Abstract
Rationale
Compensatory smoking may represent an adverse consequence of smoking reduction or the use of reduced nicotine tobacco products. Factors contributing to individual variability in compensation are poorly understood.
Objective
To examine whether severity of nicotine withdrawal as measured by elevated intracranial self-stimulation (ICSS) thresholds is related to individual differences in compensatory nicotine self-administration (NSA) following unit dose reduction.
Methods
Rats were trained for ICSS and NSA (0.06 mg/kg/inf). After stabilization, effects of reducing the nicotine unit dose to 0.03 mg/kg/inf were examined. Following reacquisition of NSA (0.06 mg/kg/inf), effects of antagonist-precipitated withdrawal and saline extinction (spontaneous withdrawal) were examined.
Results
Reducing the NSA unit dose produced partial compensation as indicated by increased infusion rates but a 35% mean decrease in daily nicotine intake. Magnitude of compensation varied considerably among rats. Dose reduction did not elicit withdrawal in rats as a group, although there were substantial increases in ICSS thresholds in some animals. Intracranial self-stimulation thresholds were consistently elevated during precipitated and spontaneous withdrawal, confirming that rats were nicotine-dependent. Individual differences in compensation were not correlated with changes in ICSS thresholds during dose reduction, precipitated withdrawal, or spontaneous withdrawal. In a secondary analysis, greater precipitated withdrawal severity predicted greater initial nicotine-seeking during extinction.
Conclusions
Severity of nicotine withdrawal was not related to the degree of compensation in this protocol. These data do not support a role for nicotine withdrawal in individual differences in compensation during reduced nicotine exposure, but do suggest that withdrawal may contribute to nicotine-seeking during early abstinence.
Keywords: Nicotine, Self-administration, Intracranial self-stimulation, Withdrawal, Tobacco harm reduction, Compensation
Introduction
Tobacco harm reduction is being evaluated as an alternative or transitional stage toward cessation for smokers who are unable or unwilling to quit (Britton and Edwards 2008; Hatsukami et al. 2007; Shiffman et al. 2002; Stratton et al. 2001). Harm reduction strategies attempt to reduce disease associated with tobacco use by reducing exposure to tobacco toxins. Approaches to harm reduction for individuals include smoking reduction (e.g., decreasing cigarettes per day) or the use of modified tobacco products containing reduced levels of toxins or nicotine (Hatsukami et al. 2004; Stead and Lancaster 2007), the primary addictive component of tobacco (e.g., Benowitz 1996; 2008; U.S. Department of Health and Human Services 1999). Population-level harm reduction strategies include smoking bans or the proposed policy of gradually decreasing the nicotine content of cigarettes to a non-addictive level (Benowitz and Henningfield 1994; Henningfield et al. 1998), an approach being considered by the Food and Drug Administration (Hatsukami et al. 2010b; Zeller and Hatsukami 2009).
The ability of certain harm reduction strategies (e.g., switching to cigarettes with reduced nicotine yields) to reduce actual toxin exposure may be limited by compensatory changes in smoking topography (e.g., increasing puff depth or frequency) that occur in an attempt to titrate nicotine intake and compensate for lower smoke delivery (Benowitz et al. 2005; Hatsukami et al. 2006; Joseph et al. 2008; Scherer 1999). The considerable degree of individual variability associated with compensation (e.g., Benowitz et al. 2006; Hecht et al. 2004) suggests that harm reduction strategies may be more effective in some smokers than in others. Characterizing the determinants of individual differences in compensation could be valuable for improving or individualizing harm reduction interventions and informing tobacco regulation policy.
The determinants of compensation have not been well established but may include or overlap factors related to smoking behavior or smoking cessation. Avoidance of the aversive aspects of nicotine withdrawal has been hypothesized to contribute to smoking behavior (e.g., Watkins et al. 2000), and nicotine withdrawal severity is a known predictor of cessation success (Hughes 2007; Piasecki et al. 1998; Piasecki et al. 2003). Although much less studied, withdrawal is also relevant to certain harm reduction interventions (e.g., switching to cigarettes with reduced nicotine yields or reduced nicotine content), in that they can elicit modest withdrawal symptoms due to decreases in nicotine intake (Benowitz et al. 2009; Benowitz et al. 2007; Hatsukami et al. 2010a; West et al. 1984; Zacny and Stitzer 1988). These findings raise the possibility that withdrawal might motivate increases in smoking in those individuals that exhibit compensation when switching to reduced-nicotine cigarettes. However, whether such a relationship exists between individual differences in severity of withdrawal and compensation during tobacco exposure reduction has not been established.
Animal models of nicotine self-administration (NSA) may be useful for modeling compensation. Numerous studies manipulating nicotine dose within-subjects have reported greater infusion rates for moderate (e.g., 0.03 mg/kg/inf) compared to high (e.g., 0.06 mg/kg/inf) NSA unit doses (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006; Donny et al. 1995; Watkins et al. 1999). While other interpretations are possible (see Discussion), most authors attribute this “inverted U” dose-response function to nicotine regulation/titration (e.g., Corrigall 1999; Corrigall and Coen 1989; Hatsukami et al. 2010b; Lynch and Carroll 1999; Matta et al. 2007; Rose and Corrigall 1997; Shoaib et al. 1997). As such, increases in NSA following unit dose reduction may be at least somewhat analogous to compensatory smoking during the use of reduced nicotine cigarettes. Whether nicotine withdrawal is associated with compensatory NSA following a reduction in unit dose is unknown.
We have previously examined correlates of compensatory increases in 23 hr/day NSA following unit dose reduction (Harris et al. 2009). As in humans, compensation (defined as a change in nicotine intake that is proportionally less than a change in nicotine unit dose; Hughes and Carpenter 2005; Scherer 1999) was associated with a considerable degree of individual variability and was only partial (i.e., baseline nicotine intake was not maintained). Whether the resulting decreases in nicotine intake were sufficient to elicit withdrawal, and the role of any withdrawal effects in individual differences in compensation, was not addressed.
The primary goal of the current study was to examine whether nicotine withdrawal is elicited by a reduction in the unit dose for NSA that is known to elicit compensation and whether individual differences in the severity of withdrawal is related to individual differences in compensation. The primary measure of withdrawal was elevation in intracranial self-stimulation (ICSS) threshold, indicative of the deficit in brain reinforcement processes (anhedonia) that accompanies withdrawal (Epping-Jordan et al. 1998; Markou and Koob 1991). To examine whether a partial reduction in nicotine intake would elicit withdrawal, ICSS thresholds were tested prior to and during unit dose reduction. Thresholds were also tested during antagonist-precipitated and spontaneous withdrawal (i.e., extinction testing) to confirm that rats were nicotine-dependent, as well as to provide measures of withdrawal sensitivity in addition to any elevations in ICSS thresholds observed during dose reduction. In a secondary analysis, the roles of several variables (e.g., baseline levels of nicotine intake) in mediating individual differences in severity of precipitated and spontaneous withdrawal were examined.
