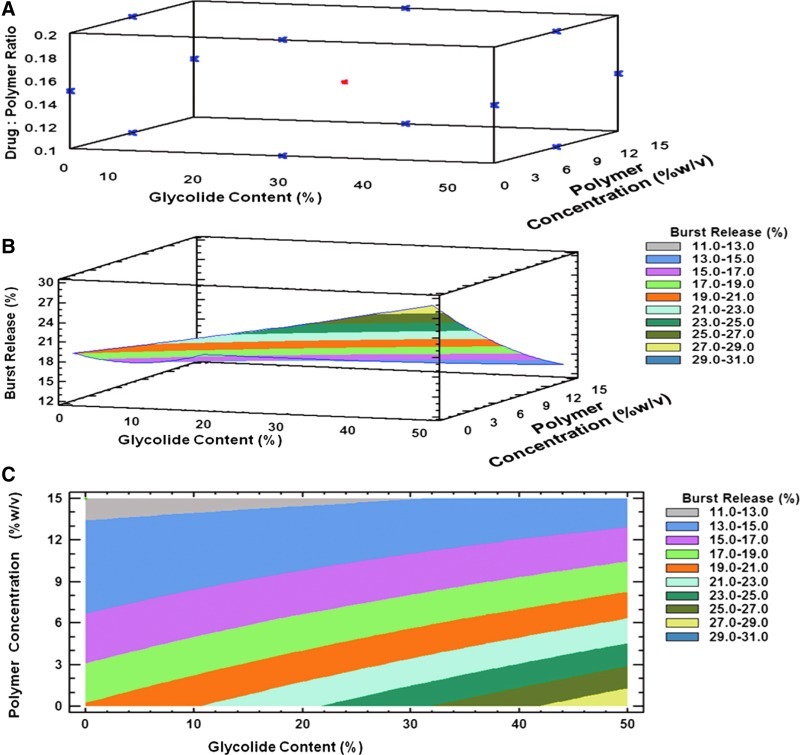
A Box-Behnken statistical design (Statgarphics Centurion XV software, Statpoint Technologies) with 3 levels and 3 factors was designed to optimize the process parameters for obtaining microspheres with high drug loading, low burst release, and optimum particle size. The independent variables and dependent variables used in the design are shown in Table 1. A total of 15 experiments for microsphere preparations were designed by the software and experiments were run in random order. The designs consisted of 3 center point replicates in the cube and the points lying at the midpoint of each edge of the cube that defines the region of interest (Fig. 2 and Table 2). Each response, including drug loading, burst release, and particle size, can be modeled using the following equation based on the Box-Behnken design.
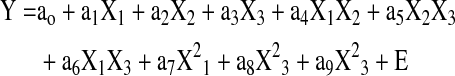 |
FIG. 2.
Box-Behnken design layout and the response surface plots to optimize process parameters for the preparation of SAR 1118-encapsulated microspheres. (A) Box-Behnken design layout with 3 factors (% glycolide, polymer concentration, and drug-to-polymer ratio) and 3 levels (low, medium, and high). (B) Three-dimensional response surface plot. (C) Three-dimensional contour plots showing the effect of % glycolide content (X1) and polymer concentration (X2) on the response variable burst release (Y1).
Table 2.
Box-Behnken Design with 3 Variables and 3 Levels and the Measured Values of Responses
| |
Independent variable |
Dependent variable |
||||
|---|---|---|---|---|---|---|
| Formulation number | Glycolide content (X1;%) | Polymer concentration (X2;% w/v) | Drug-to-polymer ratio (X3) | Burst release (Y1;%) | Drug loading (Y2;% w/w) | Particle size (Y3; μm) |
| 1 | 0 | 3 | 0.15 | 16.8 | 10.5 | 2.4 |
| 2 | 50 | 9 | 0.2 | 18.7 | 10.35 | 3.1 |
| 3 | 25 | 15 | 0.2 | 14.65 | 15 | 2.4 |
| 4 | 50 | 9 | 0.1 | 19.4 | 8.7 | 2.8 |
| 5 | 25 | 3 | 0.1 | 23.6 | 6.4 | 1.6 |
| 6 | 25 | 9 | 0.15 | 15.89 | 11.4 | 1.8 |
| 7 | 25 | 9 | 0.15 | 15.89 | 11.4 | 1.8 |
| 8 | 50 | 15 | 0.15 | 13.8 | 12.4 | 3.1 |
| 9 | 25 | 3 | 0.2 | 19.65 | 6.8 | 2.4 |
| 10 | 0 | 9 | 0.2 | 16.4 | 15.1 | 2.7 |
| 11 | 25 | 9 | 0.15 | 15.89 | 11.4 | 1.8 |
| 12 | 0 | 15 | 0.15 | 12.8 | 11.6 | 3.7 |
| 13 | 50 | 3 | 0.15 | 24.9 | 4.5 | 3.5 |
| 14 | 0 | 9 | 0.1 | 13.8 | 8.7 | 3.2 |
| 15 | 25 | 15 | 0.1 | 12.7 | 9.2 | 3.6 |
This design was used to determine the influence of glycolide content, polymer concentration, and drug-to-polymer ratio on particle size, drug loading, and burst release for SAR 1118 microspheres.
where Y is the measured response based on independent variables X1, X2, and X3; ao to a9 are the regression coefficients; and E is the error term. The 3-level and 3-factor Box-Behnken design was run and the relationship between the independent and dependent variables were elucidated using mathematical equations and the coefficient values for the factors and the P-values were determined. P-values less than 0.05 were considered to exert a significant effect, with a positive coefficient for a factor indicating an agonistic effect and a negative value indicating an antagonistic effect.
