HISTORICAL PERSPECTIVE
Although, descriptions of childhood illnesses is found in ancient Egyptian, Greek and Roman texts; there is hardly any documentation of medical research in children prior to the 18th century. Edward Jenner's smallpox vaccination experiment is, probably, the first documented study in the pediatric population. Later, in the 19th century, when pediatric medicine became a recognized specialty, children in pediatric hospitals and orphanages became a ready source of for experimentation in children. The use of this highly vulnerable section for research studies hardly caused any flutter; given the prevalent norms regarding biomedical research. The real opposition came towards the end of the 19th century, when anti-vivisectionist movement began protesting against the use of animals and children in research activities and some felt the need for regulating research involving children.[1]
During the World War II, Nazi doctors conducted several experiments of dubious scientific basis. Children were a part of some of these experiments, which resulted in pain, misery, disability and death. The Nuremberg Code that was formulated after the Nazi doctors and officials were prosecuted, insisted upon “voluntary consent by the prospective participant who had the legal capacity to do so”.[1,2] As children do not have this capacity, strict adherence to the Code would have disallowed any research in children. Since, most physicians chose to ignore the Code anyway, pediatric research continued without any regulation till 1960s. In 1964, the World Medial Association (WMA) prepared a set of guidelines to help investigators conduct ethically sound research. It allowed both therapeutic and non-therapeutic research involving children, provided consent was obtained from the child's parent or guardian.[1,3]
It is worth remembering that with other vulnerable sections of the society, children bore the brunt of ill-effects of human experimentation. Use of thalidomide by pregnant women resulted in severe birth defects. Institutionalized children in the Willowbrook State School were deliberately infected with hepatitis virus as a means of studying the potential to develop a vaccine.[1,4] The continuation of Tuskegee Syphilis study that continued even after penicillin was discovered, resulted in fetal deaths and birth of babies with congenital syphilis.[1,5] These events caused a public outcry and forced the US administration to examine the problems of research abuses and create standards for the protection of individuals participating in research. The Belmont Report justified research involving children, as it would help find better ways of treating childhood illnesses and promote their healthy development.[6] The Report categorized children as a vulnerable population with diminished autonomy and hence entitled for additional protection from undue influence and coercion. The protective approaches such as requirement of careful scrutiny of pediatric research protocol for the level of risk, entrusting the responsibility of permitting the child to enroll with parents and demanding steps for minimization of risk are some of the protective approaches described in that report.[1,6]
However, over the years; excessive concern about exposing children to molecules about which everything was not known made the society, pharmaceutical companies and regulators not undertake clinical trials in children. This resulted in many drugs being marketed without any worthwhile evidence of their safety and efficacy in children. However, pediatricians were forced to use these drugs just on the basis of data extrapolated from adult studies. This was not a happy situation, as children continued to be exposed to the new molecules without adequate pediatric data and that too without the benefit of intense monitoring that characterizes a clinical trial. In addition, pharmaceutical companies were less keen to conduct specific pediatric trials as these are more challenging and the pediatric market is smaller compared to the adult drug market.
Considering the importance of drug trials in children, the US and European countries enacted several legal provisions to encourage, entice or compel pharmaceutical companies to undertake pediatric trials.[7–12] The European Regulation of Pediatric Medicines has three major initiatives for ensuring that children will receive drugs that are safe and efficacious: the adoption of incentives for industry, the implementation of a mandatory Pediatric Investigation Plan (PIP) considering all age ranges and the creation of a Pediatric Committee (PDCO).[13] The pharmaceutical companies are obliged to submit a PIP for new indications, new routes of administration or new formulations of already patented products and for the development of new medicinal products. If information is correctly provided after conducting the required studies in compliance with the PIP, the company is rewarded with a six-month extension of the Supplementary Protection Certificate. The Regulation also intends to stimulate research for establishing safety and efficacy of drugs that are already in use in children, but without much supportive data. Under the Pediatric Use Marketing Authorization (PUMA), if studies based on pediatric indications and formulations are carried out in line with the agreed PIP; the applicant can get a PUMA approval with 10-year market exclusivity.[13] In comparison, the US approach for pediatric authorization seems pragmatic and more flexible. It asks companies to complete Pediatric Development Plan (equivalent to PIP in the EU) providing sufficient data base from adult population. When an off-label drug is used for a long period, the US authorities give a pediatric authorization based on the number of children already treated, available efficacy and safety data collected from pediatric population, life duration of the off-label product use and safety data base in adults. This is important because clinical research on off-patent drugs is rather complicated raising ethical issues and companies are generally reluctant to provide the off-label drug for research, due to thin profit margins for these products.[13]
So the events have come a full-circle. First studies in children were conducted without much oversight. This resulted in shocking the conscience of the society and pediatric trials were shunned so that children are not exposed to potentially dangerous molecules. With no safety and efficacy data, children continued to be exploited through exposure to untested drugs in the clinical practice. The stage came that regulatory authorities had to take steps to encourage the conduct of pediatric trials, but with greater regulatory and ethical oversight than that prescribed for adult clinical trials.
RATIONALE FOR CONDUCTING PEDIATRIC CLINICAL TRIALS
The Children's right to the highest attainable level of health enunciated by the Convention on the Rights of the Child[14] cannot be realized if they are provided therapy based on evidence generated through studies carried out in adults.[15,16] This is essential as children and adults differ in physiological capabilities, pharmacokinetic profile and pharmaco-dynamic characteristics. Their metabolic pathways, organic functions and metabolic rates, differ widely. Disparities also exist in terms of receptor functions, effector systems and homeostatic mechanisms. In addition, age, growth and development influence side effects,[17–19] and the dose of medications is dependent on body weight or surface area. Also, age influences the severity and type of disease, and pathological agents.[20] These differences result in complete extrapolation of adult data being appropriate in only 6% of drugs,[17,21] implying that such extrapolation will lead to inaccuracies.[20] Therefore, it is morally imperative, to formally study drugs in children so that they can enjoy appropriate access to existing and new therapeutic agents.[22]
WHEN SHOULD PEDIATRIC TRIALS BEGIN?
