Abstract
Research advancements in pharmaceutical sciences have led to the development of new strategies in drug delivery to anterior segment. Designing a new delivery system that can efficiently target the diseased anterior ocular tissue, generate high drug levels, and maintain prolonged and effective concentrations with no or minimal side effects is the major focus of current research. Drug delivery by traditional method of administration via topical dosing is impeded by ocular static and dynamic barriers. Various products have been introduced into the market that prolong drug retention in the precorneal pocket and to improve bioavailability. However, there is a need of a delivery system that can provide controlled release to treat chronic ocular diseases with a reduced dosing frequency without causing any visual disturbances. This review provides an overview of anterior ocular barriers along with strategies to overcome these ocular barriers and deliver therapeutic agents to the affected anterior ocular tissue with a special emphasis on nanotechnology-based drug delivery approaches.
Introduction
In the past two decades, significant advances have been made regarding drug delivery to targeted ocular tissues and to maintain effective drug levels in those tissues. Anterior ocular tissues are exposed to the external environment and, consequently, are subjected to inflammations due to disease, dust, and microbes such as, but not limited to, bacteria, virus, fungus, and parasites.1,2 The other pathway for inflammations may be resulting from penetration of proteins and cells from peripheral circulation. In a healthy eye, such ocular insults are normally prevented by the blood–aqueous barrier, while regulatory molecules and immune cells in the eye actively suppress the immune response. Ocular inflammation and surgical trauma induce changes in the blood–aqueous barrier.3–5 This process causes entry and accumulation of immune cells and inflammatory mediators in ocular tissues and develops redness, pain, swelling, and itching.6 Inflammation is commonly associated with ocular pathologies such as, but not limited to, uveitis, immune rejection of corneal transplants, dry eye syndrome, keratitis, age-related macular degeneration, and retinitis.7 To treat such chronic conditions, sustained drug delivery systems are required. Currently, such inflammatory conditions are treated with repeated topical applications, preferably eye drops. Absorption of topically applied agents is usually limited to the anterior ocular tissues due to ocular static and dynamic barriers. To improve drug penetration across ocular tissues, various strategies, including prodrugs and nanotechnology have been investigated. In this review, we have briefly discussed the ocular physiology and anatomy and barriers to anterior ocular drug delivery. The review is primarily focused on various prodrug- and nanotechnology-based drug delivery approaches to increase bioavailability, improve precorneal residence time, and prolonged therapeutic efficacy for anterior ocular inflammations.
Ocular physiology and anatomy relevant to anterior segment drug delivery
The globe can be broadly divided into 2 segments as anterior and posterior. The anterior segment occupies the front 1-third and the remaining 2-third is occupied by posterior ocular tissues. The cornea, conjunctiva, iris, ciliary body, tear film, and aqueous humor make up the anterior segment. Whereas, the posterior segment includes the sclera, choroid, Bruch's membrane, retinal pigment epithelium, neural retina, and vitreous humor. Barriers that pose a challenge to anterior segment drug delivery are static (corneal epithelium, corneal stroma, and blood–aqueous barrier) and dynamic barriers (conjunctival blood flow, lymph flow, and tear drainage) and metabolic barriers.8 Moreover, efflux pumps, such as MDR1 [P-glycoprotein (P-gp)], the multidrug resistance protein (MRP), and the breast cancer resistance protein (BCRP) expressed on the cell membrane constitute another significant barrier to drug delivery. In the following sections, we have attempted to describe barrier properties of the cornea and conjunctiva to anterior segment drug delivery.
Conjunctiva as a barrier
The conjunctiva is a thin, transparent, elastic, highly vascularized tissue, internally lining the upper eyelid, lower eyelid, and anterior sclera covering almost 80% of the ocular surface. This tissue is engaged in tear film formation by secreting electrolytes, fluid, and mucin.9 It is composed of 2 layers: the outer 2–10-layered epithelia made of stratified epithelial cells and the inner stroma made of substantia propria. The number of epithelial layers varies and precisely depends on its anatomical location. Embedded within the conjunctiva are several secretory cells (goblet cells) and glands, which are engaged in mucin10 and tear formation.9 Secreted mucin helps to adhere and maintain tear film, provide protection, and nourishment to the cornea.11 The outer apical epithelial cells form tight junctions (zonula adherens) with a transepithelial electrical resistance of ∼1.2 kΩcm2,12 impeding paracellular drug permeation (passive permeability) across the cell layers.13 The stroma is richly supplied with nerves, blood, and lymph vessels and is located in between the outer conjunctival epithelia and inner sclera, which may pose a barrier to hydrophobic drugs. Also, drugs may be carried away in the conjunctival lymph and blood circulation, which act as a dynamic barrier to drug absorption. Pore size analysis across freshly excised rabbit conjunctival tissues revealed the pore radius in the range of 3.0–5.5 nm.14,15 Physicochemical properties, such as hydrophilicity and molecular weight can play a major role in drug permeation across the conjunctiva. Hydrophilic drugs with less than 20-kDa molecular weights are permeable across the conjunctiva13,14 restricting transport of higher molecular weight drug molecules. The conjunctiva possesses esterase activity16 and expresses efflux proteins (P-gp)17 on the cell membrane. These efflux pumps are actively involved in drug efflux from the cell cytoplasm further reducing drug concentrations in conjunctival cells and impeding drug transport.
Another significant impediment for drug permeation from topical dosing is the tear film turnover, which is present anteriorly covering both the cornea and the conjunctiva. Tear film is engaged in protecting the corneal epithelial layer by preventing dehydration. Tear film thickness is about 3–9 μm18–20 and the cul-de-sac/precorneal pocket can hold a tear volume of ∼7 μL21,22 with an average tear flow of 1.2 μL min−1. Tear film is composed of 3 layers: an outer lipid layer, a middle aqueous layer, and an inner mucin layer (secreted by goblet cells in the conjunctiva).23 Electrolytes, glucose, immunoglobulins, lysozymes, and lactoferrin are secreted in tear fluid. These components aid in lubrication, nourishment, maintenance, and repair of the corneal epithelium.24–27 A topically applied dose into the precorneal pocket is rapidly reduced due to excessive tear production/tear reflux, thereby lowering bioavailability.28,29 Only 10%–20% is available for absorption and excess administered dose is lost due to spillage or drainage through the nasolacrimal duct.21,30,31 Precorneal drainage further lowers applied dose, thus contributing toward lower bioavailability (<5%).32–34 Other factors, such as tear turnover and limited contact time further reduce ocular bioavailability.
Cornea as a barrier
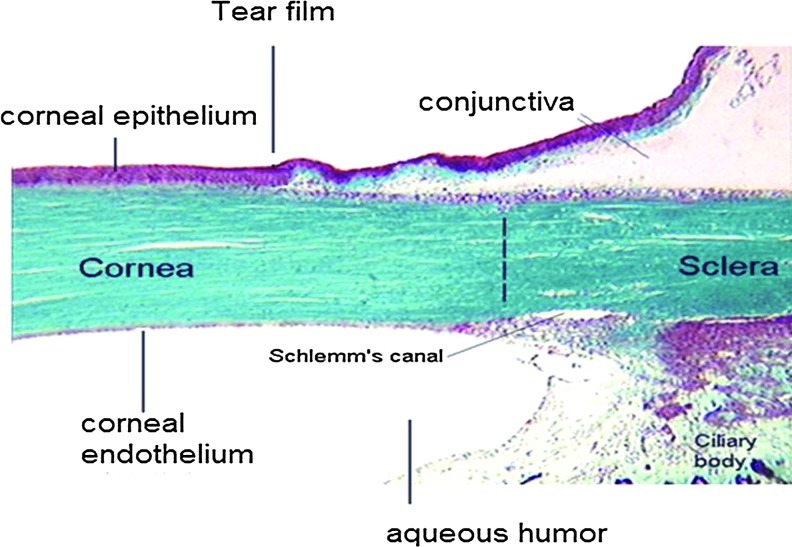
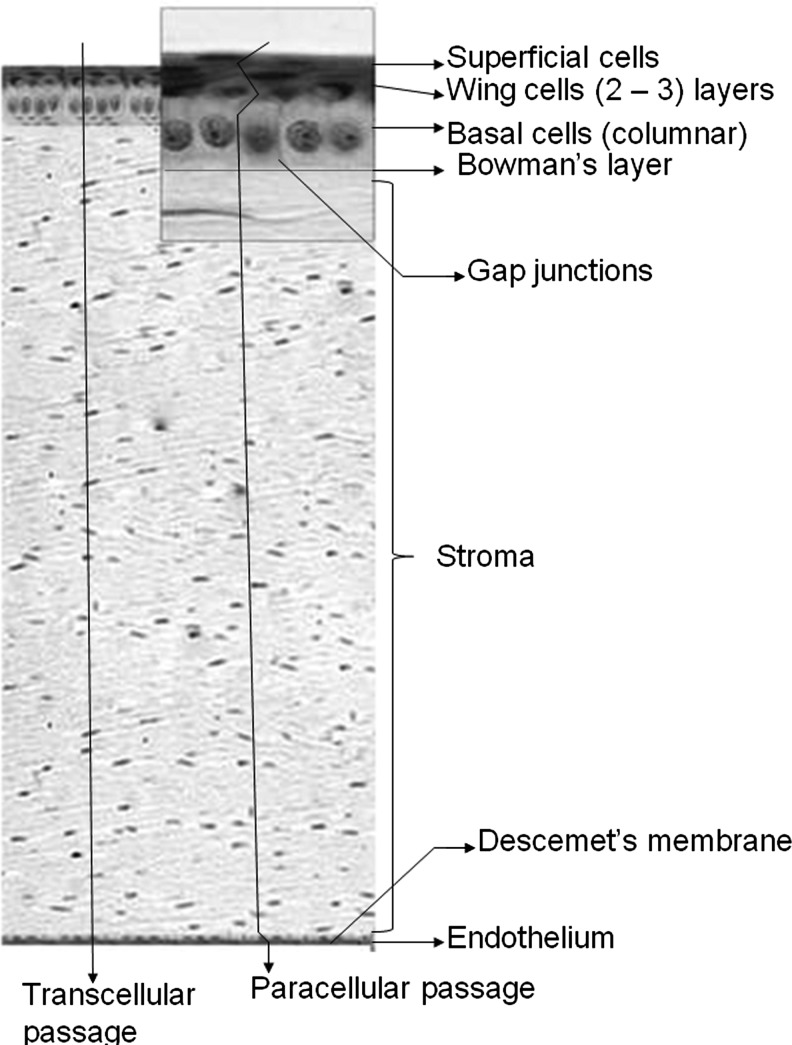
It is a multilayered, transparent, avascular, highly innervated, and most sensitive tissue,35 which hinders translocation of exogenous substances administered topically into the cul-de-sac. It is in continuation with the conjunctiva (Fig. 1) and composed of 5 different layers: the outer lipophilic epithelium, the Bowman's layer, the hydrophilic stroma, the Descemet's membrane, and the endothelium (Fig. 2). Precise arrangement of these cell layers provides rigidity, strength, and transparency. Interaction between these components is essential for maintaining tissue integrity and function. The epithelium, exposed to the outer environment, is composed of 5–6 layers of columnar cells derived from the epidermal ectoderm. It is covered on the outer surface with 3-layered tear film and the inner monolayered endothelium is in contact with aqueous humor. Mature epithelial cells are highly lipophilic with tight junction, which restricts small hydrophilic drug entry into ocular tissues36,37 following topical administration. The hydrophobic drug molecules can cross the lipophilic epithelium, but are further prevented from permeating into deeper ocular tissues due to the presence of hydrophilic stroma. The monolayered corneal endothelium is another barrier, which is sandwiched between aqueous humor and stroma. This barrier possesses leaky tight junctions allowing free movements of molecules between aqueous compartments (stroma and aqueous humor).38 Drug absorption into aqueous humor occurs by transcorneal diffusion. Another dynamic barrier that restricts drug transport across the cornea is presence of transmembrane efflux pumps. These pumps expressed on corneal surface constitute a significant barrier in ocular drug delivery by restricting drug entry into deeper ocular tissues. Expression of drug efflux pumps, such as P-gp, MRP, and BCRP on the corneal surface was reported. Transport studies conducted across the human and rabbit cornea demonstrated active involvement of drug efflux pumps at the cell surface39–42 that restricts drug penetration into ocular tissues.
FIG. 1.
Anterior ocular tissues and fluid. Color images available online at www.liebertpub.com/jop
FIG. 2.
Different layers of cornea: (1) Outer epithelium (comprising superficial cells, wing cells, and basal cells), (2) Bowman's layer, (3) Stroma, (4) Descemet's membrane, and (5) Endothelium. Reproduced and modified with permission from Barar et al.167
Topical application of ophthalmic formulation is the most convenient and patient compliant mode of drug delivery. In the past, ophthalmic drug delivery was directed to treatment of anterior chamber diseases and dominated by topical formulations, such as solutions, emulsions, suspensions, ointments, and gels. Topical instillation offers numerous advantages over systemic delivery, such as noninvasiveness, low absorption into systemic circulation, avoidance of first pass metabolism, ease of administration, and relatively small dose. However, rapid drainage, interaction with tear protein, the tear turnover rate (1 μL min−1), rapid drug removal due to reflex blinking, and rapid metabolism are the major concerns that need to be addressed during the development of any topical ocular drug delivery system.43 Such a formulation should encompass active molecules with a balance between hydrophilicity and lipophilicity and generate higher contact time.44 To improve permeability across ocular tissues, various approaches have been attempted that are discussed in the following sections.
