Abstract
The identification of biomaterials that are well tolerated in the eye is important for the development of new ocular drug delivery devices and implants, and the application of micro- and nanoengineered devices to biomedical treatments is predicated on the long-term preservation within the target organ or tissue of the very small functional design elements. This study assesses the ocular tolerance and durability of micro- and nanostructured biopolymer thin films injected or implanted into the rabbit eye. Structured poly(caprolactone) (PCL) thin films were placed in adult rabbit eyes for survival studies, with serial ophthalmic examinations over 6 months. Morphologic abnormalities and device/tissue reactions were evaluated by histologic studies, and scanning electron microscopy (SEM) of films was used to determine the structural integrity. Structured PCL thin films (20- to 40-μm thick) were constructed to design specifications with 50-μm linear microgrooves or arrays of nanopores with ∼30-nm diameters. After up to 9 months of ocular residency, SEM on devices retrieved from the eye showed preservation of micro- and nanostructural features. In ocular safety evaluations carried out over 6 months, serial examinations in 18 implanted eyes showed no evidence of chronic inflammation, cataractogenesis, or retinal toxicity. Postoperative ocular inflammation was seen in 67% of eyes for 1 week, and persistent corneal edema occurred in 1 eye. Histology revealed no ocular inflammation or morphologic abnormalities of ocular tissues. Thin-film/tissue responses such as cellular reaction, fibrosis, or surface biodeposits were not seen. Micro- and nanostructured PCL thin films exhibited acceptable ocular tolerance and maintained the structural integrity of design features while residing in the eye. Thin-film micro- and nanostructured PCL appears to be a feasible biomaterial for intraocular therapeutic applications.
Introduction
In recent years, several sustained-release drug delivery devices have been developed for treating front- and back-of-the-eye diseases.1 Typically, these systems are designed to achieve sustained therapeutic drug concentrations in ocular target tissues that are generally inaccessible by conventional means, while limiting toxicity, intraocular injection frequency, and improving patient compliance.1 However, the first step in developing a new drug delivery device for the eye is selection of a nonimmunogenic material with tunable characteristics, such as size, shape, porosity, and degradation properties.
In general, the current ocular drug delivery devices can be classified as either nondegradable or biodegradable devices. Retisert, manufactured by Bausch and Lomb, was FDA-approved in 2005 to treat uveitis2 and is currently under investigation for treatment of retinal vein occlusion and diabetic macular edema.3 It is a nondegradable implant that is surgically implanted into the vitreous and delivers fluocinolone acetonide (FA) for up to 3 years. Iluvien, manufactured by Alimera Sciences, is another nondegradable FA sustained-delivery device and delivers a low dose of FA (0.2 or 0.5 μg/day) for up to 3 years.3 Introduced in 1981, Lacrisert® (Aton Pharma, Inc.) is a sterile, translucent, rod-shaped ophthalmic insert made of hydroxypropyl cellulose, a water-soluble, biodegradable material. Physiologically inert, Lacrisert is administered daily into the inferior cul-de-sac of the eye to improve the dry-eye symptoms. More recently, Ozurdex, produced by Allergan, Inc., is a biodegradable implantable device that can deliver therapeutic concentrations of dexamethasone for up to 6 months.3 Ozurdex consists of a poly(lactic-co-glycolic acid) (PLGA) polymer that degrades by surface erosion. Other biodegradable controlled-release drug delivery devices have been studied but not yet brought to the market. Delivery platforms, such as microparticles4,5 and nanoparticles,6,7 have been used to encapsulate the drug in degradable polymers, including poly(lactic acid),8,9 PLGA,10–12 and poly(caprolactone) (PCL).13,14 Drug release from these devices can be regulated for periods of days15 to weeks,16 yet thus far, such devices have been limited to the delivery of small molecules, such as haloperidol,16 honokiol,17 dexamethasone,18 budesonide,19 or topotecan.20
Among biodegradable materials, PCL has emerged as a polymer of interest for biomedical applications. PCL is unique in its degradation behavior, as it maintains its structural properties throughout the majority of its degradation timecourse: while PCL degrades by a random hydrolytic chain scission, the poor solubility of PCL oligomers prevents dissolution of degradation products larger than the monomeric and dimeric units.21 Consequently, micro- or nanostructural features can be maintained over several months after implantation. Although PCL has been used in other implantable applications such as sutures, it has generally lacked the study in the eye, particularly in a micro- or nanostructured form. PCL–silicon nanofiber composites implanted under the conjunctiva in the rat eye and PCL-based nanofabrics were used as artificial conjunctiva with good tolerance and ocular integration.22 Also, PCL subretinal implants and scaffolds for retinal pigment epithelium and retinal progenitor cells showed good tolerance in vivo and in vitro.23–26 Similarly, the subretinal delivery of the corticosteroid triamcinolone acetonide from a PCL rod was free of complications over 4 weeks.27 Unfortunately, none of these studies evaluated PCL biocompatibility in the anterior and posterior chambers of the eye. In addition, nanostructured materials are of particular interest from the perspective of drug delivery.28 A number of nanostructured membrane drug delivery systems have recently been investigated, where zero-order controlled release can be achieved when the therapeutic size is comparable to the membrane pore size.29,30 While of great interest for the biologic delivery in particular, these materials are typically nonbiodegradable, require surgical implantation, and are rigid within the eye.31 Consequently, application of new nanostructured biodegradable materials for controlled release in the eye requires validation of ocular compatibility. Preservation of micro- and nanoscaled design features in these flexible, degradable biopolymers within the eye over the expected functional duration also requires in vivo verification.