SAR 1118-encapsulated microsphere preparation
SAR 1118-encapsulated microspheres were prepared using the o/w emulsion solvent evaporation method. For preparing microspheres, the polymer (100 mg) was dissolved in 1.5 mL of ethyl acetate and SAR 1118 was dispersed in the polymer solution and sonicated for 20 s (3 W; Misonix S 3000; Qsonica). The drug polymer dispersion was transferred to 10 mL of 2% aqueous polyvinyl alcohol solution (4°C) under homogenization at 10,000 rpm for 2 min using a VirTishear Cyclone® Homogenizer (SP Scientific). The above 2% aqueous polyvinyl alcohol solution containing the drug polymer dispersion was further transferred to 80 mL of 2% aqueous polyvinyl alcohol solution (4°C) and homogenized at 15,000 rpm for 5 min using a VirTishear Cyclone® Homogenizer. The final emulsion was stirred on a magnetic stirrer for 3 h at room temperature. The microspheres formed were centrifuged at 15,000 rpm (Sorvall RC 6 plus centrifuge, Thermo Scientific) for 20 min. The pellet of microspheres was washed twice with 50 mL distilled water each time. The final pellet was dispersed in 10 mL distilled water and lyophilized (Freezone 2.5 plus, Labconco Corporation) over 24 h. The prepared drug-loaded microspheres were characterized for drug loading, particle size, and burst release.
Based on the above studies, PLGA (50:50), PLGA (75:25), and PLGA (85:15) of inherent viscosity 0.3–0.5 dL/g and a polymer concentration of 12% w/v and a drug to polymer ratio of 0.15 were selected for preparing 1, 3, and 6 months sustained release microspheres. These optimized SAR 1118-encapsulated microspheres were characterized and assessed for complete in vitro release.
Characterization
Particle size
The SAR 1118-encapsulated microspheres were characterized for mean particle size using the dynamic light scattering measurement (Zetasizer Nano ZS, Malvern). The particles were dispersed in distilled water and measurements were taken in triplicate. The mean particle size and distribution of the 3 optimized batches of SAR 1118-encapsulated microspheres were also measured using micro-flow imaging (MFI DPA 4100; ProteinSimple).
Drug loading
The drug loading in microspheres was estimated by dissolving the drug-loaded particles (2–3 mg) in 5 mL of acetonitrile and vortexing until the microspheres were dissolved. The amount of SAR 1118 was estimated using a UV spectrophotometer set at a wavelength of 262 nm.
Evaluation of burst release
The burst release (release in 24 h) of SAR 1118 from encapsulated microspheres was determined in phosphate-buffered saline (PBS), pH 7.4. Weighed SAR 1118-encapsulated microspheres (5–10 mg) were dispersed in 1 mL of PBS pH 7.4 buffer containing 0.05% w/v sodium azide as a preservative. The dispersed particles were filled into a dialysis bag (Spectrapore, 3000 MWCO; Fisher Scientific) and the bag was placed in 25 mL of PBS pH 7.4 containing 0.05% w/v sodium azide and incubated at 37°C, while stirring at 200 rpm. After 24 h, drug release in the external PBS was estimated using the UV spectrophotometer. Burst release was estimated as the percent of loaded drug released in 24 h.
Differential scanning calorimetry
Optimized microspheres of SAR 1118 using PLGA (50:50), PLGA (75:25), and PLGA (85:15) were characterized by differential scanning calorimetry. Thermograms were recorded using Perkein Elmer Diamond DSC between 0 and 200°C under inert nitrogen atmosphere at a flow rate of 20 mL/min and at a heating rate of 10°C/min.
Surface morphology of SAR 1118 microspheres
Surface morphology of the SAR 1118 microspheres was visualized using a scanning electron microscope (LVSEM; JEOL) at different magnifications ranging from 1,000× to 5,000×. Microspheres were mounted on metal stubs using a double-sided adhesive tape. The microspheres were vacuum coated with a thin layer of gold and observed under a microscope at 5 kV.
Evaluation of in vitro drug release
The cumulative in vitro release of SAR 1118 from the 3 optimized batches was determined similar to the burst release studies for DoE batches, except that 1 mL of external PBS medium was removed periodically until nearly complete drug release occurred. Further, after each sample collection, 1 mL of blank PBS was replenished to the release compartment. An equivalent amount of plain SAR 1118 was also taken in a dialysis bag and evaluated for release under similar conditions. In the release study, the agitation of 200 rpm was selected for consistency with our earlier studies.13 The drug content in the samples was estimated using a UV spectrophotometer. All in vitro studies were carried out in triplicate.
Results
Selection of polymers by full-factorial design
A full-factorial design was applied in this study to select the polymers for preparing the SAR 1118-encapsulated microspheres with the desired in vitro release profile. The response surface plots based on the design were plotted in 3-dimensional graphs and used for the selection of polymers with a desired degradation time. The mathematical equation relating the influence of the glycolide content (B1) and inherent viscosity (B2) to the degradation time (A) is given below.
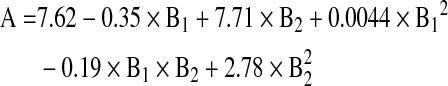 |
The coefficient values and contour plots (Fig. 1) indicate the effect of the glycolide content and inherent viscosity on polymer degradation. The positive coefficient value for B2 indicates that degradation slows down with an increase in inherent viscosity. The negative coefficient for B1 indicates that degradation is expedited with an increase in the glycolide content. The higher coefficient value for B2 (7.71) compared to B1 (0.35) indicates that inherent viscosity has a greater influence on polymer degradation time. Based on the response surface and contour plots, the polymers PLGA (50:50), PLGA (75:25), and PLGA (85:15) with an intrinsic viscosity of 0.3–0.5 dL/g were selected for preparing optimal slow release SAR 1118 microspheres capable of releasing the drug for 1, 3, and 6 months, respectively. A term comprising product of 2 factors represents an interaction term. Second order terms comprising a factor indicate a nonlinear relationship between the response and the factor.18
Optimization of process parameters for drug loading, burst release, and mean particle size
The Box-Behnken design was successfully used to optimize the process parameters for obtaining microparticles with low burst release and high drug loading. A total of 15 experiments were run in random order and the observed responses for drug content, burst release, and mean particle size are presented in Table 2. The Box-Behnken design layout and the response surface plots indicating the relationship between independent and dependent variables are shown in Figs. 2–4. The burst release varied between 13%–25% and the drug content ranged from 5% to 15% w/w, indicating that the formulation variables influence these parameters. The mean particle size of all the prepared microspheres was in the range of 1.6–4.1 μm. The model generated the following regression equations relating the independent variables glycolide content (X1), polymer concentration (X2), drug to polymer ratio (X3), and their combinations to the measured responses burst release (Y1), drug loading (Y2), and particle size (Y3).