Materials and methods
Animals
Male Holtzman rats (Harlan, Indianapolis, IN) weighing 300–325 g at arrival were maintained under a restricted feeding regimen (≈18 g/day rat chow). This strain was chosen to extend our previous studies that used the same strain to examine individual differences in compensatory NSA (Harris et al. 2008; Harris et al. 2009). Upon arrival, all rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to water under a reversed 12 h light/dark cycle (lights off at 11:00 hr). Rats in the NSA group were moved to operant conditioning chambers in a separate room under the same light/dark cycle following onset of 22 hr NSA (see below). The control group, which was not tested for NSA, remained housed in the same colony room throughout the experiment. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Apparatus
ICSS
Rats were tested in operant conditioning chambers (29 cm × 26 cm × 33 cm) (Med Associates, St. Albans, VT) placed inside sound-attenuating cubicles. A 5-cm wide metal wheel manipulandum was fixed to the front wall. Brain stimulation was administered with constant-current stimulators (Model #PHM-152, Med-Associates). Rats were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (Plastics One). MED-PC IV software was used to control stimulation parameters and for data collection.
NSA
Each operant chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, an aluminum ceiling, and a stainless steel grid floor. Two response levers were located on the front wall 10 cm above the chamber floor on either side of a food aperture located 2 cm above the floor. Stimulus lights were located 2 cm above each response lever. Water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside each cubicle delivered infusions through Tygon tubing connected to a fluid swivel mounted above the chamber, and from the swivel through a spring leash connected to a guide cannula mounted in a harness assembly on the back of the rat. MED-PC IV software was used for operating the apparatus and recording data.
Drugs
Nicotine bitartrate or mecamylamine hydrochloride (Sigma Chemical Co., St. Louis, MO) were dissolved in sterile saline. The pH of the nicotine solution was adjusted to 7.4 with dilute NaOH, and heparin (30 units/ml) was added to help maintain catheter patency. Nicotine doses are expressed as the base.
Surgery
Electrode implantation
Animals were anesthetized with i.m. ketamine (75 mg/kg) and xylazine (7.5 mg/kg) and implanted with a bipolar stainless steel electrode (Plastics One) in the medial forebrain bundle at the level of the lateral hypothalamus as described in Roiko et al. (2009). Animals were allowed to recover for at least one week prior to ICSS training. During the first two days of recovery all animals received i.m. injections of the antibiotic ceftriaxone (5.25 mg) and s.c. injections of the analgesic buprenorphine (0.1 mg/kg).
Catheter implantation
Each rat was implanted with a chronic indwelling jugular catheter under droperidol (2 mg/kg)/fentanyl (0.04 mg/kg) anesthesia (Harris et al. 2008; LeSage et al. 2002). The catheter was externalized between the scapulae and attached to a harness assembly that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least four days after surgery, during which time they received daily i.v. infusions of heparinized saline and ceftriaxone (5.25 mg) and s.c. injections of buprenorphine (0.1 mg/kg; first two days only).
Behavioral training
ICSS
Rats were trained on a modified version of the Kornetsky and Esposito (1979) discrete-trial current-threshold procedure (Harris et al. 2010; Markou and Koob 1992; Roiko et al. 2009). Each trial was initiated with presentation of a non-contingent stimulus (0.1 ms cathodal squarewave pulses at a frequency of 100 Hz for 500 ms) followed by a 7.5-sec window during which a positive response on the wheel manipulandum produced a second, contingent stimulation identical to the first. Lack of responding in the 7.5-sec window was considered a negative response. Each positive or negative response was followed by a variable inter-trial interval averaging 10 sec (range = 7.5 to 12.5 sec), during which time additional responses delayed onset of the subsequent trial by 12.5 sec. Stimulus intensities were presented in four alternating descending and ascending series (step size = 5 uA), with five trials presented at each current intensity step. The current threshold for each series was defined as the midpoint between two consecutive intensity steps that yielded three or more positive responses and two consecutive intensity steps that yielded three or more negative responses. The overall ICSS threshold for the session was defined as the mean of the current thresholds from the four alternating series. To assess performance effects (e.g., motor disruption), response latencies (time between onset of the non-contingent stimulus and a positive response) were averaged across all trials in which a positive response was made.
NSA
Rats were trained to self-administer nicotine in daily 22 hr sessions (11:00 – 09:00 hr). A similar access schedule (23 hr/day) results in patterns of nicotine intake similar to those of smokers (see Harris et al. 2009; LeSage et al. 2002). A 22 hr schedule was used to allow sufficient time for ICSS testing between NSA sessions (see below), as well as for cage maintenance, feeding and weighing of animals, and evaluation of general health. Nicotine availability was signaled by illumination of the stimulus light above the active (right) response lever. Following completion of the response requirement, the stimulus light was extinguished and nicotine (0.06 mg/kg/inf) was infused over the course of 1 sec in a volume of 50 ul heparinized saline. This training dose was chosen because it lies on the descending arm of the “inverted U” NSA dose-response curve in both limited (e.g., 1 hr) and unlimited access NSA models (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006), allowing for measurement of compensatory increases in NSA following unit dose reduction. Had the training dose been on the ascending arm or peak of the NSA dose-response curve, dose reduction would only result in decreases in infusion rates. Following a 7-sec time-out, the stimulus light turned on and the next nicotine infusion was available. Responses on the other (inactive) lever were recorded but had no programmed consequences. The response requirement was initially a fixed ratio 1 (FR 1), and was gradually increased to FR 3 across several sessions. The criteria for acquisition were a minimum of 10 infusions per day under the FR 3 schedule and a ratio of active to inactive lever presses of at least 2:1 for 5 consecutive sessions. These acquisition criteria are typical for NSA under unlimited access conditions (e.g., Brower et al. 2002; LeSage et al. 2003; Valentine et al. 1997).
Assessment of somatic withdrawal signs
Rats were habituated to clear plastic circular chambers for 10 minutes on each of 2 days prior to testing. During withdrawal tests, rats were placed in the chamber and videotaped for 10 min. Tapes were later scored for somatic signs by a blinded, trained observer using a validated checklist (Malin 2001; Roiko et al. 2009).