This is a crucial issue. As a large majority of molecules that enter phase one trials in adults never receive regulatory approval because of lack of efficacy or safety concerns; generally, it is not reasonable to enroll children in drug trials till the sufficient proof of safety and significant information about pharmacokinetics and efficacy in adults are available. Hence, generally speaking, it is appropriate to defer pediatric testing until adult testing has reached phase three or beyond.[22] This may be relaxed, if the disease exclusively occurs in children. For better understanding, the medications can be classified as follows:
Medicinal products for diseases that affect children exclusively [e.g., surfactant used for the treatment of hyaline membrane disease (HMD) in neonates]. Here, it is logical that the entire drug development program is conducted entirely in children
Medicinal products to treat diseases that mainly affect children, or are of particular gravity in children or have a different natural history in children.
Medicinal products intended to treat diseases occurring in adults and children, for which there is currently no treatment
Medicinal products to treat a disease occurring in adults and children for which treatments exist, but where there is insufficient knowledge of efficacy or toxicity in children.
For products of serious diseases in adults and children for which sufficient treatment does not exist, the development program can be conducted early in pediatric population, after safety and tolerability data have been obtained in adults. For other products, pediatric studies can be initiated once efficacy and safety have been studied and proved in adults.[20] The severity of a disease and availability or otherwise of alternative therapies influence the risk/benefit analysis. Greater severity of a disease in children or non-availability of a proven therapy could support earlier initiation of pediatric studies. Several diseases like genetic or metabolic disease that are associated with early death in childhood have no analogy in adults. Hence in such diseases there may not be close analogy in adults. And hence it may not be possible to generate adult efficacy data. Nevertheless, it may still be reasonable to obtain initial safety data in adults before the initiation of any pediatric testing.[22]
MINIMIZING RISKS
While carrying out research in children, all efforts should be made to minimize the risks. Risks include all harms, discomforts, indignities, embarrassments, and potential breaches of privacy and confidentiality associated with the research. The US federal regulations determine the type of research that can be conducted on the basis of level of risk. Four categories are described. First, research is permitted if the level of risk is no greater than minimal, regardless of whether there is a prospect of direct benefit to the child. Second, research that holds out prospect of direct benefit to individual child participants is permitted as long as the risks are minimized and justified by level of anticipated benefit. Third, research is permitted even if it involves greater than minimal risk and no prospect of direct benefit to individual children. This is provided the level of risk is a minor increase over minimal, the intervention or procedure presents experiences to subjects that are commensurate with actual or expected medical, psychological, social, or educational situations, and research is likely to yield generalizable information of vital importance about the subjects’ disorder. The last category of research is the one that is not otherwise permissible under the first three categories but presents an opportunity to understand, prevent or alleviate a serious problem affecting the health or welfare of children. This category of research can be permitted only by the Department of Health and Human Services after expert consultation and opportunity for public review.[1] Although, the Ethics Committees have to determine the magnitude of risk, this regulatory framework significantly limits the discretion of investigators, parents and ethics committees. At the same time it allows much research of importance, while ensuring that children's health and well-being are safeguarded. In all research involving children, all efforts should be made to minimize risks, irrespective of the quantum of risk. Some of the ways of minimizing risks are enlisted in Table 1.
Table 1.

DATA-AND SAFETY-MONITORING COMMITTEES
Children are a potentially fragile population. Hence, they deserve the highest standards for monitoring safety during a drug study. It is not possible to foresee all risks in children, and unexpected events can and do occur. Although some believe that an independent data-and safety-monitoring committee (DSMC) should be created for all phase three drugs and some phase one and two studies conducted in children; especially those that include blinding;[22] others believe that safety monitoring can be adequately performed by investigators and sponsors.[25] However, trials testing new interventions with few safety data available, those addressing major morbidity or mortality end points, studies carried out in high-risk populations, those with large sample size and multi-center trials in children should be monitored by an independent DSMC.[25] In a review of 739 pediatric trials performed till 2002, although 71% reported an adverse event and 20% reported a serious adverse event;[26,27] only 2% reported to have a DSMC, whereas an analysis of pediatric trials published in 2007 revealed that 4.7% had DSMC.[26,28]
It must be emphasized that establishment of DSMC does not absolve investigators or sponsors of their responsibility of safety monitoring. The role of DSMC is complementary to that of investigators, sponsors and Ethics Committees. The primary responsibility of DSMC is to regularly review of study data and make recommendations regarding the continuation of the study and suggest modifications that might be required. It is also desirable to let DSMC review and approve study protocol, especially the statistical monitoring plan and stopping rules. At times, DSMC is tasked with release of interim data and approval of manuscripts and presentations reporting trial results.[25]
PARENTAL PERMISSION AND CHILD ASSENT
Informed consent is the cornerstone of protection for human participants, even when the research participant is a child. Parents are expected to act in the best interest of their child and hence have been entrusted with the responsibility of providing permission or consent for enrolling their children in a research study. In the USA, research involving minimal risk or that providing prospect of benefit to the individual child requires consent from only one parent; while all other categories of research requires permission from both parents. Many a times, concerns have been expressed whether parents will always act in the best interests of their children. However, if there are no undue financial inducements for participation and if enough information is provided to them to make an informed choice, they can be expected to act in the best interests of their children. Many a times, the practitioners feel that information might overburden parents and find approaching families about trials to be aversive.[29] Researchers should note that when they think that the pressure may be too much on parents; the parents themselves may not mind being asked about trials or feel burdened. They may even view the trial approach as a positive and exciting opportunity. As the parents base their trial decisions on their perceptions of the trial in relation to their child's safety and well-being, potential benefits to the child and family, potential benefits to others and the practicality of participation,[29] the doctors should provide them enough information, so as to enable them to make a truly informed choice. Not inviting eligible participants is not an absence of action but a conscious organized decision based on researcher's perception.[30] In a sense it is unethical, as it deprives them of an opportunity to benefit from the trial.
