Abstract
The tumour microenvironment thwarts conventional immunotherapy through multiple immunologic mechanisms, such as the secretion of the transforming growth factor-β (TGF-β), which stunts local tumour immune responses. Therefore, high doses of interleukin-2 (IL-2), a conventional cytokine for metastatic melanoma, induces only limited responses. To overcome the immunoinhibitory nature of the tumour microenvironment, we developed nanoscale liposomal polymeric gels (nanolipogels; nLGs) of drug-complexed cyclodextrins and cytokine-encapsulating biodegradable polymers that can deliver small hydrophobic molecular inhibitors and water-soluble protein cytokines in a sustained fashion to the tumour microenvironment. nLGs releasing TGF-β inhibitor and IL-2 significantly delayed tumour growth, increased survival of tumour-bearing mice, and increased the activity of natural killer cells and of intratumoral-activated CD8+ T-cell infiltration. We demonstrate that the efficacy of nLGs in tumour immunotherapy results from a crucial mechanism involving activation of both innate and adaptive immune responses.
Metastatic melanoma is highly aggressive, leaving untreated patients with a median survival of less than 12 months1. The ineffectiveness of surgical interventions, radiation and cytotoxic chemotherapies has resulted in immunotherapy as a primary treatment modality. Approximately 5–6% of patients with metastatic melanoma will achieve durable complete remissions when treated with high doses of IL-2, a cytokine that induces or expands activation of melanoma-specific T-cell responses1–3. Data from clinical trials demonstrate the substantial potential for immunotherapy to provide meaningful benefit for patients with advanced melanoma2,3. However, the majority of malignancies from melanoma patients remain refractory to this treatment approach.
One mechanism behind how melanomas and other cancers evade immunotherapy has been postulated to be the inability of the immune system to recognize the tumour as ‘non-self’4. This may occur owing to the secretion of a number of immunosuppressive factors by tumour cells, including TGF-β (ref. 5), a pleiotropic cytokine that decreases the number and activity of natural killer (NK) cells,6 and that reduces the activity of cytotoxic T lymphocytes7 while increasing the number of regulatory T lymphocytes (Tregs)7,8. TGF-β activity has been extensively evaluated in a number of animal disease systems, including murine tumour models5,8–10. Its secretion is suspected to thwart high-dose IL-2 therapy, which is supposed to enhance NK and cytotoxic T lymphocyte activity against melanomas and renal cell cancers11 but lacks efficacy in the majority of patients. This has led to the evaluation of strategies that counteract immunosuppressive factors secreted from tumours, including TGF-β (reviewed elsewhere4,7). Although the exact source of intratumoral TGF-β has not been well established, the cytokine has been found at high levels in a large number of different tumours, including melanomas. It is believed that TGF-β is pivotal for tumour cell growth and differentiation, as well as for maintaining an immunosuppressive environment to protect an established tumour from the host immune response, rendering it an ideal target for cancer therapies9,10,12,13. In particular, its suppressive effect on the number of NK cells present in tumour beds may be crucial for immune tolerance14, as these cells play an important role in the anti-tumour response15.
Little is known about the immunoprotective mechanisms behind TGF-β signalling blockade in the tumour microenvironment when administered together with an immunostimulant such as IL-2. It has been demonstrated that the combination of a TGF-β receptor-I inhibitor (LY364947) and the cytotoxic chemotherapeutic agent, doxorubicin, can be effective against pancreatic and gastric carcinomas13; however, these studies did not explore the immunoprotective mechanisms behind TGF-β signalling blockade or the potential for synergizing this effect with immunostimulatory molecules such as IL-2. Given that the high-dose-related toxicity of IL-2 can hamper its therapeutic benefits, newer approaches aim to reduce the administered dose by increasing the half-life of the cytokine in circulation. Some examples include fusion proteins (IL-2/Ig; ref. 16), PEGylated (PEG is poly(ethylene glycol)) IL-2 (ref. 17), IL-2/anti-IL-2 complexes18, viral and plasmid vectors19, and liposomal formulations20,21. However, the relatively low efficacy of single-agent immunotherapies and the non-responsiveness of melanomas to chemotherapies suggested that combination immunotherapy might be effective for treating melanomas.
We therefore chose to evaluate the ability of a commercially available TGF-β receptor-I inhibitor, SB505124 (refs 7,10,22,23; SB), in combination with IL-2 to induce anti-tumour responses in the murine melanoma B16 model. Critical to the success of this combination therapy is a safe and flexible delivery platform that releases effector molecules with different physiochemical properties to tumour beds in a sustained fashion. Cytokines, including IL-2, represent a complex network of soluble proteins critical for immunological and effector cell function. In a similar fashion, small molecule hydrophobic drugs, such as TGF-β antagonists, represent a class of pleiotropic immunomodulators that can overcome barriers posed by tumours to escape the immune response. The sustained local delivery of these agents, in combination, can induce localized therapeutic immune responses while reducing the immune-resistant nature of the tumour microenvironment.
The initial goal of this study is to determine the efficacy of simultaneous, sustained release of IL-2 and SB against tumours. Given the vastly different physiochemical properties of IL-2, a soluble 17 kDa protein, and SB, a small hydrophobic drug (Log P = 4.33), co-encapsulation for sustained release of both agents presents a challenge for conventional particle technologies. For example, liposomes are easily modified for encapsulation of small hydrophilic molecules, and have long been used for delivery of hydrophilic and amphiphilic drugs such as doxorubicin to tumours24 and even proteins, but the stability of these formulations and the release profiles of encapsulated agents are not easily controlled25. Biodegradable solid particles, on the other hand, such as those fabricated from poly(lactic-co-glycolic acid; PLGA), are highly stable and have controllable release characteristics, but pose complications for facile encapsulation and controlled release of therapeutic cytokines26 or for combinatorial delivery, and have lagged in their utility owing to relatively poorer biodistribution and clearance24,26–30. Combined synergistic or additive delivery of both hydrophobic drugs and hydrophilic cytokines from either platform is difficult to achieve.