Protocol
NSA group
Figure 1 shows a timeline for the experimental procedure for the NSA group (n = 14). Rats were initially trained for ICSS in 1 hr sessions conducted 5 days a week (Mon-Fri) until ICSS thresholds were stable (i.e., less than 10% coefficient of variation over a 5-day period and no apparent trend). Rats were subsequently implanted with i.v. catheters and continued to be tested for ICSS. When ICSS thresholds were again stable, rats were concurrently trained for NSA (0.06 mg/kg/inf) in 22 hr/day sessions conducted 7 days a week. ICSS continued to be tested 5 days a week beginning 15 min after each NSA session. When both ICSS thresholds and NSA were stable (stability criteria for ICSS as above; criteria for NSA = no trend in infusion rates across 5 consecutive session and a coefficient of variation < 15%), the nicotine unit dose was reduced to 0.03 mg/kg/inf for at least 12 NSA sessions (corresponding to 10 ICSS sessions due to weekend breaks in ICSS testing) and until both infusion rates and ICSS thresholds were stable. This unit dose was used because it is near the peak of the NSA dose-response curve (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006). The unit dose was always reduced on a Monday. Rats were subsequently allowed to reacquire NSA (0.06 mg/kg/inf) for at least 12 NSA sessions (10 ICSS sessions) and until infusion rates and ICSS thresholds were stable, at which point antagonist-precipitated withdrawal was tested to confirm that animals were nicotine-dependent. Injections during this phase typically occurred on Tues and Fri, provided that infusion rates and ICSS thresholds were within baseline range on intervening days. On each of two habituation days, rats were injected with s.c. saline immediately after NSA testing and, 15 min later, tested for ICSS. On each of two test days, this procedure was repeated with the exception that rats received either saline or the nicotinic antagonist mecamylamine (1.5 mg/kg, s.c.), with the injection order counterbalanced. This mecamylamine dose reliably precipitates increases in ICSS thresholds and somatic signs in rats dependent on a chronic nicotine infusion while having no effects on these measures in drug-naïve rats (Markou and Paterson 2001; O'Dell et al. 2006; Watkins et al. 2000). To confirm further that animals were nicotine-dependent, a subset (n = 8) of rats was tested for somatic withdrawal signs immediately after each precipitated withdrawal test. Following precipitated withdrawal testing, rats continued to be tested for at least 7 NSA sessions (5 ICSS sessions) and until both infusion rates and ICSS thresholds were stable. To assess spontaneous withdrawal, a period of extinction was subsequently arranged in which saline was substituted for the NSA unit dose. Stimuli that had been paired with nicotine infusions continued to be presented during extinction, allowing for isolation of the effects of nicotine from the effects of nicotine-associated cues on drug-seeking. Extinction conditions were always introduced on a Monday. Effects of extinction on infusion rates and ICSS thresholds were tested for a total of 9 NSA sessions (7 ICSS sessions). Somatic withdrawal signs were not measured during extinction. Extended access (6–23 hr) NSA at a range of unit doses remains stable over prolonged periods of time (e.g., months) (Harris et al. 2008; Harris et al. 2009; Kenny and Markou 2006; O'Dell et al. 2007; Paterson and Markou 2004). Therefore, we did not include a separate group tested on the training dose throughout the protocol to control for changes (e.g., escalation) in NSA due to long-term drug exposure.
Fig 1.
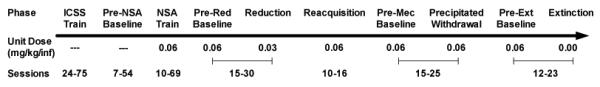
Timeline for experimental procedure for the NSA group. Also shown is the nicotine unit dose (mg/kg/inf) available and number of ICSS sessions conducted (range) during each experimental phase. Pre-Red, Pre-Mec, and Pre-Ext Baselines = Pre-reduction, premecamylamine, and pre-extinction baselines, respectively.
Control group
An additional group was used to examine whether ICSS thresholds remained stable over time in the absence of the NSA protocol described above. Rats (n = 11) were trained for ICSS until ICSS thresholds were stable, catheterized, and then tested until ICSS thresholds were again stable. Animals continued to be tested for ICSS 5 days a week for an additional 87 sessions (i.e., the average number of ICSS sessions that the NSA group required to complete all phases of the NSA protocol). Rats were tested during a similar time of day as the NSA group, but were returned to their home cages between ICSS sessions. This control condition is commonly used in studies examining effects of drug self-administration on ICSS (e.g., Ahmed et al. 2002; Kenny et al. 2009; Kenny et al. 2006; Kenny and Markou 2006). Beginning on session 60 (i.e., the average ICSS session in which precipitated withdrawal testing began for rats in the NSA group), effects of s.c. saline and mecamylamine (1.5 mg/kg) on ICSS thresholds and somatic signs were tested as for the NSA group.
Data Analysis
Baseline measures
Baseline ICSS thresholds (in μA), response latencies (sec), daily (22 hr) infusion rates, and total daily nicotine intake (mg/kg/day) in the NSA group for each experimental phase were defined as the mean during the last 5 sessions prior to the beginning of that phase. Baseline periods in the control group corresponded to the average ICSS sessions (in relation to achievement of stability) in which each baseline period occurred in the NSA group. Baseline ICSS data were compared between groups during each baseline period using separate independent sample t-tests to confirm that they did not differ. All ICSS and NSA data were computed as percentage of the appropriate baseline for subsequent analyses.
Effects of dose reduction and reacquisition on NSA and ICSS
Daily NSA and ICSS data in the NSA group during reduction and reacquisition were compared to baseline using separate one-way repeated measure ANOVAs followed by Dunnett's post hoc tests. Compensation occurs when changes in nicotine intake are proportionally less than changes in nicotine unit dose (Scherer 1999). To confirm the presence of compensation, single-sample t-tests with Bonferroni correction were used to compare total daily nicotine intake during reduction to a theoretical mean of 50%, which represents the predicted nicotine intake if intake decreased proportionally to unit dose (i.e., if no compensation had occurred). ICSS data in the control group during comparable sessions were analyzed in the same manner as for the NSA group in this and subsequent analyses.
Effects of mecamylamine on ICSS and somatic sign
Paired-sample t-tests were used to compare the effects of acute mecamylamine versus saline injection on ICSS data and somatic withdrawal signs.
Effects of extinction on infusion rates and ICSS
Overall (i.e. 22 hr) infusion rates during pre-extinction and each day of extinction in the NSA group were analyzed as above. To examine within-session patterns of responding on the first day of extinction, infusion rates during pre-extinction and on extinction day 1 were separated into 2-hr blocks (Harris et al. 2007). Data were analyzed using a two-way repeated measures ANOVA with phase (pre-extinction versus extinction day 1) and 2-hr block as factors, followed by post-hoc paired t-tests with Bonferroni correction.
Intracranial self-stimulation threshold and latency data during extinction were analyzed using separate two-way ANOVAs with group (i.e., NSA versus control) and extinction session as factors. To explore the significant interaction between group and session on ICSS thresholds (see Results), data were averaged across extinction sessions 1–3 (i.e., the sessions in which ICSS thresholds in the NSA group were elevated compared to baseline, see Fig 4C) and compared between groups using an independent samples t-test. In addition, ICSS thresholds during extinction within each group were analyzed using separate one-way ANOVAs followed by Bonferroni post tests.