Novel Drug Delivery Approaches
Prodrugs
Prodrugs are bioreversible derivatives of drug molecules and are designed to be therapeutically inactive until enzymatic and/or chemical bioreversion. In vivo bioreversion generates an active parent drug, which can then exert a desired pharmacological response.44–47 The rate of bioreversion depends on the capacity and turnover rate of enzyme processes as well as affinity of the prodrug toward hydrolyzing enzymes. These derivatives are generally synthesized by chemical conjugation of a specific promoiety to the parent drug via an ester, amide, or other enzymatically cleavable linkages. Esterases and amidases expressed in most of the biological fluids and tissues are primarily responsible for the hydrolysis of ester and amide prodrugs, respectively. Among the ocular tissues, the highest esterase activity has been found in the iris-ciliary body followed by the cornea and aqueous humor. Prodrugs containing ester or amide linkages can undergo varying extents of esterase and amidase-mediated hydrolysis, while permeating through the cornea/conjunctiva and subsequent entry into the aqueous humor, iris, and ciliary body. The highest aminopeptidase activity has been reported in lipophilic corneal epithelium and the iris-ciliary body followed by the conjunctiva and the hydrophilic corneal stroma.48–50
Various strategies have been explored to improve drug absorption in the cornea and conjunctiva following topical administration (Table 1). Lipophilic ester and transporter-targeted prodrug approaches have been investigated for many drugs, which suffer from poor ocular absorption. Several prodrug derivatives of prostaglandin (PGF2α) analogs have been synthesized, which resulted in blockbuster drugs with an enhanced potency.51 These prodrugs include isopropyl ester prodrugs known as latanoprost, travoprost, and isopropyl unoprostone and the ethyl amide prodrug, such as bimatoprost. All these prodrugs exhibit significant improvement in permeability across the cornea, thereby providing successful delivery. This strategy has been very successful in lipophilic prodrug derivatization of timolol, which suffered from high incidence of cardiovascular and respiratory side effects due to systemic exposure from topical application. Acetyl-, propionyl-, and butyryl- ester prodrugs of timolol exhibited an increase in corneal permeability (2–3-folds higher) and the aqueous humor concentration (4–6-fold higher). Such an improvement resulted in 2-fold reduction in topical dose leading to subsequent reduction in cardiovascular and respiratory side effects.52,53 A similar approach has been extended to antiviral drugs, such as acyclovir (ACV) and ganciclovir (GCV). Acylation of ACV exhibited a significant improvement in the permeation of ACV across the cornea. A linear relationship between apparent permeability coefficient and the octanol/water partition coefficient was observed. Surprisingly, ACV-isobutyrate showed a lower corneal permeability despite enhanced lipophilicity. Authors hypothesized that this anomalous behavior may be due to enhanced enzymatic stability of these prodrugs toward esterases.54,55 Many reports suggest that an increase in the carbon chain length decreases aqueous solubility of lipophilic ester prodrugs, thus posing a challenge to formulating these prodrugs into aqueous eye drops.
Table 1.
Summary of Prodrugs Developed for Anterior Segment Ocular Drug Delivery
| Drug | Prodrugs | Conclusion/remarks | References |
|---|---|---|---|
| PGF2alpha | Lipophilic esters: PGF2alpha 1-isopropyl, 1,11-lactone, 15-acetyl, 15-pivaloyl, 15-valeryl, and 11,15-dipivaloyl esters | Improvement in rabbit corneal permeation by 4- to 83-fold | 51 |
| Timolol | Lipophilic esters: O-acetyl, propionyl, butyryl, and pivalyl ester prodrugs of timolol | Improved corneal penetration to reduce systemic drug load | 53 |
| ACV and ganciclovir | Aliphatic acyl esters of ACV | Significant improvement in the corneal permeation of ACV except for ACV-isobutyrate | 54,55 |
| Amino acid prodrugs: alanine-ACV, SACV, isoleucine-ACV, and γ-glutamate-ACV | SACV demonstrated excellent stability, higher AUC, Cmax and Clast values in comparison to ACV | 65 | |
| Peptide prodrugs: valine-valine-ACV, glycine-valine-ACV, tyrosine-valine-ACV, valine-tyrosine-ACV, tyrosine-valine-ganciclovir, and valine-valine-ganciclovir | Enhanced transcorneal permeability, higher ocular bioavailability, higher antiviral efficacy against herpes simplex virus epithelial and stromal keratitis | 57–61 | |
| Stereoisomeric dipeptide prodrugs: L-Valine-L-valine-ACV, L-valine-D-valine-ACV, D-valine-L-valine-ACV and D-valine-D-valine-ACV | L-valine-L-valine-ACV and L-valine-D-valine-ACV were found to be optimum in terms of enzymatic stability, uptake, and cytotoxicity | 158 | |
| Targeted lipid prodrugs: biotin-ricinoleicacid-ACV and biotin-12hydroxystearicacid-ACV | Biotinylated lipid prodrugs exhibited much higher cellular accumulation than ACV and demonstrated enhanced affinity toward sodium-dependent multivitamin transporter | 159 | |
| Dexamethasone | Dexamethasone esters: 21-sodium phosphate, 21-metasulfobenzoate, 21-acetate, 17-propionate, 21-propionate, 21-butyrate, 21-valerate, and 21-palmitate | Increase in permeability was observed with increase in lipophilicity until dexamethasone butyrate | 66 |
| Flurbiprofen | Flurbiprofen axetil | Flurbiprofen axetil emulsion displayed low irritancy and improved anti-inflammatory effect | 160 |
| CP-544326 | PF-04217329 (Taprenepag isopropyl) | Increased corneal permeability and ocular bioavailability, reduced intraocular pressure in preclinical models of glaucoma | 161 |
| CS-A | Phosphate ester: OPPH 088 | Generated therapeutic concentrations of CS-A in the precorneal area immediately after administration | 162,163 |
| Resolvin E1 (RX-10001) | RX-10005, methyl ester prodrug of Resolvin E1 | Reduced corneal epithelial barrier disruption and protected against goblet cell loss in a murine model of dry eye | 164 |
| Mycophenolic acid | Mycophenolate mofetil | Currently indicated for high-risk keratoplasties and ocular immune-mediated diseases | 165 |
ACV, acyclovir; PGF2alpha, prostaglandin F2alpha; SACV, serine-ACV.
Research during the past decade is focused on transporter-targeted prodrug approach for delivery of drugs, including ACV and GCV. Presence of the peptide transporter on the corneal epithelium has encouraged researchers to develop novel peptidomimetic prodrugs to improve ocular bioavailability.56 Valacyclovir and valganciclovir, L-valyl ester prodrugs of ACV and GCV revolutionized the era of peptide prodrug design targeting oligopeptide transporters. Consequently, a series of water soluble dipeptide ester prodrugs, such as valine-valine-acyclovir, glycine-valine-acyclovir, tyrosine-valine-acyclovir, valine-tyrosine-acyclovir, tyrosine-valine-ganciclovir, and valine-valine-ganciclovir have been synthesized in an effort to improve ocular bioavailability.57,58 All the dipeptide prodrugs demonstrated an enhanced transcorneal permeability achieving a higher ocular bioavailability. These prodrugs also exhibited a higher antiviral efficacy against herpes simplex virus (HSV)-mediated epithelial and stromal keratitis with less cytotoxicity. Interestingly, these prodrugs appear to be more effective than trifluorothymidine—the current gold standard treatment.59–61
Besides peptide transporters, amino acid transporters, such as LAT1 (Na+-independent large neutral amino acid transporter), ASCT1 (Na+-dependent amino acid transporter), and B(0,+) (Na+-dependent neutral and cationic amino acid transporter) have been characterized and utilized for corneal drug delivery.62–64 A series of amino acid prodrugs, including alanine-ACV, serine-ACV (SACV), isoleucine-ACV, and γ-glutamate-ACV (EACV), were evaluated for in vivo corneal absorption in rabbits. Among these amino acid prodrugs, enhanced stability of SACV resulted in higher area under the curve (AUC), Cmax, and Clast values in comparison to ACV. This prodrug appears to be a promising candidate for the treatment of ocular HSV infections.65
The prodrug approach for conjunctival drug delivery has not been exploited to a great extent relative to drug delivery to the cornea. In this article, we have discussed some of the important studies regarding targeted as well as nontargeted prodrug strategy to enhance drug uptake across the conjunctiva. Prostaglandins are widely indicated in the treatment of open angle glaucoma. Although effective in reducing intraocular pressure (IOP), the therapeutic concentration is not achieved in the target site due to the low permeability of PGF2α across the cornea following topical application. Several lipophilic ester prodrugs of PGF2α have been designed and synthesized to improve permeability of the parent drug. Prodrugs, such as 1-isopropyl, 1,11-lactone, 15-acetyl, 15-pivaloyl, 15-valeryl, and 11,15-dipivaloyl esters were evaluated for transport and bioconversion.51 All the prodrugs except the 15-acetyl ester prodrug of PGF2α exhibited a greater increase in permeability across the cornea and the conjunctiva. Permeabilities of the most lipophilic prodrugs, 15-valeryl and 11,15-dipivaloyl esters were lower than other lipophilic prodrugs (with less lipophilicity), thus negating the direct relationship between log P and permeability. 1,11-lactone was observed to be the most metabolically stable prodrug, followed by 11,15-dipivaloyl, 15-pivaloyl, 15-acetyl, 1-isopropyl, and 15-valeryl esters, the latter was rapidly converted to PGF2α. Bulky pivaloyl groups in 15-pivaloyl and 11,15-dipivaloyl ester conjugates improved prodrug stability against the enzymatic activity relative to other prodrugs. This study suggested that the size and branching arrangement of the conjugated promoiety can modulate the rate of enzymatic hydrolysis and alter permeability across the conjunctiva and cornea. Adequate penetration of these prodrugs may result a lowering in dose that may help to lower conjunctival hyperemia, ocular discomfort, and other adverse effects.
Also, several lipophilic esters of dexamethasone, a corticosteroid indicated for the treatment of uveitis and postsurgical inflammations, were developed.66 These prodrugs were evaluated for permeability and bioreversion across the rabbit cornea and bovine conjunctival epithelial cells (BCEC). This study was directed at selecting an optimum prodrug that can reach the target tissue with minimal permeation across other tissues causing much reduced side effects. Permeability values across BCEC and the rabbit cornea correlate well with the lipophilicity of dexamethasone prodrugs and reached a maximum value in correspondence with dexamethasone butyrate (Log P=3.95). The permeability of sodium phosphate and metasulfobenzoate esters of dexamethasone was restricted across BCEC due to their hydrophilic and ionic nature. In contrast, the other prodrugs, including acetate, propionate, and butyrate esters demonstrated a higher permeability with increase in lipophilicity. The valerate ester conjugate being highly lipophilic easily traverses the corneal epithelium, but the hydrophilic stroma acts as a barrier. This layer forms a depot to such lipophilic drugs until hydrolysis to parent dexamethasone. Hydrolysis of valerate ester is very slow in the cornea suggesting possible utilization of this prodrug for sustained drug release. This slow release may reduce the drug amount in aqueous humor and other tissues, thus reducing the side effects. Butyrate ester of dexamethasone was found to be completely hydrolyzed both in the BCEC and cornea along with remarkable improvement in dexamethasone permeability. These studies demonstrated the importance of promoiety for optimal screening and development of prodrugs in the ophthalmic drug design.
Several esters of timolol (alkyl, aryl, and cycloalkyl esters) were also synthesized and evaluated for their bioreversion across the cornea and conjunctiva.52 Rapid hydrolysis of straight-chain alkyl and unsubstituted cycloalkyl esters was evident relative to their corresponding branched-chain and/or substituted alkyl and aryl esters. Unlike the ease of accessibility of the ester linkages to esterases in straight-chain alkyl esters, the branched-chain alkyl esters are slowly hydrolyzed due to steric hindrance. A similar trend was observed across the conjunctiva and cornea.
A transporter-targeted approach has also been extended to drug delivery across the conjunctiva. Expression of various transporters on conjunctival cells and tissues may be utilized to enhance drug permeation across the conjunctiva. However, this strategy has not been investigated to a great extent for conjunctival drug delivery. Amino acid transporters, including B,(0 +) has been characterized on the apical side of rabbit conjunctiva. Presence of a functionally active, sodium-dependent, monocarboxylate transport system on the mucosal side of the rabbit conjunctival epithelium has been reported. This transport system is able to translocate topically administered nonsteroidal anti-inflammatory drugs (NSAID) and fluoroquinolones.67 Also, the sodium-dependent glucose transporter and the nucleoside transporter were characterized.67,68 Among various transporters, peptide transporters have gained immense popularity due to their high capacity and wide substrate specificity. Even though, proton coupled dipeptide transport systems has been reported on the rabbit conjunctival epithelial cells and pigmented rabbit conjunctiva, the expression levels of these transporters were found to be rather low in conjunctival cells and tissues. Nevertheless, the molecular and functional presence of various influx nutrient transporters on the conjunctiva may be exploited for targeted delivery.69,70
Additives
Several attempts have been made to improve drug availability into the anterior chamber either by utilizing enhancers to increase drug permeability across ocular tissues or by employing mucoadhesives to enhance precorneal residence time of the applied dose.