To overcome these limitations, hybrid platforms integrating features of different materials offer advantages for combinatorial encapsulation and delivery. One example is in core–shell systems, in which an organic or inorganic mesoporous or nanoporous core, entrapping molecules of interest, is coated with a lipid or polymer shell. Such hybrid systems can enhance encapsulation and release of a wide variety of agents, such as small-molecule drugs, proteins and nucleic acids, while promoting favourable pharmacokinetics and biodistribution of the encapsulant31–34. In this work we developed a biodegradable core–shell platform that combines features of both liposomal and solid polymer systems, that enables sustained and simultaneous release of both hydrophobic drugs and hydrophilic cytokines, and that incorporates the advantageous pharmacokinetic properties of PEGylated liposomes. We demonstrate that the sustained delivery of both IL-2 and SB from this system induces potent anti-tumour immune responses in a B16/B6 mouse model of melanoma after intratumoral or systemic administration. Furthermore, we show induction of remission with this combination therapy through activation of the innate and adaptive arms of the immune response, with the innate arm playing a critical role in mediating anti-tumour activity in vivo.
nLG design for co-delivery of TGF-β inhibitor and IL-2
We term the delivery vehicle a ‘nLG’ particle owing to its nature as a lipid bilayer surrounding a hydrogel core fabricated from a degradable polymer (Fig. 1)35. Liposomes were used as nanoscale molds for photo-initiated hydrogel formation30,36. To achieve sustained release of the hydrophobic drug in conjunction with encapsulated proteins, we incorporated methacrylate-conjugated β-cyclodextrins (CDs; refs 37,38) into the interior of the liposomes. β-CDs have a long history as solubilization agents for hydrophobic compounds, and are key excipients in various pharmaceutical formulations37,38. This formulation procedure thus enabled co-encapsulation of both proteins as well as small hydrophobic drugs within the interior of the lipid bilayer (Fig. 1). Conjugated CDs were created by reaction of succinylated-CDs with photosensitive methacrylate groups through hydrolysable ester groups (Supplementary Fig. S1a; ref. 36). Complexation of SB or rhodamine (for imaging) with functionalized CD (f-CD) was verified using 1H NMR (Supplementary Fig. S1b,c). The f-CD becomes covalently bound to the liposome-encapsulated polymer matrix during photoinduced polymerization, thus the SB can only be released on f-CD/SB hydrolysis of the polymer ester groups and subsequent diffusion out of the nLG, enabling sustained release compared to the burst-dominated release of SB in the absence of gelled CD (Supplementary Fig. S2a). This system enabled control over the release of remotely loaded IL-2 without compromising its bioactivity (Supplementary Fig. S2b). Loading of IL-2 in the polymer-hydrogel space outside of the CD enabled simultaneous release of both protein and drug (Fig. 2a). The decreased total release of both components (Fig. 2a) compared with single-component release (Supplementary Fig. S2a,b) was probably due to steric limitations within the interior of the nLG, or decreased loading efficiency of SB and IL-2. The release profile of SB/IL-2-loaded nLGs was not altered by incubation in serum (Supplementary Fig. S2c), and release was substantively completed by seven days (Supplementary Fig. S2d,e).
Figure 1. Fabrication of the nLG particle system.
The synthesis approach consists of two steps. a, In the first step, methacrylate-f-CD was used to solubilize the TGF-β inhibitor (SB505124). NHS, N-Hydroxysuccinimide. b, In the second step, nLGs were formulated from lyophilized liposomes loaded with biodegradable crosslinking polymer, acrylated-CD-SB505 complex, and IL-2 cytokine. This core–shell structure facilitated entrapment of the drug-loaded CD (blue) and the IL-2 (green) in a biodegradable polymer matrix (red) with a PEGylated liposomal coating (grey). The degradable gel consists of central water-soluble PEG groups (n = 200 repeats), lactide groups (m ~ 2.5), and terminal acrylate groups. After loading, photoinduced polymerization of the polymer and acrylated-CD results in gel formation.
Figure 2. Controlled release clearance and biodistribution in healthy animals.
a, Cumulative IL-2 and drug released from co-loaded nLGs normalized by carrier mass. Error bars in all plots represent ± 1 s.d. All experiments were repeated at least twice with similar results. b, Clearance of drug dose: encapsulation in nLGs significantly increased the remaining percentage of initial dose in the blood at 1 and 24 h post-injection (two population t-test, P < 0.01, ###). Mice received a single dose of rhodamine-loaded nLG or soluble rhodamine (in saline) via intravenous tail-vein injection. Animals were euthanized at 1, 24, 48 and 72 h post-injection for extraction and quantification of fluorescence. c, Whole body biodistribution: significantly higher (two population t-test, P < 0.01) amounts of rhodamine were detected in the major organs of nLG-treated animals (top panel) compared with animals injected with free dye (bottom panel). Data are presented as mean percentage of initial dose given and error bars represent ± 1 s.d. averaged across at least three mice per time point. d, Time-dependent accumulation in subcutaneous tumour: cumulative rhodamine tumour penetration (red) after B16 peritumoral injection in B6 mice. Peritumoral tissue was collected to quantify the remaining dose of nLG surrounding the tumour (black).
To demonstrate the impact of polymerization in the nLG on the release profile of SB and IL-2 we compared release kinetics of both agents with release from liposomes and solid PLGA nanoparticles encapsulating both agents. Incorporation of photo-cured polymer in the nLG vehicle enabled a more sustained release of SB compared with liposomes, and a more complete release compared with conventional 50:50 (PLGA nanoparticles) of the same diameter (Supplementary Fig. S2d). The release kinetics of the drug is seen to be intermediate between the diffusion-dependent release kinetics from liposomes and the hydrolysis-dependent release kinetics from PLGA. Comparative cumulative release of IL-2 from liposomes, nLGs, and PLGA nanoparticles demonstrated that encapsulation of IL-2 in nLGs enabled better-sustained release of cytokine (Supplementary Fig. S2e).
The bioactivities of the SB (Supplementary Fig. S3a) and IL-2 (Supplementary Fig. S3b) were unaffected by nLG incorporation. Encapsulation of IL-2 (80%) and/or drug (36%) (Supplementary Fig. S4C) did not significantly affect the nLG diameter; dynamic light scattering analysis revealed a mean diameter of 120 nm and polydispersity index of 0.2 (Supplementary Fig. S4a). Liposomes and nLGs incorporating amine-terminated PEGylated phosphatidyl ethanolamine (PEG-PE) demonstrated a neutral zeta potential, compared to the −22 ± 10 mV zeta potential of liposomes formulated with only phosphatidyl choline and cholesterol (Supplementary Fig. S4b). Cryotransmission electron microscopy of nLGs showed the formation of spherical liposomal structures (Supplementary Fig. S4e), detectable by light scattering even after disruption of the liposomal exterior by detergent (Supplementary Fig. S4f), validating an inner gel core with approximately the same diameter as the intact nLG. The in vitro cytotoxicity of this system was negligible (Supplementary Fig. S5).