Understanding the body's reaction to polymeric implants is nontrivial: the host response is affected not only by the chemical properties of the polymer but also by the physical properties of the implant (size, shape, and surface characteristics). In the context of an ocular implant, smooth materials can have very different immunogenic properties compared to micro- or nanostructured materials.32 Although the anterior chamber, vitreous cavity, and subretinal space are known to display immune privilege, synthetic biomaterials may still cause long-term inflammation and associated immunogenicity that can lead to detrimental effects on the eye. Therefore, it is important to characterize new potential ocular-compatible biomaterials.
In this article, we investigate the ocular biocompatibility of micro- and nanostructured thin films of PCL, which have potential applications in the eye such as tissue transplantation, cell-based therapies, and drug delivery. We describe approaches to fabricate micro- and nanostructured PCL thin films (20- to 40-μm total thickness) toward the development of ophthalmologic implants. The biocompatibility of these thin films is evaluated by a standard needle injection or incisional implantation into adult rabbit eyes for up to 9 months. Prototype thin films implanted into the anterior chamber or the vitreous cavity are evaluated using serial in vivo ophthalmologic examinations, ocular histology studies, and scanning electron microscopy (SEM) on retrieved polymer devices after prolonged implantation.
Methods
Microgrooved thin-film fabrication
Microgrooved PCL thin films were fabricated using a mold-transfer process in 3 stages. This process was described and investigated in detail previously,24 and will be briefly outlined here for the benefit of the reader. First, a standard photolithography was used to define a silicon and SU-8 master mold. A PMW32 spin coater (Headway Research) was used to spin-cast SU-8 2010-negative photoresist (Microchem) at 1,000 rpm for 30 s onto 3″ silicon wafers (Addison Engineering). The wafer was prebaked (95°C for 3.5 min), followed by UV exposure on a Karl Suss MJB 3 mask aligner (Hoffmann Instruments). Exposure of 365-nm light at 5 mW cm2 for 30 s through a photomask generated the patterned array of microgrooves. The patterned SU-8 was postbaked (95°C for 4.5 min) and developed for 2 min with an SU-8 Developer (Microchem).
The second stage of this process involved transferring the pattern in the rigid SU-8 mold to a flexible polymer. For this, poly(dimethylsiloxane) (PDMS) (Sylgard 184; Dow Corning) was mixed (10:1 v/v base:curing agent), degassed, and poured onto the SU-8 molds. PDMS was cured (65°C for 2 h), and upon peeling the solid PDMS from the SU-8 mold, a flexible mold was ready to facilitate fabrication of structured PCL thin films.
For the final stage of microgroove fabrication, PCL (Mn 70,000–90,000; Sigma-Aldrich) was dissolved in 2,2,2-trifluoroethanol (Sigma-Aldrich) at a concentration of 100 mg/mL (mixed at 65°C until completely dissolved). A model P6700 spin coater (Specialty Coating Systems) was used to spin-cast PCL solutions onto the PDMS molds at 500 rpm for 30 s, followed by 1,500 rpm for 30 s. Microgrooved PCL thin films were then peeled from the PDMS molds using forceps. Thin films were cut to 4 mm by 4 mm for implantation.
Nanostructured thin-film fabrication
A template-based approach was used to fabricate nanostructured PCL and was selected to mimic the processes used to generate nanoporous membranes.33 All processes utilized chemicals obtained from Sigma-Aldrich. Silicon substrates were cleaned before use with a mixture of sulfuric acid and hydrogen peroxide (3:1 v/v) for at least 30 min, followed by a deionized water rinse and nitrogen dry. An oxygen plasma clean (5 min at 200 W and 0.5 mTorr) with a Plasmaline plasma cleaner (Plasmatek Labs) was used to further clean the silicon substrates. Next, a zinc acetate (ZnAc2) seed layer (0.75 M ZnAc2 and ethanolamine in 2-methoxyethanol) was spin-cast using the model P6700 spin coater (1,000 rpm for 60 s) and annealed at 400°C for 30 min to convert ZnAc2 to ZnO. Nanorods of ZnO were grown hydrothermally in a 5-mM solution of ZnAc2 at 85°C–90°C for 4 h (growth solution replenished once). The ZnO nanorod templates were coated with PCL, spin-cast from 2,2,2-trifluoroethanol (300 mg/mL) at 500 rpm for 30 s, followed by 1,500 rpm for 30 s. Substrates were heated at 130°C, and subsequently ZnO was removed with 10 mM H2SO4, which allowed the nanostructured PCL to float off. Thin films were cut to 4 mm by 4 mm for implantation.
Animal studies
All studies were approved by the University of California San Francisco Committee on Animal Research. New Zealand White female rabbits (2.5–3.5 kg) from the Western Oregon Rabbit Company were anesthetized by inhalation of 2%–4% isoflurane. Pupils were dilated with 1% tropicamide, 2.5% phenylephrine hydrochloride, and 0.5% proparacaine drops administered to each eye. A surgical microscope (Carl Zeiss Surgical GmbH) was used with a silicone flat lens (Dutch Ophthalmics) to visualize the retina. After disinfection with 5% povidone iodine, the thin-film devices were implanted into the anterior chamber at the limbus in 3 eyes via a 20-gauge needle injection on a 3-mL syringe backloaded with 0.2 to 0.5 mL of balanced saline at the corneal limbus, and into the vitreous cavity 2 mm posterior to the limbus in 15 eyes via a 20-gauge transconjunctival needle injection (Fig. 2), or by an incisional sclerotomy using a 20-gauge microvitreoretinal blade after a small conjunctival peritomy. Through sclerotomies, unfurled devices could be inserted with microforceps into the peripheral vitreous cavity. Incisions were closed with 7-0 vicryl sutures (Ethicon, Inc.). Subconjunctival cefazolin (150 mg in 0.5 mL) was given after the procedure. In all cases, no more than one PCL thin film was implanted per eye. Unoperated eyes served as controls.
FIG. 2.