Viscosity enhancing polymers can be incorporated into ophthalmic formulations to improve residence time in the precorneal area and to increase absorption of the therapeutic agents into and across the cornea. Various polymers, such as hydroxypropyl methyl cellulose (HPMC),71,72 hyaluronic acid (HA),73,74 polyvinyl alcohol (PVA),71,72 hydroxyethyl cellulose,72 and methylcellulose75 have been investigated to determine their potential in improving the bioavailability of drugs following topical applications.
Pluronics
Pluronics can significantly improve drug solubility and enhance the viscosity of topical formulations. Pluronic™ F68 (15%) and Pluronic F127 (10%) were more effective as indomethacin solubilizers and viscosity enhancers relative to polyols and polysorbate 80.76 Moreover, Pluronic solutions were well tolerated on rabbit eyes than commercial formulations, Indocid™ suspension. Poloxamer 407 [poly(oxyethylene)- poly(oxypropylene) block copolymer] was evaluated as a solubilizer for topical application of indomethacin.77 This formulation has significantly elevated indomethacin levels in aqueous humor (AUC=∼21 h·μg·mL−1) relative to the marketed solution (AUC=∼8 h·μg·mL−1). In addition, anti-inflammatory studies performed on an immunogenic uveitis model demonstrated comparatively more rapid resolution of the symptoms. In another study, PVA and HPMC caused improvement in viscosity and stability of indomethacin topical ocular suspension.71 Mucoadhesive eye drops of tolmetin (pyrrole-acetic acid derivative) exhibited higher drug levels in aqueous humor in comparison to the aqueous solution, in both inflamed and uninflamed rabbit eyes.78
A novel approach of a drug-embedded thermosensitive gel has shown some promising results for topical ophthalmic drug delivery. In a recently published study, Gao et al. have prepared and evaluated a dexamethasone-loaded poly(lactide-co-glycolide)-polyethylene glycol-poly(lactide-co-glycolide), ((PLGA)-PEG_PLGA) thermosensitive gelling solution.79 This formulation improved precorneal residence time and demonstrated 7-fold higher drug levels in aqueous humor (Cmax 125.2 μg/mL) relative to eye drops. This hydrogel system showed good biodegradability, sustained release, and ocular biocompatibility, indicating that the system is a safe candidate for sustained ophthalmic drug delivery. Many other in situ polymeric gelling systems, such as chitosan,80 poloxamer,81 HPMC,82 PEG-poly-ɛ caprolactone (PCL)-PEG,83 poly(N-isopropylacrylamide)/chitosan,84 poloxamer/chitosan,85 pluronic F-127/chitosan,86 and poloxamer/carbopol87 were explored for topical ocular applications.
Cyclodextrins
Almost all NSAIDs and corticosteroids are highly lipophilic molecules, which exhibit very poor aqueous solubility, and therefore it is very challenging to formulate them in aqueous eye drops. Cyclodextrins (CD) are known to improve aqueous solubility of poorly soluble therapeutic agents. In a recently published manuscript, Valls et al. demonstrated improved solubility and a higher ocular bioavailability of diclofenac.88 Six times higher transport of diclofenac across the corneal tissue was observed with β- CD/diclofenac complex treatment relative to free drug. Loftsson et al. have examined the effects of various CDs [randomly methylated β-CD (RMβCD) and 2-hydroxypropyl-β CD (HPβCD)] on ocular delivery of dexamethasone.89 Results from in vivo ocular tissue distribution studies illustrated that both lipophilic RMβCD and hydrophilic HPβCD have improved dexamethasone levels in rabbit eyes. However, RMβCD delivered higher amounts of dexamethasone relative to other CDs.
These investigators recently published a patent, demonstrating the role of CDs [RMβCD and γ-cyclodextrin (γCD)] in the delivery of corticosteroids to various ocular tissues.90 Nanoparticulate formulation of γCD-drug conjugates were able to deliver corticosteroids more efficiently to the back of the eye, contrary to the RMβCD drug solution, which causes localization of more drug into the anterior chamber of the rabbit eyes. Cyclooxygenase-2 (COX-2) inhibitors are also indicated in the treatment of ocular inflammations. However, poor aqueous solubility of these agents limits their topical application. In an attempt to improve ocular bioavailability, nanoparticulate formulation of valdecoxib with HPβCD was evaluated.91 As anticipated, levels of valdecoxib in the cornea and conjunctiva were significantly higher in nanoparticle-treated rabbit eyes relative to control.
In vivo ocular bioavailability of 3 different hydrocortisone (HC) formulations (1% HC solution with HPβCD, 1% HC solution with HPβCD along with sodium hyaluronate or carbopol 934P, and 1% HC suspension) was evaluated in New Zealand White rabbit.92 Incorporation of HPβCD in formulation improved HC solubility and bioavailability in the cornea and aqueous humor by 75% and 55%, respectively, relative to aqueous suspension. Interestingly, inclusion of hyaluronate or carbopol in the HPβCD solution did not alter ocular bioavailability.
In clinical trials, topical delivery of the dexamethasone/HPβCD solution exhibited a significantly higher aqueous humor concentration (2.6-fold higher AUC) in human subjects relative to suspension.93 Currently, several CD containing formulations are marketed in Europe, such as Indocid® (indomethacin with HPβCD) and Voltaren® (diclofenac-Na with HPβCD), for the treatment of anterior chamber inflammations. It may not be surprising if CD containing ophthalmic topical formulations enter the US market in the near future.
Colloidal dosage forms for drug delivery to anterior segment of the eye
Various colloidal dosage forms have been explored to overcome some of the constraints of conventional ocular drug delivery systems. Such dosage forms include nanoparticles, nanosuspension, nanoemulsion, liposomes, dendrimers, nanomicelles, and drug–polymer conjugates. Colloidal dosage forms offer several advantages over conventional dosage forms, such as sustained and tissue targeted drug delivery, ability to overcome drug efflux, increase drug stability, and reduce dosing frequency. Recently, published patents on nanotechnology-based ocular drug delivery have been discussed in various review articles.94,95 In the current review, research articles and patents on colloidal dosage forms in the treatment of anterior chamber inflammations are discussed in the following section of this review article.
Polymeric nanoparticles
The polymeric nanoparticulate system comprises particles in the range of 1–1,000 nm in which the parent drug is adsorbed, entrapped, conjugated, or encapsulated. The nanoparticulate system can be an alternative to address irritation and toxicity related issues of liposomes and dendrimers. Aqueous or nonaqueous suspension of drug-loaded nanoparticles can be administered in the cul-de-sac to achieve sustained drug delivery, which can eliminate frequent drug administration. Moreover, the active drug can be slowly released by diffusion, dissolution, or mechanical disintegration and/or erosion of the polymer matrix. Moreover, nanoparticles can circumvent the limitation of poor solubility of anti-inflammatory agents, and also protect an active agent from chemical and enzymatic degradations. These advantages make the nanoparticle a better candidate for anterior segment delivery of the anti-inflammatory agent.
Various biodegradable and nonbiodegradable polymeric systems have been developed for sustained delivery of NSAIDs, such as ibuprofen, flurbiprofen, and indomethacin in the treatment of anterior chamber inflammations (Table 2). In a recently published study, investigators have utilized Eudragit RS100® to prepare ibuprofen nanoparticles for inhibition of an inflammatory response to surgical trauma.96 Results from in vivo efficacy studies performed on the rabbit eye model demonstrated a significantly higher aqueous humor concentration than the control aqueous eye drop. Similar studies were performed with flurbiprofen as an active agent for the prevention of myosis induced by extracapsular cataract surgery.97 A higher interaction of positively charged nanoparticles (zeta potential+40–60 mV) with an anionic corneal surface was observed.98,99 A higher precorneal retention achieved with controlled release formulation was noted to be the primary reason for improvement in flurbiprofen ocular bioavailability. Ocular applications of indomethacin are overshadowed by its poor availability. In an attempt to improve ocular bioavailability, Calvo et al. have examined 3 different colloidal carrier systems, that is, nanoparticles, nanocapsules, and nanoemulsions.100 Results of ex vivo transport studies across the excised rabbit cornea demonstrated higher indomethacin ocular bioavailability, due to the colloidal nature of the carrier system. Two different biodegradable polymers (PLGA and PCL) were utilized to formulate flurbiprofen-encapsulated nanoparticles to improve ocular availability.88 Significantly enhanced corneal transport of flurbiprofen was observed in case of a nanocarrier system relative to free drug. Moreover, PLGA nanoparticles demonstrated ∼2-fold higher transport of flurbiprofen compared with the PCL nanoparticles. Subsequently, flurbiprofen-loaded PLGA nanoparticles were prepared and evaluated by Vega et al.101 Incorporation of Poloxamer 188 in nanoparticle preparation has significantly improved stability of nanoparticles. Moreover, topical instillation of nanoparticle formulation in the rabbit eye, enhanced anti-inflammatory efficacy without any signs of irritation or toxicity to ocular tissues. Improved efficacy could be due to an improvement in the bioadhesive property of nanoparticles.
Table 2.
Summary of Delivery Systems Investigated for Anterior Ocular Inflammations
| Delivery system | Drug | Key component | Conclusions/remarks | References |
|---|---|---|---|---|
| Nanoparticles | Ibuprofen | Eudragit RS100® | Significant improvement of drug bioavailability in rabbit model, relative to control aqueous drops | 96 |
| Flurbiprofen | Eudragit RS100, Eudragit RL100® | Improved ocular bioavailability was observed due to strong interaction between+ve charged nanoparticle to the anionic corneal surface | 97 | |
| Indomethacin | PCL, Migliol 840, Poloxamer 188 | Colloidal formulation showed 3-fold higher ex vivo penetration than commercial eye drops | 100 | |
| Flurbiprofen | PLGA, PCL | Colloidal systems have improved drug ocular bioavailability, moreover, PLGA nanoparticles showed ∼2-fold higher drug transport than that of PCL nanoparticles | 88 | |
| Flurbiprofen | PLGA, Poloxamer 188 | Enhanced anti-inflammatory effect was demonstrated and that may be due to improved bioadhesiveness of nanoparticles | 101 | |
| CS-A | Chitosan | Chitosan nanoparticles have selectively improved CS-A concentration in cornea (∼2-folds) and in conjunctiva (∼4-folds) relative to topical drops of chitosan solution or CS-A suspension | 102 | |
| CS-A | Cholesterol-conjugated chitosan | Improved precorneal retention of CS-A-loaded nanoparticles was observed | 103 | |
| Rapamycin | Chitosan, Poly lactic acid | Corneas of the rapamycin-loaded nanoparticle-treated rabbit group were found clear and no blood vessels or inflammation were observed | 104 | |
| Prednisolone, Gatifloxacin | Eudragit RS100, Eudragit RL100, Hyaluronic acid | Ocular bioavailability of gatifloxacin was significantly enhanced | 105 | |
| Indomethacin, Cs-A | PCL, Chitosan, Lecithin | Specific ingredients like, chitosan and lecithin have provided+ve charge to nanoparticles and also contributed in the stabilization of nanoparticles | 106 | |
| Nanosuspension | Indomethacin | Sesame oil | Both 1% aqueous and 1% oil suspension demonstrated higher concentrations of indomethacin in human aqueous humors, noticeably, oil suspension has shown higher aqueous humor levels (429 ng/mL) relative to aqueous suspension (198 ng/mL) | 122 |
| Prednisolone, Hydrocortisone, Dexamethasone | Pluronic F68 | Delivery of nanosuspensions demonstrated higher levels of glucocorticoids in aqueous chamber compared to solutions or microcrystalline suspension of respective drugs | 123 | |
| Diclofenac, CS-A | Sophisen | Nanosuspension of diclofenac has improved precorneal residence time of therapeutic agent and nanosuspension of CS-A has improved tear production from baseline 5 mm to 11 mm | 124 | |
| Rofecoxib | Polystyrene, Poloxamer-407, Hydroxypropylmethyl cellulose | Drug levels in anterior chamber ocular tissues, such as cornea (∼6610 ng/gram tissue) and aqueous humor (∼251 ng/gram tissue), were significantly higher after topical instillation of nanosuspension | 125 | |
| Nanoemulsion | Indomethacin | Chitosan | Application of nanoemulsion has significantly improved levels of indomethacin in cornea and aqueous humor of rabbit eyes | 126 |
| CS-A | Kelcogel® | Microemulsion-Kelcogel system demonstrated ∼3-fold higher levels of CS-A relative to CS-A microemulsion | 127 | |
| Diclofenac | N-octenylsuccinate starch | Improved permeability across excised porcine cornea was observed | 128 | |
| Indomethacin | Lauroamphodiacetate | Nanoemulsion has enhanced transcorneal permeability of indomethacin by 3.8 times relative to marketed formulation (Indocollyre®) | 129 | |
| CS-A | Stearylamine | Positively charged lipid increased residence time of the drug | 130 | |
| Nanomicelles | Dexamethasone | Pluronic/chitosan system | Nanomicelles entrapping dexamethasone have significantly improved bioavailability to anterior ocular tissues by 2.4-fold relative to unformulated dexamethasone | 113 |
| Voclosporine, Dexamethasone, Rapamycin | Vitamin E TPGS and octoxynol-40 | In vivo studies showed improved bioavailability with topical dosing of dexamethasone- and rapamycin-loaded mixed nanomicellar system | 166 | |
| Improved bioavailability of voclosporine with mixed nanomicellar system | 120 | |||
| CS-A | methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) | Transparent, highly stable, biocompatible formulation | 112 | |
| Pilocarpine | pluronic F127 (poly(oxyethylene)/poly(oxypropylene)/poly (oxyethylene) | Significant prolongation of miotic activity with an improved area under the curve of 64% | 117 | |
| Plasmid DNA with lacZ gene | PEO-PPO-PEO | Significant elevation of β-gal activity, transgene expression marker, elevated mRNA levels of bcl-x(L) by 2.2-fold and reduced corneal apoptosis in mouse and rabbit cornea. | 115 | |
| Liposomes | C6-ceramide | 1,2-disteoroyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy PEG(2000)], and PEG(750)-C6-ceramide | Significantly efficacious in reducing corneal inflammation | 135 |
| Dexamethasone | human serum albumin; bis(sulfosuccinimidyl) suberate; Tris(hydroxymethyl) aminomethane; 3,3-dithiobis-(sulfosuccinimidylpropionate); SLX, Sialyl Lewis X | Significantly higher drug concentrations in the eye (∼13.5 ng mg tissue−1) than unformulated drug (2.4 ng mg tissue−1) | 136 | |
| Cubosomes | Dexamethasone | Poloxamer 407, Carboxy methyl cellulose sodium, Carbopol 974 | After delivery of dexamethasone cubosomes, apperent permeability of drug through cornea was increased up to 3.5–4.5 times, moreover AUC0→240min of dexamethasone in aqueous humor was enhanced up to 1.8- and 8-folds relative to drug solution and suspension, respectively. | 144 |
AUC, area under the curve; CS-A, cyclosporin-A; PCL, poly-ɛ caprolactone; PEG, Polyethylene glycol; PLGA, poly(lactide-co-glycolide).