In addition to obtaining parental permission, researchers must solicit the child's assent; which has been described as “affirmative agreement to participate in research.” The ICMR Guidelines state that assent should be obtained from children aged 7-18 years.[31] In some countries, the Ethics Committees are expected to determine whether assent will be required or can be waived after taking into account the age, maturity and psychological state of the prospective child participants. The ECs may determine if all children in a particular research should assent or that children above a particular age should assent. Assent process must be age-and developmentally appropriate. It should be an empowering and respectful experience. Although, the components of information required to be provided to the prospective child participant have not been clearly described; It is reasonable to include information about their condition, about what will happen and what to expect and then asking them whether they would like to participate.[1,32] Separate age-appropriate information sheets and consent and assent forms should be developed for informing parents and for children about the trial.[20] Although, assent need not include a written form or signature; several investigators and Ethics Committees prefer to have a written documentation. The operationalization of the assent process has been left to the discretion of the ECs; leading to a great variability in practices implemented.[33] Assent need not be sought if the beneficial intervention is available only on participation in the research study. However, even in such a situation, it is prudent to inform them about the research study and procedures.
The issue of assent is contentious. At one level, it is argued that there is no consensus amongst various international and national guidelines regarding what an ‘assent’ really means.[34] It has also been argued that young children are not competent to make significant decisions in their lives. Choices are made for incompetent children by their parents, or their parents confirm choices that incompetent children make, or adults guide the incompetent children to come to the right decision.[34] Parents make these choices, often in the interests of their child, but they do consider the interests of others in the family.[34] In addition, there is no unanimity amongst various experts that age of seven years that is generally accepted, is the appropriate age.[34–36] Also, insisting on chronological age is considered strange. How can a child aged seven years with mental retardation and mental age of three years be expected to assent to participation? Some also point to the paradox that assent is emphasized in research, but is largely ignored in medical treatment for children. When parent and child provide incompatible responses, one of them gets over-ruled. The requirement for assent may cause other moral problems, too. When child's assent contains a veto over the parents’ consent, we may be introducing tensions into the decision-making within a family, which itself may harm relationships with children.[34]
INCENTIVES, COMPENSATION AND PAYMENT FOR PARTICIPATION
Providing compensation to children for participating in clinical trial is a highly contentious issue. One extreme opinion is that children should never be paid for such participation, because this may have undue influence on parental decision. Some believe that extra-care is required to be taken in pediatric clinical trials, because it is parents, who are not at any risk of physical injury, provide permission to include their children in the study.[37] In such a situation, compensation amount could act as bait and induce them to overlook the risks involved and use children as a commodity.[1] At the other extreme is the opinion that providing compensation is a must to facilitate enrollment and enhance retention of participants in the trial. For greater clarity, we can classify the compensation for participation into two categories. The first one consists of providing reimbursement of costs of participation in research such as for travel and meals. It is unfair to ask parents and children to bear additional costs resulting from participation in research. It is, therefore, generally accepted that they should be reimbursed on the basis of actual expenses incurred or a realistic estimate of such expenditure.[1] The second category of compensation can be sub-divided into three types: compensation for time spent in participation, enticements for recruitment and retention; and gifts of appreciation at the completion of the study.[33] A small token gift to the child as a means to say “thank you” for participation is not uncommon. The value of the gift could be varied as per the length of time spent in research-related activities. It should never be determined on the basis of level of risk.[1] The American Academy of Pediatrics suggests that if remuneration is to be given directly to the child in research, it is best not discussed until after the study so as not to affect voluntary participation.[32] Keeping the compensation amount reasonable and minimal will ensure that the participation is voluntary.[22] The regulators have delegated the responsibility of determining the quantum of compensation for participation to the Ethics Committees, since it is the responsibility of the ECs to ensure that participation in research studies is voluntary, unpressured and not unduly influenced by external factors (such as payment). It is also because the quantum of compensation would be dependent upon the local population characteristics (at times even individual family characteristics), number of visits envisaged in the individual protocols, etc. Thus, it is the prerogative of the ECs determine the quantum, methods and timings of compensation and make sure that these payments would not compromise voluntariness of participation. It must, however, be conceded that this unlimited latitude given to the ECs for determining compensation leads to great variability in practices related to and amounts of compensation approved by the ECs.[33] The basic concern is to strike the right balance between the need to make participation in studies attractive to children and their parents and ensuring that the compensation does not pose undue influence on parents’ decisions about interests of their children.
RECRUITMENT ISSUES
Recruitment in pediatric clinical trials is a major issue that investigators and sponsors have to tackle. There are several reasons for difficulties faced in recruitment:[20,38]
Fear of harming or hurting children, objections to using children as “guinea pigs”, misconceptions regarding the need for placebos and the increasing complexities of information sheets, contribute to parents’ reluctance.[20]
Parents seem to be reluctant to enroll children in research studies that do not offer perceivable immediate benefit.[20]
The childhood population is smaller and healthier than the adult population and generally, diseases in children are less commonly associated with adverse outcomes.
There are complex ethical issues associated with pediatric research studies
The regulatory oversight is significantly more restrictive
The additional requirement of obtaining parental permission as well as participants’ assent
The consequences of poor recruitment could be disastrous. Many trials are abandoned due to poor recruitment. Thus, several important research questions remain unanswered, efforts and resources get wasted and more importantly, risks and inconveniences suffered by participating children go in vain. These issues need to be tackled with multi-pronged approach. Public confidence in clinical research is integral to improving participation in research. The people need to be assured that studies have been carried out only when necessary, adequate steps are being taken to minimize risks involved, the regulatory oversight is ensuring that studies are being conducted in a scientific and ethical manner and the results would be available in the public domain so that other children would benefit.[39] There is also a need to improve research infrastructure, including funding systems, for pediatric studies. More pediatricians should undergo GCP training and efforts should be taken to maintain the trial sites.[40] A posse of trained pediatric pharmacologists should be created, too.
Recruitment can be improved through advertisements. However, these should be used judiciously. These should give introductory factual information requesting interested parents to contact the investigator for more details. In no case should benefits be exaggerated or risks downplayed. Their content should be reviewed and approved by the Ethics Committees. The practice of paying healthcare workers in the hospital a direct financial incentive for enrolling research participants (finder's fee) should, however, be shunned; as it has the potential of coercion or undue influence.[22]
Networking and getting into newer geographical areas are two ways of increasing the accessible population.[23] Pediatric clinical research networks (PCRNs) have been existence for over five decades with pediatric oncology community establishing the first networks in the 1950s.[38] Now networks exist across continuum of care (primary, secondary and tertiary care), across specialties (oncology, nephrology, neurology, etc) and across several countries. It is necessary to strengthen networking which is helpful not only for recruitment but also for exchange of ideas and views regarding new research proposals, discussing strategies for enhancing recruitment and in dealing with Ethics Committee related issues.[41] While doing international studies, several ethical issues might crop up [Table 2].