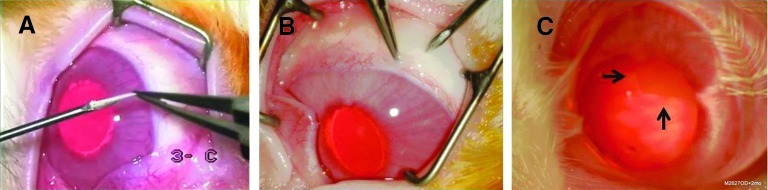
Injection of thin films into rabbit eyes. (A) The furled thin film is loaded into a 20-gauge needle for in vivo implantation (‘3-C’ is an artifact of the video capture used during surgery) and (B) inserted into the vitreous cavity via a needle injection. (C) Implanted thin films dwell in the periphery of the vitreous cavity. Arrows indicate location of implanted film. Color images available online at www.liebertpub.com/jop
Ocular tolerance was evaluated with serial ophthalmic examinations at 1 day, weeks 1–4, and months 1 to 6, including pneumotonometry (Mentor, Inc.), slit-lamp microscopy (handheld slit lamp; Kowa Company), and indirect ophthalmoscopy. Eyes were evaluated for evidence of ocular inflammation, corneal edema, cataract, intraocular hemorrhage, endophthalmitis, and retinal tears or detachment. For SEM evaluations of the device structure, in vivo studies were prolonged up to 9 months.
Histology
At intervals of 1, 2, 3, 4, 5, and 6 months after surgery, rabbits were euthanized under deep anesthesia by an intravenous overdose of pentobarbital sodium. The eyes were enucleated, and the implants were retrieved by dissection before preservation of ocular tissues in 2.5% glutaraldehyde and 1.5% paraformaldehyde in a 0.1 M sodium cacodylate buffer solution for 24 h at 4°C. The ocular tissue was embedded in paraffin, and the representative central and peripheral zones were used for serial sections at 5 μm. Slices were mounted on glass slides, and standard staining with hematoxylin and eosin was used to delineate ocular structures. Digital images were obtained with light microscopy (Zeiss Axiophot) and analyzed by imaging software (Spot v4.5; Diagnostic Instruments, Inc.).
Scanning electron microscopy
Retrieved thin-film devices were evaluated by SEM. Thin films were exposed to 3% glutaraldehyde (Sigma-Aldrich) in a 0.1 M sucrose–cacodylate (Sigma-Aldrich) buffer for 72 h at room temperature to fix any cells or debris accumulated during ocular residence. After fixation, the samples were rinsed 3 times in the 0.1 M sucrose–cacodylate buffer for 5 min. Samples were then dehydrated by exposure to a series of ethanol solutions in water with increasing concentration as follows: 35%, 50%, 70%, 95%, and 100% for 10 min each, and the final pure ethanol solution was applied twice. Films were exposed to hexamethyldisilazane (PolySciences, Inc.) for 10 min and promptly removed to air-dry. Microstructured thin films were imaged using a Novelx mySEM field-emission scanning electron microscope (Agilent) operated at a 1-kV beam voltage. Nanostructured films were coated with gold–palladium and imaged using a Sirion scanning electron microscope (FEI) operated at a 5-kV beam voltage. The pore size and density were analyzed using a series of characteristic SEM images (n=4, magnification=100,000×) for each condition, and the threshold and particle analysis tools in ImageJ (National Institutes of Health) were used to determine the size and number of pores for each image.
Results
Micro- and nanofabricated polymer device construction
Structured PCL thin-film prototypes were fabricated using a combination of photolithography and molding techniques for microstructured films, and self-assembled nanostructure growth and templating for nanostructured films. The microstructured molds and nanostructured templates determine the uniformity and reproducibility of the features produced in our implants. For microstructure fabrication, photolithography was chosen, given its high degree of precision and reproducibility based on the particular exposure mask selected. For nanostructure fabrication, the ZnO template was selected for its largely monodispersed collection of rods.34 Spin-casting onto these molds and templates was used to generate final micro- and nanostructured films, which is a technique known to provide a high degree of uniformity in the film thickness. PCL was cast into thin films 20- to 40-μm thick and cut manually to the desired dimensions for implantation (4×5 mm; Fig. 1). The microstructured thin films were patterned with 50-μm-wide grooves with 10-μm depth and 50-μm spacing. Nanostructured films exhibited partial thickness pores ∼30 nm in diameter at a density of roughly 1.7×1010 cm2 (Table 1). Inherent flexibility of PCL at this film thickness allowed furling and insertion into a 20-gauge standard needle to facilitate the intraocular injection (Fig. 2). Furled films did not necessarily completely unfurl after implantation, even though well hydrated. A comparison of furled versus unfurled thin films will need to be examined in further device functionality studies.
FIG. 1.
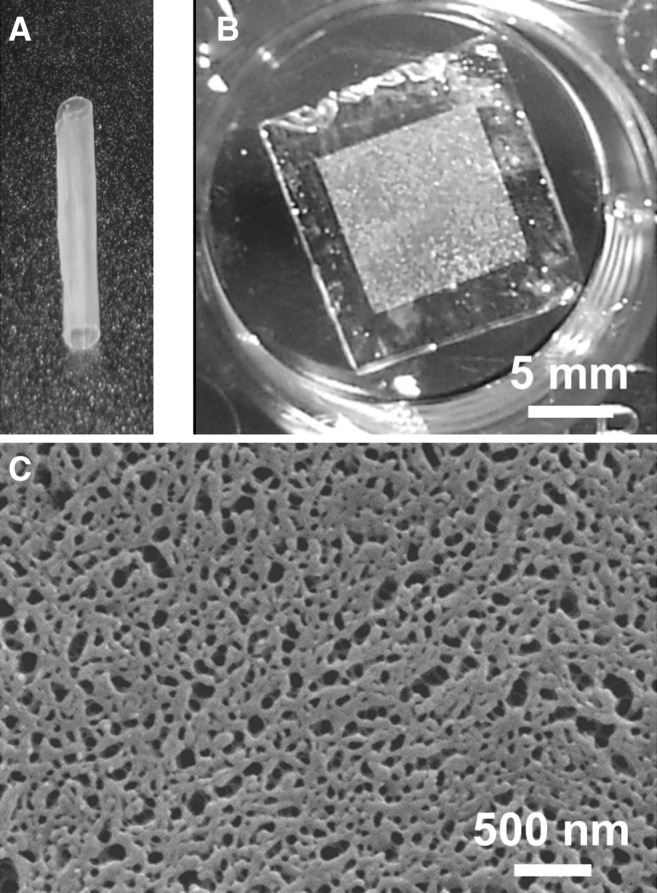
Biopolymer thin films. Examples of (A) a furled poly(caprolactone) (PCL) film and (B) an unfurled PCL film in a phosphate buffered saline solution (scale bar shown for both A and B). (C) Scanning electron microscopy (SEM) showing a prototype nanostructured PCL thin film.