Cyclosporin-A (CS-A)-loaded chitosan nanoparticles were successfully prepared and evaluated for topical ocular applications.102 Significantly positive zeta potential and a smaller particle size improved precorneal retention of nanoparticles. In vivo studies have revealed that topical instillation of chitosan nanoparticles can selectively increase CS-A levels in the cornea (2-fold higher) and in the conjunctiva (∼4-fold higher) relative to topical eye drops of the chitosan solution or aqueous suspension of CS-A. Cholesterol-conjugated hydrophobically modified chitosan was utilized to prepare CS-A encapsulated nanoparticles.103 Higher nanoparticle retention at the precorneal surface was confirmed by single photon emission computed tomography and scintillation counter measurement. In another study, the same research group has disclosed physical mixture of poly lactic acid (PLA)/chitosan to prepare rapamycin-loaded nanoparticles.104 Incorporation of PLA significantly improved nanoparticle encapsulation efficiency (∼13-fold) due to stronger hydrophobic interactions. In vivo studies were conducted in rabbits with topical dosing of rapamycin-loaded nanoparticles, empty nanoparticles, and no treatment. Post-treatment, inflammation or blood vessel development was monitored. Results for treatment with rapamycin-loaded nanoparticles demonstrated clear and transparent corneas. On the contrary, corneas, which received no treatment or empty nanoparticle treatment were found opaque with stromal edema and/or neovascularization, within first 10 days. In addition, rapamycin suspension exhibited some degree of inhibitory effect on neovascularization.
Prednisolone is one of the most effective agents in a group of glucocorticoids, and it is marketed as ocular suspensions and drops. This product inhibits a wide variety of inflammatory responses, such as fibrin disposition, leukocyte migration, fibroblast proliferation, edema, capillary dilation, and capillary proliferation. Gatifloxacin and prednisolone were simultaneously incorporated in mucoadhesive polymer (HA)-coated Eudragit nanoparticles (RS 100 and RL 100) in the treatment of bacterial keratitis.105 Noticeably, improved ocular bioavailability (corneal and aqueous humor) for gatifloxacin was observed after topical instillation of nanoparticle suspension. However, investigators did not evaluate ocular tissue distribution of prednisolone.
Recently, Alonso et al. have patented CS-A and indomethacin encapsulated PCL nanoparticles for ocular drug delivery.106 Specific ingredients such as chitosan and lecithin have provided positive charge to nanoparticles and also improved stability of the formulation. Recently, Mitra and Mishra developed pentablock copolymer system with both nanoparticle forming and thermosensitive gelling ability as a function of the polymer block ratio.107 These polymeric systems may be employed for treatment of chronic anterior ocular diseases.
Role of CD44 HA receptors, located on human corneal and conjunctival cells, in the uptake of hyaluronic acid-chitosan oligomer based nanoparticles (HA-CSO NPs) have been studied. As shown in Fig. 3, plasmid-loaded HA-CSO NPs undergo active transport mediated by CD44 HA receptors via caveolin-dependent endocytosis pathway.108 Confocal studies demonstrated involvement of CD44 HA receptors mediated fluidic endocytosis, internalizing the plasmid encapsulated HA-chitosan NPs.109 Similarly, Enriquez de Salamanca et al. documented the contribution of active transport mechanism for internalization of chitosan nanoparticles by human conjunctival epithelial cells.110 Current research is focused on utilizing various transporters or receptors expressed on the cell surface for active targeting. Targeting specific transporters or receptors with functionalized nanoparticles may facilitate enhanced uptake into ocular tissues. Kompella et al. studied the effect of surface functionalization on the uptake of nanoparticles employing ex-vivo bovine eye model.111 Nanoparticles surface functionalized with deslorelin, a luteinizing hormone-releasing hormone agonist, or transferrin demonstrated 64% and 74% higher transport respectively, relative to nonfunctionalized nanoparticles.
FIG. 3.
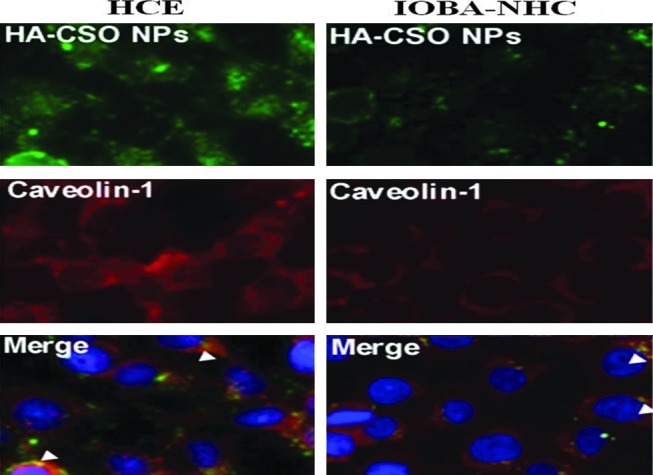
Caveolin-1 immunofluorescence in HCE (human corneal epithelial cell line) and IOBA-NHC (conjunctival epithelial cell line) cells after HA-CSO NP incubation. Merged images showed colocalization of HA-CSO NP with caveolin (staining at arrowheads). Reproduced from Contreras-Ruiz et al.108 HA, hyaluronic acid; HA-CSO NP, hyaluronic acid-chitosan oligomer-based nanoparticle. Color images available online at www.liebertpub.com/jop
Nanomicelles
Nanomicellar formulation is the most frequently utilized approach to formulate therapeutic agents in to a clear aqueous solution. Nanomicelles made from polymers such as N-isopropylacrylamide, vinyl pyrrolidone, acrylic acid, polyhydroxyehtyl aspartamide, poly(ethylene glycol)-hexylsubstituted poly(lactides) and surfactants such as Pluronic F127, Vitamin E TPGS and octoxynol-40 have been employed to encapsulate and deliver drugs and/or genes to anterior ocular tissues.112–119 In a recently published review Cholkar and Mitra have discussed in detail about methods for nanomicelle preparation and their applications in ocular drug delivery.94
Recently, Pepic et al. attempted to deliver the dexamethasone-encapsulated Pluronic/chitosan micellar system.113 It was observed that addition of chitosan to the pluronic micelle system improved in vitro drug release properties. In vivo studies conducted in rabbits demonstrated nanomicellar formulation increased AUC by 2.4-fold relative to dexamethasone suspension. Mitra et al. attempted to encapsulate and deliver voclosporin, dexamethasone, and rapamycin in a mixed nanomicellar system prepared from vitamin E TPGS and octoxynol-40. These formulations were clear/transparent and carried high drug payload. In vivo studies conducted in rabbits and canines exhibited enhanced drug bioavailability to anterior segment ocular tissues with no sign of ocular irritation or toxicity.94,120 In vivo single-dose pharmacokinetic studies with voclosporine containing nanomicellar formulation were conducted in rabbit animal models. Results demonstrated a better pharmacokinetic profile with mixed nanomicellar formulation in the anterior ocular tissues (Fig. 4).121
FIG. 4.
Anterior chamber pharmacokinetic study with voclosporin in New Zealand white rabbits with single topical drop administration of mixed nanomicellar formulation.121
In another study, methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) was employed to encapsulate and deliver CS-A. Results from in vitro and in vivo studies showed that the formulation was transparent, highly stable, biocompatible, and may be employed as eye drop formulations. Pilocarpine-entrapped pluronic F127 nanomicellar formulation was evaluated for anterior chamber treatment. This formulation showed significant prolongation of miotic activity with improvement in AUC by 64%.113
The nanomicellar approach to deliver genes to anterior ocular tissues is an emerging and promising area of research. Liaw and Robinson have explored the nonionic copolymeric system (PEO-PPO-PEO) for ocular gene delivery.93 Encapsulation of plasmid DNA with the lacZ gene showed stable and efficient delivery of cargo inside the cell. A polymeric system composed of PEO-PPO-PEO was also employed to deliver genes for cornea-specific promoters (keratin 12 and keratocan). In vivo studies with 6 doses of DNA-encapsulated micellar system demonstrated elevated levels of β-gal activity and transgene expression marker in the mouse and rabbit cornea. Following topical drop administration, the gene-encapsulated polymeric micellar system improved the mRNA levels of bcl-x(L) by 2.2-fold and reduced corneal apoptosis.115 From these studies, it is evident that an aqueous nanomicellar formulation of drug or genes can improve permeability in anterior tissues.
Nanosuspension
Nanosuspension is a colloidal dispersion of nanosized particles, which is stabilized by other excipients, such as surfactants, viscosity enhancers, or charge modifiers. Such a formulation can be prepared by pearl milling, high-pressure homogenization, and precipitation techniques. Topical delivery of 1% aqueous suspension and 1% oil suspension in human subjects have shown higher levels of indomethacin in aqueous humor relative to oral delivery.122 Notably, oil suspension has shown higher aqueous humor levels (429 ng mL−1) relative to aqueous suspension (198 ng mL−1).
Glucocorticoids are widely prescribed in the treatment of ophthalmic inflammations. However, poor aqueous solubility poses a challenge to ophthalmic formulation development. In a recently published report, Kassem et al. have prepared and evaluated nanosuspension formulation of prednisolone, hydrocortisone, and dexamethasone for topical ocular delivery.123 In vivo tissue distribution studies of the glucocorticoids nanosuspensions demonstrated significantly higher levels in anterior chamber tissues relative to solution and microcrystalline suspension of similar compounds. Moreover, investigators also showed direct relationship for nanosuspension viscosity and ocular bioavailability. Recently, a randomized double-blind clinical study of Sophisen derivatives, 3A Ofteno™ (1.0% diclofenac sodium w/v), and Modusik-A Ofteno (0.1% CS-A w/v) were performed in 120 healthy volunteers.124 Topical instillation of 3A Ofteno- diclofenac nanosuspension remained on the ocular surface for longer periods with less annoying sensation and irritation. Also, Modusik-A Ofteno- CS-A nanosuspension caused significant improvement in tear production from baseline 5 mm to 11 mm.
In a recent patent (WO 2006/062875) entitled, “Ophthalmic nanoparticulate formulation of a COX-2 selective inhibitor,” investigators have incorporated rofecoxib (COX-2 inhibitor) in the polystyrene nanoparticulate system for ophthalmic applications.125 Formulation prepared with Poloxamer-407 (0.05% w/w) and HPMC remained physically stable, without any change in particle size up to 4 weeks. Drug levels in anterior chamber ocular tissues, such as the cornea (∼6,610 ng gram tissue−1) and aqueous humor (∼251 ng/gram tissue) were significantly higher after topical instillation of nanosuspension. Readers are advised to read patent number WO 2006/062875 for more detailed information of in vivo tissue distribution studies.
Nanoemulsion
Nanoemulsion offers several advantages in ocular drug delivery, such as high capacity to dissolve both hydrophilic and lipophilic drugs, stability, improved bioavailability, and good spreadability. In addition, surfactants used in formulating emulsions can also act as penetration enhancers, thereby improving drug permeability across the cornea.
Chitosan, a cationic polymer, finds application in the field of ocular drug delivery due to its potential ability to enhance corneal drug permeability by opening tight junctions. It strongly interacts with negatively charged mucin and improves residence time on the precorneal surface. Indomethacin-embedded chitosan nanoemulsion was evaluated for its residence time and ability to deliver therapeutics into the anterior chamber of the eye.126 Topical application of nanoemulsion has significantly improved indomethacin levels in the cornea and aqueous humor of rabbit eyes relative to the indomethacin solution. Drug levels in the cornea and aqueous humor were about 12-fold higher for nanoemulsion relative to solution-treated eyes.