Table 2.

Traditionally, pediatric oncology has a high accrual to trials. In most oncology trials, the treating physician is the one performing the research and this fact could be at least partly responsible for better accrual rates. This creates a situation wherein the parents struggle to distinguish between research and treatment. This may vitiate the consent process and raise a question whether high accrual rates are attained at the expense of voluntariness.[43,44] A balance of ethical recruitment and high accrual is necessary for optimal recruitment to clinical trials.[43] Higher accrual rates can be attained if the research question is considered important by both researchers and parents, if both the parties are comfortable, with clinical and personal equipoise; if there is a constant communication channel between the researcher and family, and information provided about the trial is personal, tailored and timely. The retention is better; if throughout the recruitment process, the parents feel that the doctor gives priority to their child's care over the scientific imperative of the trial and that if the trial continuation brought significant physical or emotional cost, the doctor would withdraw the child.[43]
For certain uncommon diseases, the number of prospective participants available is smaller than the number of participants required for several simultaneously ongoing clinical trials testing various therapeutic interventions for that condition. This situation where an individual patient could be eligible for enrollment for several trials creates a unique ethical dilemma.[45] The researchers have to choose from one of the three approaches: Full disclosure, paternalistic and random assignment. The researcher taking the full disclosure approach provides information about all ongoing concurrent trials, allowing parents and participants to make the decision regarding the trial to enroll with. In the paternalistic approach the researcher, considering that he/she knows what is in the best interest of the patient, decides which trial the patient should join. This approach introduces bias besides compromising parental autonomy. The random approach randomly allocates patients to each trial. This strategy may erode patient autonomy.[45] Pediatric treatment outcome research focusing on the physical and mental health of children living in rural areas is limited, despite the immense need. Challenges to recruitment include researchers being viewed as outsiders by rural community members, population size and density of rural communities, unique aspects of rural culture and higher rates of poverty and lower educational achievement in rural areas.[46]
There is a need for investigators, journalists and public to be knowledgeable about the various ethical issues involved in pediatric research, in order to engage in a dialogue about balancing research risks and benefits and to be able to distinguish fact from distortion in an era of multiple and rapid transmission of information.[47] Most of the times, the considerations that go into the decisions are not only not clear-cut but are also contrasting, if not conflicting. For example, consider the conflict between protecting subjects from research risk while allowing them access to the benefits of research and the blurring of potentially conflicting roles that treating doctors don when they also act as researchers.[47] The media should always provide the true information, act as a watchdog and ensure that enough pressure is built to punish the wrong-doers. However, they should also play a role in educating the general public of the necessity of carrying out ethical pediatric research. While reporting mishaps, they should be objective and should not indulge in “manufacturing mistrust” that would make the general public over-apprehensive creating insurmountable hurdles in pediatric research. This would hinder development of new therapies for children and thereby hurting the interests of the very children, we all profess to protect.
SCIENTIFIC INTEGRITY
Bad science is bad ethics. Hence, every care should be taken that pediatric clinical trials depict robust science. Some of the specific issues [Table 3] in this regard are discussed below.
Table 3.
Threats to scientific integrity of pediatric research studies, their impact and possible solutions[17,22,26,39,48–55]
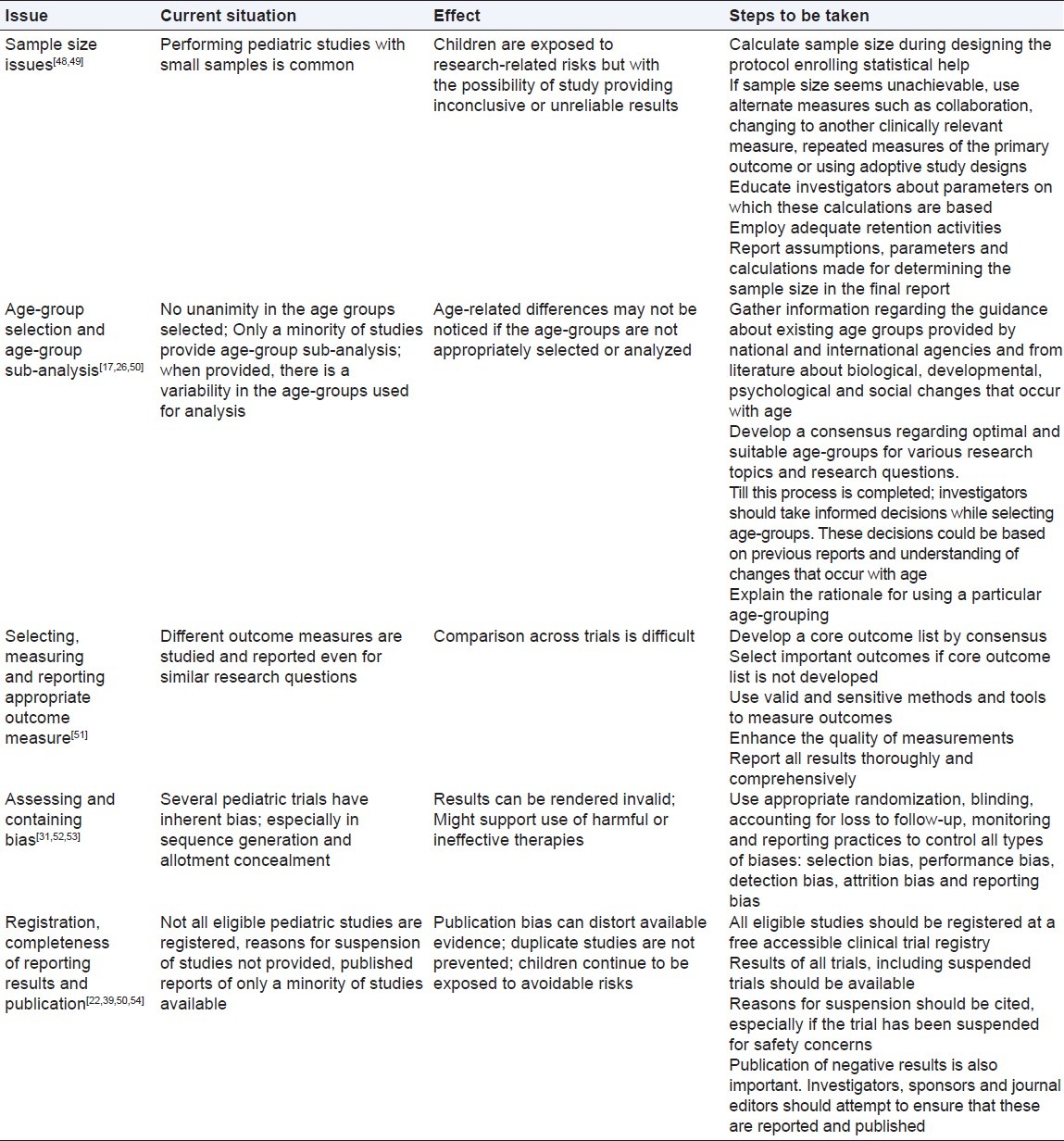
The research design should be scientifically sound and significant, with value to children in general and, in most cases, to the individual child participant. The design should take into consideration the unique physiology, pharmacology, psychology, social milieu and special needs of children and their families and should minimize risks while maximizing benefits. It should take into account the racial, ethnic, gender, and socioeconomic characteristics of children and their parents. When necessary, inputs from the community or appropriate advocacy representatives should be obtained. It should conform to all local, regional and national regulatory guidelines and laws.[22] Star Child Health was founded in 2009 to address the paucity and shortcomings of pediatric clinical trials. There is a need to develop practical, evidence-based standards to enhance the reliability and relevance of pediatric clinical research. It is recognized that the quantity, quality and relevance of data involving children are substantially lower than for adults.[26,56,57] There is a need to improve the design, conduct and reporting of pediatric research through the development and dissemination of evidence based standards. This should involve a systematic ‘knowledge to action’ process, which includes the following: identifying problems that need to be addressed; generating knowledge where gaps exist; adapting knowledge to relevant context; assessing barriers to knowledge implementation; designing knowledge transfer strategies and promoting best practice and evaluating knowledge uptake and impact on practice.[26]
EMERGENCY PEDIATRIC RESEARCH