Table 1.
Nanostructured Thin Films: Average Pore Diameter and Density
| Diameter (nm) | Density (1010 cm−2) | |
|---|---|---|
| Initial | 31.1±18.1 | 1.66±0.22 |
| 6 months | 27.1±17.0 | 1.73±0.04 |
| 9 months | 29.5±18.1 | 1.69±0.12 |
(Mean±standard deviation).
Clinical examinations
Eighteen eyes of 11 rabbits (3 anterior chamber implants, 15 vitreous implants; 4 control eyes) were studied for ocular tolerance toward the PCL thin films over a period up to 6 months (Fig. 3). Postoperative corneal edema was present in 4 eyes, persisting more than 1 week in 1 eye with an anterior chamber device insertion (Table 2). Postoperative ocular inflammation was seen over 1 week in 2 out of 3 anterior chamber insertion procedures; beyond 1 week postoperatively, significant chronic inflammation was absent in all eyes for up to 6-month follow-up periods (Table 3). Pneumotonometry measurements of intraocular pressure were variable and poorly reproducible in both implanted and control rabbit eyes, precluding the assessment of IOP effects. Adverse events related to the vitreous cavity device injection or incisional insertion included 3 eyes with posterior capsular cataract from lens trauma during vitreous insertion (Table 2); progressive noniatrogenic cataract formation was not seen in any eyes up to 6 months after device insertion. Other iatrogenic events related to the posterior cavity insertion included 3 eyes with an intraoperative retinal tear or trauma, and 2 eyes with postoperative vitreous hemorrhage that resolved at 1 week (Table 4). No eyes developed endophthalmitis or clinical signs of retinal degeneration. At implantation and over the ensuing months, devices were positioned in the peripheral vitreous cavity or over the peripheral iris, outside of the central visual axis. Device migration exceeding 1 clock hour or posterior dislocation was not observed for either anterior or posterior devices over 6 months. In the setting of the formed vitreous of the rabbit eye, posterior devices were in contact with the posterior lens in some cases; the contact with retinal tissues was not seen in any cases throughout the follow-up period.
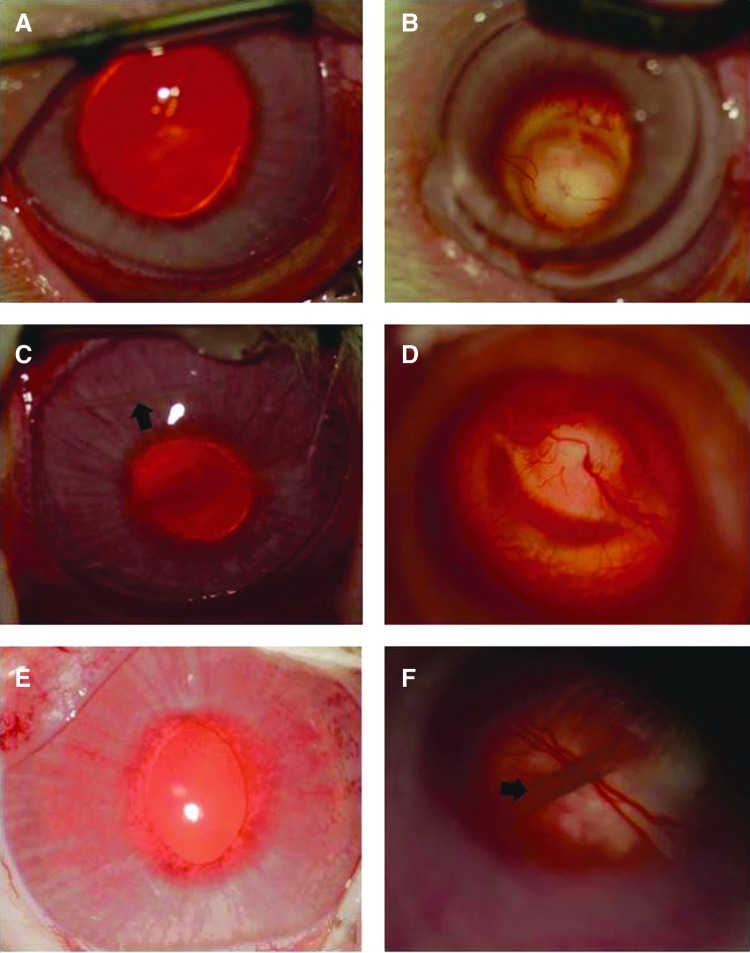
FIG. 3.
Ocular tolerance of indwelling PCL thin films in rabbit eyes (left column: anterior segment photos; right column: posterior segment photos). (A, B) Control eyes. (C, D) Anterior chamber thin-film implantation at 4 months. (E, F) Vitreous cavity thin-film implantation at 6 months. (A, C, E) Anterior segment of the eye demonstrating normal examination with no inflammation, corneal abnormalities, or cataract. (B, D, F) Posterior segment of the eye demonstrating the normal appearance of the retina and vitreous. Arrows indicate the location of the thin film. Magnification 4X. Color images available online at www.liebertpub.com/jop
Table 2.