nLG in vivo safety and toxicological profile
To examine the in vivo safety of nLG particles, C57/BL6 mice were administered a single intravenous dose of nLGs and acute toxicology was measured seven days later. No statistically significant toxic effects were observed from the administration of empty nLGs, or nLGs co-encapsulated with SB505124 (SB) or IL-2. No hepatoxicity was observed, as measured by serum levels of alkaline phosphatase (Supplementary Fig. S6a) and alanine aminotransferase (Supplementary Fig. S6b). Normal physiological reference ranges given by the IDEXX VetTest system for mouse alkaline phosphatase and alanine aminotransferase were 62–209 IU/L and 28–132 IU/L, respectively. Furthermore, no renal toxicity was observed, as blood urea nitrogen levels were within the normal mouse reference range of 18–29 mg dl−1 (Supplementary Fig. S6c). A complete blood count was also performed to identify any haematological toxicity. Leukocyte counts (Supplementary Fig. S6d), platelet counts (Supplementary Fig. S6e), and haemoglobin content (Supplementary Fig. S6f) were all within normal physiological ranges for mouse (leukocytes: 1.8–10.7 × 103 cells μl−1; platelets: 592–2,971 × 103 cells μl−1; haemoglobin: 11.0–15.1 g dl−1). Lung toxicity was evaluated by histology to determine if systemically administered nLGs induced any acute inflammation. Haematoxylin and eosin staining of lungs demonstrated no obvious pulmonary toxicity (Supplementary Fig. S6g). Bronchiolar and alveolar structures seemed normal, and no disruption to epithelial layers or inflammatory infiltrates were observed in lung sections. Serum IL-4 (Supplementary Fig. S6h), a proxy for potential allergic responses, and tumour necrosis factor-α (TNF-α; Supplementary Fig. S6I) for inflammatory responses were measured, and no statistically significant increases in these cytokines were detected with empty nLG treatment compared to buffer. However, IL-4, but not TNF-α, was elevated after administration with nLGs-encapsulating SB and IL-2. This is to be expected because IL-2 can play a dominant role in stabilizing the IL-4 gene, and thus may enhance the in vivo priming of IL-4 production39.
In vivo systemic biodistribution and clearance of nLGs
To investigate the biodistribution and clearance of this platform, we used CD-solubilized rhodamine as a fluorescent surrogate marker model for SB; rhodamine complexation with CD had been previously used to qualify guest–host interactions with CDs (refs 40, 41) and was confirmed here by 1H NMR (Supplementary Fig. S1). The in vivo pharmacokinetics of rhodamine following systemic administration was evaluated in healthy mice receiving a single intravenous administration of rhodamine-loaded nLG (nLG-rhod), an equivalent dose of free rhodamine, or phosphate-buffered saline (PBS) control via tail-vein injection. Spectrofluorometric analysis of rhodamine extracted from blood showed 15.7 ± 4.1% and 7.7 ± 3.7% (mean ± s.d.) of the initial dose of nLG remaining at 1 and 24 h, respectively, post-injection (Fig. 2b). Free rhodamine was rapidly cleared and was not detectable in blood at any of the time points taken following injection. Analysis of the biodistribution to major organs showed that the lungs, liver and kidney were primary sites of accumulation of both nLG-encapsulated rhodamine and free rhodamine (Fig. 2c). Encapsulation in nLG increased both the total initial dose to most tissues as well as the cumulative dose over three days (Fig. 2c).
To understand nLG-mediated delivery in the tumour microenvironment after a local injection, nLG-rhod was injected peritumorally, and accumulation was evaluated by comparative measurement of rhodamine concentrations in tumours versus peritumoral tissues (Fig. 2d). The pharmacokinetic profile suggested sustained delivery of drug from the localized depot of nLG: at 24 h after peritumoral administration, only 3 ± 1% of the initial dose had penetrated into the tumour mass, and 36 ± 17% of the initial dose remained in the surrounding tissues (Fig. 2d). Over the course of seven days, the cumulative rhodamine concentration in the tumour increased to 25 ± 0.5% of the initial dose, while total rhodamine concentration in the peritumoral tissue decreased to 4 ± 2% of the initial dose (Fig. 2d). Because the diffusion profiles of SB, IL-2 and rhodamine are not identical (Supplementary Figs S2d,e and S7c, respectively) the kinetics and level of accumulation of IL-2 and SB in the tumour may be slightly different from those observed with the fluorescent surrogate.
Intratumoral and systemic nLG therapy
We evaluated the effects of IL-2 and TGF-β antagonist monotherapies to enhance the anti-tumour responses against B16 melanomas. As encapsulation in nLGs decreased clearance of free drug and improved biodistribution (Fig. 2b,c), localized therapy of subcutaneous tumours was evaluated first to assess therapeutic efficacy. Weekly intratumoral administration of soluble SB alone failed to delay tumour growth (Fig. 3a), consistent with previous results using LY364947 in preclinical prostate and animal cancer models13. A similar null effect was observed when both soluble SB and IL-2 were co-administered in weekly doses (Fig. 3a). The nLG-encapsulated SB administered individually (nLG–SB) significantly delayed tumour growth (Fig. 3a), resulting in mice with smaller tumours after one week of therapy (Fig. 3b). Although nLG-encapsulated IL-2 administered individually (nLG–IL-2) did not significantly delay tumour surface area (Fig. 3a), the tumour masses at one week, which take into account inwards tumour penetration, were significantly smaller than those of tumours in the control group (Fig. 3b). These results are in accord with prior studies demonstrating the efficacy of sustained release of either IL-2 or small molecules inhibiting TGF-β signalling over pulses of these agents for enhancement of anti-tumour responses13,21. When comparing all treatment groups, the most striking and significant reduction in both tumour growth rate and tumour mass after one week of therapy was observed in the mice receiving simultaneous sustained delivery of SB and IL-2 (Fig. 3a,b).