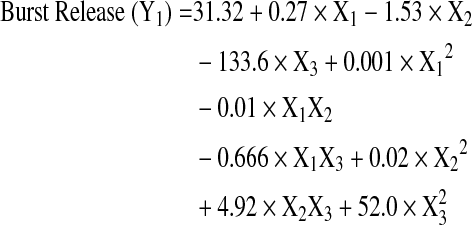 |
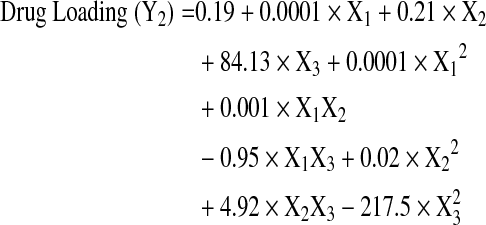 |
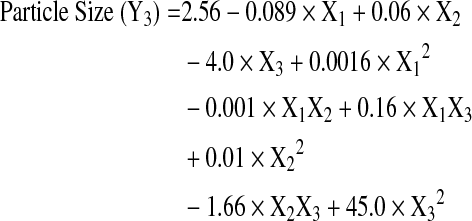 |
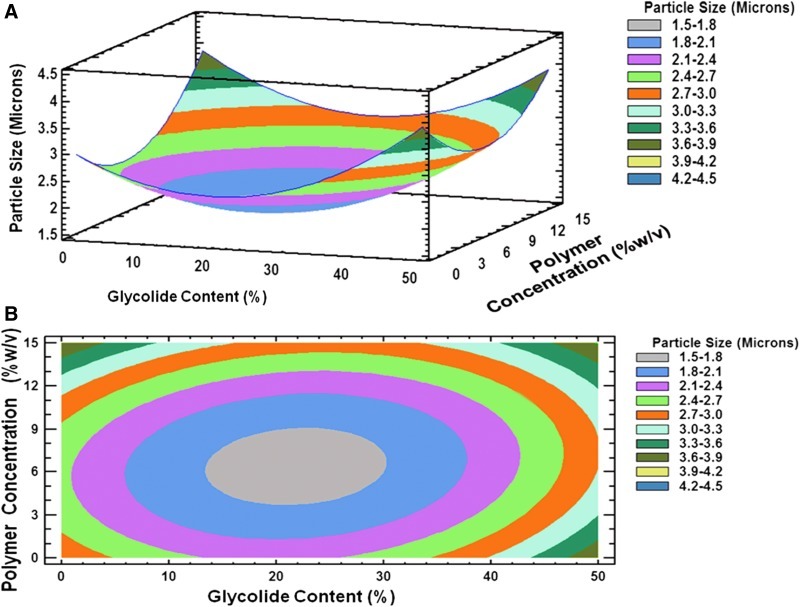
FIG. 4.
(A) Three-dimensional response surface plot and, (B) contour plot showing the effect of % glycolide content (X1) and polymer concentration (X2) on mean particle size (Y3).
The regression coefficients and probability values for various coefficients based on a quadratic model for drug loading, burst release, and mean particle size are listed in Table 3. The quadratic models show excellent fit for the burst release, drug loading, and particle size responses as demonstrated by high correlation coefficient values (R2 of 0.99).
Table 3.
The Coefficients of the Quadratic Models to Predict Particle Size, Drug Loading, and Burst Release
| Response Model | Burst release (Y1;%) Regression coefficient | P-value | Drug loading (Y2;%w/w) Regression coefficient | P-value | Particle size (Y3; μm) Regression coefficient | P-value |
|---|---|---|---|---|---|---|
| Coefficient | 31.32 | 0.19 | 2.56 | |||
| X1 | 1.53 | 0.0003 | 0 | 0.0005 | −0.089 | 0.077 |
| X2 | 0.27 | <0.0001 | 0.21 | <0.0001 | 0.06 | 0.002 |
| X3 | −133.6 | 0.9592 | 84.13 | 0.0001 | −4 | 0.4169 |
| X12 | 0 | 0.4135 | 0 | 0.5548 | 0.001 | 0.0004 |
| X1×2 | −0.01 | 0.0029 | 0.01 | 0.0006 | −0.001 | 0.2042 |
| X1×3 | −0.66 | 0.0539 | −0.95 | 0.0028 | 0.16 | 0.1562 |
| X22 | 0.02 | 0.0499 | −0.04 | 0.0012 | 0.01 | 0.0053 |
| X2X3 | 4.92 | 0.0065 | 4.5 | 0.0016 | −1.6 | 0.0087 |
| X32 | 352 | 0.0499 | −217.5 | 0.0622 | 45 | 0.4086 |
| R2 | 0.99 | 0.99 | 96.7 | |||
| R2 (Adj) | 0.97 | 0.98 | 90.77 |
P<0.05 represents significance and significant coefficient values are marked in bold.
