INTRODUCTION
Over the last decade, India has become an important country for clinical trials of international pharmaceutical companies. India's potential for fast recruitment of patients and reduction of clinical trial cost made the country one of the most attractive strategic imperatives for global clinical trials. However, globally, there has been a concern about ethical and scientific implications of globalization of clinical trials to developing countries.[1,2] These concerns have also been reflected in Indian literature and media stories, which have highlighted issues of vulnerability, consent deviations, compensation for patients, Ethics Committee (EC) training and functioning, placebo, post-trial access etc.[3–6] These individual opinions could have an impact in shaping the perceptions of clinical research professionals. This survey attempts to understand the overall perceptions of clinical research professionals about ethics of clinical research in India.
METHODS
The objective of this survey was to seek opinion from clinical research professionals on their perceptions of ethical issues in clinical research in India. To achieve the same, a survey questionnaire was developed and mailed to about 500 clinical research professionals. A follow-up to receive the response was also done by way of mails/telephone calls.
The survey questionnaire covered 12 items which were:
Listing the top three ethical issues in the conduct of clinical trials in India.
Readiness of the ECs on six parameters (rating on a scale of 1-10; 1 being non-competent and 10 being competent).
Independence as the hallmark of EC functioning (rating on a scale of 1-10; 1 being non-independent and 10 being independent) and factors that posed barriers to independence.
Whether GCP training should be mandatory for all EC members and requirement of other training.
Contribution of the lay person during EC meetings and the challenges that the lay person may perceive/face during the meetings.
Adequacy of the informed consent process on seven parameters and suggestions for process improvement.
Adequacy of regulations to safeguard the trial participants and measures for improvement.
Adequacy of safety review by ECs and additional measures for improvement.
Justification of compensation for clinical trial related injuries; factors determining the compensation amount and the body deciding the compensation.
Justification for use of placebos in clinical research and measures to ensure patient rights and wellbeing.
Post-trial compassionate use of investigational drugs by trial subjects.
Vulnerability of patients participating in trials and measures to mitigate their vulnerability.
Most of the questions were of the ‘Yes/No’ format. Some subjective questions were also incorporated so as to obtain solution-based suggestions. No confidential data was requested or received during this process and anonymity of the responders had been maintained. Descriptive analysis was employed to present the data.
RESULTS
A total of 34 responses received included: Sponsor/Contract Research Organization (CRO)-27 (79.4%); ECs-06 (17.7 %); and investigator-01 (2.9%).
Ethical issues in the conduct of clinical trials in India
The top three issues which surfaced maximum number of times from the responses were:
Informed consent process and documentation (n = 18)
Empowerment of ECs to be independent and competent (n = 10)
Patient awareness about safety and compensation rights (n = 9).
Some of the other issues highlighted were: a) economically/educationally vulnerable population's participation in trials; b) patient eligibility; c) EC functioning and training; d) awareness across the society about clinical research; and e) patients being tested for drugs which would never be affordable or made available in India.
Readiness of ethics committees
Opinion was sought on the perception of readiness of ECs for the listed parameters [Table 1].
Table 1.
Readiness of ethics committees (n=33)
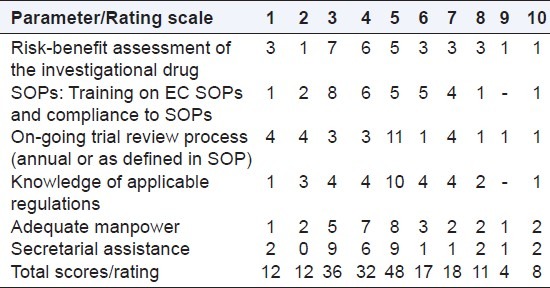
For all the 6 parameters, majority of scores were < 5. Out of a total score of 198 responses for 06 parameters, the total score of ratings < 5 was 140.
Independence of ethics committees
Of the 33 responses, only 3 felt that ECs function independently [Table 2].
Table 2.