Fig 4.
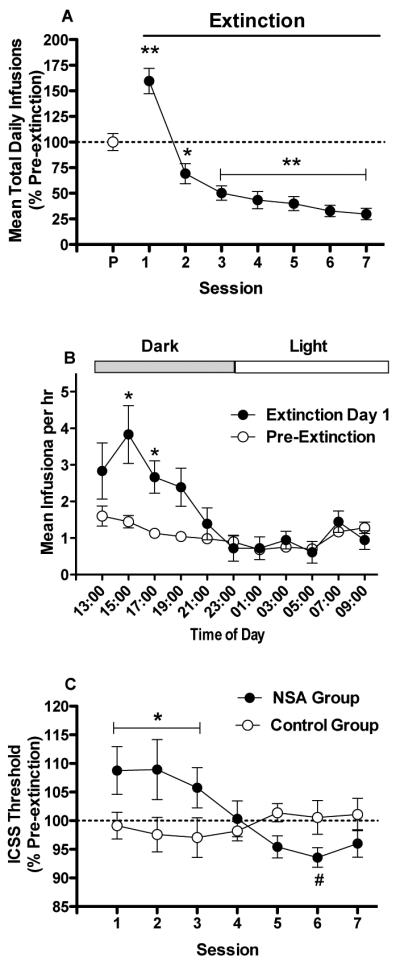
(A) Mean (±SEM) number of infusions per 22 hr session (expressed as % of pre-extinction baseline) during pre-extinction (P) and extinction in the NSA group. (B) Mean (±SEM) hourly infusion rate per 2-hr block during pre-extinction and on extinction day 1. Dark = dark phase of the light/dark cycle. Light = light phase of the light/dark cycle. *,** Significantly different from the pre-extinction baseline during that session (A) or at that 2-hr block (B), p < 0.05 or 0.01. (C) ICSS thresholds (expressed as percent of pre-extinction baseline, mean ± SEM) during each extinction session in the NSA and control groups. *Significantly different from the control group (averaged across extinction sessions 1–3), p < 0.05. #Significantly different from extinction session 1, p < 0.05.
Compensation indices and correlates
A Compensation Index (CI) analogous to a measure of compensation used in smokers (Scherer 1999) was calculated for each rat using the formula: 1 – (% decrease in total daily nicotine intake following reduction ÷ % decrease in nicotine unit dose following reduction). A CI of 0 indicates no compensation (total daily nicotine intake decreased proportionally to the reduction in unit dose), while a CI of 1.0 indicates full compensation (total daily nicotine intake was unchanged following dose reduction). Separate CIs were computed for each rat during the first (initial CI) and final (stable CI) 5 days of reduction. The initial CI reflects a period in which any withdrawal effects would be most likely to occur, while the stable CI represents the final degree of compensation achieved. Each CI was compared to a theoretical mean of 0 using a single-sample t-test. Linear regression was then used to examine the relationship between each rat's initial and stable CI and the following factors: daily infusion rates during the pre-reduction baseline, changes in ICSS thresholds during either the first or final 5 days of reduction testing, magnitude of mecamylamine-precipitated withdrawal (percent change in ICSS thresholds compared to baseline following s.c. mecamylamine), and magnitude of spontaneous withdrawal (peak percent change in ICSS thresholds compared to baseline during the first 3 extinction sessions, regardless of the session in which the peak occurred). This measure accounts for the substantial between-subject differences in the time course of changes in ICSS thresholds during extinction.
Additional correlates of withdrawal magnitude
In a secondary analysis, linear regression was used to examine the relationship between magnitude of precipitated and/or spontaneous withdrawal and the following factors: baseline daily infusion rates, cumulative nicotine exposure (total mg/kg) prior to withdrawal testing, magnitude of the “overall extinction burst” (i.e., percent change in 22 hr infusion rate between pre-extinction and extinction day 1), and magnitude of the “peak extinction burst” (i.e., percent change in infusion rates collapsed across the 2 hr blocks ending at 15:00 and 17:00, the only within-session intervals in which the extinction burst was statistically significant, see Fig 4B).
Results
Attrition and baseline ICSS measures
Several animals were lost to attrition during either mecamylamine-precipitated withdrawal testing (n = 3 for NSA group, n = 2 for Control group) or extinction/spontaneous withdrawal testing (n = 2 for NSA group, n = 1 for Control group) due to loss of catheter patency, loss of ICSS headcap, or other procedural problems. Data for these animals are analyzed only for those phases they completed. ICSS thresholds and response latencies did not differ between the NSA group and the control (i.e., nicotine-naïve) group during any baseline period (Table 1), indicating that 22 hr NSA (0.06 mg/kg/inf) did not affect brain reinforcement function.
Table 1.
Mean (±SEM) ICSS thresholds (in μA) and response latencies (in sec) in the NSA group and control group during each baseline period.
| ICSS Thresholds (μa) | ICSS Latencies (sec) | |||
|---|---|---|---|---|
|
| ||||
| NSA Group | Control Group | NSA Group | Control Group | |
| Pre-NSA | 100.7 ± 5.6 | 115.0 ± 11.8 | 2.6 ± 0.2 | 2.8 ± 0.1 |
| Pre-Reduction | 96.8 ± 4.2 | 109.2 ± 10.4 | 2.4 ± 0.1 | 2.8 ± 0.2 |
| Pre-Mecamylamine | 100.8 ± 4.6 | 107.4 ± 9.1 | 2.5 ± 0.1 | 2.7 ± 0.2 |
| Pre-Extinction | 98.4 ± 5.7 | 105.3 ± 10.7 | 2.6 ± 0.1 | 2.7 ± 0.2 |
Effects of dose reduction and reacquisition on NSA and ICSS
Reduction in nicotine unit dose elicited partial compensation (i.e., compensatory increases in NSA were not sufficient to maintain baseline levels of intake) but did not precipitate withdrawal as measured by elevations in ICSS thresholds (Fig 2A). There was an effect of session on daily infusion rates (F (10, 130) = 4.2, p < .0001), with infusion rates increased compared to baseline on all days of reduction (p < .05 or 0.01). Within-session patterns of responding during baseline and reduction were similar to those reported previously in this model (see Harris et al. 2009). Despite the increase in infusion rates, there was an effect of session on total daily nicotine intake (F (10, 130) = 11.0, p < .0001), with reduced intake compared to baseline on all days of reduction (all p values < 0.01). However, decreases in nicotine intake were proportionally less than the 50% decrease in unit dose on nearly all days of reduction (Fig 2A; t(13) = 3.5 – 5.1, all p values < .05), indicating partial compensation. During the final 5 days of reduction, the mean percent increase in infusion rates ± SEM was 30.9 ± 6.3% (range = −13.0 to 78.4%) while the mean percent decrease in nicotine intake ± SEM was 34.6 ± 3.2% (range = 10.8 to 56.5%). The decrease in nicotine intake was not sufficient to induce withdrawal, as there was no effect of session on ICSS thresholds during reduction. During reacquisition of NSA (0.06 mg/kg/inf), infusion rates and total daily nicotine intake immediately returned to baseline levels, while ICSS thresholds remained unchanged. There was no effect of session on any of these measures during reacquisition. There was also no effect of session on ICSS response latencies during either reduction or reacquisition (data not shown), indicating that ICSS threshold data were not influenced by non-specific (e.g., motor) effects.