Surface morphology of the SAR 1118 microspheres
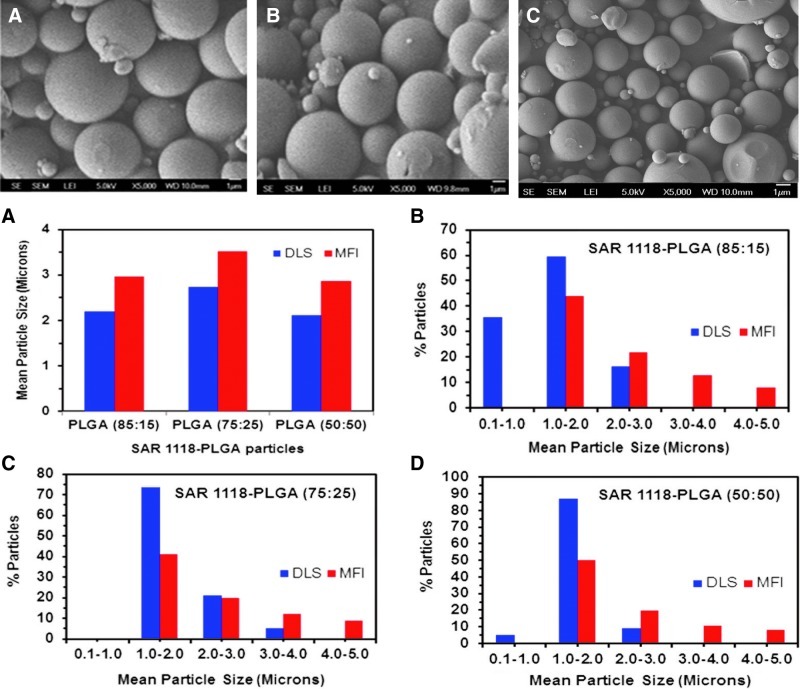
The scanning electron microscopic pictures of the 3 optimized SAR 1118-loaded PLGA microsphere batches are shown in Fig. 5. The microspheres prepared were spherical and had smooth surfaces. A similar appearance was observed for all 3 batches.
FIG. 5.
(1) Scanning electron microscopy images showing the external surface structure of poly (lactide-co-glycolide) (PLGA) microspheres. Pictures were obtained using a scanning electron microscopy (JEOL). (A) PLGA (85:15), (B) PLGA (75:25), and (C) PLGA (50:50). (2) Mean particle size and distribution data of SAR 1118-encapsulated PLGA microspheres measured using dynamic light scattering and micro-flow imaging techniques. (A) Mean particle size of optimal batches. Particle size distribution of (B) SAR 1118-PLGA (85:15) microspheres, (C) SAR 1118-PLGA (75:25) microspheres, and (D) SAR 1118-PLGA (50:50) microspheres.
Differential scanning calorimetry for the SAR 1118-loaded PLGA microspheres
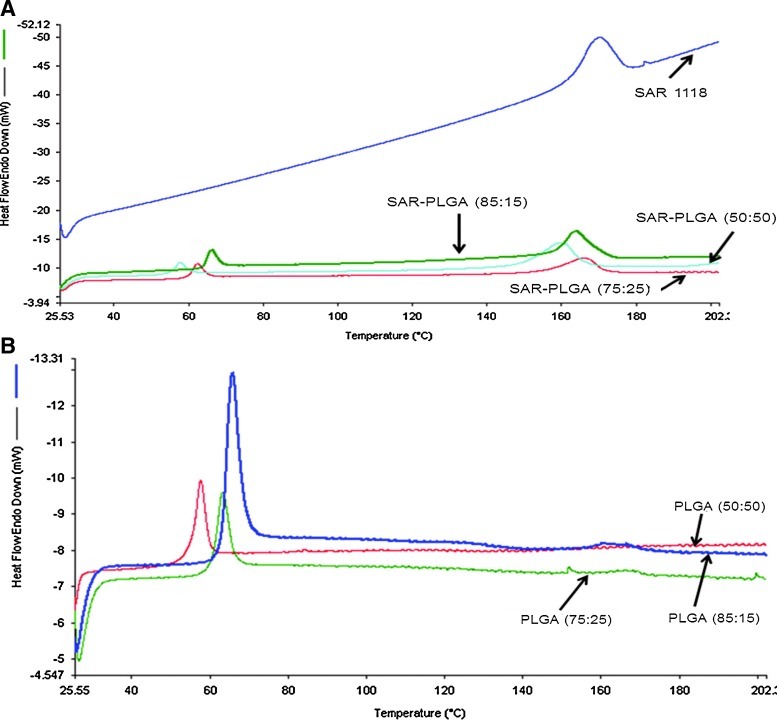
The thermograms of the SAR 1118, SAR 1118-encapsulated PLGA microspheres, and drug-free PLGA microspheres are presented in Fig. 6. The thermograms indicated a distinct melting point of SAR 1118 at 155°C–160°C and after encapsulation into microspheres, the melting point of SAR 118 was slightly reduced to 150°C–155°C. The thermograms of SAR 1118-encapsulated PLGA microspheres have shown additional glass transition temperature of PLGA polymers at 55°C–60°C, 60°C–65°C, and 65°C–70°C for PLGA (50:50), PLGA (75:25), and PLGA (85:15), respectively. The thermograms have confirmed the crystalline nature of the SAR 1118 and indicated that encapsulation slightly changes the crystalline nature of the drug.
FIG. 6.
Differential scanning calorimetry thermograms of (A) SAR 1118 and SAR 1118-encapsulated PLGA microspheres and (B) drug-free PLGA microspheres. Thermograms were recorded between 0 and 200°C under inert nitrogen atmosphere at a flow rate of 20 mL/min and at a heating rate of 10°C/min using the Perkin Elmer, Diamond DSC with hyper-DSC™.