Several diagnostic and therapeutic interventions used in life-threatening and emergency situations in children may not have adequate evidence to back their continued use. Hence, there is a need to subject them to rigorous investigation to determine their safety, efficacy, cost-benefit ratio and utility. It is obvious that in such situations, the patient is in no condition to understand research and provide valid consent and there may not be enough time to find and explain the research to the parents and obtain their consent. The US federal regulations allow the conduct of research studies to test emergency treatments, only if they hold out the prospect of direct benefit to the subject. The exception for obtaining informed consent applies to emergency research that involves human subjects who have life-threatening medical conditions for which available treatments are unproven or unsatisfactory, who, cannot give informed consent because of their condition, and when the intervention (to be effective), has to be initiated before consent can be obtained from parents. The regulations also require that these studies engage in community consultation and public disclosure before the study is initiated. The studies should also have a mechanism of contacting and providing information to the child's parents at the earliest opportunity; so that their consent can be obtained. If a child participant is enrolled before consent is obtained, the family members should have an opportunity to object to the child's continued participation in the study.[22] Proxy, deferred and retrospective consent have all been advocated as solutions.[58]
NEONATAL RESEARCH
Neonatal research is a special and priority area of pediatric research. And the reasons are not far to seek. Newborn babies may have conditions, such as Hyaline Membrane Disease, Meconium Aspiration Syndromes and necrotizing enterocolitis that exclusively occur in them or are rarely seen at other ages. Neonates are constantly undergoing maturation and differentiation, which can alter pharmacokinetics and drug responses.[59] Hence, even results of pediatric trials cannot be extrapolated to neonates. The off-label use is more rampant in newborns than that even in older children. However, it must be conceded that neonatal trials raise quite a few peculiar ethical issues:[59]
Ethical issues arising from the research design
Consider a situation where a drug is being used for a neonatal condition, in an off-label manner, without high level of evidence. If clinical equipoise, is supposed to exist and a clinical trial is planned; the issue of an appropriate comparator (placebo or active drug; for Placebo-controlled trial, PCT or Active controlled trial, ACT, respectively) needs to be tackled. One uses an active comparator only if the study drug has been proved to be more efficacious than placebo. For a drug used without such evidence, undertaking ACT is fraught with problems. If both the study drug and active comparator are shown to be equivalent; it is possible that both are ineffective or only marginally effective. Such trials, therefore, could perpetuate the use of therapeutic agents that are ineffective or have small benefit-to-risk ratio. Some recommend that except in life-threatening situations, ACT should only be undertaken when the superior efficacy of the active control over the placebo has been established.[59] If this has not been demonstrated, one can conduct a three-arm trial (administration of a placebo, administration of experimental drug and administration of an active comparator) or an “add-on” trial.[59]
PCTs can be justified, when no proven active treatment exists, or the standard treatment is extremely toxic and many parents refuse therapy because of its toxicity. Even when proven therapy does not exist, since the study drug has been used for a considerable proportion of doctors for a considerable period of time, researchers think that the patients in placebo arm are receiving an ‘inferior treatment’, and this raises an ethical dilemma. This can be addressed by using a fixed randomization scheme that has unequal allocation; or using a fully sequential design with equal group allocation or using one of the response adoptive designs (“play-the-winner” or “drop-the-loser” technique). In these techniques the probability of being assigned to the (currently) superior treatment is greater than 50%. When the standard treatment is effective and is not associated with any serious side effects, a PCT can be justified only if the risk of placebo is limited to minor and temporary discomfort and proper informed consent is obtained; and there exists a compelling scientific justification to conduct the study using a placebo and if valuable knowledge can be gained and investigators have disclosed the administration of a placebo. The most challenging ethical dilemma in conducting PCTs of drugs used off-label arises when only Grade II-III evidence for efficacy exists. One of the controversial aspects of the use of placebo in a given situation is the trade-off between the risks to the subjects and the potential benefit to society. The question remains whether we should err on the side of caution when dealing with vulnerable neonates and not routinely recommend PCTs for drugs used off-label with Grade II evidence supporting their efficacy.[59]
Ethical issues with enrollment of subjects
Individual equipoise may or may not coincide with the prevailing ‘clinical equipoise’ of the medical community. For example, there may be a situation, wherein the neonatologist believes that a particular drug is effective and uses it in his or her practice to treat a particular neonatal condition. However, the general opinion in the scientific community is that equipoise exists because of only level II/III evidence of efficacy. In such a situation, the neonatologist might feel obligated to inform the baby's parents regarding his or her preference for a drug in the baby's best medical interests; creating a barrier to enrollment in the trial. This can be resolved by the doctor explaining to the parents that although he/she prefers a particular drug, there is insufficient evidence to support its use and that there is a lot of disagreement in this regard in the expert medical community. Therefore, it is necessary to conduct a formal study to settle this dispute. From an ethical viewpoint, the neonatologist is obligated to offer the parents the opportunity to enroll their baby in an RCT.[59]