Figure 3. Clinical effects of nLG therapy on subcutaneous and metastatic melanoma.
a, Plot of tumour area versus time (day 0 was the day of tumour cell injection). Red arrows indicate treatments (via intratumoral injection). Mice bearing subcutaneous tumours were euthanized either when the greatest tumour dimension was larger than 15 mm or when exhibiting signs of illness. Tumour areas of deceased mice were not included after the day of death. Each group initially contained five mice, except for the nLG–SB + IL-2 group, which contained four. Error bars represent ± 1 s.d. Tumours in the nLG–SB and nLG–SB + IL-2 groups were significantly smaller when compared against all other groups from day 12 to day 22 (P < 0.05, *, for nLG–SB, and P < 0.001, * * *, for nLG–SB-IL+2, versus no treatment, soluble SB, soluble SB + IL-2, and nLG–IL-2 groups by ANOVA with Tukey’s multiple comparison test). Tumours in the nLG–SB + IL-2 group were also significantly smaller than in the nLG–SB group from day 12 until day 26 (p < 0.05, #, by two-tailed t-test). b, Tumour masses of nLG-treated groups seven days after treatment. Mice were euthanized directly before tumour mass determination. Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. The nLG–SB + IL-2 group had significantly lower tumour masses than those of the nLG–empty (P < 0.001, * * *), nLG–IL-2 (P < 0.01, **) and nLG–SB (P < 0.01, **) groups. Tumour masses in the nLG–IL-2 and nLG–SB groups were also significantly lower than those in the nLG–empty (P < 0.05, *) group. All statistical comparisons were performed with an ANOVA using Tukey’s post-test. c, Survival plot of mice from the same study given in a. Red arrows denote treatment days. The survival of mice treated with nLG–SB was significantly longer by Mantel–Cox and Gehan–Breslow–Wilcoxon analyses (P < 0.01), and nLG–SB + IL-2 significantly extended survival by both analyses (P < 0.001). Studies were repeated 2–3 times with similar results. d, Survival plot of mice after systemic therapy. Red arrows denote treatment days. The survival of mice treated with nLG–SB + IL-2 was significantly higher by Gehan–Breslow–Wilcoxon analysis (P < 0.05). Studies were repeated 2–3 times with similar results. e, Number of tumours counted by blind observers in lungs of mice 14 days after initiation of treatment. Lung metastatic regions ranged in size from 0.5–2 mm in diameter. Treatment with nLG–SB, nLG–IL-2 and nLG–SB + IL-2 significantly reduced the number of metastases (P < 0.05, *, or P < 0.01, **, by ANOVA with Tukey’s post-test) when compared with soluble SB. Data represent mean ± 1 s.d. averaged across at least six mice per treatment group. f, Representative lung images from mice immediately before collection of lung-infiltrating lymphocytes. g, Visualization of nanopliogel trafficking to metastatic tumours. The distribution of both nLG carrier and encapsulated payload was investigated using dual-labelled NLG; fluorescein-labelled phosphoethanolamine was incorporated into the lipid component of CD-rhodamine-loaded nLGs. h, Analysis of lung tissues under bright field and fluorescent microscopy demonstrate the presence of both lipid carrier (green) and rhodamine payload (red) around individual lung tumours at 2 h post injection. Lipid fluorescence was significantly diminished four days after injection.
Tumour size in treated and untreated mice correlated with their survival. Administration of soluble SB or SB in combination with IL-2 did not improve survival over untreated controls, whereas nLG formulations of IL-2 or SB alone modestly increased average survival times (Fig. 3c). In contrast, the nLG-delivered combination immunotherapy dramatically increased survival (Fig. 3c). As observed with the tumour kinetics data, administration of particles releasing each agent slightly improved survival; however, mice receiving combination therapy via particles releasing both agents demonstrated markedly smaller tumours and longer survival compared to the other treatment groups. Of the animals receiving nLG–SB + IL-2, 100% survived through the study endpoint at 35 days after initial tumour implantation. Complete tumour regression and survival was observed in a cohort (40%) of the group through 60 days (not shown).
A significant unmet need is improved biodistribution of short-lived cytokines11,21 and hydrophobic drugs42,43 in the treatment of distant metastatic tumours. We therefore evaluated the effect of systemic nLG therapy against highly aggressive B16 lung metastases. It has previously been shown that intravenous injection of B16 cells results in rapid metastatic tumour growth in the lungs of B6 mice5,44. Furthermore, genetically modified mice containing T cells resistant to TGF-β signalling abrogated the development of metastatic B16 melanoma deposits in the lungs5, providing additional motivation for assessing TGF-β blockade with and without IL-2 therapy in this tumour model. In this study, treatments were given by tail-vein injection, and were initiated one week after cell injection to assess efficacy against growing tumours. As was the case in subcutaneous tumours, maximum survival benefit was observed in the group receiving the nLG-encapsulated combination therapy; Mantel–Cox analysis demonstrated a statistically significant (P < 0.01) increase in survival over animals receiving saline alone (Fig. 3d). A small cohort (50%) of animals receiving nLG–SB + IL-2 survived through the study endpoint at 45 days. To examine the effect of treatment on tumour burden, animals were sacrificed two weeks after initial treatment, and whole lung samples inspected visually for melanoma deposits. Administration of soluble SB with or without IL-2 co-therapy failed to reduce the number of lung tumours at three weeks (Fig. 3e). Maximum tumour burden reduction was observed in animals receiving the nLG-delivered combination therapy (Fig. 3e,f).
Comparative biodistribution was repeated in mice bearing B16 metastatic lung tumours. To assess trafficking of the particles versus trafficking of the payload, we used dual-labelled nLGs formulated by incorporating fluorescein-labelled PEG-phosphoethanolamine into the lipid membrane of rhodamine-loaded nLGs (Fig. 3g). Fluorescein-labelled PEG did not interfere with detection or release of rhodamine (Supplementary Fig. S7b,c). Mice with lung tumours received a single intravenous (tail vein) dose of dual-labelled nLG. B16 metastases were often visible as 0.5–2 mm irregular nodules (yellow arrows) under bright-field observation, whereas the fluorescein-labelled lipid of the delivery vehicle (green) and rhodamine (red) were detected under fluorescent filters up to 24 h post administration (Fig. 3h). Fluorescent detection of lipid was significantly diminished by four days after administration (Fig. 3h).