CS-A-loaded microemulsion in situ electrolyte triggered gelling system was developed by Gan et al.127 for the treatment of corneal allograft rejection. Microemulsion was dispersed in the Kelcogel® (deacetylated gellan gum) solution, which provided in situ gelling property when applied to the corneal surface. In vivo studies suggested that the microemulsion-Kelcogel system can generate ∼3-fold higher levels of CS-A relative to CS-A microemulsion even at 32 h postdosing. Moreover, concentration of CS-A in Kelcogel system-treated corneas were maintained at therapeutic levels with no ocular irritation, even after 32 h. In another study, n-octenylsuccinate starch was utilized to prepare the diclofenac solution and emulsion, and these formulations exhibited improved permeability across the excised porcine cornea compared to the commercial product Voltaren Ophtha.128 Indomethacin nanoemulsion prepared with amphoteric surfactant (lauroamphodiacetate) also improved corneal permeability by 3.8 times relative to a marketed product (Indocollyre®).129
In a recently published patent, CS-A was successfully incorporated in a nanoemulsion, utilizing positively charged polar lipid, such as stearylamine.130 Mean droplet size of the formulation was within the range of 150–250 nm, with zeta potential of 34–45 mV. Gan et al. have recently patented nanoemulsion-based in situ gelling system for topical ocular delivery of flurbiprofen.131 CS-A-loaded nanoemulsion (NOVA22007) containing cationic lipid formulation has just completed Phase III studies for dry eye.132 The same emulsion was applied for vernal keratoconjunctivitis treatment and this study has recently completed phase II/III.133
Liposomes
Liposomes are one of the effective drug carrier systems consisting of 2 compartments, that is, inner hydrophilic and peripheral lipophilic. This carrier system holds the ability to entrap both hydrophilic and lipophilic drugs and also allows surface modification with targeting agents. Although liposomes could entrap a wide range of molecules, their use is limited in topical ocular delivery because of short half life and relatively poor stability.134 Sun et al. attempted to entrap short-chain-conjugated ceramide, C6-ceramide, in nanoliposomes and utilized this formulation to treat corneal inflammations in murines.135 Ceramides are known for antiproliferative and proapoptotic sphingolipid metabolism. However, their role in inflammation is not yet clear. In this report, initially in vitro and in vivo formulation toxicities were reported in human corneal epithelial cells and a murine animal model. Results demonstrate that these formulations are safe and nontoxic. To test formulation efficacy in wound healing and lowering inflammations, corneal inflammation was induced with LPS or S. aureus stimulation after wounding corneas. Animals were treated with formulations before and after developing corneal wound and inducing inflammation. Treatment was given by administering blank liposomes or C6-ceramide-loaded liposomes. The C6-ceramide liposomal formulation showed significant efficacy in reducing corneal inflammation, whereas it did not show any effect in corneal wound healing. These studies indicated the positive role of short-chain ceramide-loaded liposomes in the treatment of anterior chamber inflammations.
In another study, Arakawa et al. investigated dexamethasone-loaded sugar-chain surface-modified liposomes for anterior chamber ocular inflammations.136 Because of specific recognition and binding between lectin and sugar chain,137 authors tried to utilize sugar as a targeting moiety toward ocular inflammations. Tumor inflammation targeting by sugar molecule was shown by Yamazaki et al.138 The Sialysl-Lewis X (sLex)-conjugated liposomal carrier system was developed for anterior chamber delivery of dexamethasone. In vivo biodistribution studies were conducted in 2 mice models (normal and inflammation induced mice) with intravenously administration of sLex surface-modified dexamethasone liposomes and unformulated dexamethasone (1 mg) as control. Administration of dexamethasone suspension was found to be nonspecific in its distribution and produced levels in all tissues (eye, brain, heart, lung, liver, kidney, spleen, and intestine). In both models, significantly low dexamethasone concentrations were detected in eye and all corresponding tissues. On the contrary, sugar-conjugated surface-modified dexamethasone-loaded liposomes demonstrated significantly higher drug concentrations in the eye (∼13.5 ng/mg tissue) with no drug detected in other body tissues. These results suggest the potential role of surface-modified liposomes in drug delivery to the anterior chamber for the treatment of ocular inflammations.136
Niosomes
Niosomes are a special type of nanovesicular carrier systems, similar to liposomes. These vesicles are comprised of amphiphilic nonionic surfactants with a size range of 10 to 1,000 nm. Such surfactants are biodegradable, biocompatible, and nonimmunogenic.139 Examples include, but not limited to, are poly(caprolactone) grafted silylated dextran,139 Tween 60, Tween 80, Brij 35,140 polyglycerol alkyl ether, glucosyl dialkyl ethers, ester linked surfactants, polyoxyethylene alkyl ether, crown ethers, and span 60.141,142 Niosomes may encapsulate both the hydrophilic and hydrophobic drugs and provide better chemical stability to the enclosed molecules. For topical ocular drug delivery, niosomes are highly preferable over other carrier systems. The reasons may be attributed to their high chemical stability, very low toxicity because of their nonionic nature, and easy handling. Also, nanovessels may improve bioavailability and control drug delivery at a specific ocular site.
Aggarwal and Kaur141 studied niosomal formulations of timolol maleate (TM) with surface coating of TM niosomes with chitosan and carbopol. The parameters under consideration were in vitro release and IOP lowering effect. In vitro release studies demonstrated 50% burst release followed by sustained release. A comparative in vivo pharmacodynamics study with unformulated TM and noisome-encapsulated TM for reducing IOP in albino rabbits was studied. High IOP reduction for up to 8 h was achieved with niosomal TM; whereas, the effect with the TM solution lasted for up to 2 h suggest a controlled release and a higher IOP reducing effect of TM when encapsulated in niosomes. Abdelbary and El-Gendy,140 developed niosomal formulations of a hydrophilic antibiotic, gentamicin, for topical ocular delivery. In vitro results revealed a high encapsulation efficiency of 92% with a prolonged release relative to the gentamicin solution (control). Three surfactants, namely, Tween 60, Tween 80, and Brij 35 were incorporated by varying the ratios of cholesterol and dicetyl phosphate (charge inducer). The encapsulation efficiency and gentamicin release rate were dependent on 3 factors, type of surfactant used, cholesterol content, and presence of dicetyl phosphate. Furthermore, in vivo toxicity studies were conducted in albino rabbits for 48 h. Results demonstrated no signs of redness, irritation, inflammation, or increased tear production. All the formulations were well tolerated suggesting that gentamicin niosomes may be used as a safe topical ocular drug delivery system. To treat glaucoma and to determine the IOP lowering effect, Prabu et al.139 studied feasibility of liposome and niosome as a vesicular carrier system for brimonidine tartarate and compared their efficacy with the marketed solution. Brimonidine tartarate carrier systems were in the size range of 210–245 nm, with a high drug loading of 42 wt%. Although these formulations were not as effective as the marketed solution in lowering IOP, they sustained brimonidine tartarate release and improved duration of activity (∼8-folds) relative to the marketed drug solution. Abdelkader et al.143 studied niosomes and discomes as carrier systems for naltrexone hydrochloride. The entrapment efficiency of niosomal formulation improved 5 times with the use of additives. In vitro results suggest that the ingredients played a significant role on controlling the size and drug release from niosomes. Ex vivo transcorneal permeability studies conducted across the bovine cornea exhibited that niosomes are capable of controlling drug release and improving corneal permeability. An ocular irritancy test of niosomes and discomes were conducted with hen's chorioallontoic membrane assay (HET-CAM assay). Results demonstrated no sign of irritation suggesting that niosomes can be regarded as safe carrier systems for controlled transcorneal delivery of drugs.
Cubosomes
Novel dexamethasone-embedded self-assembled liquid crystalline particles (cubosomes) were developed and investigated for precorneal retention and ocular tissue distribution.144 Apparent permeability coefficient of dexamethasone, delivered in cubosomes was 3.5–4.5 times higher than dexamethasone eye drops. In addition, precorneal retention of cubosmes was significantly longer than carbopol gel or solution. In vivo microdialysis studies were performed to evaluate pharmacokinetics of dexamethasone in aqueous humor. Delivery of cubosomes exhibited 1.8-fold and 8-fold higher AUC0→240min of dexamethasone in aqueous humor relative to eye drops and suspension, respectively. Moreover, tissue integrity and corneal structure indicated good biocompatibility of cubosome formulation.
Ocular implants and inserts
To overcome low availability and short activity of topical ophthalmic administration problems, implants were investigated as vehicles for sustained and local drug release. Drug release is influenced by the surface area of the implant and degradation pattern of polymers. These devices can be implanted into the eyelid or periocular muscle to treat conjunctival disorders, or directly into the anterior chamber. A variety of therapeutic agents, such as NSAIDs, angiogenesis inhibitors, and glucocorticoids, have been incorporated into polymeric implants.
Diclofenac sodium-loaded ophthalmic inserts prepared with sodium CMC (4%) and MC (1%) demonstrated controlled zero order in vivo release without any symptoms of irritation and inflammation.145 Baeyens et al. have simultaneously incorporated gentamicin and dexamethasone in ocular inserts and these devices were evaluated for in vivo drug release kinetics.146 This new drug delivery system ensured concomitant release of both drugs for the first 10 h of treatment and it was followed by gentamicin release to maintain levels above minimum inhibitory concentration for 50 h.
The CS-A-loaded polymeric implants inserted in the anterior chamber of rat eyes have a significantly improved corneal graft survival rate by ∼2 times compared to untreated and 1% CS-A solution-treated eyes. It even proved more effective than subconjunctival implantation of the same formulation.147 CS-A-loaded PLGA devices were implanted in the anterior chamber of rabbit eyes and evaluated for pharmacokinetic profiles.148 In vivo tissue distribution studies revealed significantly higher drug concentrations in the cornea (epithelium, corneal stroma, and endothelium) throughout the 3-month study period with no symptoms of adverse reactions.
Corneal transplantation was performed on the rat eye model and effects of dexamethasone-loaded ocular implants on the allograft survival rate were evaluated.149 Almost all the corneal grafts survived following treatment with dexamethasone ocular implants relative to betamethasone topical drops treated and untreated rat groups. A randomized comparative human clinical study was performed after intraocular lens implantation and phacoemulsification. Surodex® (dexamethasone-loaded biodegradable PLGA implants) was placed either in the anterior chamber (36 eyes) or in the sulcus (35 eyes) and results were compared with 0.1% dexamethasone eye drop (4 times/day) treatment. However, the outcome of the study demonstrated no significant differences in complications or efficacy between the anterior chamber or sulcus implantations.150–151 In another study, Kodama et al. investigated anti-inflammatory activity of Surodex in 2 experimental intraocular inflammation model; experimental autoimmune uveoretinitis and endotoxin-induced uveitis.152 Treatment with Surodex exhibited significantly reduced inflammation in both animal models.
FK-506 is a macrolide antibiotic indicated in the regulation of immune response during human organ transplantation and is 10–100 times more potent than CS-A.153,154 FK-506-loaded ocular implants were evaluated for corneal allograft rejection in the rabbit eye model and results were compared with the untreated, FK-506 eye drops treated and placebo-implanted animals.155 A biopolymer utilized in implant backbone was biodegradable, disintegrated slowly with time and eventually maintained sustained drug release. Levels of FK-506 in aqueous humor were maintained for at least 168 days without any adverse reactions. More importantly, mean graft survival time was longest (>180 days) in FK-506-loaded implants in comparison to other treatments. As anticipated, untreated and placebo-implanted animals showed opacity and neovascularization within 28 days.
In a recently published patent, US 7846468, Wong et al. have discussed methods for preventing or reducing ocular transplant rejection, by implantation of immunosuppressive agent-embedded biopolymeric implants.156 For example, inventors have prepared dexamethasone-embedded implants with PLGA and HPMC as polymeric matrices. These devices were implanted into the anterior chamber and evaluated for its efficacy to prevent corneal allograft rejection (neovascularization and edema) in a rat eye model. Moreover, results were compared with efficacy of dexamethasone topical drops and also with the control group (without any treatment). After 2 weeks, the control group showed 100% corneal rejection and after 6 weeks, 80% of corneal rejections were observed in topical eye drop-treated animals. Contrary to this observation, animals treated with dexamethasone implant showed no signs of neovascularization or edema even after 8 weeks of surgery. In a novel approach patented by Robinson et al., a rapid release loading dose and sustained release maintenance dose were simultaneously incorporated in a single device.157 The release kinetics of maintenance dose was controlled by superhydrolyzed PVA. According to inventors, CS-A-loaded device can be implanted subconjunctivally to reduce rejection rates. Collectively, these patents show that ocular implants can maintain therapeutic levels in the anterior chamber and reduce or prevent corneal allograft rejection and/or postoperative inflammations.