Ethical Issues with the consent processs
In many neonatal trials, the enrollment should occur at or soon after birth. This raises ethical issues similar to those in emergency research. Seeking consent within a short period of time not only causes parental distress;[59,60] it might violate the principle of autonomy. Concern for parental burden, might tempt investigators to offer incomplete information, questioning the validity of the consent process.[60] The ethical issue may be resolved by seeking exception from informed consent process requirements (by invoking the regulations for emergency research); obtaining waiver of consent (from the EC) or by obtaining consent during the antenatal period. The last option seems to be most appropriate, when the relevant national research guidelines do not provide detailed safeguards or steps for invoking the first two options. Even when the consent has been obtained during the antenatal period, the parents should be informed as soon as the baby is involved in the trial. In a less studied opt-out system, the parents’ consent is presumed following antenatal discussion unless they had refused to participate antenatally or after inclusion of their baby in the trial. Some argue that such a process will lessen parental distress and will be socially acceptable when conducted during less hurried and frightful circumstances than when conducted after delivery of a sick infant. The opt-out system may be kinder by allowing more than enough time to opt-out and by decreasing the burden of having to decide whether or not to consent.[59] Many neonatal trials are associated with high rate of mortality. However, few trial teams have had responses to bereavement in place. It may be a good idea for research teams to develop and assess responses to bereavement.[61]
CONCLUSIONS
It is essential to carry out research in children to ensure that better therapies become available to them. However, additional safeguards are necessary to guarantee the rights of children and their families. All the stake-holders: Regulators, Parent Groups, Ethics Committees, Research institutions, Practitioners, Academia, media, pharmaceutical companies and scientists have to collaborate to ensure that ethical pediatric research is promoted.
Footnotes
Source of Support: Nil
Conflict of Interest: The author is a pediatrician, member of institutional and independent ethics committees and has been associated with clinical trials conducted in children
REFERENCES
- 1.Fleischman AR, Collogan LK. Research with Children. In: Emanuel EJ, Grady C, Crouch RA, Lie RK, Miller FG, Wendler D, editors. The Oxford Textbook of Clinical Research. New York: Oxford University Press; 2008. pp. 446–60. [Google Scholar]
- 2.Nuremberg Code. Directives for experimentation. [Last accessed on 2012 Oct 4]. Available from: http://ori.dhhs.gov/education/products/RCRintro/c03/b1c3.html .
- 3.World Medical Association. BMJ. Vol. 2. Helsinki, Finland: WMA; 1964. Jun, Code of Ethics of the World Medical Association: Declaration of Helsinki; p. 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krugman S, Giles JP, Hammond J. Infectious hepatitis: Evidence for two distinct clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365–73. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 5.Tuskegee University. About the USPHS Study. [Last accessed on 2012 Oct 4]. Available from: http://www.tuskegee.edu/about_us/centers_of_excellence/bioethics_center/about_the_usphs_syphilis_study.aspx .
- 6.The National Commission for the Protection of the Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for Protection of Human Subjects of Research. HHS.gov. US Department of Health and Human Services. [Last accessed on 2012 Sep 19]. Available from: http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.html .
- 7.US Department of Health and Human Services. US Food and Drug Administration Modernization Act (FDMA) of 1997. [Last accessed on 2012 Sep 19]. Available from: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDAMA/FullTextofFDAMAlaw/default.htm#SEC.111 .
- 8.National Institutes of Health. NIH Policy and Guidelines on the inclusion of children as participants in research involving human subjects. [Last accessed on 2012 Sep 19]. Available from: http://grants.nih.gov/grants/guide/noticefiles/not98-024.html .
- 9.US Department of Health and Human Services. Best Pharmaceuticals for Children Act. [Last accessed on 2012 Sep 19]. Available from: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148011.htm .
- 10.US Congress. Pediatric Research Equity Act of 2003. [Last accessed on 2012 Sep 19]. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM077853.pdf .
- 11.European Public Health Alliance. The EU adopts a regulation on medicines for children. [Last accessed on 2012 Sep 19]. Available from: http://www.epha.org/a/1451 .
- 12.Regulation (EC) no 1901 / 2006 of the European Parliament and of the Council on medicinal products for paediatric use and amending Regulation (EEC) No 1768 / 92, Directive 2001 / 20/EC, Directive 2001 / 83/EC and Regulation (EC) No 726 / 2004. [Last accessed on 2012 Sep 19]. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:378:0001:0019:en:PDF .
- 13.Knellwolf AL, Bauzon S, Alberight OD, Lutsar I, Bacsy E, Alfarez D, et al. Framework conditions facilitating paediatric clinical research. Italian J Pediatr. 2011;37:12. doi: 10.1186/1824-7288-37-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.General Assembly of the United Nations. Convention on the Rights of the Child, 20 November 1989. [Last accessed on 2012 Sep 19]. Available from: http://www.unicef.org/crc/crc.htm .