Localization and delivery in the tumour microenvironment
To test for accumulation of nLG and drug in distant tumours beyond lung tissue following intravenous injection, biodistribution experiments were repeated in mice bearing distant subcutaneous tumours and in mice with metastatic lung melanoma. In one cohort inoculated with subcutaneous tumours following nLG intravenous injection, lipid and rhodamine concentrations were quantified in the tumour and in homogenized tissues at various time points after administration. Peak tumoral concentrations of lipid and rhodamine, 8.8 ± 4.0% and 2.5 ± 0.8% per gram of tumour, respectively, were observed one day after administration (Fig. 4a). In another cohort, after intravenous injection in mice bearing metastatic melanoma, an analysis of all tissues confirmed biodistribution patterns that were similar for both the lipid and rhodamine components of the nLG system (Supplementary Fig. S7d,e), indicating that the drug payload was associated with the particles during biodistribution. Imaging the subcutaneous tumour cohort with time-resolved two-photon laser scanning intravital microscopy after nLG intravenous injection validated trafficking of nLGs and payload within the vasculature of tumours. Accumulation of fluorescein-labelled nLGs along the vasculature was detected both in the areas surrounding tumours (Fig. 4b) as well as within the tumour itself within 30 min post-intravenous injection (Fig. 4c). Particle trafficking in the tumour vasculature (green) was accompanied by an increase in the encapsulant (red) fluorescence in the tumour microenvironment (Fig. 4d). Extravasation of rhodamine was evident in the interstitial spaces between tumour cells outside of the vasculature (Supplementary Fig. S8).
Figure 4. Biodistribution to subcutaneous tumours after systemic administration.
a, Rhodamine and lipid were extracted from subcutaneous tumours following intravenous tail-vein injection at day 0. Experiments were repeated at least twice and data represent mean ± s.d. of 3–4 mice per time point. b, Whole-mount wide-field images demonstrating accumulation of both lipids and rhodamine in the peritumoral space. BF, Bright Field; FL, Fluorescence. c, Demonstration of nLG diffusion within the subcutaneous tumour by time-resolved intravital two-photon laser scanning microscopy after intravenous injection. Images presented are an extended focus projection of two optical sections at three separate time points after intravenous injection and are representative of those obtained in three separate experiments. d, Mean pixel fluorescein and rhodamine intensities of the entire imaged tissue volume represented in c.
Activation of innate and adaptive immunity
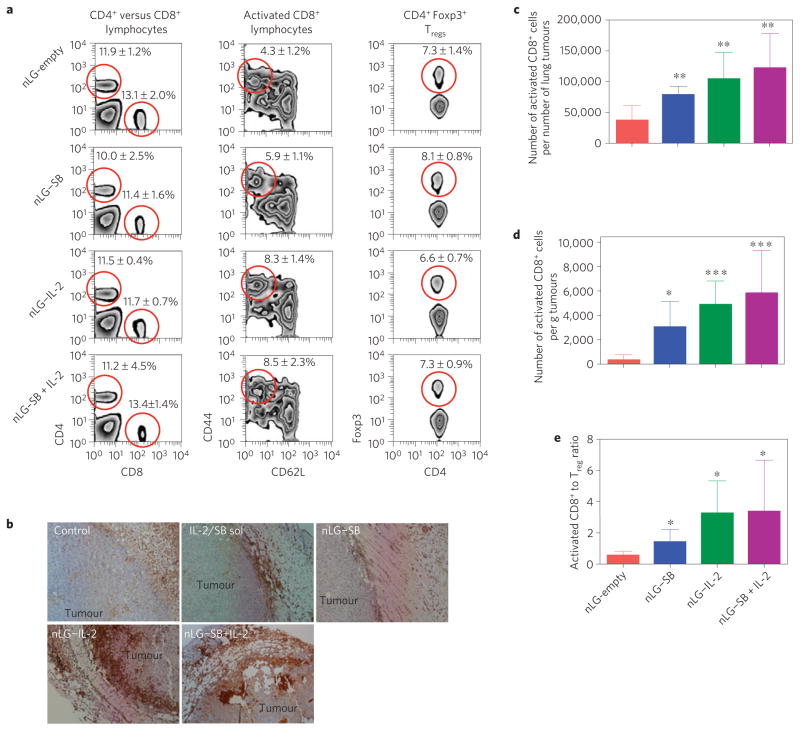
To elucidate the immunologic mechanisms behind the synergistic therapeutic effects of the sustained release combination therapy and the relative contribution of each agent delivered as monotherapies, tumour-infiltrating lymphocytes (TILs) were harvested and evaluated in mice euthanized one to two weeks after the initial therapeutic dose. This time point was chosen based on when mice in all groups had developed either subcutaneous tumours of sufficient size (up to 10 mm in the greatest dimension), or sufficient numbers of lung tumours (more than five), to isolate adequate number of TILs for analysis. Administered in nLGs alone or in combination with SB, IL-2 increased both the percentage (Fig. 5a, middle panel) and absolute numbers (Fig. 5c,d) of activated CD8+ T cells in tumours with minimal impact on overall CD4/CD8 ratios and Tregs, results that were consistent with reported clinical outcomes3. Representative histological images of tumours showed that IL-2 significantly increased lymphocyte infiltration into tumours (Fig. 5b). Sustained administration of this cytokine also increased activated CD8+: Treg ratios in TIL populations (Fig. 5e).
Figure 5. The adaptive immune response and mechanism of nLG–SB + IL-2 action.
a, Representative FACS analyses from subcutaneous tumours receiving intratumoral injections (see Fig. 3). Panels represent, from left to right, percentage of CD4+ versus CD8+ cells in lymphocyte gate, percentage of CD8+ that are activated (CD44+CD62L−), and percentage of CD4+ that are Foxp3+ regulatory T cells. Each group contained six mice and studies were repeated 2–3 times with similar results. Data represent mean ± 1 s.d. The red circles highlight the population that is being quantitated in the charts. b, Haematoxylin (light blue) and anti-Leukocyte Common Antigen (brown) staining shows relative increases in TIL infiltration in tumours with nLG treatments. c, Absolute number of activated CD8+ cells present in lung tumours (normalized per number of tumours) for the study shown in a. All groups have significantly greater numbers (P < 0.01) compared with empty nLGs. d, Absolute number of activated CD8+ cells present in tumours (normalized per tumour mass) removed from mice seven days after treatment (same study as in Fig. 3b). Treatment with nLG–SB significantly increased activated CD8+ populations (P < 0.05), as did treatment with nLG–IL-2, or nLG–SB + IL-2 (P < 0.001), over unloaded particles (nLG–empty). Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. e, Activated CD8+: Treg ratio in TILs for the study shown in c. All groups have significantly greater ratios (P < 0.05) compared with empty nLGs.