Conclusions
Topical administration is a widely acceptable method of drug delivery to treat anterior chamber inflammations. For topically administered drug solutions/formulations, the amount of administered drug loss overweighs the amount of drug absorbed into ocular tissues. Such loss is attributed due to static (corneal epithelium, corneal stroma, and blood–aqueous barrier) and dynamic (tear drainage, conjunctival blood and lymph flow) barriers. To overcome these ocular barriers, research is being conducted to derivatize parent drug to generate a therapeutically inactive, but more bioavailable prodrug. Application of nanotechnology to protect active molecule and/or to sustain drug delivery is being actively pursued. Nanotechnology and prodrug-based drug delivery strategies offer considerable therapeutic benefits in terms of reduced dosing frequency, avoid/minimize drug-induced systemic toxicity, and nonspecific drug delivery. Within past 2 decades, significant progress in ocular research has been made to develop successful, patient-compliant drug delivery systems, such as targeted prodrugs, nanoparticles, thermosensitive gels, nanomicelles, niosomes, liposomes, nanosuspensions, nanoemulsions, cubosomes, and ocular implants. With the advent of nanotechnology in ocular research, patient body is benefited with minimal/no drug-induced toxicities and vision loss. However, there is still a need for developing an efficient formulation strategy to sustain drug delivery in the treatment of chronic anterior chamber inflammatory diseases. With the current pace of ophthalmic drug delivery research, an efficient delivery system may be available in the near future to treat various anterior chronic ocular inflammations.
Acknowledgments
This work was supported by NIH (grants R01 EY 09171-16 and R01 EY 10659-14).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McCannel C.A. Holland G.N. Helm C.J. Cornell P.J. Winston J.V. Rimmer T.G. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am. J. Ophthalmol. 1996;121:35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 2.Ueta M. Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog. Retin. Eye Res. 2012;31:551–575. doi: 10.1016/j.preteyeres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Stein-Streilein J. Streilein J.W. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int. Rev. Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 4.Lapalus P. Moulin G. Bayer V. Fredj-Reygrobellet D. Elena P.P. Effects of a new anti-allergic agent: the magnesium salt of N-acetyl-aspartyl-glutamic acid on experimental allergic inflammation of the rabbit eye. Curr. Eye Res. 1986;5:517–522. doi: 10.3109/02713688608996374. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson V.M. Spalton D.J. Recovery of the blood-aqueous barrier after cataract surgery. Br. J. Ophthalmol. 1991;75:106–110. doi: 10.1136/bjo.75.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abelson M.B. Schaefer K. Conjunctivitis of allergic origin: immunologic mechanisms and current approaches to therapy. Surv. Ophthalmol. 1993;38(Suppl):115–132. doi: 10.1016/0039-6257(93)90036-7. [DOI] [PubMed] [Google Scholar]
- 7.Aswani Dutt Vadlapudi A.P. Cholkar K. Ashim K. Mitra recent patents on emerging therapeutics for the treatment of glaucoma, age related macular degeneration and uveitis. Recent Pat. Biomed. Eng. 2012;5:83–101. doi: 10.2174/1874764711205010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvvuri S. Majumdar S. Mitra A.K. Role of metabolism in ocular drug delivery. Curr. Drug Metab. 2004;5:507–515. doi: 10.2174/1389200043335342. [DOI] [PubMed] [Google Scholar]
- 9.Dartt D.A. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog. Retin. Eye Res. 2002;21:555–576. doi: 10.1016/s1350-9462(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee V.H. Robinson J.R. Prelminary examination of rabbit conjunctival mucins. J. Pharm. Sci. 1980;69:430–438. doi: 10.1002/jps.2600690418. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan B.D. Jakobiec F.A. Iwamoto T. Conjunctiva. Philadelphia: Harper and Row; 1982. [Google Scholar]
- 12.Shi X.P. Candia O.A. Active sodium and chloride transport across the isolated rabbit conjunctiva. Curr. Eye Res. 1995;14:927–935. doi: 10.3109/02713689508995132. [DOI] [PubMed] [Google Scholar]
- 13.Huang A.J. Tseng S.C. Kenyon K.R. Paracellular permeability of corneal and conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 1989;30:684–689. [PubMed] [Google Scholar]
- 14.Horibe Y. Hosoya K. Kim K.J. Ogiso T. Lee V.H. Polar solute transport across the pigmented rabbit conjunctiva: size dependence and the influence of 8-bromo cyclic adenosine monophosphate. Pharm. Res. 1997;14:1246–1251. doi: 10.1023/a:1012123411343. [DOI] [PubMed] [Google Scholar]
- 15.Hamalainen K.M. Kananen K. Auriola S. Kontturi K. Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest. Ophthalmol. Vis. Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- 16.Gukasyan H.J. Yerxa B.R. Pendergast W. Lee V.H. Metabolism and transport of purinergic receptor agonists in rabbit conjunctival epithelial cells. Adv. Exp. Med. Biol. 2002;506:255–259. doi: 10.1007/978-1-4615-0717-8_35. [DOI] [PubMed] [Google Scholar]
- 17.Yang J.J. Kim K.J. Lee V.H. Role of P-glycoprotein in restricting propranolol transport in cultured rabbit conjunctival epithelial cell layers. Pharm. Res. 2000;17:533–538. doi: 10.1023/a:1007508714259. [DOI] [PubMed] [Google Scholar]
- 18.Azartash K. Kwan J. Paugh J.R. Nguyen A.L. Jester J.V. Gratton E. Pre-corneal tear film thickness in humans measured with a novel technique. Mol. Vis. 2011;17:756–767. [PMC free article] [PubMed] [Google Scholar]
- 19.Prydal J.I. Campbell F.W. Study of precorneal tear film thickness and structure by interferometry and confocal microscopy. Invest. Ophthalmol. Vis. Sci. 1992;33:1996–2005. [PubMed] [Google Scholar]
- 20.Prydal J.I. Muir M.G. Dilly P.N. Comparison of tear film thickness in three species determined by the glass fibre method and confocal microscopy. Eye (Lond). 1993;7(Pt 3):472–475. doi: 10.1038/eye.1993.96. [DOI] [PubMed] [Google Scholar]
- 21.Mishima S. Gasset A. Klyce S.D., Jr. Baum J.L. Determination of tear volume and tear flow. Invest. Ophthalmol. 1966;5:264–276. [PubMed] [Google Scholar]
- 22.Scherz W. Doane M.G. Dohlman C.H. Tear volume in normal eyes and keratoconjunctivitis sicca. Albrecht Von Graefes Arch. Klin. Exp Ophthalmol. 1974;192:141–150. doi: 10.1007/BF00410700. [DOI] [PubMed] [Google Scholar]
- 23.Holly F.J. Lemp M.A. Tear physiology and dry eyes. Surv. Ophthalmol. 1977;22:69–87. doi: 10.1016/0039-6257(77)90087-x. [DOI] [PubMed] [Google Scholar]
- 24.van Setten G.B. Schultz G.S. Macauley S. Growth factors in human tear fluid and in lacrimal glands. Adv. Exp. Med. Biol. 1994;350:315–319. doi: 10.1007/978-1-4615-2417-5_53. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi Y. Motokura M. Kinoshita Y. Mano T. Watanabe H. Kinoshita S. Manabe R. Oshiden K. Yanaihara C. Presence of epidermal growth factor in human tears. Invest. Ophthalmol. Vis. Sci. 1989;30:1879–1882. [PubMed] [Google Scholar]
- 26.van Setten G.B. Tervo T. Viinikka L. Perheentupa J. Tarkkanen A. Epidermal growth factor in human tear fluid: a minireview. Int. Ophthalmol. 1991;15:359–362. doi: 10.1007/BF00137945. [DOI] [PubMed] [Google Scholar]
- 27.Prost M. [Formation and structure of lacrimal film on the ocular surface] Klin. Oczna. 1989;91:29–31. [PubMed] [Google Scholar]
- 28.Hopkins G.A. Pearson R.M. General Pharmacological Principles. United Kingdom, Oxford: Butterworth-Heinemann; 1988. [Google Scholar]
- 29.Hitoshi Sasaki K.Y. Nishida K. Nakamura J. Ichikawa M. Delivery of drugs to the eye by topical application. Prog. Retin. Eye Res. 1996;15:583–620. [Google Scholar]
- 30.Schoenwald R.D. Ocular drug delivery. Pharmacokinetic considerations. Clin. Pharmacokinet. 1990;18:255–269. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lee V.H. Robinson J.R. Topical ocular drug delivery: recent developments and future challenges. J. Ocul. Pharmacol. 1986;2:67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 32.Chrai S.S. Patton T.F. Mehta A. Robinson J.R. Lacrimal and instilled fluid dynamics in rabbit eyes. J. Pharm. Sci. 1973;62:1112–1121. doi: 10.1002/jps.2600620712. [DOI] [PubMed] [Google Scholar]
- 33.Chrai S.S. Makoid M.C. Eriksen S.P. Robinson J.R. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J. Pharm. Sci. 1974;63:333–338. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed I. The Noncorneal Route in Ocular Drug Delivery. New York: Marcel Dekker, Inc.; 2003. [Google Scholar]
- 35.Rozsa A.J. Beuerman R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 36.Hornof M. Toropainen E. Urtti A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005;60:207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Huang H.S. Corneal penetration behavior of beta-blocking agents III: in vitro-in vivo correlation. J. Pharm. Sci. 1983;72:1279–1281. doi: 10.1002/jps.2600721110. [DOI] [PubMed] [Google Scholar]
- 38.Jorge F. The Corneal Endothelium. Elsevier; Amsterdam, the Netherlands: 2005. [Google Scholar]
- 39.Karla P.K. Earla R. Boddu S.H. Johnston T.P. Pal D. Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr. Eye Res. 2009;34:1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karla P.K. Pal D. Quinn T. Mitra A.K. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 2007;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karla P.K. Pal D. Mitra A.K. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp. Eye Res. 2007;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Dey S. Patel J. Anand B.S. Jain-Vakkalagadda B. Kaliki P. Pal D. Ganapathy V. Mitra A.K. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 43.Kaur I.P. Kanwar M. Ocular preparations: the formulation approach. Drug Dev. Ind. Pharm. 2002;28:473–493. doi: 10.1081/ddc-120003445. [DOI] [PubMed] [Google Scholar]
- 44.Anand B.S. Dey S. Mitra A.K. Current prodrug strategies via membrane transporters/receptors. Expert Opin. Biol. Ther. 2002;2:607–620. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- 45.Rautio J. Kumpulainen H. Heimbach T. Oliyai R. Oh D. Jarvinen T. Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 46.Majumdar S. Mitra A.K. Chemical modification and formulation approaches to elevated drug transport across cell membranes. Expert Opin. Drug Deliv. 2006;3:511–527. doi: 10.1517/17425247.3.4.511. [DOI] [PubMed] [Google Scholar]
- 47.Majumdar S. Duvvuri S. Mitra A.K. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv. Drug Deliv. Rev. 2004;56:1437–1452. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Stratford R.E., Jr. Lee V.H. Ocular aminopeptidase activity and distribution in the albino rabbit. Curr. Eye Res. 1985;4:995–999. doi: 10.3109/02713689509000007. [DOI] [PubMed] [Google Scholar]
- 49.Lee V.H. Esterase activities in adult rabbit eyes. J. Pharm. Sci. 1983;72:239–244. doi: 10.1002/jps.2600720310. [DOI] [PubMed] [Google Scholar]
- 50.Talluri R.S. Hariharan S. Karla P.K. Mitra A.K. Patricia D'Amore. Reza Dana. “Drug delivery to cornea and conjunctiva-esterase and protease directed prodrug design, in Ocular Periphery and Disorders,”. In: Darlene A. Dartt., editor; Peter Bex., editor; Linda Mcloon., editor; Jerry Niederkorn., editor. San Deigo, California, USA: Elsevier, Academic Press; 2011. pp. 42–53. [Google Scholar]
- 51.Chien D.S. Tang-Liu D.D. Woodward D.F. Ocular penetration and bioconversion of prostaglandin F2alpha prodrugs in rabbit cornea and conjunctiva. J. Pharm. Sci. 1997;86:1180–1186. doi: 10.1021/js950373b. [DOI] [PubMed] [Google Scholar]
- 52.Chien D.S. Sasaki H. Bundgaard H. Buur A. Lee V.H. Role of enzymatic lability in the corneal and conjunctival penetration of timolol ester prodrugs in the pigmented rabbit. Pharm. Res. 1991;8:728–733. doi: 10.1023/a:1015845916293. [DOI] [PubMed] [Google Scholar]
- 53.Chang S.C. Bundgaard H. Buur A. Lee V.H. Improved corneal penetration of timolol by prodrugs as a means to reduce systemic drug load. Invest. Ophthalmol. Vis. Sci. 1987;28:487–491. [PubMed] [Google Scholar]
- 54.Hughes P.M. Mitra A.K. Effect of acylation on the ocular disposition of acyclovir. II: Corneal permeability and anti-HSV 1 activity of 2′-esters in rabbit epithelial keratitis. J. Ocul. Pharmacol. 1993;9:299–309. doi: 10.1089/jop.1993.9.299. [DOI] [PubMed] [Google Scholar]
- 55.Hughes P.M. Krishnamoorthy R. Mitra A.K. Effect of acylation on the ocular disposition of acyclovir. I: Synthesis, physicochemical properties, and antiviral activity of 2′-esters. J. Ocul. Pharmacol. 1993;9:287–297. doi: 10.1089/jop.1993.9.287. [DOI] [PubMed] [Google Scholar]
- 56.Anand B.S. Mitra A.K. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm. Res. 2002;19:1194–1202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- 57.Gunda S. Hariharan S. Mitra A.K. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): in vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J. Ocul. Pharmacol. Ther. 2006;22:465–476. doi: 10.1089/jop.2006.22.465. [DOI] [PubMed] [Google Scholar]