Safety Studies: Anterior Segment Findings
| |
|
Intraocular pressure |
|
Corneal edema |
Cataract |
||||
|---|---|---|---|---|---|---|---|---|---|
| Insertion site | <10 mmHg | >22 mmHg | >30 mmHg | Wound leak | Any event | Beyond >1 weeka | Iatrogenic trauma | Nontraumatic | |
| # of eyes (%) | A/C devices (3 eyes) | 1 (33%) | 1 (33%) | 0 | 0 | 1 (30%) | 1 (30%) | 0 | 0 |
| Vitreous devices (15 eyes) | 1 (7%) | 10 (67%) | 3 (20%) | 0 | 3 (20%) | 0 | 3 (20%) | 0 | |
Finding persisting 1 week or longer after insertion procedure.
Table 3.
Safety Studies: Ocular Inflammation
| |
|
Conjunctival injection |
A/C Cell/Fibrin |
|
|
||
|---|---|---|---|---|---|---|---|
| Insertion site | Any event | Grade >2, >1 weeka | Any event | Grade >2, >1 weeka | Vitreous cell/fibrin | Endophthalmitis | |
| # of eyes (%) | A/C devices (3 eyes) | 3 (100%) | 0 | 2 (67%) | 0 | 0 | 0 |
| Vitreous devices (15 eyes) | 9 (60%) | 0 | 0 | 0 | 0 | 0 | |
Graded 0 to 4 in severity, with finding persisting more than 1 week after the insertion procedure.
Table 4.
Safety Studies: Ocular Hemorrhage and Adverse Events
| |
|
|
Vitreous hemorrhage |
Retinal tear or trauma |
|
||
|---|---|---|---|---|---|---|---|
| Hyphema | Any event | Persist ≥1 weeka | During insertion | Postinsertion | Retinal Detachment | ||
| # of eyes (%) | A/C devices (3 eyes) | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitreous devices (15 eyes) | 0 | 2 (13%) | 0 | 3 (20%) | 0 | 0 | |
Finding persisting 1 week or longer after the insertion procedure.
Histological evaluation
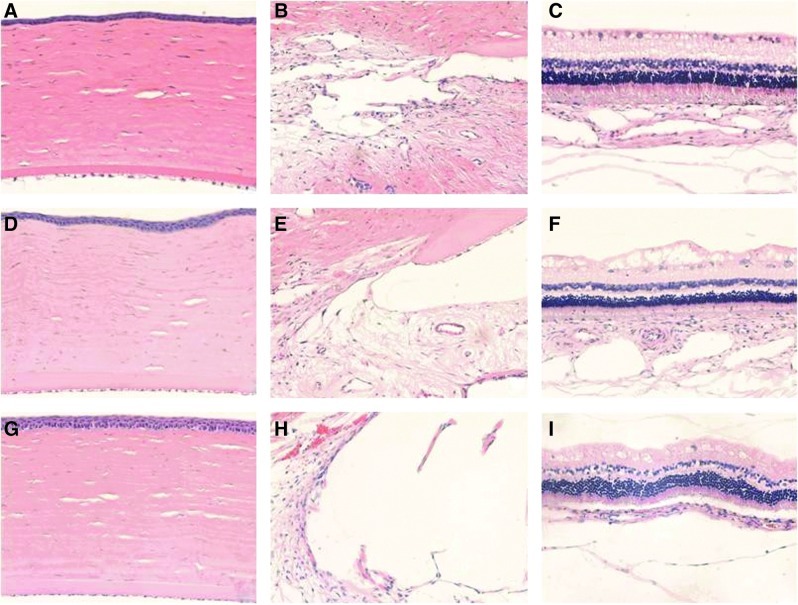
Histology of 10 eyes (8 vitreous implants, 1 anterior chamber implant, and 1 control) showed no inflammation or morphologic abnormalities over 1 to 6 months of follow-up at the ocular sites, including the cornea and anterior segment, trabecular meshwork, retina, uvea, and vitreous, and the local sites of anatomic residence of the implanted device (Fig. 4). Retinal degeneration and device/tissue responses such as fibrotic encapsulation of the device were not seen in any study eyes over the 1 to 6 months of follow-up. Figure 4 shows the preservation of the iris angle and the trabecular meshwork, as well as an absence of inflammatory cells.
FIG. 4.
Hematoxylin–eosin staining of the rabbit eyes after implantation of an intraocular thin film. (A–C) Control eyes. (D–F) Thin film implanted into the anterior chamber (4 months). (G–I) Thin film implanted into the vitreous cavity (6 months). The corneal anatomy and endothelium are preserved (A, D, G). No inflammation or degeneration is observed in the anterior segment of the eyes (B, E, H), or at the level of the retina or choroid (C, F, I). Magnification 20X. Color images available online at www.liebertpub.com/jop
SEM evaluation of device integrity and durability
SEM studies demonstrated that micro- and nanostructured biopolymer thin films were maintained up to 9 months, and no substantial cellular debris, inflammatory cells, or fibrosis was observed on the thin-film surfaces. Microstructured thin films featuring a 50-μm groove array showed no structural damage or degradation after implantation and ocular residency. Microscale grooves were found to maintain their definition and designed width and regularity (Fig. 5). Similarly, nanostructured thin films maintained the integrity of the smallest-scale design features through 9 months of ocular residence (Table 1; Fig. 6). Compared to preimplantation, the thin films had no discernable alteration of the 30-nm-diameter pores or their spacing and regularity after 6 and 9 months in the eye. Films were not seen to be coated or occluded with cells or other deposits while exposed to the vitreous gel.