Independence of ethics committee functioning (n=33)
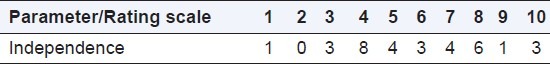
The factors thought to pose barrier to independence were: a) conflicts of interest; b) internal pressure from senior management of institution for revenue generation from trials; c) lack of awareness about independence and its implications in safeguarding patient safety; and d) dependence on explanations provided by the investigator/sponsor/CRO.
Training for ethics committee members
All the responders agreed that GCP training must be mandatory for EC members, along with training on other topics such as: a) applicable regulations; b) compulsory continuing medical education; c) protocol review process; d) ethics and ethical thinking; e) EC standard operating procedures (SOPs); and f) roles and responsibilities of each quorum member.
Additional training topics suggested were:
Legal aspects of trials
Compensation
Structure/design of study documents
General understanding of the patient population visiting the site in terms of literacy/culture/socio-economic status
Appropriate methods for consent process.
Contribution of lay person in ethics committee
About two thirds of the responders (23) opined that the lay person is unable to contribute adequately in the EC meetings. Some of the perceived challenges for the lay person were: a) lack of medical/technical knowledge to keep pace with and decipher the discussions; b) diffidence to speak and too many power centers in the meeting to overturn his/her voice; c) the lay person is unaware about the importance of his/her role; d) role of the lay person is considered more so for the sake of meeting quorum; and e) inadequate exposure or training on clinical research, human rights, and compensation.
Informed consent process
The responders shared their thoughts [Table 3] on the current informed consent process on a set of given parameters. Majority felt that the subject is offered the opportunity to ask questions and is able to refuse participation in clinical research, and also favored the need to record the consent process.
Table 3.
Informed consent process in India (n=34)
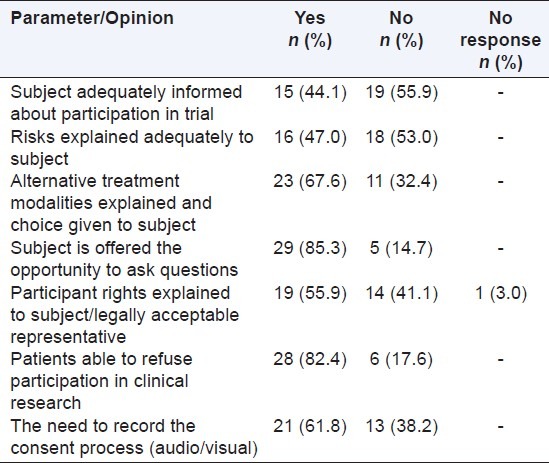
Steps suggested to improve the consent process were: a) improved awareness to be created among the patients, society and medical fraternity on the need for clinical trials and patient's rights; b) the EC or an independent committee or a patient research advocate should interview the subjects randomly or monitor the consent process; c) the audio-visual recording of the consent administration process may bring transparency, however this method should be considered more so to sensitize the researcher than to do policing; and d) the consent form to be simplified and include pictorial images for better patient understanding.
Adequacy of regulations to safeguard the clinical trial participants
Of 34 responders, 18 expressed satisfaction with the current regulations, 15 opined that regulations are inadequate, and one did not opine.
The respondents, satisfied with the current regulations, felt that the following can be further improved: a) regular site inspections by authorities and publishing critical findings for public access; b) more transparency in publishing trial outcomes; c) clarity of guidelines for reimbursement and alternative treatment; d) accountability of the investigators to hospital administration; e) appointment of a supervisory body for trials; f) education on implementation of regulations; g) intermittent regulatory oversight (e.g. meeting the subjects); and h) inspections of all stakeholders with appropriate warnings/sanctions/penalties for non-compliances.
The respondents, dissatisfied with the current regulations suggested: a) faster turn-around time; b) to upgrade the regulations per need basis based on opinions from stakeholders; c) need for regular monitoring of trials by trained GCP experts; and d) need for more transparency in the consenting process for patients.
Safety review by ethics committee
Out of 34, 14 responders felt that safety review by ECs was adequate but 20 responders did not feel so.