These data, however, did not fully explain the observed results in mice receiving particles releasing both IL-2 and SB (Fig. 3), suggesting that another mechanism may be involved in the enhanced anti-tumour effects observed in the treated mice. As TGF-β can also regulate NK cell number and function14, we evaluated whether this cell type involved in the innate arm of the immune system was present in TILs. In stark contrast to the relative number of TIL Tregs observed in all tumour-bearing mice, sustained administration of SB in combination with IL-2 resulted in substantially increased percentage (Fig. 6a) and absolute numbers (Fig. 6b,c) of NK cells present in tumour beds compared to groups receiving either ‘empty’ particles or particles releasing either IL-2 or SB alone. To validate that the therapeutic benefit observed in mice treated with particles releasing both agents was NK-dependent, studies were performed in NK-depleted mice. NK1.1 antibodies were used to deplete mice of NK cells (Supplementary Fig. S9; ref. 14), and tumour cells were injected in NK-depleted mice and mice retaining NK cells. Mice were again euthanized one week after the initial treatment, and tumour masses were measured. NK depletion did not affect the sizes of tumours in mice receiving particles releasing IL-2 alone (Fig. 6d). In contrast, absence of these cells abrogated the delay in tumour growth in animals receiving particles releasing SB and IL-2 (Fig. 6d). Interestingly, there was a modest therapeutic benefit in mice receiving particles releasing drug alone, which was abrogated by NK depletion (Fig. 6d). We observed a modest, statistically significant increase in NK cells in the TILs of mice treated with particles releasing SB alone (Fig. 6b). Thus, the maximum therapeutic benefit observed in mice treated with particles simultaneously delivering SB and IL-2 therapies was probably related to enhanced numbers of NK cells at the tumour site, resulting in increased effector cell populations in the tumour.
Figure 6. Role of NK cells in tumour immunotherapy after combination delivery.
a, Representative FACS analyses from subcutaneous tumours receiving intratumoral injections in Fig. 3. Panels represent percentage of lymphocytes that are NK cells (NK1.1+). Data represent mean ± 1 s.d. Each group contained six mice and studies were repeated 2–3 times with similar results. The red circles highlight the population that is being quantitated in the charts. b, Absolute number of NK cells present in tumours (normalized per number of tumours) for the study shown in a. Compared with the empty particle group, significantly more NKs were present in the lungs following treatment by nLG–SB + IL-2 (P < 0.05, *), nLG–SB (P < 0.05, *), and nLG–IL-2 (P < 0.01, **). c, Absolute number of NK cells present in tumours (normalized per tumour mass) for the same study as in Fig. 3b. The nLG–SB + IL-2-treated group has significantly more NKs than the control group (P < 0.01, **), the SB-treated group (P < 0.05, *), and the IL-2-treated group (P < 0.01, *). Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. d, Comparison of tumour masses from wild type (WT) or NK-depleted (NKD) mice euthanized seven days after initial treatment. The nLG–SB + IL-2-treated WT group has significantly smaller tumours than all other treatment groups (P < 0.001, ###). The NKD nLG–SB and nLG–SB + IL-2 groups have significantly larger tumours than their WT counterparts (both P < 0.001, * * *). Studies were repeated 2–3 times with similar results.
Importantly, we note that the clinical effects following systemic therapy were consistent with the results of localized therapy and drug biodistribution. We found that encapsulation in nLG increased both the initial dose to the lungs as well as dose persistence over a three-day period; on the third day after administration, 9.0 ± 0.8% (mean ± s.d.) of the initial dose of nLGs in healthy animals, 38 ± 8% in the metastatic animals, was measured in the lungs compared with 1.5 ± 0.7% of soluble drug (Fig. 2c and Supplementary Fig. S7e). This pharmacokinetic effect correlates with increased survival (Fig. 3d) and a significant decrease in the number of tumours (Fig. 3e). Analysis of lung-infiltrating lymphocytes demonstrated that, as observed in subcutaneous tumours receiving intratumoral nLG–SB + IL-2 treatments, enhanced numbers of activated CD8+ (Fig. 5c) and NK (Fig. 6b) effector cells mediated tumour abrogation and increased survival. These data indicate that significant anti-tumour responses against metastatic melanoma can indeed be achieved by the sustained, combined delivery of a TGF-β inhibitor drug and IL-2 in a clinically relevant mode of administration.
Our results in the B16 melanoma model suggest that the difficulties in ensuring simultaneous, synergistic delivery of both labile proteins and small hydrophobic molecules can be addressed with rational engineering of a nanoscale delivery system fabricated from inert, biodegradable components with a history of use, individually, in different drug-delivery applications. This study shows that the activation of the innate arm of the immune system is a critical immunologic mechanism underlying the synergistic effects of simultaneously delivering IL-2 and SB, resulting in delayed tumour growth and enhanced survival of tumour-bearing mice. Administration of SB in combination with IL-2 stimulated the innate immune system, greatly increasing the number of NKs in tumours in mice receiving this combination. Absence of therapeutic efficacy following NK depletion demonstrated that stimulation of the innate arm by nanoparticles releasing both agents was crucial for achieving an improvement in survival in this model. Particles releasing SB, IL-2 alone or the combination also stimulated the adaptive immune system, enhancing activated CD8+: Treg ratios. These results suggest that combination therapy can stimulate both arms of the immune system simultaneously.
Aggressive tumours such as melanoma inherently develop leaky vasculature with 100–800 nm pores due to rapid vessel formation to sustain a rapidly growing tumour. This defect in vasculature and poor lymphatic drainage results in enhanced permeation and retention (EPR) of nanoparticles within the tumour bed (Fig. 4c), and is a form of ‘passive targeting’. The basis for increased accumulation of drug-loaded nanoparticles in tumours over normal tissues is that, unlike tumour beds supplied by leaky vasculature, normal tissues contain capillaries with tight junctions that are less permeable to nanosized particles. Passive targeting can therefore result in several-fold increases in particulate concentrations in solid tumours compared to free administration of antibodies or other drugs29, and may explain the increased survival and effective treatment of metastasis observed after intravenous injection (Fig. 3d). Potential strategies to further increase this survival index may include increasing the frequency of injections, dosage per injection, or inclusion of tumour retention ligands on the surface of the nanoparticle to improve the selective delivery of agents and retention in the tumour microenvironment. It is also possible that the activity of tumour-infiltrating lymphocytes and NK cells can be enhanced by the delivery of additional cytokines such as IL-15 (ref. 45) or its agonists46. IL-15 belongs to the IL-2 family of cytokines and can function to enhance the survival of IL-2 activated cells45.