FIG. 5.
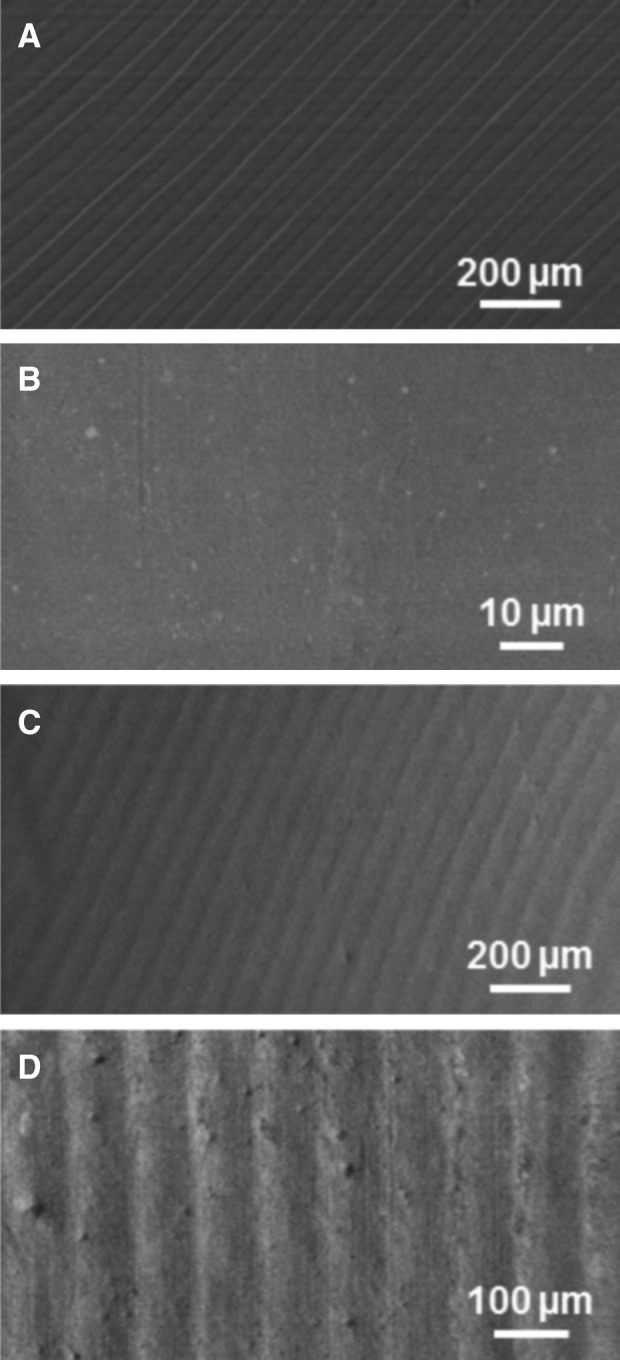
SEM images of microstructured thin films before and after ocular implantation. (A) Microstructured thin films before implantation, and (B) unstructured and (C, D) microstructured thin films after 4 months in the vitreous cavity. Microstructural features are preserved over this time course and lack debris, fibrosis, or cellular reaction.
FIG. 6.
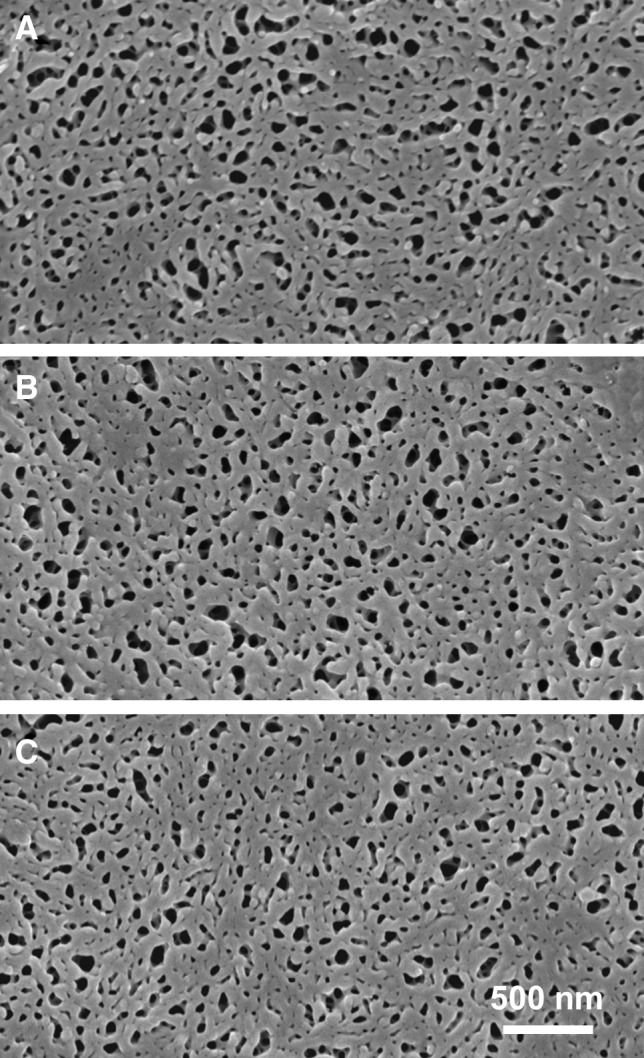
SEM images of nanostructured thin films retrieved after ocular implantation. Nanostructured films (A) before implantation, (B) after 6 months in the vitreous cavity, and (C) after 9 months in the vitreous cavity. Nanostructural features are preserved over this time course with no pore disruption or surface deposits.