- 58.Katragadda S. Talluri R.S. Mitra A.K. Modulation of P-glycoprotein-mediated efflux by prodrug derivatization: an approach involving peptide transporter-mediated influx across rabbit cornea. J. Ocul. Pharmacol. Ther. 2006;22:110–120. doi: 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- 59.Vadlapudi A.D. Vadlapatla R.K. Mitra A.K. Current and emerging antivirals for the treatment of cytomegalovirus (CMV) retinitis: an update on recent patents. Recent Pat. Antiinfect. Drug Discov. 2012;7:8–18. doi: 10.2174/157489112799829765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majumdar S. Nashed Y.E. Patel K. Jain R. Itahashi M. Neumann D.M. Hill J.M. Mitra A.K. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. J. Ocul. Pharmacol. Ther. 2005;21:463–474. doi: 10.1089/jop.2005.21.463. [DOI] [PubMed] [Google Scholar]
- 61.Anand B. Nashed Y. Mitra A. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. Curr. Eye Res. 2003;26:151–163. doi: 10.1076/ceyr.26.3.151.14893. [DOI] [PubMed] [Google Scholar]
- 62.Katragadda S. Talluri R.S. Pal D. Mitra A.K. Identification and characterization of a Na+-dependent neutral amino acid transporter, ASCT1, in rabbit corneal epithelial cell culture and rabbit cornea. Curr. Eye Res. 2005;30:989–1002. doi: 10.1080/02713680500306439. [DOI] [PubMed] [Google Scholar]
- 63.Jain-Vakkalagadda B. Pal D. Gunda S. Nashed Y. Ganapathy V. Mitra A.K. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol. Pharm. 2004;1:338–346. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- 64.Jain-Vakkalagadda B. Dey S. Pal D. Mitra A.K. Identification and functional characterization of a Na+-independent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest. Ophthalmol. Vis. Sci. 2003;44:2919–2927. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- 65.Katragadda S. Gunda S. Hariharan S. Mitra A.K. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int. J. Pharm. 2008;359:15–24. doi: 10.1016/j.ijpharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Civiale C. Bucaria F. Piazza S. Peri O. Miano F. Enea V. Ocular permeability screening of dexamethasone esters through combined cellular and tissue systems. J. Ocul. Pharmacol. Ther. 2004;20:75–84. doi: 10.1089/108076804772745482. [DOI] [PubMed] [Google Scholar]
- 67.Horibe Y. Hosoya K. Kim K.J. Lee V.H. Carrier-mediated transport of monocarboxylate drugs in the pigmented rabbit conjunctiva. Invest. Ophthalmol. Vis. Sci. 1998;39:1436–1443. [PubMed] [Google Scholar]
- 68.Turner H.C. Alvarez L.J. Bildin V.N. Candia O.A. Immunolocalization of Na-K-ATPase, Na-K-Cl and Na-glucose cotransporters in the conjunctival epithelium. Curr. Eye Res. 2000;21:843–850. doi: 10.1076/ceyr.21.5.843.5532. [DOI] [PubMed] [Google Scholar]
- 69.Hosoya K. Lee V.H. Kim K.J. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur. J. Pharm. Biopharm. 2005;60:227–240. doi: 10.1016/j.ejpb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Prausnitz M.R. Noonan J.S. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 71.Vulovic N. Primorac M. Stupar M. Brown M.W. Ford J.L. Some studies on the preservation of indometacin suspensions intended for ophthalmic use. Pharmazie. 1990;45:678–679. [PubMed] [Google Scholar]
- 72.Meseguer G. Buri P. Plazonnet B. Rozier A. Gurny R. Gamma scintigraphic comparison of eyedrops containing pilocarpine in healthy volunteers. J. Ocul. Pharmacol. Ther. 1996;12:481–488. doi: 10.1089/jop.1996.12.481. [DOI] [PubMed] [Google Scholar]
- 73.Lapcik L., Jr. Lapcik L. De Smedt S. Demeester J. Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem. Rev. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 74.Kaur I.P. Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002;28:353–369. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 75.Gebhardt B.M. Varnell E.D. Kaufman H.E. Cyclosporine in collagen particles: corneal penetration and suppression of allograft rejection. J. Ocul. Pharmacol. Ther. 1995;11:509–517. doi: 10.1089/jop.1995.11.509. [DOI] [PubMed] [Google Scholar]
- 76.Dimitrova E. Bogdanova S. Mitcheva M. Tanev I. Minkov E. Development of model aqueous ophthalmic solution of indomethacin. Drug Dev. Ind. Pharm. 2000;26:1297–1301. doi: 10.1081/ddc-100102312. [DOI] [PubMed] [Google Scholar]
- 77.Chetoni P. Panichi L. Burgalassi S. Benelli U. Saettone M.F. Pharmacokinetics and anti-inflammatory activity in rabbits of a novel indomethacin ophthalmic solution. J. Ocul. Pharmacol. Ther. 2000;16:363–372. doi: 10.1089/jop.2000.16.363. [DOI] [PubMed] [Google Scholar]
- 78.Bucolo C. Spadaro A. Pharmacological evaluation of anti-inflammatory pyrrole-acetic acid derivative eye drops. J. Ocul. Pharmacol. Ther. 1997;13:353–361. doi: 10.1089/jop.1997.13.353. [DOI] [PubMed] [Google Scholar]
- 79.Gao Y. Sun Y. Ren F. Gao S. PLGA-PEG-PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev. Ind. Pharm. 2010;36:1131–1138. doi: 10.3109/03639041003680826. [DOI] [PubMed] [Google Scholar]
- 80.Chen X. Li X. Zhou Y. Wang X. Zhang Y. Fan Y. Huang Y. Liu Y. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: preparation, characterization, and in vivo evaluation. J. Biomater. Appl. 2012;27:391–402. doi: 10.1177/0885328211406563. [DOI] [PubMed] [Google Scholar]
- 81.Qian Y. Wang F. Li R. Zhang Q. Xu Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev. Ind. Pharm. 2010;36:1340–1347. doi: 10.3109/03639041003801893. [DOI] [PubMed] [Google Scholar]
- 82.Boddu S.H. Jwala J. Vaishya R. Earla R. Karla P.K. Pal D. Mitra A.K. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J. Ocul. Pharmacol. Ther. 2010;26:37–48. doi: 10.1089/jop.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin H. Gong C. Shi S. Liu X. Wei Y. Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J. Biomed. Mater. Res. B Appl. Biomater. 2010;92:129–137. doi: 10.1002/jbm.b.31498. [DOI] [PubMed] [Google Scholar]
- 84.Cao Y. Zhang C. Shen W. Cheng Z. Yu L.L. Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release. 2007;120:186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Gratieri T. Gelfuso G.M. de Freitas O. Rocha E.M. Lopez R.F. Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and poloxamer/chitosan in situ forming gel. Eur. J. Pharm. Biopharm. 2011;79:320–327. doi: 10.1016/j.ejpb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Gupta H. Jain S. Mathur R. Mishra P. Mishra A.K. Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–515. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 87.Cao F. Zhang X. Ping Q. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Deliv. 2010;17:500–507. doi: 10.3109/10717544.2010.483255. [DOI] [PubMed] [Google Scholar]
- 88.Valls R. Vega E. Garcia M.L. Egea M.A. Valls J.O. Transcorneal permeation in a corneal device of non-steroidal anti-inflammatory drugs in drug delivery systems. Open Med. Chem. J. 2008;2:66–71. doi: 10.2174/1874104500802010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loftsson T SH. Hreinsdottir D. Konradsdottir F. Stefansson E. Dexamethasone delivery to posterior segment of the eye. J Incl. Phenom. Macrocycl. Chem. 2007;57:585–589. [Google Scholar]
- 90.Loftsson T. Stefánsson E. Cyclodextrin nanotechnology for ophthalmic drug delivery. 2011. p. US7893040.
- 91.Kararli T.T. Bandyopadhyay R. Singh S.K. Hawley L.C. Ophthalmic formulation of a selective cyclooxygenase-2 inhibitory drug. 2002. p. WO02005815.
- 92.Bary A.R. Tucker I.G. Davies N.M. Considerations in the use of hydroxypropyl-beta-cyclodextrin in the formulation of aqueous ophthalmic solutions of hydrocortisone. Eur. J. Pharm. Biopharm. 2000;50:237–244. doi: 10.1016/s0939-6411(00)00108-9. [DOI] [PubMed] [Google Scholar]
- 93.Kristinsson J.K. Fridriksdottir H. Thorisdottir S. Sigurdardottir A.M. Stefansson E. Loftsson T. Dexamethasone-cyclodextrin-polymer co-complexes in aqueous eye drops. Aqueous humor pharmacokinetics in humans. Invest. Ophthalmol. Vis. Sci. 1996;37:1199–1203. [PubMed] [Google Scholar]
- 94.Cholkar K. Patel A. Vadlapudi A.D. Mitra A.K. Novel Nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat. Nanomed. 2012;2:82–95. doi: 10.2174/1877912311202020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel S. Mitra A.K. An overview of recent patents on nanocarrier based ophthalmic drug delivery systems. Recent Pat. Nanomed. 2012;2:2–16. doi: 10.2174/1877912311202020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pignatello R. Bucolo C. Ferrara P. Maltese A. Puleo A. Puglisi G. Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur. J. Pharm. Sci. 2002;16:53–61. doi: 10.1016/s0928-0987(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 97.Pignatello R. Bucolo C. Spedalieri G. Maltese A. Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–3255. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 98.Liaw Y.R.J. Robinson J.R. The effect of drug charge type and charge density on corneal transport. Int. J. Pharm. 1992;88:111–124. [Google Scholar]
- 99.Rojanasakul Y. Wang L.Y. Bhat M. Glover D.D. Malanga C.J. Ma J.K. The transport barrier of epithelia: a comparative study on membrane permeability and charge selectivity in the rabbit. Pharm. Res. 1992;9:1029–1034. doi: 10.1023/a:1015802427428. [DOI] [PubMed] [Google Scholar]
- 100.Calvo P. Vila-Jato J.L. Alonso M.J. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J. Pharm. Sci. 1996;85:530–536. doi: 10.1021/js950474+. [DOI] [PubMed] [Google Scholar]
- 101.Vega E. Egea M.A. Valls O. Espina M. Garcia M.L. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J. Pharm. Sci. 2006;95:2393–2405. doi: 10.1002/jps.20685. [DOI] [PubMed] [Google Scholar]
- 102.De Campos A.M. Sanchez A. Alonso M.J. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001;224:159–168. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 103.Yuan X.B. Li H. Yuan Y.B. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohydr. Polym. 2006;65:337–345. [Google Scholar]
- 104.Yuan X.B. Yuan Y.B. Jiang W. Liu J. Tian E.J. Shun H.M. Huang D.H. Yuan X.Y. Li H. Sheng J. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int. J. Pharm. 2008;349:241–248. doi: 10.1016/j.ijpharm.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 105.Ibrahim H.K. El-Leithy I.S. Makky A.A. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol. Pharm. 2010;7:576–585. doi: 10.1021/mp900279c. [DOI] [PubMed] [Google Scholar]
- 106.Alonso F.M.J. Calvo S.P. Remunan L.C. Vila J.J.L. Stabilization of colloidal systems by the formation of ionic lipid-polysaccharide complexes. 2006. p. EP0771566.
- 107.Mitra A.K. Mishra G.P. Pentablock polymers, U.S. 2011/0250283. 2010. p. A1.
- 108.Contreras-Ruiz L. de la Fuente M. Parraga J.E. Lopez-Garcia A. Fernandez I. Seijo B. Sanchez A. Calonge M. Diebold Y. Intracellular trafficking of hyaluronic acid-chitosan oligomer-based nanoparticles in cultured human ocular surface cells. Mol. Vis. 2011;17:279–290. [PMC free article] [PubMed] [Google Scholar]
- 109.de la Fuente M. Seijo B. Alonso M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest. Ophthalmol. Vis. Sci. 2008;49:2016–2024. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 110.Enriquez de Salamanca A. Diebold Y. Calonge M. Garcia-Vazquez C. Callejo S. Vila A. Alonso M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest. Ophthalmol. Vis. Sci. 2006;47:1416–1425. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]
- 111.Kompella U.B. Sundaram S. Raghava S. Escobar E.R. Luteinizing hormone-releasing hormone agonist and transferrin functionalizations enhance nanoparticle delivery in a novel bovine ex vivo eye model. Mol. Vis. 2006;12:1185–1198. [PubMed] [Google Scholar]
- 112.Di Tommaso C. Torriglia A. Furrer P. Behar-Cohen F. Gurny R. Moller M. Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int. J. Pharm. 2011;416:515–524. doi: 10.1016/j.ijpharm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 113.Pepic I. Hafner A. Lovric J. Pirkic B. Filipovic-Grcic J. A nonionic surfactant/chitosan micelle system in an innovative eye drop formulation. J. Pharm. Sci. 2010;99:4317–4325. doi: 10.1002/jps.22137. [DOI] [PubMed] [Google Scholar]
- 114.Civiale C. Licciardi M. Cavallaro G. Giammona G. Mazzone M.G. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009;378:177–186. doi: 10.1016/j.ijpharm.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 115.Tong Y.C. Chang S.F. Liu C.Y. Kao W.W. Huang C.H. Liaw J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J. Gene Med. 2007;9:956–966. doi: 10.1002/jgm.1093. [DOI] [PubMed] [Google Scholar]
- 116.Usui T. Sugisaki K. Amano S. Jang W.D. Nishiyama N. Kataoka K. New drug delivery for corneal neovascularization using polyion complex micelles. Cornea. 2005;24:S39–S42. doi: 10.1097/01.ico.0000178738.29459.59. [DOI] [PubMed] [Google Scholar]
- 117.Pepic I. Jalsenjak N. Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int. J. Pharm. 2004;272:57–64. doi: 10.1016/j.ijpharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 118.Liaw J. Chang S.F. Hsiao F.C. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001;8:999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 119.Gupta A.K. Madan S. Majumdar D.K. Maitra A. Ketorolac entrapped in polymeric micelles: preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000;209:1–14. doi: 10.1016/s0378-5173(00)00508-1. [DOI] [PubMed] [Google Scholar]
- 120.Velagaleti P.R. Anglade E. Khan J. Gilger B.C. Mitra A.K. Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior and posterior segments of the eye. Drug Deliv. Technol. 2010;10:43–47. [Google Scholar]
- 121.Mitra A.K. Velagaleti P.R. Natesan S. Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors, US2011/0300195. 2011. p. A1.