In vitro drug release of SAR 1118 from PLGA microspheres
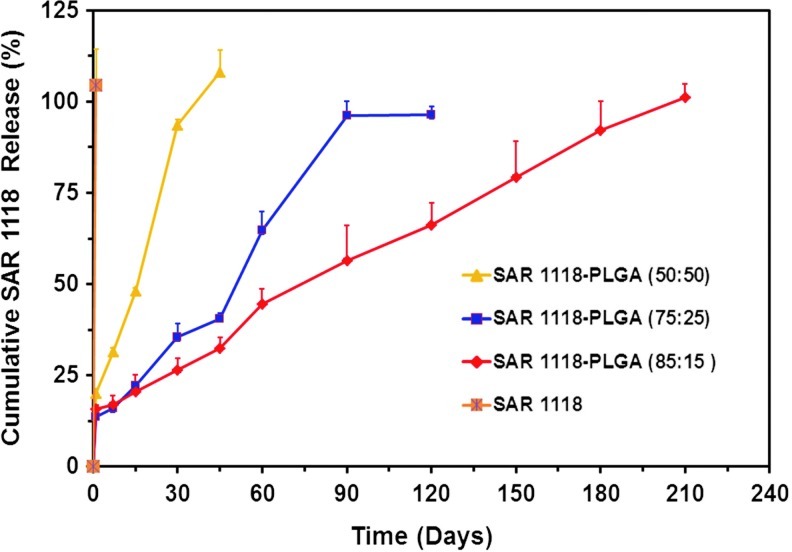
In vitro release of SAR 1118 from PLGA (50:50), PLGA (75:25), and PLGA (85:15) microspheres is shown in Fig. 7. The formulations showed a burst release in the range of 14–20%, with the highest burst release occurring with PLGA (50:50) microspheres. The microspheres prepared using PLGA (50:50) showed 90% release in 1 month and complete release in 1.5 months. Microspheres prepared with PLGA (75:25) showed 90% release in 3 months and complete release of SAR 1118 in 4 months. Microspheres prepared with PLGA (85:15) exhibited 90% release in 6 months. The plain SAR 1118 release from the dialysis membrane was observed to be complete by the end of day 1.
FIG. 7.
In vitro drug release from 3 optimized microsphere formulations of SAR 1118. The release study was conducted in phosphate-buffered saline at 37°C. Data is expressed as mean±SD for n=3.
Discussion
In the present study, we prepared SAR 1118-encapsulated sustained release microspheres with low burst release and high drug loading using the full factorial and Box-Behnken statistical designs along with the response surface methodology. A variety of lactic and glycolic acid polymers and their copolymers with different degradation times are commercially available. Recently, a PLGA (50:50)-based intravitreally injectable implant20,21 capable of sustaining the release of dexamethasone was approved by the US FDA for human use. Given the earlier success of this PLGA polymer, in the present study, we used the DoE approach in selecting PLGA polymers capable of degrading in 1, 3, and 6 months. Although the release of drug from the microspheres depends on various factors, such as the geometry of the device, drug properties, and particle preparation method, polymer degradation is a critical contributing factor. Although the above factors, including device geometry, can influence the degradation of the delivery system, the polymer glycolide content and its intrinsic viscosity or molecular weight are critical factors influencing polymer degradation in a delivery system.21 Therefore, the initial objective was the use of DoE for the selection of PLGA polymers suitable for SAR 1118 microsphere preparation. The surface response contour plots from the full factorial design have indicated a clear dependence of polymer degradation on the glycolide content and inherent viscosity (Fig. 1). From the contour plots, it was observed that the polymers PLGA (50:50), PLGA (75:25), and PLGA (85:15) with an intrinsic viscosity of 0.3–0.5 dL/g were suitable for the microsphere formulations capable of degrading in 1, 3, and 6 months, respectively. Microspheres made using such polymers will likely sustain SAR 1118 release for 1, 3, and 6 months.
In the present study, the variables in the preparation of SAR 1118-encapsulated microspheres, polymer concentration, glycolide content in polymer, and drug to polymer ratio were optimized using the Box-Behnken experimental design with an objective of achieving high drug loading, low burst release, and micron size particles. The relationship between the dependent and independent variables in optimizing the microspheres are depicted in the contour plots and response surface plots shown in Figs. 2–4. These plots identify the design space that is useful in selecting values of independent variables suitable for desired response values. From Fig. 2, it is clear that the glycolide content and polymer concentration influence the burst release of SAR 1118 from the microspheres. Further, based on the coefficient values, the burst release increases with an increase in the glycolide content (coefficient=+0.27) and decreases with an increase in the polymer concentration (coefficient=−1.53), with the polymer concentration being more influential. In an earlier report, it was shown that highly porous microspheres were obtained with a low polymer concentration compared to the higher polymer concentration, when visualized using confocal microscopy.22 By increasing the polymer concentration, the viscosity will also be increased, reducing the tendency of the drug to be carried toward particle surface, and hence, burst release. With an increase in the glycolide content in PLGA, the polymer takes up more water from the release medium, which in turn, might explain the higher burst release23 observed for SAR 1118 microspheres with the higher glycolide content.
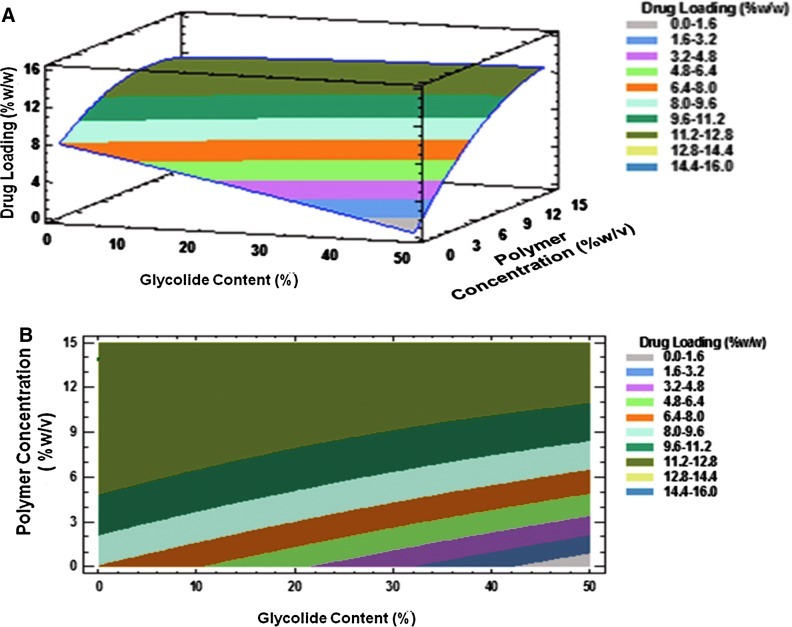
Drug loading is another important factor to be considered in the development of microsphere formulations, with higher drug loading reducing the administered polymer content. Similar to the burst release, all the studied factors and their interaction terms have shown statistically significant influence on the drug loading response. The polymer concentration, drug to polymer ratio, and the product of glycolide content and drug-to-polymer ratio positively influenced drug loading. The influence of the polymer concentration and glycolide content on drug loading is shown in Fig. 3. Relative to the glycolide content (coefficient value=0.0001), the influence of the polymer concentration (coefficient value=0.21) on drug loading was dominant. It was noticed that the drug loading increased with an increase in the polymer concentration. This can be explained by the increased viscosity of the organic phase at a higher polymer concentration, which might result in a denser internal structure that prevents the drug diffusion across the phase boundary during the evaporation process. Moreover, the high polymer concentration increases the viscosity of the solution and delays the drug diffusion within the polymer droplet.24 The drug loading was found to be higher with the decreasing glycolide content. This might be because water penetration during the solvent evaporation process is lower in the low glycolide content polymers, thereby minimizing the drug loss during the preparation of microspheres.