- 15.Bavdekar SB, Sadawarte PA, Gogtay NJ, Jain SS, Jadhav S. Off-label drug use in a Pediatric Intensive Care Unit. Indian J Pediatr. 2009;76:1113–8. doi: 10.1007/s12098-009-0238-3. [DOI] [PubMed] [Google Scholar]
- 16.Jain SS, Bavdekar SB, Gogtay NJ, Sadawarte PA. Off-label drug use in children. Indian J Pediatr. 2008;75:1133–6. doi: 10.1007/s12098-008-0188-1. [DOI] [PubMed] [Google Scholar]
- 17.Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JP, Curtis S, et al. Standard 6: Age Groups for Pediatric Trials. Pediatrics. 2012;129:S153–60. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin DK, Jr, Smith PB, Sun MJ, Murphy MD, Avant D, Mathis L, et al. Safety and transparency of pediatric drug trials. Arch Pediatr Adolesce Med. 2009;163:1080–6. doi: 10.1001/archpediatrics.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PB, Benjamin DK, Jr, Murphy MD, Johann-Liang R, Iyasu S, Gould B, et al. Safety monitoring of drugs receiving pediatric marketing exclusivity. Pediatrics. 2008;122:e628–33. doi: 10.1542/peds.2008-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill D, Kurz R. Practical and Ethical Issues in Pediatric Clinical Trials. Appl Clin Trials. 2003;12:41–4. [Google Scholar]
- 21.Dunne J, Rodriguez WJ, Murphy MD, Beasely BN, Burckart GJ, Filie JD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128:e1242–9. doi: 10.1542/peds.2010-3487. [DOI] [PubMed] [Google Scholar]
- 22.Shaddy RE, Denne SC. The Committee on Drugs and Committee on Pediatric Research. Clnical Report- Guidelines for the Ethical Conduct of Studies to Evaluate Drugs in Pediatric Populations. [Last accessed 2012 Sep 5]. Available from: http://www.pediatrics.org/cgi/doi/10.1542/peds.2010-0082 . [DOI] [PubMed]
- 23.Arenas-Lopez S, Fajardo C, Vallis I, Soler A, Garcia-Corza JR, Lima-Rogel MV, et al. Pediatric Clinical Trials in Latin America and Guyana. Pediatr Drugs. 2011;15:257–66. doi: 10.2165/11590350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Ethical Considerations for Clinical trials on medicinal products conducted with the paediatric population. [Last accessed on 2012 Sep 20]. Available from: ftp://ftp.cordis.europa.eu/pub/fp7/docs/ethical-considerations-paediatrics_en.pdf . [DOI] [PubMed]
- 25.Ellenberg S, Fernandes RM, Saloojee H, Bassier D, Askie L, Vandermeer B, et al. Standard 3: Data Monitoring Committees. Pediatrics. 2012;129:S133–7. doi: 10.1542/peds.2012-0055F. [DOI] [PubMed] [Google Scholar]
- 26.Hartling L, Wittmeier KD, Caldwell P, van der Lee H, Klassen TP, Craig JC, et al. for the Star Child Health Group. StaR Child Health: Developing evidence-based guidance for the design, conduct, and reporting of pediatric trials. Pediatrics. 2012;129:S112–7. doi: 10.1542/peds.2012-0055C. [DOI] [PubMed] [Google Scholar]
- 27.Sammons HM, Gray C, Hudson H, Cherril J, Choonara I. Safety in paediatric clinical trials- a 7 year review. Acta Paediatr. 2008;97:474–7. doi: 10.1111/j.1651-2227.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 28.Hamm MP, Hartling L, Milne A, Tjosvold L, Vandermeer B, Thomson D, et al. A descriptive analysis of a representative sample of pediatric randomized controlled trials published in 2007. BMC Pediatr. 2010;10:96. doi: 10.1186/1471-2431-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shilling V, Williamson RP, Hickey H, Sowden E, Smyth RL, Young B. Processes in recruitment to randomized controlled trials of medicines for children (RECRUIT): A qualitative study. Health Technol Assess. 2011;15:1–116. doi: 10.3310/hta15150. [DOI] [PubMed] [Google Scholar]
- 30.Amiel P, Moreau D, Vincent-Genod C, Alberti C, Hankard R, Ravaud P, et al. Noninvitation of eligible individuals to participate in pediatric studies: A qualitative study. Arch Ped Adolesc Med. 2007;161:446–50. doi: 10.1001/archpedi.161.5.446. [DOI] [PubMed] [Google Scholar]
- 31.Ethical Guidelines for Biomedical Research on Human Participants. New Delhi: Indian Council of Medical Research; 2006. [Last accessed 2012 Oct 4]. Indian Council of Medical Research. Available from: http://icmr.nic.in/ethical_guidelines.pdf . [Google Scholar]
- 32.American Academy of Pediatrics Committee on Drugs. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics. 1995;95:286–94. [PubMed] [Google Scholar]
- 33.Kimberly MB, Hoehn KS, Feudtner C, Melson RM, Schreiner M. Variation in standards of research compensation and child assent practices: A comparison of 69 institutional review board-approved informed permission and assent forms for 3 Multicenter Pediatric Clinical Trials. Pediatrics. 2006;117:1706–11. doi: 10.1542/peds.2005-1233. [DOI] [PubMed] [Google Scholar]
- 34.Baines P. Assent for Children's participation in research is incoherent and wrong. Arch Dis Child. 2011;96:960–2. doi: 10.1136/adc.2011.211342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Lourdes Levy M, Larcher V, Kurz R Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP) Informed Consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP) Eur J Pediatr. 2003;162:629–33. doi: 10.1007/s00431-003-1193-z. [DOI] [PubMed] [Google Scholar]
- 36.Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research? Am J Bioeth. 2003;3:1–7. doi: 10.1162/152651603322614382. [DOI] [PubMed] [Google Scholar]
- 37.Grady C. Payment of clinical research subjects. J Clin Invest. 2005;115:1681–7. doi: 10.1172/JCI25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tishler CL, Reiss NS. Pediatric drug-trial recruitment: Enrollment without coercion. Pediatrics. 2011;127:949–54. doi: 10.1542/peds.2010-2585. [DOI] [PubMed] [Google Scholar]