- 122.Sanders D.R. Goldstick B. Kraff C. Hutchins R. Bernstein M.S. Evans M.A. Aqueous penetration of oral and topical indomethacin in humans. Arch. Ophthalmol. 1983;101:1614–1616. doi: 10.1001/archopht.1983.01040020616024. [DOI] [PubMed] [Google Scholar]
- 123.Kassem M.A. Abdel Rahman A.A. Ghorab M.M. Ahmed M.B. Khalil R.M. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007;340:126–133. doi: 10.1016/j.ijpharm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Quintana-Hau J.D. Cruz-Olmos E. Lopez-Sanchez M.I. Sanchez-Castellanos V. Baiza-Duran L. Gonzalez J.R. Tornero M. Mondragon-Flores R. Hernandez-Santoyo A. Characterization of the novel ophthalmic drug carrier Sophisen in two of its derivatives: 3A Ofteno and Modusik-A Ofteno. Drug Dev. Ind. Pharm. 2005;31:263–269. doi: 10.1081/ddc-52058. [DOI] [PubMed] [Google Scholar]
- 125.Santipharp P. Alani Laman L. Ophthalmic nanoparticulate formulation of a cyclooxygenase-2 selective inhibitor. 2006. WO 2006/062875.
- 126.Badawi A.A. El-Laithy H.M. El Qidra R.K. El Mofty H. El dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch. Pharm. Res. 2008;31:1040–1049. doi: 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- 127.Gan L. Gan Y. Zhu C. Zhang X. Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int. J. Pharm. 2009;365:143–149. doi: 10.1016/j.ijpharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Baydoun L. Muller-Goymann C.C. Influence of n-octenylsuccinate starch on in vitro permeation of sodium diclofenac across excised porcine cornea in comparison to Voltaren ophtha. Eur. J. Pharm. Biopharm. 2003;56:73–79. doi: 10.1016/s0939-6411(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 129.ShaulMuchtar M. Frucht-Pery J. Benita S. Ex-vivo permeation study of indomethacin from a submicron emulsion through albino rabbit cornea. J. Control. Release. 1997;44:55–64. [Google Scholar]
- 130.Benita S. Lambert G. Method and composition for dry eye treatment. 2003. p. US0108626.
- 131.Gan Y. Gan L. Shen J. Zhu C. A Ophthalmic flurbiprofen ester nano-emulsion in situ gel formulation and the preparation method thereof. 2010. p. WO2010048788.
- 132.Double-masked trial of NOVA22007 (Ciclosporin 0.1%) versus vehicle in patients with moderate to severe dry eye syndrome. http://www.clinicaltrials.gov/ct2/show/NCT00814515. Nov 27, 2012. http://www.clinicaltrials.gov/ct2/show/NCT00814515
- 133.Efficacy, tolerance of Nova22007 versus vehicle in patients with vernal keratoconjunctivitis (VKC) http://clinicaltrials.gov/ct2/show/NCT00328653. Nov 27, 2012. http://clinicaltrials.gov/ct2/show/NCT00328653
- 134.Law S.L. Huang K.J. Chiang C.H. Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and absorption. J Control. Release. 2000;63:135–140. doi: 10.1016/s0168-3659(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 135.Sun Y. Fox T. Adhikary G. Kester M. Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J. Leukoc. Biol. 2008;83:1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arakawa Y. Hashida N. Ohguro N. Yamazaki N. Onda M. Matsumoto S. Ohishi M. Yamabe K. Tano Y. Kurokawa N. Eye-concentrated distribution of dexamethasone carried by sugar-chain modified liposome in experimental autoimmune uveoretinitis mice. Biomed. Res. 2007;28:331–334. doi: 10.2220/biomedres.28.331. [DOI] [PubMed] [Google Scholar]
- 137.Hirai M. Minematsu H. Kondo N. Oie K. Igarashi K. Yamazaki N. Accumulation of liposome with Sialyl Lewis X to inflammation and tumor region: application to in vivo bio-imaging. Biochem. Biophys. Res. Commun. 2007;353:553–558. doi: 10.1016/j.bbrc.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 138.Yamazaki N. Kojima S. Yokoyama H. Biomedical nanotechnology for active drug delivery system by applying sugar-chain molecular functions. Curr. Appl. Phys. 2005;5:112–117. [Google Scholar]
- 139.Prabu P. Chaudhari A.A. Aryal S. Dharmaraj N. Park S.Y. Kim W.D. Kim H.Y. In vitro evaluation of poly(caporlactone) grafted dextran (PGD) nanoparticles with cancer cell. J. Mater. Sci. Mater. Med. 2008;19:2157–2163. doi: 10.1007/s10856-007-3307-z. [DOI] [PubMed] [Google Scholar]
- 140.Abdelbary G. El-Gendy N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech. 2008;9:740–747. doi: 10.1208/s12249-008-9105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aggarwal D. Kaur I.P. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int. J. Pharm. 2005;290:155–159. doi: 10.1016/j.ijpharm.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 142.Prabhu P. Nitish K.R. Koland M. Harish N. Vijayanarayan K. Dhondge G. Charyulu R. Preparation and evaluation of nano-vesicles of brimonidine tartrate as an ocular drug delivery system. J. Young Pharm. 2010;2:356–361. doi: 10.4103/0975-1483.71623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abdelkader H. Ismail S. Kamal A. Alany R.G. Design and evaluation of controlled-release niosomes and discomes for naltrexone hydrochloride ocular delivery. J. Pharm. Sci. 2011;100:1833–1846. doi: 10.1002/jps.22422. [DOI] [PubMed] [Google Scholar]
- 144.Gan L. Han S. Shen J. Zhu J. Zhu C. Zhang X. Gan Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010;396:179–187. doi: 10.1016/j.ijpharm.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 145.Sankar A.K.C.V. Durga S. Design and evaluation of diclofenac sodium ophthalmic inserts. Acta Pharm. Sci. 2006;48:5–10. [Google Scholar]
- 146.Baeyens V. Kaltsatos V. Boisrame B. Varesio E. Veuthey J.L. Fathi M. Balant L.P. Gex-Fabry M. Gurny R. Optimized release of dexamethasone and gentamicin from a soluble ocular insert for the treatment of external ophthalmic infections. J. Control. Release. 1998;52:215–220. doi: 10.1016/s0168-3659(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 147.Xie L. Shi W. Wang Z. Bei J. Wang S. Prolongation of corneal allograft survival using cyclosporine in a polylactide-co-glycolide polymer. Cornea. 2001;20:748–752. doi: 10.1097/00003226-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 148.Theng J.T. Ti S.E. Zhou L. Lam K.W. Chee S.P. Tan D. Pharmacokinetic and toxicity study of an intraocular cyclosporine DDS in the anterior segment of rabbit eyes. Invest. Ophthalmol. Vis. Sci. 2003;44:4895–4899. doi: 10.1167/iovs.02-1112. [DOI] [PubMed] [Google Scholar]
- 149.Kagaya F. Usui T. Kamiya K. Ishii Y. Tanaka S. Amano S. Oshika T. Intraocular dexamethasone delivery system for corneal transplantation in an animal model. Cornea. 2002;21:200–202. doi: 10.1097/00003226-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 150.Tan D.T. Chee S.P. Lim L. Theng J. Van Ede M. Randomized clinical trial of Surodex steroid drug delivery system for cataract surgery: anterior versus posterior placement of two Surodex in the eye. Ophthalmology. 2001;108:2172–2181. doi: 10.1016/s0161-6420(01)00839-9. [DOI] [PubMed] [Google Scholar]
- 151.Chang D.F. Garcia I.H. Hunkeler J.D. Minas T. Phase II results of an intraocular steroid delivery system for cataract surgery. Ophthalmology. 1999;106:1172–1177. doi: 10.1016/S0161-6420(99)90262-2. [DOI] [PubMed] [Google Scholar]
- 152.Kodama M. Numaga J. Yoshida A. Kaburaki T. Oshika T. Fujino Y. Wu G.S. Rao N.A. Kawashima H. Effects of a new dexamethasone-delivery system (Surodex) on experimental intraocular inflammation models. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241:927–933. doi: 10.1007/s00417-003-0753-2. [DOI] [PubMed] [Google Scholar]
- 153.Reis A. Reinhard T. Sundmacher R. Braunstein S. Godehardt E. A comparative investigation of FK506 and cyclosporin A in murine corneal transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236:785–789. doi: 10.1007/s004170050159. [DOI] [PubMed] [Google Scholar]
- 154.Ho S. Clipstone N. Timmermann L. Northrop J. Graef I. Fiorentino D. Nourse J. Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 155.Shi W. Liu T. Xie L. Wang S. FK506 in a biodegradable glycolide-co-clatide-co-caprolactone polymer for prolongation of corneal allograft survival. Curr. Eye Res. 2005;30:969–976. doi: 10.1080/02713680500320752. [DOI] [PubMed] [Google Scholar]
- 156.Wong V.G. Methods for reducing or preventing transplant rejection in the eye and intraocular implants for use therefor. 2010. p. US7846468B2.
- 157.Robinson M.R. Csaky K.G. Nussenblatt R.B. Smith J.A. Yuan P. Sung C. Fronheiser M.P. Kim H. Ocular therapeutic agent delivery devices and methods for making and using such devices. 2010. p. US0272777.
- 158.Jwala J. Boddu S.H. Shah S. Sirimulla S. Pal D. Mitra A.K. Ocular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovir. J. Ocul. Pharmacol. Ther. 2011;27:163–172. doi: 10.1089/jop.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vadlapudi A.D. Vadlapatla R.K. Kwatra D. Earla R. Samanta S.K. Pal D. Mitra A.K. Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int. J. Pharm. 2012;434:315–324. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen J. Gan L. Zhu C. Zhang X. Dong Y. Jiang M. Zhu J. Gan Y. Novel NSAIDs ophthalmic formulation: flurbiprofen axetil emulsion with low irritancy and improved anti-inflammation effect. Int. J. Pharm. 2011;412:115–122. doi: 10.1016/j.ijpharm.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 161.Prasanna G. Carreiro S. Anderson S. Gukasyan H. Sartnurak S. Younis H. Gale D. Xiang C. Wells P. Dinh D. Almaden C. Fortner J. Toris C. Niesman M. Lafontaine J. Krauss A. Effect of PF-04217329 a prodrug of a selective prostaglandin EP(2) agonist on intraocular pressure in preclinical models of glaucoma. Exp. Eye Res. 2011;93:256–264. doi: 10.1016/j.exer.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 162.Lallemand F. Furrer P. Felt-Baeyens O. Gex-Fabry M. Dumont J.M. Besseghir K. Gurny R. A novel water-soluble cyclosporine A prodrug: ocular tolerance and in vivo kinetics. Int. J. Pharm. 2005;295:7–14. doi: 10.1016/j.ijpharm.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 163.Lallemand F. Varesio E. Felt-Baeyens O. Bossy L. Hopfgartner G. Gurny R. Biological conversion of a water-soluble prodrug of cyclosporine A. Eur. J. Pharm. Biopharm. 2007;67:555–561. doi: 10.1016/j.ejpb.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 164.de Paiva C.S. Schwartz C.E. Gjorstrup P. Pflugfelder S.C. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- 165.Knapp S. Bertelmann E. Hartmann C. Keipert S. Pleyer U. Intraocular availability of topically applied mycophenolate mofetil in rabbits. J. Ocul. Pharmacol. Ther. 2003;19:181–192. doi: 10.1089/108076803321637717. [DOI] [PubMed] [Google Scholar]
- 166.Mitra A.K. Velagaleti P.R. Grau U.M. Topical drug delivery systems for ophthalmic use. 2010. US 2010/0310642 A1.
- 167.Barar M.A.J. Mortazavi-Tabatabaei S.A. Omidi Y. Ocular drug delivery; Impact of in vitro cell culture models. J. Ophthalmic. Vis. Res. 2009;4:238–252. [PMC free article] [PubMed] [Google Scholar]