Discussion
Both microstructural (50-μm grooves) and nanostructural (30-nm-diameter pores) design features were successfully constructed in PCL thin films, a degradable medical biopolymer previously used for ocular application.35,36 The size scales of structural features on these films offer novel functional properties and potential biomedical applications; microgrooved films are scaled for applications involving cell-based therapies, and nanoporous films can serve as diffusion control membranes for drug delivery applications. However, the translation of this technology to ophthalmic devices is predicated on (1) the structural integrity of micro- and nanoscaled design elements during long-term residence in the intraocular environment, and (2) the tolerance and safety of biopolymer materials for the delicate tissues of the eye.
SEM studies performed on thin-film devices implanted into the anterior chamber or the vitreous cavity of an adult rabbit demonstrated that the structural integrity of the micro- and nanostructural architecture was maintained up to 9 months in the eye. This is fundamental to the use of this technology in the eye, since a distinguishing feature of micro- and nanoengineered devices is that unique functional properties are determined by the structure. For both micro- and nanostructured films, the back of the structured film served as an internal control, since the reverse side of these films was unstructured and received an identical casting procedure. No observable changes in the surface structure were noted on the flat side of the PCL films. Because these films were cast from solution, the polymer is extremely contiguous, which logically contrasts to pressed PCL pellets, where inherent cracks and grain boundaries are present, and significant surface roughening is observed.35,37 Microscale features were maintained without alteration of the fine structure of the 50-μm surface grooves after up to 6 months in the vitreous cavity, with no evidence of adverse tissue–device reactions or deposits (Fig. 5). Similarly, no visible alteration in the nanostructured PCL was found between the preimplanted and postimplanted devices, with nanopores maintaining the patency and stable 30-nm pore diameters (Fig. 6). Further, the thin films did not elicit an observable cellular reaction, fibrosis, encapsulation, or biodeposits during residence in the rabbit eyes. These findings are necessary for the use of this material in intraocular applications such as drug delivery. Additionally, in this in vivo eye model, no such structural disruption is seen over 9 months.
An acceptable ocular safety profile of these biopolymer films was shown in toxicity studies in adult rabbit eyes using serial ophthalmologic examinations and ocular histology. Microstructured and nanostructured PCL thin films were implanted into the anterior chamber or the vitreous cavity for 1 week to 6 months (Tables 2–4). After the device implantation procedure, typical postoperative signs of low-grade conjunctival injection, corneal edema, and anterior chamber inflammation were seen in some eyes, resolving within 1 week. Persistent corneal edema was rare. Longer-term ocular inflammation, iritis, or vitritis was not seen, nor was cataractogenesis observed. No retinal degeneration or retinopathy was seen clinically or histologically. Complications associated with the device insertion procedure included vitreous hemorrhage, iatrogenic cataract, and retinal tear. While the 4-×5-mm thin films were scaled for the human eyes, this rabbit eye model is limited by small eye size and a large spheroidal lens, which constrains the vitreous cavity space, increasing the risk of inadvertent surgical trauma.
A limitation of these toxicity studies is that these devices were composed of relatively high-molecular-weight PCL (initial Mn 70–90 kDa) and did not undergo complete physical degradation during the 6-month implantation period, with minimal gross breakdown observed ophthalmoscopically. PCL degrades by a random chain scission at its ester linkages; only mono- and dimeric caprolactic acid has significant aqueous solubility, and consequently, the bulk physical structure in PCL is maintained until the final stages of degradation.21 For experiments involving smaller thin-film pieces (∼1–2 mm), film migration was not detected, indicating that degraded fragments are likely to behave similarly to the large films presented here. Further experiments with lower-molecular-weight PCL are underway to fully assess any ocular toxicity that may be associated with PCL breakdown products released into the vitreous during complete device degradation, as well as to determine the potential impacts of film fragmentation.
Conclusions
The data presented here indicate the feasibility of micro- and nanostructured PCL for ophthalmologic applications, providing the first demonstration to our knowledge that nanoscale design elements in biodegradable polymers can maintain the structural integrity when dwelling in the eye: in particular, this result establishes the groundwork for a class of devices that rely on the preservation of the membrane architecture over time.
Acknowledgments
The National Institutes of Health/National Eye Institute RO1 Grant EY021574-01A1 and the NIH/NEI Core Grant EY002162; the Wallace H. Coulter Foundation; the That Man May See Foundation; the Allergan Horizon Fellowship; the Research to Prevent Blindness unrestricted grant; the UCSF Micro and Nanofabrication Core Grant. DAB was funded by a Genentech Postdoctoral Fellowship.
Author Disclosure Statement
U.S. Provisional Patent 61/475,373 (TAD, MRS, RBB, and DAB); Consultants for Santen, Inc. (RBB and TAD), ActiveSite Pharmaceuticals, and ISTA Pharmaceuticals (RBB); Speakers Bureau for Genentech, Inc. (RBB and TAD).