Fig 2.
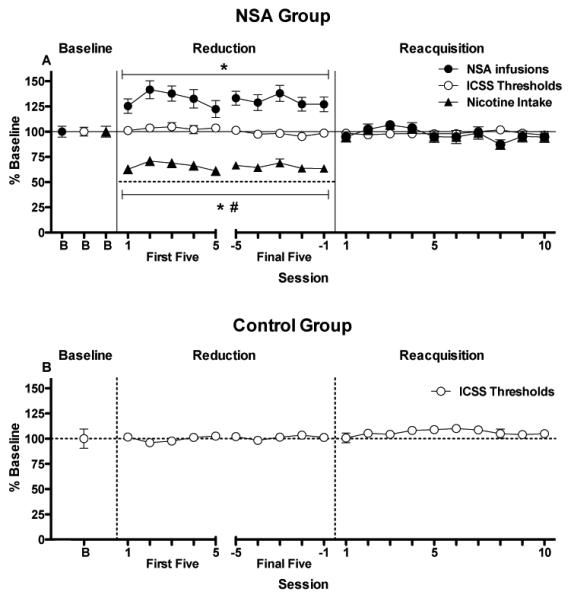
(A) Mean (±SEM) total number of infusions earned per daily session, total daily nicotine intake, and ICSS thresholds (expressed as percent of pre-reduction baseline) during baseline (0.06 mg/kg/inf), the first and final five days of reduction (0.03 mg/kg/inf), and reacquisition of NSA (0.06 mg/kg/inf) in the NSA group. The dotted line during the reduction phase represents predicted total daily nicotine intake if intake had decreased proportionally to reduction in nicotine unit dose (i.e., no compensation had occurred). * Significantly different from the pre-reduction baseline, p < 0.05 or 0.01. # Significantly different from predicted total daily nicotine intake if no compensation had occurred (reduction day 5 excluded), p < 0.05 or 0.01. Intracranial self-stimulation threshold data during comparable periods in the control group are shown in (B).
There was no effect of session on ICSS thresholds (Fig 2B) or response latencies (data not shown) in the control group during the ICSS sessions corresponding to either reduction or reacquisition testing in the NSA group, confirming that these measures remained stable over time in the absence of NSA testing.
Effects of mecamylamine on ICSS and somatic signs
Mecamylamine increased ICSS thresholds (t(10) = 2.7, p = .02) and somatic signs (t(7) = 2.7, p = .03) compared to saline in the NSA group, reflecting precipitated withdrawal, but did not affect these measures in the control group (Fig 3A and 3B). Mecamylamine also increased response latencies in the NSA group (mean percent change in latency following mecamylamine ± SEM = 113.9 ± 5.8%; saline = 99.4 ± 3.6%, t(10) = 2.3, p < .05) but not in controls (data not shown).
Fig 3.
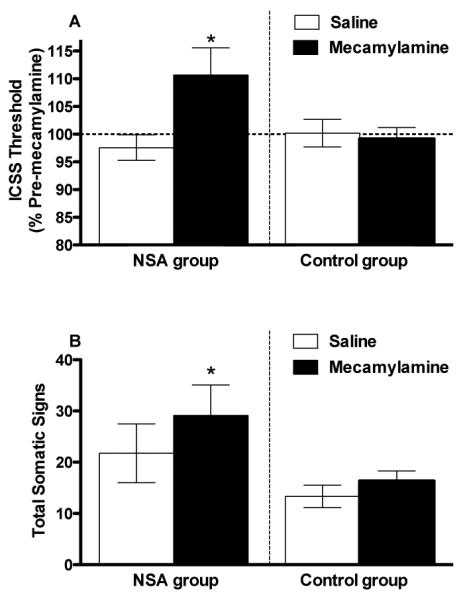
Mean (±SEM) ICSS thresholds (expressed as percent of pre-mecamylamine baseline; A) and total somatic signs (B) following s.c. injection of saline or 1.5 mg/kg mecamylamine in the NSA group and control groups. *Significantly different from saline for that group, p < 0.05.
Effects of extinction on infusion rates and ICSS
Infusion rates
There was an effect of session on daily infusion rates during extinction (F(7, 56) = 31.5, p < .0001), with infusion rates increased compared to the pre-extinction baseline on extinction day 1 (Fig 4A; p < .01) and decreased compared to pre-extinction on subsequent days (p < .05 or 0.01). Magnitude of the “extinction burst” on extinction day 1 was greatest early within the session (Fig 4B). A within-session analysis indicated effects of phase (i.e., pre-extinction versus extinction day 1) (F(1, 8) = 26.6, p < .0001), 2 hr block (F(10, 80) = 6.6, p < .0001), and an interaction (F(10, 80) = 4.1, p < .0001). Infusion rates were increased compared to pre-extinction during the 2 hr blocks ending at 15:00 and 17:00 hr (ps < 0.05).
ICSS
There was no effect of group or session on ICSS thresholds during extinction, but there was a significant interaction (F(6, 90) = 4.2, p < .001). Intracranial self-stimulation thresholds were elevated in the NSA group compared to the control group during extinction sessions 1–3 (t(15) = 2.3, p < .05), reflecting spontaneous withdrawal (Fig 4C). There was also an effect of session in the NSA group (F(7, 56) = 3.2, p < .01), with ICSS thresholds differing significantly between extinction day 1 and extinction day 6 (p < 0.05). In contrast, there was no effect of session on ICSS thresholds in the control group. There was also no effect of group, session, or interaction on response latencies.
Compensation indices and correlates (Primary outcomes)
Compensation in this model was partial and associated with a considerable degree of individual variability. The average initial and stable CI (i.e., CI during the first and final 5 days of reduction, respectively) for the NSA group (Mean ± SEM) were 0.32 ± 0.07 (range = −0.06 to 0.91) and 0.31 ± 0.06 (range = −0.13 to 0.78), respectively. One rat had negative CIs because its reduction in nicotine intake was greater than the reduction in nicotine unit dose. Initial and stable CIs were correlated with each other (r= 0.78, p < 0.01), and were greater than 0 (a CI representing no compensation; t(13) = 4.6 or 4.9, ps < .001), but less than 1.0 (a CI representing complete compensation).