FIG. 3.
(A) Three-dimensional response surface plot and, (B) contour plot showing the effect of % glycolide content (X1) and polymer concentration (X2) on drug loading (Y2).
Using DoE, we were able to identify process conditions suitable for preparing particles with a similar size, irrespective of the polymer chosen. Parameters that can precisely control particle size are useful for the final performance of the particles. The particle size is a critical parameter that controls the surface area, and hence, burst release.25 The coefficient values showed the significant influence of the polymer concentration on mean particle size and this could be because an increase in the polymer concentration increases the viscosity of the internal polymer phase, requiring a higher energy input to obtain smaller size particles.26
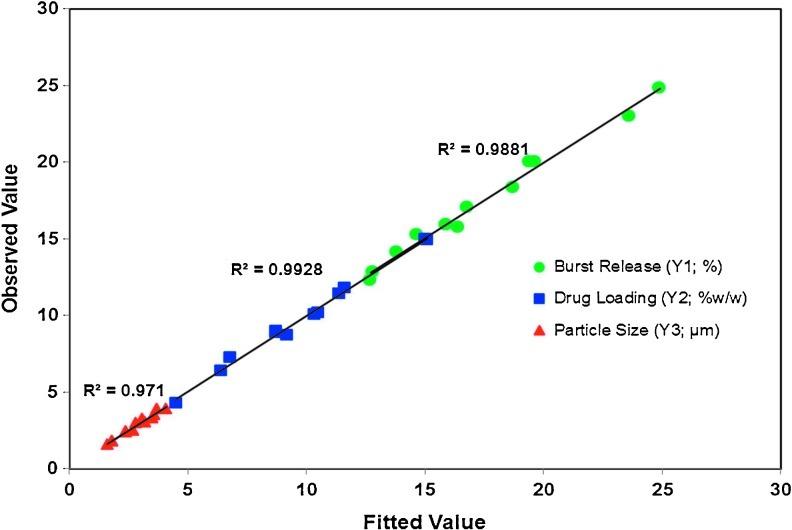
The use of the polynominal equation was verified by substituting the values of independent variables, glycolide content, polymer concentration, and drug-to-polymer ratio from all the experimental runs in the previously mentioned mathematical equations to predict responses. The predicted values were in good agreement with observed values as indicated by the regression coefficients and low residual values (Table 4 and Fig. 8). The regression coefficients (R2) were 0.9922, 0.9884, and 0.9675 for drug loading, burst release, and mean particle size, respectively, indicating the suitability of the model in this design.
Table 4.
The Observed and Predicted Values of the Particle Size, Drug Loading, and Burst Release
| |
Burst release (Y1;%) |
Drug loading (Y2;%w/w) |
Particle size (Y3; μm) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Row | Observed value | Fitted value | Residual | Observed value | Fitted value | Residual | Observed value | Fitted value | Residual |
| 1 | 13.8 | 13.55 | 0.25 | 12.4 | 12.71 | −0.31 | 2.4 | 2.58 | −0.18 |
| 2 | 12.7 | 12.31 | 0.39 | 9.2 | 8.72 | 0.48 | 3.1 | 3.26 | −0.16 |
| 3 | 24.9 | 24.85 | 0.05 | 4.5 | 4.31 | 0.19 | 2.4 | 2.41 | −0.01 |
| 4 | 16.4 | 15.76 | 0.64 | 15.1 | 14.93 | 0.17 | 2.8 | 3.01 | −0.21 |
| 5 | 15.89 | 15.89 | 0 | 11.4 | 11.4 | 0 | 1.6 | 1.59 | 0.01 |
| 6 | 14.65 | 15.24 | −0.59 | 15 | 14.98 | 0.02 | 1.8 | 1.80 | 0.00 |
| 7 | 15.89 | 15.89 | 0 | 11.4 | 11.4 | 0 | 1.8 | 1.80 | 0.00 |
| 8 | 19.4 | 20.04 | −0.64 | 8.7 | 8.88 | −0.18 | 4.1 | 3.93 | 0.18 |
| 9 | 19.65 | 20.04 | −0.39 | 6.8 | 7.28 | −0.48 | 2.4 | 2.44 | −0.04 |
| 10 | 13.8 | 14.14 | −0.34 | 8.7 | 8.99 | −0.29 | 2.7 | 2.49 | 0.21 |
| 11 | 15.89 | 15.89 | 0 | 11.4 | 11.4 | 0 | 1.8 | 1.80 | 0.00 |
| 12 | 12.8 | 12.85 | −0.05 | 11.6 | 11.79 | −0.19 | 3.7 | 3.90 | −0.20 |
| 13 | 16.8 | 17.05 | −0.25 | 10.5 | 10.19 | 0.31 | 3.5 | 3.30 | 0.20 |
| 14 | 18.7 | 18.36 | 0.34 | 10.35 | 10.06 | 0.29 | 3.2 | 3.04 | 0.16 |
| 15 | 23.6 | 23.01 | 0.59 | 6.4 | 6.42 | −0.02 | 3.6 | 3.56 | 0.04 |
FIG. 8.