Those who were satisfied with the safety review, suggested to: a) improve the review process viz. conduct of actual site visits for close monitoring by EC members; b) audits of EC performance by third parties; c) EC minutes of meeting to reflect safety review; d) face-to-face discussions with study team while evaluating safety reports and comparison of data with frequency of similar events at other sites/countries; e) have a safety committee with experts to review the adverse events and suggest appropriate safety management plan; and f) EC to query the site or place a hold on recruitment if subjects are exposed to undue safety risk.
Those who were not satisfied with the safety review suggested that: a) ECs should vigilantly scrutinize the adverse events for protocol deviations or negligence on the part of the investigator; and b) the total number of trials handled by the EC should be limited to only a certain number at any given time.
Compensation for clinical trial related injuries
Thirty out of 34 responders were in favor of compensation to trial subjects for trial related injuries and four responders felt that compensation is not justified.
Opinion was also sought on the amount and the factors to be considered to determine the same. Majority of responses (n = 12) suggested that the amount should be commensurate as per the recent Central Drugs Standard Control Organization guidelines. It was also mentioned that the number of dependents of the trial subject should be considered and the amount should be adequate to compensate the subject's contribution to scientific improvement. A word of caution was raised by one respondent that the amount should not act as an inducement for trial participation such that relatives would end up taking advantage for seriously ill patients (e.g. oncology studies).
Opinion was also sought on who should be deciding the compensation [Table 4]. Twenty eight of 34 responders suggested that multiple authorities should join hands to collectively decide the compensation amount.
Table 4.
Deciding body for the compensation

Six of 34 responders opined that only a single body should have the authority to decide the amount rather than a conglomeration of multiple bodies, and responses were as follows:
Regulators-1
Ethics Committee-3
Others-2
Responders who recorded responses for ‘Others’ category suggested that an independent trained third party committee (comprising of community person/social worker/lawyer/hospital administrator/national level independent body) should decide the compensation.
Justification for placebo
Thirty out of 34 responses were in favor of the use of placebo, two were against the use of placebo and two did not opine.
The use of placebo was justified, subject to the condition that: a) the said disease had no defined/established standard of care; b) adequate rescue procedures for patient withdrawal and safety management were ensured; c) back-up investigators present at the site for additional oversight; and d) additional monitoring ensured by the sponsor/CRO. It was also opined that the EC should review the scientific soundness of the placebo-controlled trial in greater depth and have an increased level of subject education at screening stage.
Other miscellaneous opinions suggested that standard treatment must also be provided along with the placebo since putting the patient only on placebo would be unethical; also, wherever possible, patient should be in-patient so that emergency medical care could be made available; and placebo should be used only for proof of concept trials.
Post-trial access to investigational drug
Out of 34 responders, 21 were in favor and 13 were against the investigational drug being made available to patients post end of trial.
The responders who answered ‘Yes’ expressed the following: a) on humanitarian grounds only in case of terminal illness; b) if the investigational drug is found to be beneficial and not going to be marketed in India; c) patients may benefit and get used to the drug wherein it would be difficult for the investigator to withdraw the drug post trial completion; and d) trial subjects may not afford the commercial drug and it could be given at no cost to reciprocate their contribution to science.
The responders who answered ‘No’ for the drug being made available, presented the following justification: a) drug may not have adequate safety/stability and additional further studies post end of study could change the efficacy/safety information to preclude such treatment; b) the drug is not known to be reacting in uncontrolled conditions post study without adequate oversight and more importantly, if there was any adverse event/death during the window period of trial conclusion and marketing authorization, the investigator would be charged of using an unapproved drug; and c) it could also happen that some trial subjects could be benefitted from the drug during the trial and may insist on continued use post trial, but the regulatory authority could end up to refuse marketing approval for the drug.
Vulnerability of low literacy patients
Out of 34, 26 responders felt that Indian patients were vulnerable and 8 responders felt otherwise.