There was a negative correlation between daily infusion rates during the pre-reduction baseline and both initial CI (Table 2) and stable CI (Table 2 and Fig 5A). Rats with the highest baseline infusion rates exhibited the lowest levels of compensation throughout reduction. Changes in ICSS thresholds during dose reduction, antagonist-precipitated withdrawal, or spontaneous withdrawal were not correlated with initial or stable CIs (Table 2 and Fig 5B–5D). These measures were also not correlated with more acute changes in CIs calculated across the first 1 or 3 days of dose reduction (data not shown).
Table 2.
Pre-reduction baseline infusion rates (inf/22hr) and ICSS thresholds (expressed as percent of the appropriate baseline) during reduction and withdrawal testing for rats in the NSA group. Correlation coefficients between these measures and the two Compensation Indices are also shown.
| Measure | Mean ± SEM (Range) | Initial CI | Stable CI |
|---|---|---|---|
|
| |||
| Baseline Rate | 26.8 ± 5.4 (16.6–35.3) | *r = −0.62, p = 0.02 | *r = −0.66, p = 0.01 |
| Thresh - Reduction | |||
| First 5 sessions | 103.1 ± 2.5% (85.5–118.3%) | r = −0.16, p = 0.58 | |
| Final 5 sessions | 98.2 ± 2.0% (88.5–116.6%) | r = 0.17, p = 0.56 | |
| Thresh - Withdrawal | |||
| Precipitated | 110.7 ± 4.5% (85.9–135.4%) | r = 0.03, p = 0.92 | r = 0.25, p = 0.43 |
| Spontaneous | 113.7 ± 4.4% (98.6–137.2%) | r = −0.61, p = 0.08 | r = −0.53, p = 0.14 |
Significantly correlated, p < 0.05.
Fig 5.
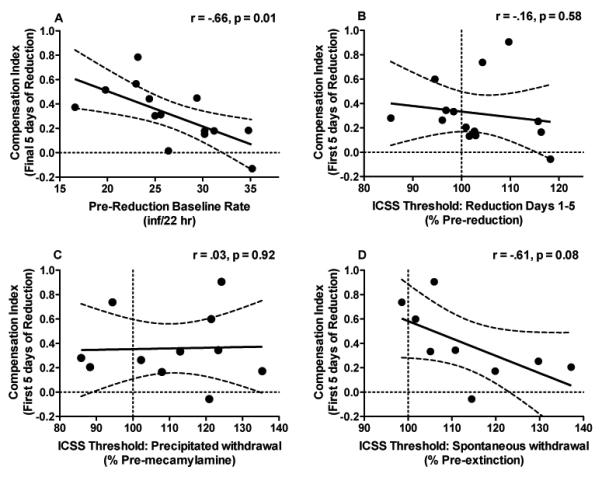
(A) Correlation between each rat's stable CI (i.e. CI during the final 5 days of reduction) and daily infusion rates during the pre-reduction baseline. Also shown is the correlation between each rat's initial CI (i.e. CI during the first 5 days of reduction) and changes in ICSS thresholds during that same period of reduction testing (B), magnitude of mecamylamine-precipitated withdrawal (C), and magnitude of spontaneous withdrawal (D; see text for definitions of these terms). Fig 5A – 5D also illustrate the considerable degree of between-subject variability associated with compensation in this model.
Additional correlates of withdrawal magnitude (Secondary outcomes)
Magnitude of the peak extinction burst, but not the overall (22 hr) extinction burst, was correlated with magnitude of precipitated withdrawal (Table 3 and Fig 6). There were no significant relationships between any other variables examined and withdrawal magnitude (Table 3).
Table 3.
Infusion rates during the pre-mecamylamine and pre-extinction baseline periods (inf/22hr), cumulative nicotine intake (mg/kg) prior to precipitated and spontaneous withdrawal testing, and overall and peak extinction burst magnitude (infusion rates during appropriate period expressed as percent of baseline). Correlation coefficients between these measures and magnitude of precipitated and/or spontaneous withdrawal are also shown.
| Measure | Mean ± SEM (Range) | Precipitated Withdrawal | Spontaneous Withdrawal |
|---|---|---|---|
|
| |||
| Baseline Rate | |||
| Pre-Mec | 26.3 ± 1.8 (16.6–34.6) | r = 0.21, p = 0.51 | |
| Pre-Extinction | 23.4 ± 1.9 (14.8–32.0) | r = 0.05, p = 0.89 | |
| Cumulative Intake | |||
| Precipitated | 147.7 ± 11.8 (108.3–206.2) | r = 0.09, p = 0.78 | |
| Spontaneous | 189.1 ± 16.7 (126.9–266.9) | r = 0.34, p = 0.40 | |
| Extinction Burst | |||
| Overall | 159.6 ± 12.3% (113.3–242.9%) | r = 0.54, p = 0.14 | r = −0.32, p = 0.40 |
| Peak | 147.0 ± 28.8% (50.0–283.5%) | *r = 0.74, p = 0.02 | r = −0.36, p = 0.35 |
Significantly correlated, p < 0.05.
Fig 6.
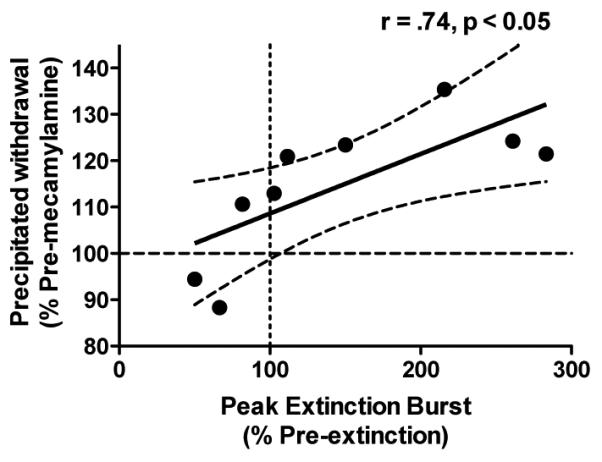
(A) Correlation between magnitude of precipitated withdrawal and magnitude of the peak extinction burst (see text for definition of these terms).