References
- 1.Lee S.S. Hughes P. Ross A.D. Robinson M.R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010;27:2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 2.Mohammad D.A. Sweet B.V. Elner S.G. Retisert: is the new advance in treatment of uveitis a good one? Ann. Pharmacother. 2007;41:449–454. doi: 10.1345/aph.1H540. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.S. Hughes P.M. Robinson M.R. Recent advances in drug delivery systems for treating ocular complications of systemic diseases. Curr. Opin. Ophthalmol. 2009;20:511–519. doi: 10.1097/ICU.0b013e328330ccb9. [DOI] [PubMed] [Google Scholar]
- 4.Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi H. Functional polymer microspheres. Prog. Polym. Sci. 2000;25:1171–1210. [Google Scholar]
- 6.Ravi Kumar M.N. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 7.Soppimath K.S. Aminabhavi T.M. Kulkarni A.R. Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 8.Conti B. Pavanetto F. Genta I. Use of polylactic acid for the preparation of microparticulate drug delivery systems. J. Microencapsul. 1992;9:153–166. doi: 10.3109/02652049109021231. [DOI] [PubMed] [Google Scholar]
- 9.Lassalle V. Ferreira M.L. PLA nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol. Biosci. 2007;7:767–783. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 10.Allison S.D. Analysis of initial burst in PLGA microparticles. Expert Opin. Drug Deliv. 2008;5:615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- 11.Giteau A. Venier-Julienne M.C. Aubert-Pouëssel A. Benoit J.P. How to achieve sustained and complete protein release from PLGA-based microparticles? Int. J. Pharm. 2008;350:14–26. doi: 10.1016/j.ijpharm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Wischke C. Schwendeman S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Aishwarya S. Mahalakshmi S. Sehgal P.K. Collagen-coated polycaprolactone microparticles as a controlled drug delivery system. J. Microencapsul. 2008;25:298–306. doi: 10.1080/02652040801972004. [DOI] [PubMed] [Google Scholar]
- 14.Luciani A. Coccoli V. Orsi S. Ambrosio L. Netti P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials. 2008;29:4800–4807. doi: 10.1016/j.biomaterials.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Wei X. Gong C. Gou M., et al. Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009;381:1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Budhian A. Siegel S.J. Winey K.I. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int. J. Pharm. 2008;346:151–159. doi: 10.1016/j.ijpharm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Wei X. Gong C. Shi S., et al. Self-assembled honokiol-loaded micelles based on poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) copolymer. Int. J. Pharm. 2009;369:170–175. doi: 10.1016/j.ijpharm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Loftsson T. Hreinsdóttir D. Stefánsson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007;59:629–635. doi: 10.1211/jpp.59.5.0002. [DOI] [PubMed] [Google Scholar]
- 19.Kompella U.B. Bandi N. Ayalasomayajula S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 20.Carcaboso A.M. Chiappetta D.A. Opezzo JAW, et al. Episcleral implants for topotecan delivery to the posterior segment of the eye. Invest. Ophthalmol. Vis. Sci. 2010;51:2126–2134. doi: 10.1167/iovs.09-4050. [DOI] [PubMed] [Google Scholar]
- 21.Labet M. Thielemans W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 2009;38:3484–3504. doi: 10.1039/b820162p. [DOI] [PubMed] [Google Scholar]
- 22.Kashanian S. Harding F. Irani Y., et al. Evaluation of mesoporous silicon/polycaprolactone composites as ophthalmic implants. Acta Biomaterialia. 2010;6:3566–3572. doi: 10.1016/j.actbio.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Redenti S. Tao S. Yang J., et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Inform. 2008;1:19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steedman M.R. Tao S.L. Klassen H. Desai T.A. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed. Microdevices. 2010;12:363–369. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sodha S. Wall K. Redenti S. Klassen H. Young M.J. Tao S.L. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011;22:443–456. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen A.T. Tao S.L. Smith M., et al. Subretinal implantation of electrospun, short nanowire, and smooth poly(ɛ-caprolactone) scaffolds to the subretinal space of porcine eyes. Stem Cells Int. 2012;2012:454295. doi: 10.1155/2012/454295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeley N.R.F. Rossi J.V. Mello-Filho P.A.A., et al. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J. Biomed. Mater. Res.Part A. 2005;73A:437–444. doi: 10.1002/jbm.a.30294. [DOI] [PubMed] [Google Scholar]
- 28.Zarbin M.A. Montemagno C. Leary J.F. Ritch R. Nanomedicine in ophthalmology: the new frontier. Am. J. Ophthalmol. 2010;150:144–162. doi: 10.1016/j.ajo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Bernards D.A. Desai T.A. Nanoscale porosity in polymer films: fabrication and therapeutic applications. Soft Matter. 2010;6:1621–1631. doi: 10.1039/B922303G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon G. Yang S.Y. Kim J.K. Functional nanoporous membranes for drug delivery. J. Mater. Chem. 2012;22:14814–14834. [Google Scholar]
- 31.Edelhauser H.F. Rowe-Rendleman C.L. Robinson M.R., et al. Ophthalmic Drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest. Ophthalmol. Vis. Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ainslie K.M. Tao S.L. Popat K.C., et al. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J. Biomed. Mater. Res. Part A. 2009;91A:647–655. doi: 10.1002/jbm.a.32262. [DOI] [PubMed] [Google Scholar]
- 33.Bernards D.A. Desai T.A. Nanotemplating of biodegradable polymer membranes for constant-rate drug delivery. Adv. Mater. 2010;22:2358–2362. doi: 10.1002/adma.200903439. [DOI] [PubMed] [Google Scholar]
- 34.Wang C.H. Wong A.S.W. Ho G.W. Facile solution route to vertically aligned, selective growth of ZnO nanostructure arrays. Langmuir. 2007;23:11960–11963. doi: 10.1021/la702296q. [DOI] [PubMed] [Google Scholar]
- 35.Silva-Cunha A. Fialho S.L. Naud M-C. Behar-Cohen F. Poly-epsilon-caprolactone intravitreous devices: an in vivo study. Invest. Ophthalmol. Vis. Sci. 2009;50:2312–2318. doi: 10.1167/iovs.08-2969. [DOI] [PubMed] [Google Scholar]
- 36.Natu M.V. Gaspar M.N. Fontes Ribeiro C.A. Cabrita A.M. de Sousa H.C. Gil M.H. In vitro and in vivo evaluation of an intraocular implant for glaucoma treatment. Int. J. Pharm. 2011;415:73–82. doi: 10.1016/j.ijpharm.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Fialho S.L. Behar-Cohen F. Silva-Cunha A. Dexamethasone-loaded poly(epsilon-caprolactone) intravitreal implants: a pilot study. Eur. J. Pharm. Biopharm. 2008;68:637–646. doi: 10.1016/j.ejpb.2007.08.004. [DOI] [PubMed] [Google Scholar]