Particulate formulations that can increase the cytokine half-life in circulation, but also co-deliver in a sustained manner a potent pleiotropic inhibitor (TGF-β inhibitor), are thus attractive new immunotherapies. Such core–shell systems are multifunctional drug-delivery alternatives offering exciting new possibilities for combinatorial delivery, and can work synergistically in cancer therapy31,33,34. Systems engineered with a fluid lipid bilayer have desirable circulation properties, and offer the potential for targeting while enabling the delivery of agents of different physical properties. For example, in a manner similar to the nLGs, lipid bilayers circumscribing colloidal mesoporous31 or nanoporous34 cores may facilitate the tunable delivery of small-molecule drugs and bioactive cytokines for combined drug-delivery. Incorporation of cationic polymers such as polyethyleneimine33 can potentially enable the delivery of complex oligonucleotides such as siRNA, which may be used to silence the tumour’s immune inhibitory microenvironment, and/or plasmids that express immunostimulatory cytokines. Other platforms are also attractive for this purpose, such as crosslinked multilamellar vesicles47, which can be loaded with multiple agents for sustained release and stable delivery to immune cells and tumours. A critical prerequisite in platform selection for immunotherapy, however, is a complete understanding of how material composition may activate or deactivate innate/adaptive immune responses, as clearly this will dictate the magnitude and direction of the anti-tumour immune response.
Our results demonstrate a potential NK-mediated mechanism of anti-tumour activity. Thus, this work provides an essential proof-of-concept demonstration of a biodegradable nanoparticle technology that facilitates sustained delivery of hydrophilic and hydrophobic immunomodulators to enhance anti-tumour activity against subcutaneous and metastatic melanomas. This therapeutic approach warrants further preclinical evaluation, as sustained delivery of immunotherapeutics such as IL-2 and SB by hybrid, core–shell, nanoparticle technology could ultimately provide a novel, clinically relevant approach to treat metastatic melanoma patients.
Methods
Polymer synthesis
Polylactide-PEG-polylactide diacrylate was synthesized in two steps according to a previously reported procedure35 (see Supplementary Methods).
nLG formulation
All lipids were obtained from Avanti Polar Lipids and used without further preparation. Phosphatidyl choline (PC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG) and cholesterol were mixed in chloroform in a 3:1:1 molar ratio, and liposomes were formulated using a remote loading technique48. Lipid-labelled fluorescent liposomes were formulated by incorporation of 10% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N -[poly(ethylene glycol)2000-N 3-carboxyfluorescein] (DSPE-PEG-Fluorescein). Briefly, the dissolved lipids were mixed in a glass scintillation vial, followed by complete solvent removal with a directed nitrogen stream. This formed a thin lipid film on the inner glass surfaces, which was rehydrated by the addition of 1X PBS. Cycles of 30 s vortexing followed by 5 min idle sitting at room temperature were repeated 10 times, and the resulting multilamellar liposomes were extruded 10 times through a 5 μm polycarbonate membrane (Whatman), 10 times through a 1 μm membrane and finally 11 times through a 100 nm membrane using a LIPEX extruder (Northern Lipids). The resulting unilamellar liposomes were then frozen and lyophilized. Lyophilized liposomes were reconstituted with a solution containing 5% (w/v) polymer (Fig. 1) and 2.5 mg ml−1 Ciba Irgacure 2959 as the photoinitiator and: no other additive (nLG–empty), 9 mg f-CD-solubilized SB/100 mg nLG (nLG–SB; SB505124, Sigma), 1 μg IL-2/100 mg lipids (LG-IL-2; Aldesleukin Proleukin, Novartis), or both f-CD-solubilized SB and IL-2 (nLG–SB + IL-2). CD (randomly succinylated β-CD) was functionalized with 2-aminoethyl methacrylate (Supplementary Fig. S1) by stirring a 1:3 molar ratio of the compounds in 1X PBS for 1 h at room temperature. SB was incorporated into f-CD by adding the drug dissolved in methanol to the f-CD. After 20 min of vigorous stirring at room temperature to form the complexes, the methanol was evaporated with a directed stream of nitrogen. The reconstitution step proceeded with 30 min of vortexing to rehydrate the liposomes. The liposomes were then irradiated under ultraviolet light for 8 min with a Blak-Ray long-wave ultraviolet lamp (Model B 100) at a 10 cm working distance. Directly prior to ultraviolet irradiation, the samples were diluted five-fold to prevent macroscale gelation. The resulting nLGs were pelleted by centrifugation (5 min at 7,200 rcf) and resuspended in 1X PBS (see Supplementary Methods).
In vitro release studies and bioactivity of encapsulants
Release studies were performed at 37 °C with constant agitation in 1X PBS + 10% fetal bovine serum (see Supplementary Methods).
In vivo subcutaneous tumour studies
B16-F10 cells (ATCC) were cultured in DMEM (Gibco) and suspended at 2×106 cells ml−1 in 1X PBS (kept on ice) directly before injection. For subcutaneous tumour studies, female 6–8 week-old B6 albino mice were sedated with AErrane (isofluorane; Baxter) and the right hind flank was shaved before a subcutaneous injection of 50 μl of the cellular suspension. Tumours were monitored and treatment began when the average tumour area reached ~5.5 mm2 (8–10 days after B16 injection); mice were rearranged to normalize tumour sizes across groups (see Supplementary Methods).
In vivo nLG biodistribution, toxicity and metastatic lung tumour studies
Metastatic B16 melanomas were established in female 6–8 week old B6 mice by intravenous (tail vein) administration of 50 μl of B16 cellular suspension, as previously described5. Treatment was initiated seven days later, with each dose consisting of 5 mg nLGs administered intravenously via tail-vein injection (see Supplementary Methods).
Time-resolved two-photon laser scanning microscopy
An Olympus BX61WI fluorescence microscope in combination with a ×20, 0.95NA Olympus objective and LaVision Biotec two-photon microscopy system was used for imaging tumour vasculature and nLG accumulation in tumours. Briefly, incisions were made to expose skin flaps surrounding subcutaneous tumours on anaesthetized C57BL/6 mice. Intravital image acquisition was started 5 min after intravenous administration of nLGs (see Supplementary Methods).
FACS analyses of TILS
A Becton Dickenson LSRII flow cytometer was used to quantify the phenotype of isolated tumour infiltrating lymphocytes (see Supplementary Methods). The percentage of immune effector cells (CD4+, CD8+ T cells and NK cells) as well as Tregs in the tumour-bearing mice was evaluated by staining for the cell-surface expression of CD4, CD8, NK1.1 and TCR-β, as well as intracellular FoxP3, as per the manufacturer’s instructions (eBioscience).