Discussion
As in prior animal studies (e.g., Corrigall and Coen 1989; DeNoble and Mele 2006; Harris et al. 2009), decreases in nicotine intake following NSA unit dose reduction were proportionally less than the decrease in unit dose. The overall magnitude and degree of individual variability in compensation (mean initial CI = 0.32, range = −0.06 to 0.91) was similar to that observed in smokers (e.g,. Benowitz et al. 1986; Scherer 1999; Hecht et al. 2004). Rats were clearly nicotine-dependent as shown by increases in ICSS thresholds and somatic signs during antagonist-precipitated withdrawal and increases in ICSS thresholds during extinction (i.e., spontaneous withdrawal). Reducing the nicotine unit dose resulted in a 35% decrease in total daily nicotine intake but did not elicit withdrawal as measured by the mean change in ICSS thresholds (i.e., compensation in most animals was sufficient to avoid withdrawal). There was individual variability in ICSS thresholds after nicotine dose reduction, with substantial (i.e., >20%) increases in some rats (see Fig 5B), but no correlation was found between changes in ICSS thresholds and degree of compensation. The lack of a robust withdrawal effect may have limited our ability to detect a relationship between changes in ICSS thresholds during reduction and degree of compensation. However, there was no correlation between compensation and elevated ICSS thresholds during antagonist-precipitated or spontaneous withdrawal, effects that were more robust. Taken together, these findings suggest that severity of withdrawal is not a determinant of individual differences in compensation in this model.
Interpretation of compensation
Compensation occurs when a change in nicotine intake is proportionally less than a change in nicotine unit dose (see Hughes and Carpenter 2005; Scherer 1999). Compensation clearly occurred in this study, as nicotine intake was reduced by only 35% when the unit dose was reduced by 50%, and was achieved via an increase in infusion rates. While the definition of compensation is simply descriptive and does not allude to any underlying mechanism(s), compensation is typically interpreted as an attempt to titrate nicotine intake (e.g., Corrigall and Coen 1989; Matta et al. 2007). An alternative explanation is that the 0.06 mg/kg/inf unit dose (i.e., training dose) produces aversive or motoric effects that suppress infusion rates, and that increased responding following dose reduction reflects a reduction or elimination of these non-specific effects. Several findings argue against this interpretation. First, a 0.06 mg/kg/inf unit dose supported higher infusion rates than a 0.03 mg/kg/inf unit dose on a progressive ratio schedule of reinforcement (Donny et al. 1999; Paterson et al. 2004), suggesting greater reinforcing efficacy for the higher unit dose rather than aversive effects. Second, experimenter-administered i.v. nicotine infusions (0.06 mg/kg/inf) typically do not suppress locomotor activity or operant responding (Chaudhri et al. 2006; Cohen et al. 2009). Third, in the current study, rates of inactive lever pressing (a measure of non-specific motor effects) during access to the 0.06 and 0.03 mg/kg/inf unit doses did not differ (data not shown). Finally, initiation of NSA training with the 0.06 mg/kg/inf unit dose did not elevate ICSS thresholds or response latencies, effects that are thought to measure drug-induced aversion and motor suppression, respectively (Markou and Koob 1992; Spiller et al. 2009). It is therefore highly unlikely that aversive or motoric effects of the training dose can account for the effects of dose reduction in this study. Therefore, it seems most likely that compensation in this study reflected nicotine-seeking, an interpretation supported by similarities between compensation in this model and compensatory smoking in humans (see above).
Potential for nicotine exposure reduction to induce withdrawal
The lack of withdrawal following dose reduction for rats as a group is consistent with studies reporting only moderate or no withdrawal symptoms in humans smoking cigarettes with reduced nicotine yields or reduced nicotine content (e.g., Benowitz et al. 2007; West et al. 1984). Together, these data suggest that withdrawal is not a prominent consequence of partial reductions in nicotine intake for most individuals. These findings also complement a previous report that a large but partial reduction in brain nicotine levels achieved via administration of nicotine-specific antibodies was not sufficient to elicit withdrawal in rats dependent on a chronic nicotine infusion (Roiko et al. 2009).
A potential limitation of the current study design is that the times at which ICSS was tested during reduction (i.e., at 24 hr intervals) may not have coincided with the times at which withdrawal occurred. A more detailed characterization of the time course of ICSS thresholds following dose reduction was not conducted because the sequential nature of the ICSS and NSA assessment would have required repeated interruption of the NSA sessions. Methods that allow concurrent measurement of NSA and ICSS are needed to address this issue.
The current approach may not have been optimal for detecting a robust withdrawal effect during reduction, as most rats appeared to have self-administered enough nicotine to alleviate any withdrawal before the first withdrawal measurement. Of course, rats must have access to the lower unit dose before the effects of reduced nicotine exposure can be measured, but it is unclear how much exposure should be allowed before assessment begins. We chose a 24 hr interval for assessing withdrawal because it is analogous to human studies examining the effects of reduced nicotine cigarettes. These studies also involved assessment of withdrawal at only limited (i.e., daily to weekly) intervals and continued access to nicotine during reduction (e.g., Benowitz et al. 2007; West et al. 1984). That mild withdrawal symptoms were reported in several of these human studies (e.g., West et al. 1984; Zacny and Stitzer 1988) supports the feasibility of our approach. Therefore, despite its limitations, the current study represents an important initial step in modeling the consequences of reduced nicotine intake that parallels human studies addressing the same issue.
Factors contributing to individual differences in compensation
The considerable degree of between-subject variability in compensation in this model was exploited to examine potential correlates of compensation. We have previously reported that rats with higher baseline infusion rates compensate less in this dose-reduction model (Harris et al., 2009). The current study confirms this finding and extends its generality to the experimental conditions unique to this study (e.g., assessment of ICSS in the same animals). High baseline infusion rates were also correlated with less compensation following reduction in the duration of daily access to NSA (Harris et al. 2008). Consistent with these animal studies, higher levels of baseline nicotine intake were associated with lower compensation in humans (Benowitz et al. 1986). While the mechanism underlying this relationship is not yet clear (see Harris et al. 2009 for discussion), a smoker's baseline level of nicotine intake prior to nicotine exposure reduction may provide an easily obtained predictor of the magnitude of compensation during reduction.
The current findings suggest that severity of withdrawal, as measured using ICSS, does not mediate individual differences in compensation in this model of nicotine dose reduction. However, withdrawal-induced elevations in ICSS thresholds may be correlated with compensation elicited in a different manner (e.g., reduction in access; Harris et al. 2008). Examining whether severity of withdrawal assessed using different measures (e.g., conditioned place aversion) is related to individual differences in compensation in this and other models would also be of interest.
Additional correlates of withdrawal magnitude
While not a primary focus, this study provided the opportunity to examine the role of several factors in individual differences in withdrawal severity. Baseline infusion rates and cumulative nicotine intake were not related to magnitude of either precipitated or spontaneous withdrawal. This contrasts with a report (O'Dell et al. 2007) that greater cumulative nicotine intake in an unlimited access NSA model was associated with greater precipitated withdrawal severity as measured by somatic signs. Methodological factors that could account for this difference include withdrawal measure (i.e., ICSS versus somatic signs), rat strain, and history of nicotine exposure prior to withdrawal testing. In addition, data in O'Dell et al. (2007) were pooled across separate groups responding for different nicotine unit doses. This resulted in a larger overall sample size and a wider range of baseline levels of nicotine intake than in the current study, and may have facilitated detection of a significant relationship between nicotine intake and withdrawal severity.