Linear regressions plots correlating the observed values and fitted values for burst release, drug loading, and mean particle size.
After identifying the polymers for the release of SAR 1118 for 1, 3, and 6 months and optimizing the factors for low burst release, higher drug loading, and micron size particles, microsphere batches were prepared with each polymer and characterized for drug loading, mean particle size, burst release, and in vitro cumulative release. The drug loading and mean particle size were observed to be in the range of 11.5–13.0%w/w and 2–3 μm, respectively, for these batches. These observed values are in very close agreement with the predicted values from the Box-Behnken design (Table 5). The measured burst release from PLGA (85:15), PLGA (75:25), and PLGA (50:50) microspheres was 15.9%, 13.7%, and 20.1%, respectively, whereas the respective predicted values were 13.6%, 14.5%, and 17.6%. This demonstrated the reliability of the DoE procedure in optimizing the process parameters.
Table 5.
The Observed and Predicted Values of the Particle Size, Drug Loading, and Burst Release for SAR 1118-Encapsulated PLGA (85:15), PLGA (75:25), and PLGA (50:50) Microspheres Prepared Using Optimized Parameters Based on Box-Behnken Design
| |
Predicted value |
Actual value |
||||
|---|---|---|---|---|---|---|
| Formulation | Drug loading (%w/w) | Burst release (%) | Particle size (μm) | Drug loading (%w/w) | Burst release (%) | Particle size (μm) |
| PLGA(85:15) | 20.23 | 13.55 | 2.41 | 18.35±0.3 | 15.87±1.1 | 2.74±0.6 |
| PLGA(75:25) | 18.89 | 14.47 | 2.28 | 16.86±2.6 | 13.73±1.2 | 2.12±0.8 |
| PLGA(50:50) | 15.89 | 17.58 | 2.38 | 14.92±3.0 | 20.09±0.3 | 2.20±0.6 |
PLGA, poly (lactide-co-glycolide).
The in vitro cumulative release data indicated that complete release of SAR 1118 from the polymers PLGA (50:50), PLGA (75:25), and PLGA (85:15) occurred close to 1, 3, and 6 months, respectively. The SEM images indicated spherical SAR 1118-loaded microspheres, with smooth surfaces (Fig. 5). The decline in the melting point of SAR 1118 in the PLGA microspheres might be due to an amorphous or disordered crystalline phase of molecular dispersion of SAR 1118 in the microspheres (Fig. 6).
Under the conditions of our study, during the formulation optimization, mean particle sizes of SAR 1118-PLGA particles were in a narrow size range of 1.8–3.7 μm, as measured by dynamic light scattering (DLS) (Malvern Zetasizer ZS). Malvern Zetasizer ZS measures mean particle size in the range of 0.3 nm to 10 μm. We validated the Malvern Zetasizer ZS with NIST standard latex beads (Coulter CC standards) obtained from Beckman Coulter Inc. Standard latex beads of 2, 5, and 10 microns were measured for mean particle size using Zetasizer. The results indicated that the differences in mean sizes were within±7% of those indicated by the manufacturer. The mean sizes measured for all SAR 1118-PLGA microspheres, including the final 3 batches, were within this range of standards. For size distribution analysis, DLS measurements may not be adequate since large particles, if any, may not be monitored by DLS. At sizes >3 microns, particles may distort the measurements and alternative size measurement techniques are needed.27 Since our particle size distribution is anticipated to be in a range that no single instrument can accurately determine, to complement our DLS data, we measured the mean particle size of the 3 optimized SAR 1118 microsphere batches using the micro-flow imaging technique (MFI DPA 4100; ProteinSimple), an image-based size analysis. Although the 2 instruments provided comparable mean sizes, the size distributions provided by the 2 instruments differed (Fig. 5). MFI can measure particle size in the range of 1–300 microns precisely and our MFI results demonstrated that all particles are below 5 μm. Zetasizer indicated that all particles are above 100 nm in size.
In this study, DoE was employed to prepare 3 different microsphere formulations releasing the drug for 1, 3, and 6 months. This is the first study based on DoE for sustained drug release systems intended for the eye. This is the first study to incorporate burst release in DoE for microspheres. SAR 1118 used in this study is undergoing clinical investigation as an ophthalmic drop formulation; we anticipate that the sustained release microsphere formulations developed in this study will have a high translational relevance in the long run. Thus, DoE is a useful technique for the selection of the polymers and process parameters in developing SAR 1118-loaded PLGA microspheres.
Conclusions
The performance of sustained release PLGA microspheres can be influenced by a variety of factors. This study predicted the polymer degradation using a factorial design and optimized microsphere process parameters using the Box-Behnken design. In this study, we have shown the usefulness of DoE in designing the sustained release microspheres of SAR 1118. The quantitative effects of the glycolide content, polymer concentration, and drug to polymer ratio on measured responses were predicted by polynomial equations and contour plots. Observed response values were very close to the predicted values from the model. The polymer concentration and glycolide content were shown to be having a maximum influence on drug loading and burst release. The durations of SAR 1118 release from the prepared microspheres were close to the predicted values, indicating the value of DoE. Thus, DoE is useful in identifying and selecting polymers and optimization of process parameters in the preparation of SAR 1118 microspheres with desired slow release properties.
Acknowledgments
This work was supported, in part, by a research grant from the SARcode Corporation and, in part, by the NIH grants EY018940 and EY017533.
Authors' Contributions
Participated in research design: Sarath Yandrapu and Uday B. Kompella
Conducted experiments: Sarath Yandrapu.
Performed data analysis and/or interpretation: Sarath Yandrapu and Uday B. Kompella.