- 39.Shamliyan T. Reporting of results of interventional studies by the information services of the National Institutes of Health. Clin Pharmacol Adv Appl. 2010;2:169–76. doi: 10.2147/CPAA.S12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserman R, Bocian A, Harris D, Slora E. Limited capacity in US pediatric drug trials: Qualitative analysis of expert interviews. Paediatr Drugs. 2011;13:119–24. doi: 10.2165/11584240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Siora EJ, Harris DL, Bocian AB, Wasserman RC. Pediatric clinical research networks: Current status, common challenges, and potential solutions. Pediatrics. 2010;126:740–5. doi: 10.1542/peds.2009-3586. [DOI] [PubMed] [Google Scholar]
- 42.Levine RJ. Ethical issues in International vaccine research and development. Yale J Biol Med. 2005;78:227–33. [PMC free article] [PubMed] [Google Scholar]
- 43.Byrne-Davis LM, Salmon P, Gravenhorst K, Eden TO, Young B. Balancing high accrual and ethical recruitment in pediatric oncology; a qualitative study of the ‘look and feel’ of clinical trial discussions. BMC Med Res Methodol. 2010;10:101. doi: 10.1186/1471-2288-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasan AD, Taubenberger SP, Robinson WM. Reasons for participation in pain research: can they indicate a lack of informed consent? Pain Med. 2009;10:111–9. doi: 10.1111/j.1526-4637.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim GM, Chung C, Bernstein M. Competing for patients: An ethical framework for recruiting patients with brain tumors into clinical trials. J Neurooncol. 2011;104:623–7. doi: 10.1007/s11060-011-0536-2. [DOI] [PubMed] [Google Scholar]
- 46.Lim CS, Follansbee-Junger KW, Crawford MS, Janicke DM. Treatment outcome research in rural pediatric populations: the challenge of recruitment. J Pediatr Psychol. 2011;26:696–707. doi: 10.1093/jpepsy/jsr018. [DOI] [PubMed] [Google Scholar]
- 47.Slomka J. Manufacturing mistrust: Issues in the controversy regarding foster children in the pediatric HIV/AIDS clinical trials. Sci Eng Ethics. 2009;15:503–16. doi: 10.1007/s11948-009-9179-5. [DOI] [PubMed] [Google Scholar]
- 48.van der Tweel I, Askie L, Vandermeer B, Ellenberg S, Fernandes RM, Saloojee H, et al. Standard 4: Determining Adequate Sample Sizes. Pediatrics. 2012;129:S138–45. doi: 10.1542/peds.2012-0055G. [DOI] [PubMed] [Google Scholar]
- 49.Campbell H, Surry SA, Royle EM. A review of randomised controlled trials published in Archives of Diseases in Childhood from 1982-96. Arch Dis Child. 1998;79:192–7. doi: 10.1136/adc.79.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Contopoulos-Ioannidis DG, Seto I, Hamm MP, Thomson D, Hartling L, Ioannidis JP, et al. Empirical evaluation of age groups and age-subgroup analyses in pediatric randomized trials and pediatric meta-analyses. Pediatrics. 2012;129:S161–84. doi: 10.1542/peds.2012-0055J. [DOI] [PubMed] [Google Scholar]
- 51.Sinha IP, Altman DG, Beresford MW, Boers M, Clarke M, Craig J, et al. Standard 5: Selection, measurement, and reporting of outcomes in clinical trials in children. Pediatrics. 2012;129:S146–52. doi: 10.1542/peds.2012-0055H. [DOI] [PubMed] [Google Scholar]
- 52.Hartling L, Hamm M, Klassen T, Chan AW, Meremikwu M, Moyer V, et al. Standard 2: Containing risk of bias. Pediatrics. 2012;129:S124–34. doi: 10.1542/peds.2012-0055E. [DOI] [PubMed] [Google Scholar]
- 53.Crocetti MI, Amin DD, Scherer R. Assessment of risk of bias among pediatric randomized controlled trials. Pediatrics. 2010;126:298–305. doi: 10.1542/peds.2009-3121. [DOI] [PubMed] [Google Scholar]
- 54.Satyanarayana K, Sharma A, Parikh P, Vijayan VK, Sahu DK, Nayak BK, et al. Statement on publishing clinical trials in Indian biomedical journals. J Postgrad Med. 2008;54:78–9. doi: 10.4103/0022-3859.40766. [DOI] [PubMed] [Google Scholar]
- 55.Shamilyan T, Kane RI. Clinical research involving children: Registration, completeness, and publication. Pediatrics. 2012;129:e1291–300. doi: 10.1542/peds.2010-2847. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: The gap in high quality study design. Pediatrics. 2008;122:52–7. doi: 10.1542/peds.2007-2849. [DOI] [PubMed] [Google Scholar]
- 57.Cohen E, Goldman RD, Ragone A, Uleryk E, Atenafu EG, Siddiqui U, et al. Child vs.adult randomized controlled trials in specialist journals: A citation analysis of trends, 1985-2005. Arch Pediatr Adolesc Med. 2010;164:283–8. doi: 10.1001/archpediatrics.2009.291. [DOI] [PubMed] [Google Scholar]
- 58.Brierley J, Larcher V. emergency research in children: options for ethical recruitment. J Med Ethics. 2011;37:429–37. doi: 10.1136/jme.2010.040667. [DOI] [PubMed] [Google Scholar]
- 59.Amin SB, McDermott MP, Shamoo AE. Clinical trials of drugs used off-label in neonates: Ethical issues and alternative study designs. Account Res. 2008;15:168–87. doi: 10.1080/08989620802194392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stenson BJ, Becher JC, McIntosh N. Neonatal research: te parental perspective. Arch Dis Child Fetal Neonatal Ed. 2004;89:F321–4. doi: 10.1136/adc.2002.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snowdon C, Harvey SE, Brocklehurst P, Tasker RC, Platt MP, Allen E, et al. The BRACELET Study: Survey of mortality in UK neonatal and paediatric intensive care trials. Trials. 2010;11:65. doi: 10.1186/1745-6215-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]