Greater severity of precipitated withdrawal was associated with a greater magnitude of the “peak extinction burst” (i.e., increases in infusion rates early within the session on extinction day 1), a novel finding suggesting that withdrawal processes may motivate increases in drug-seeking during the early stages of extinction. Future studies are needed to examine whether similar factors influence withdrawal-induced elevations in ICSS thresholds and extinction-induced bursts in nicotine-seeking, which may help elucidate neural mechanisms mediating extinction of NSA. To the extent that response rate during extinction is indicative of the motivation to engage in drug-seeking and relapse during drug abstinence (Epstein et al. 2006; Markou et al. 1993), these findings also complement human data indicating that greater withdrawal severity is associated with reduced smoking cessation success (Piasecki et al. 1998; Piasecki et al. 2003).
Effects of unlimited access NSA on ICSS
This study is the first to examine unlimited access NSA and ICSS within-subjects, and provides a model for studying the effects of self-administered nicotine and withdrawal on brain reinforcement function. While 22 hr/day NSA had no acute effects on ICSS in the current study, limited access (1, 6, or 12 hr/day) NSA produced a significant reduction in ICSS thresholds (Kenny et al. 2009; Kenny and Markou 2006; Paterson et al. 2008). A nicotinic antagonist blocked the ICSS threshold-reducing effects of NSA in Kenny and Markou (2006), but did not elevate ICSS thresholds above baseline levels (i.e., precipitated withdrawal did not occur). Kenny and Markou (2006) also reported decreases in ICSS thresholds following suspension of NSA sessions (rats were not tested under conditions of saline extinction, see Kenny and Markou 2006). The modest decrease in ICSS thresholds on extinction days 5–7 in the current study (See Fig 4B) may parallel the latter finding, but in general the effects of NSA on ICSS in this study (i.e., no acute effects on ICSS thresholds, elevations in ICSS thresholds during withdrawal) differed considerably compared to those reported in Kenny and Markou (2006). While there are a variety of methodological differences (e.g., rat strain, nicotine training dose, manner in which spontaneous withdrawal was assessed) between studies, these findings raise the possibility that duration of access (i.e., limited versus unlimited) may influence the effects of NSA on brain reinforcement function.
Conclusion
Nicotine dose reduction in smokers has been studied in a variety of ways including 1) use of commercial cigarettes which are highly ventilated, achieve lower nicotine yield through increasing ventilation, and readily allow compensation through changes in smoking topography (e.g., Benowitz et al. 2005), and 2) use of reduced nicotine content research cigarettes which are poorly ventilated, achieve lower nicotine delivery through their reduced nicotine content, and which do not readily allow compensation due to these differences in product design (Benowitz et al. 2007; Hatsukami et al. 2010a; Rose and Behm 2004). The current study most closely models reduction via commercial cigarettes because both these cigarettes and the rodent NSA paradigm allow compensation to be readily expressed. The rodent data in the present study suggest that nicotine withdrawal is not a major determinant of individual differences in compensation under these conditions. However, the current data do suggest that withdrawal may mediate nicotine-seeking during the early stages of abstinence.
Acknowledgements
Supported by NIH grants T32 DA 07097 (NIDA), F32 DA021935 (NIDA), and P50-DA013333 (NIDA/NCI) and the Minneapolis Medical Research Foundation Translational Addiction Research Program. We would also like to thank Dr. Athina Markou for her helpful advice at early stages of this research and for providing the opportunity to train with members of her laboratory (especially Jessica Chevrette) in the ICSS methodology.
Footnotes
No conflicts of interest.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–6. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3rd Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:876–83. doi: 10.1158/1055-9965.EPI-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3rd Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Bernert JT, Wilson M, Wang L, Allen F, Dempsey D. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:1376–83. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315:1310–3. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- Britton J, Edwards R. Tobacco smoking, harm reduction, and nicotine product regulation. Lancet. 2008;371:441–5. doi: 10.1016/S0140-6736(07)61482-2. [DOI] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology (Berl) 2009;204:541–9. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology (Berl) 2006;184:266–72. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–94. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–42. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16:86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Harris AC, Mattson C, LeSage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010;96:217–27. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology (Berl) 2007;194:395–402. doi: 10.1007/s00213-007-0848-2. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology (Berl) 2009;205:599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Henningfield JE, Kotlyar M. Harm reduction approaches to reducing tobacco-related mortality. Ann Rev Public health. 2004;25:1–19. doi: 10.1146/annurev.publhealth.25.102802.124406. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Joseph AM, Lesage M, Jensen J, Murphy SE, Pentel PR, Kotlyar M, Borgida E, Le C, Hecht SS. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9(Suppl 4):S537–53. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010a;105:343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Le CT, Zhang Y, Joseph AM, Mooney ME, Carmella SG, Hecht SS. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiol Biomarkers Prev. 2006;15:2355–8. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010b;19:e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. 1998;7:281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction. 2005;100:1074–89. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Lando H, Carmella SG, Gross M, Bliss R, Le CT, Hatsukami DK. Smoking reduction fails to improve clinical and biological markers of cardiac disease: a randomized controlled trial. Nicotine Tob Res. 2008;10:471–81. doi: 10.1080/14622200801901948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–81. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–6. [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–86. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–89. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of intravenously self-administered nicotine in rats. Exp Clin Psychopharmacol. 1999;7:198–207. doi: 10.1037//1064-1297.7.3.198. [DOI] [PubMed] [Google Scholar]
- Malin DH. Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav. 2001;70:551–9. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–9. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–73. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–82. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–93. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–86. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–51. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112:14–27. [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Behm F. Effects of low nicotine content cigarettes on smoke intake. Nicotine Tob Res. 2004;6:309–19. doi: 10.1080/14622200410001676378. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gitchell JG, Warner KE, Slade J, Henningfield JE, Pinney JM. Tobacco harm reduction: conceptual structure and nomenclature for analysis and research. Nicotine Tob Res. 2002;4(Suppl 2):S113–29. doi: 10.1080/1462220021000032717. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr., Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–6. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007:CD005231. doi: 10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10:189–95. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Nicotine addiction: Health consequences of smoking. DHHS; 1999. [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–51. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–64. [PubMed] [Google Scholar]
- West RJ, Russell MA, Jarvis MJ, Feyerabend C. Does switching to an ultra-low nicotine cigarette induce nicotine withdrawal effects? Psychopharmacology (Berl) 1984;84:120–3. doi: 10.1007/BF00432039. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Stitzer ML. Cigarette brand-switching: effects on smoke exposure and smoking behavior. J Pharmacol Exp Ther. 1988;246:619–27. [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–32. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]