Data and statistical analyses
ANOVA and paired t-tests (two-tailed) were used to analyse differences in tumour areas and masses. We used OriginPro version 8.1 (Microcal) and Prism version 5.01 (GraphPad Software) for the analyses. Survival studies were analysed using the Kaplan–Meier and Wilcoxon–Gehan tests with Origin.
Supplementary Material
Acknowledgments
The authors would like to thank R. Murelli and D. Spiegel for assistance with polymer synthesis; P. McEnaney, S. Royce-Hynes, J. Bertram and Q. Wang for helpful discussions and assistance with the procedures. The work was supported in part by NIH through grants R01-HL085416 and R01-EB008260 (T.M.F.); an NIH Autoimmunity Center of Excellence Pilot Award (U19AI082713; T.M.F); a Public Health Grant (HL-55397; T.M.F.); an NSF CAREER grant (T.M.F.); a Yale Skin SPORE Career Development Award (S.H.W.); the Howard Hughes Medical Institute (R.A.F.); the Yale Cancer Center (S.H.W.), and a post-doctoral fellowship from the Pew Charitable Trust: Pew Latin American Fellow Program in Biomedical Sciences (P.L.L.) and the Yale Rheumatologic Disease Research Core Center P30AR053495.
Footnotes
Author contributions
J.P., E.S., S.H.W., R.A.F. and T.M.F. designed all of the experiments for this study. R.R helped synthesize the polymer with J.C., J.P. and E.S. T.M.F. conceived the formulation and E.S., J.P., S.M.J., A.A. and T.M.F. developed and characterized the particles. J.P., E.S., S.H.W., P.L.L. and S.L.D. performed in vivo experiments. J.P., S.H.W. and P.L.L. performed FACS analyses and in vitro characterization experiments. M.L. performed toxicology experiments, nLG characterization experiments and part of the statistical analysis. R.A.F. and T.M.F. initiated the research and supervised the program. A.H. and D.S. helped perform and analyse the intravital imaging microscopy work together with J.P. J.P., E.S., S.H.W. and T.M.F. wrote the manuscript. T.M.F. edited the manuscript.
The authors declare no competing financial interests.
Supplementary information accompanies this paper on www.nature.com/naturematerials. Reprints and permissions information is available online at www.nature.com/reprints.
References
- 1.Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Semin Oncol. 2007;34:532–545. doi: 10.1053/j.seminoncol.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 3.Acquavella N, et al. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008;31:569–576. doi: 10.1097/CJI.0b013e318177a4ba. [DOI] [PubMed] [Google Scholar]
- 4.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nature Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 6.Smyth MJ, et al. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 7.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: Implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 8.Liu VC, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: Role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 9.Fan TM, Kranz DM, Roy EJ. Enhancing antitumor immunity: combining IL-12 with TGFβ1 antagonism. J Immunother. 2007;30:479–489. doi: 10.1097/CJI.0b013e318031a2b2. [DOI] [PubMed] [Google Scholar]
- 10.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-β signaling inhibitors for cancer therapy. Nature Rev Drug Disc. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 11.Neville ME, Boni L, Pflug L, Popescu MC, Robb RJ. Biopharmaceutics of liposomal IL-2. Oncol Cytok. 2000;12:1691–1701. doi: 10.1006/cyto.2000.0769. [DOI] [PubMed] [Google Scholar]
- 12.Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 13.Kano MR, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. Proc Natl Acad Sci USA. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta control T helper type 1 cell development through regulation of natural killer interferon-gamma. Nature Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 15.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies SD, et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011;17:3673–3685. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 17.Teppler H, et al. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin 2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993;177:483–492. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letourneau S, et al. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci USA. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman HL, Flanagan K, Lee CS, Perretta DJ, Horig H. Insertion of interleukin-2 (IL-2) and interleukin-12 (IL-12) genes into vaccinia virus results in effective anti-tumor responses without toxicity. Vaccine. 2002;20:1862–1869. doi: 10.1016/s0264-410x(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 20.Kanaoka E, et al. A significant enhancement of therapeutic effect against hepatic metastases of M5076 in mice by a liposomal interleukin-2 (mixture) J Control Rel. 2002;82:183–187. doi: 10.1016/s0168-3659(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 21.Neville ME, Robb RJ, Popescu MC. In situ vaccination against a non-immunogenic tumour using intratumoural injections of liposomal interleukin 2. Cytokine. 2001;16:239–250. doi: 10.1006/cyto.2001.0963. [DOI] [PubMed] [Google Scholar]
- 22.Town T, et al. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nature Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byfield SD, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol Pharm. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 24.Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. Semin Oncol. 2004;31:5–15. doi: 10.1053/j.seminoncol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Rev Drug Disc. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 26.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Thevenot J, Troutier A-L, David L, Delair T, Ladavire C. Steric stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromole. 2007;8:3651–3660. doi: 10.1021/bm700753q. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 29.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 30.Kazakov S, Levon K. Liposome-nanogel structures for future pharmaceutical applications. Curr Pharm Des. 2006;12:4713–4728. doi: 10.2174/138161206779026281. [DOI] [PubMed] [Google Scholar]
- 31.Meng H, et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng H, et al. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liong M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashley CE, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(α-hydroxy acid) diacrylate macromers. Macromole. 1993;26:581–587. [Google Scholar]
- 36.Van Thienen TG, Raemdonck K, Demeester J, De Smedt SC. Protein release from biodegradable dextran nanogels. Langmuir. 2007;23:9794–9801. doi: 10.1021/la700736v. [DOI] [PubMed] [Google Scholar]
- 37.Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 38.Stella J, He Q. Cyclodextrins. Toxicol Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 39.Cote-Sierra J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Soufi W, et al. Fluorescence correlation spectroscopy, a tool to investigate supramolecular dynamics: inclusion complexes of pyronines with cyclodextrin. J Am Chem Soc. 2005;127:8775–8784. doi: 10.1021/ja0508976. [DOI] [PubMed] [Google Scholar]
- 41.Wu S, et al. Photoreversible fluorescence modulation of a rhodamine dye by supramolecular complexation with photosensitive cyclodextrin. Angew Chem Int Ed. 2007;46:7015–7018. doi: 10.1002/anie.200701396. [DOI] [PubMed] [Google Scholar]
- 42.Farokhzad OC, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 44.Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hori Y, Stern PJ, Hynes RO, Irvine DJ. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials. 2009;30:6757–6767. doi: 10.1016/j.biomaterials.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.