The responders who answered ‘Yes’, suggested the following measures to mitigate the risk: a) educate potential subjects; b) Indian doctors being considered equivalent to ‘Gods’, should not take undue advantage of this belief; c) ECs to play a larger role by creating patient support groups who could have proactive discussions with subjects on clinical research and patient rights; d) ECs to conduct inspections of sites to confirm adequacy of consenting process and contact subjects to confirm their voluntary participation; e) media and non-government organizations to shoulder the responsibility of spreading awareness about clinical research rather than the media highlighting any negative aspect; and f) selection of investigators to be based on their interest in research rather than monetary gains.
The eight responders who felt that patients are not vulnerable expressed: a) literacy rates are increasing and patients are asking questions or having discussions with the investigators; and b) patients have a choice to go to other doctor/hospitals as affordability to health care has increased. An interesting thought was that vulnerability due to illiteracy/low literacy is only a myth since illiteracy does not mean naivety.
DISCUSSION
The survey reflects that professionals in clinical research are aware of the ethical issues of clinical research in India. In spite of varied perceptions, the main areas of concern appear to be informed consent process and documentation, empowerment of ECs based on independency and competency, and patient awareness about safety and compensation rights.
The survey participants identified several reasons why lay person is unable to participate effectively in EC proceedings. The opinion on adequacy of safety review by EC was divided. However, several useful suggestions were made to improve the safety review process e.g. EC audit, separate committee for safety review, limiting number of trials reviewed by EC and face-to-face meeting with study team. The respondents recommended several areas for trainings of EC members such as GCP, regulations, SOPs, and consent process, with a stress on ethical thinking.
The regulatory process appeared adequate to majority of respondents. However, there were suggestions to improve the process e.g. trained GCP experts to inspect/monitor trials, clarity in guidelines, and regulatory bodies meeting the subjects.
Majority participants felt that during the informed consent process: a) alternative treatment modalities are explained and choice given to subject; b) subject is offered the opportunity to ask questions; c) participant rights are explained to subject/legally acceptable representative; and d) patients are able to refuse participation in clinical research. As the majority of survey participants were from industries, who are not involved directly in the consent process, these perceptions require confirmation by survey of investigators, ECs and trial participants.
About three fourths of the responders felt that low literacy levels increased the vulnerability levels for patients and suggested measures for its mitigation e.g. EC oversight for consent process, creation of patient support groups, role of media in creating awareness about clinical research.
A large majority favored compensation for trial subjects for trial related injury, and felt that multiple stakeholders could collectively decide the compensation. Majority of participants favored the use of placebo with adequate safeguards to protect the trial participants. Post-trial access to investigational drug was acceptable to a majority.
The main limiting aspect of this survey is a small sample size. Besides, majority (79.4%) of respondents are from industry. A large survey with adequate representation of all stakeholders-investigators, EC members, media, and patient groups is required to validate the survey findings.
DISCLAIMER
The views expressed in this article are those of the individual and may not necessarily represent their organization.
ACKNOWLEDGMENTS
We would like to thank Deven Babre (PharmaNet-i3) for his assistance in the compilation and analysis of this survey.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–23. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 2.Nundy S, Gulhati CM. A new colonialism?--Conducting clinical trials in India. N Engl J Med. 2005;352:1633–6. doi: 10.1056/NEJMp048361. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt A. Indian clinical trials: Paradigm shift from speed to quality? Perspect Clin Res. 2012;3:1–3. doi: 10.4103/2229-3485.92299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmachari B, Bhatt A. Clinical research ethics in developing countries: Challenges and the way forward Pharma Times. 2010;42:27–9. [Google Scholar]
- 5.Bavdekar SB, Thatte UM. Compensation for research-related injury. J Postgrad Med. 2009;55:87–8. doi: 10.4103/0022-3859.52836. [DOI] [PubMed] [Google Scholar]
- 6.Taur SR, Bavdekar SB, Thatte UM. Survey of ethics committee protocol approval letters: Compliance with Schedule Y/ICMR guidelines 2006. Indian J Med Ethics. 2011;8:214–6. doi: 10.20529/IJME.2011.083. [DOI] [PubMed] [Google Scholar]