Wrote or contributed to the writing of the manuscript: Sarath Yandrapu and Uday B. Kompella
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Del Amo E.M. Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Herrero-Vanrell R. Refojo M.F. Biodegradable microspheres for vitreoretinal drug delivery. Adv. Drug Deliv. Rev. 2001;52:5–16. doi: 10.1016/s0169-409x(01)00200-9. [DOI] [PubMed] [Google Scholar]
- 3.Ayalasomayajula S.P. Kompella U.B. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005;511:191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Kompella U.B. Kadam R.S. Lee V.H. Recent advances in ophthalmic drug delivery. Ther. Deliv. 2011;1:435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shive M.S. Anderson J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 6.Short B.G. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H. Ogura Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica. 2001;215:143–155. doi: 10.1159/000050849. [DOI] [PubMed] [Google Scholar]
- 8.Semba C.P. Torkildsen G.L. Lonsdale J.D. McLaurin E.B. Geffin J.A. Mundorf T.K. Kennedy K.S. Ousler G.W. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am. J. Ophthalmol. 2012;153:1050–1060. doi: 10.1016/j.ajo.2011.11.003. e1051. [DOI] [PubMed] [Google Scholar]
- 9.Gadek T.R. Burdick D.J. McDowell R.S. Stanley M.S. Marsters J.C., Jr. Paris K.J. Oare D.A. Reynolds M.E. Ladner C. Zioncheck K.A. Lee W.P. Gribling P. Dennis M.S. Skelton N.J. Tumas D.B. Clark K.R. Keating S.M. Beresini M.H. Tilley J.W. Presta L.G. Bodary S.C. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science. 2002;295:1086–1089. doi: 10.1126/science.295.5557.1086. [DOI] [PubMed] [Google Scholar]
- 10.Rao V.R. Prescott E. Shelke N.B. Trivedi R. Thomas P. Struble C. Gadek T. O'Neill C.A. Kompella U.B. Delivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR) Invest. Ophthalmol. Vis. Sci. 2010;51:5198–5204. doi: 10.1167/iovs.09-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B. Kumar R. Ahuja N. Optimizing drug delivery systems using systematic “design of experiments.”. Part I: fundamental aspects. Crit. Rev. Ther. Drug Carrier Syst. 2005;22:27–105. doi: 10.1615/critrevtherdrugcarriersyst.v22.i1.20. [DOI] [PubMed] [Google Scholar]
- 12.Singh B. Dahiya M. Saharan V. Ahuja N. Optimizing drug delivery systems using systematic “design of experiments.”. Part II: retrospect and prospects. Crit. Rev. Ther. Drug Carrier Syst. 2005;22:215–294. doi: 10.1615/critrevtherdrugcarriersyst.v22.i3.10. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira S.L. Bruns R.E. Ferreira H.S. Matos G.D. David J.M. Brandao G.C. da Silva E.G. Portugal L.A. dos Reis P.S. Souza A.S. dos Santos W.N. Box-Behnken design: an alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597:179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Araujo P.W. Brereton R.G. Experimental design I. Screening. Trends Anal. Chem. 1996;15:26–31. [Google Scholar]
- 15.Zidan A.S. Sammour O.A. Hammad M.A. Megrab N.A. Habib M.J. Khan M.A. Quality by design: understanding the formulation variables of a cyclosporine A self-nanoemulsified drug delivery systems by Box-Behnken design and desirability function. Int. J. Pharm. 2007;332:55–63. doi: 10.1016/j.ijpharm.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 16.Box G.E.P. Behnken D.W. Some new three-level designs for the study of quantitative variables. Technometrics. 1960;2:455–475. [Google Scholar]
- 17.Shelke N.B. Kadam R. Tyagi P. Rao V.R. Kompella U.B. Intravitreal poly(L-lactide) microparticles sustain retinal and choroidal delivery of TG-0054, a hydrophilic drug intended for neovascular diseases. Drug Deliv. Transl. Res. 2011;1:76–90. doi: 10.1007/s13346-010-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo C. Stine K.J. Kauffman J.F. Doub W.H. Assessment of the influence factors on in vitro testing of nasal sprays using Box-Behnken experimental design. Eur. J. Pharm. Sci. 2008;35:417–426. doi: 10.1016/j.ejps.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Singare D.S. Marella S. Gowthamrajan K. Kulkarni G.T. Vooturi R. Rao P.S. Optimization of formulation and process variable of nanosuspension: an industrial perspective. Int. J. Pharm. 2010;402:213–220. doi: 10.1016/j.ijpharm.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Ozurdex (dexamethasone intravitreal) implant. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm295117.htm http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm295117.htm
- 21.Wischke C. Schwendeman S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y.Y. Chung T.S. Ng N.P. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22:231–241. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 23.Malaekeh-Nikouei B. Sajadi Tabassi S.A. Jaafari M.R. The effect of different grades of PLGA on characteristics of microspheres encapsulated with cyclosporine A. Curr. Drug Deliv. 2006;3:343–349. doi: 10.2174/156720106778559074. [DOI] [PubMed] [Google Scholar]
- 24.Bodmeier R. McGinity J.W. Polylactic acid microspheres containing quinidine base and quinidine sulphate prepared by the solvent evaporation method. III. Morphology of the microspheres during dissolution studies. J. Microencapsul. 1988;5:325–330. doi: 10.3109/02652048809036729. [DOI] [PubMed] [Google Scholar]
- 25.Jain V. Jain D. Singh R. Factors effecting the morphology of eudragit S-100 based microsponges bearing dicyclomine for colonic delivery. J. Pharm. Sci. 2010;100:1545–1552. doi: 10.1002/jps.22360. [DOI] [PubMed] [Google Scholar]
- 26.Mittal G. Sahana D.K. Bhardwaj V. Ravi Kumar M.N. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control Release. 2007;119:77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Shekunov B.Y. Chattopadhyay P. Tong H.H. Chow A.H. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm. Res. 2007;24:203–227. doi: 10.1007/s11095-006-9146-7. [DOI] [PubMed] [Google Scholar]