Abstract
The purpose of this prospective, randomized, single-blind study was to determine the anesthetic efficacy of 127.2 mg lidocaine with 50 μg epinephrine compared to 127.2 mg lidocaine with 50 μg epinephrine plus 0.5 M mannitol in inferior alveolar nerve (IAN) blocks. Forty subjects randomly received 2 IAN blocks consisting of a 3.18 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine and a 5 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine (3.18 mL) plus 0.5 M mannitol (1.82 mL) in 2 separate appointments spaced at least 1 week apart. Mandibular anterior and posterior teeth were blindly electric pulp tested at 4-minute cycles for 60 minutes postinjection. Pain of solution deposition and postoperative pain were also measured. No response from the subject to the maximum output (80 reading) of the pulp tester was used as the criterion for pulpal anesthesia. Total percent pulpal anesthesia was defined as the total of all the times of pulpal anesthesia (80 readings) over the 60 minutes. One hundred percent of the subjects had profound lip numbness with both inferior alveolar nerve blocks. The results demonstrated that a 5 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine plus 0.5 M mannitol was significantly better than the 3.18 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine for all teeth. Solution deposition pain and postoperative pain were not statistically different between the lidocaine/mannitol formulation and the lidocaine formulation without mannitol. We concluded that adding 0.5 M mannitol to a lidocaine with epinephrine formulation was significantly more effective in achieving a greater percentage of total pulpal anesthesia than a lidocaine formulation without mannitol.
Key Words: Inferior alveolar nerve block, Lidocaine, Mannitol, Epinephrine
The inferior alveolar nerve (IAN) block is the most frequently used injection technique for achieving local anesthesia for mandibular restorative and surgical procedures. However, the IAN block does not always result in successful pulpal anesthesia.1–6 Failure rates (never achieving 2 consecutive 80 readings with the electric pulp tester) of 10 to 39% have been reported.1 A possible reason for failure is the perineurial barrier around the nerve may not allow complete diffusion of the anesthetic solution into the nerve trunk.
A previous study by Wolf et al7 demonstrated that lidocaine in combination with mannitol significantly improved the success of the IAN block. The proposed mechanism is that mannitol opens the perineurial membrane to allow for enhanced penetrability for lipophilic compounds8 (such as lidocaine), and it may also directly affect nerve conduction.9
While Wolf et al7 demonstrated that 2.84 mL of 36 mg lidocaine with 18 μg epinephrine (1.80 mL) plus 0.5 M mannitol (1.04 mL) was significantly better than 1.8 mL of 36 mg lidocaine with 18 μg epinephrine for the IAN block in the molars and premolars, a 5 mL formulation of 63.6 mg lidocaine with 32 μg epinephrine (3.18 mL) plus 0.5 M mannitol (1.82 mL) was significantly better than 2.84 mL of lidocaine with epinephrine plus 0.5 M mannitol for all teeth except the central incisor. Therefore, increasing the amount of lidocaine plus mannitol increased success.
Rood10 and Eldridge and Rood11 have shown that a 5% lidocaine solution with 1 : 80,000 epinephrine increased the success rate of an IAN block when compared to a 2% lidocaine solution with 1 : 80,000 epinephrine. Rood and Sowray12 also described a series of cases where 5% lidocaine provided adequate pain control when 2% lidocaine was inadequate. Lastly, Sandy and Rood13 discussed the use of 5% lidocaine in children. They showed a 5% lidocaine solution was helpful in achieving anesthesia when 2% lidocaine was inadequate.
Because total pulpal anesthesia was not obtained by Wolf et al,7 further increasing the amount of lidocaine when combined with 0.5 M mannitol could potentially allow the anesthetic solution to permeate the nerve trunk in greater amounts, thereby increasing anesthetic efficacy. The purpose of this prospective, randomized, single-blind study was to determine the anesthetic efficacy of 127.2 mg lidocaine with 50 μg epinephrine compared to 127.2 mg lidocaine with 50 μg epinephrine plus 0.5 M mannitol in IAN blocks. Pain of injection and postinjection pain were also studied.
MATERIALS AND METHODS
Forty adult subjects participated in this study. The subjects were in good health and were not taking any medications that would alter pain perception. Exclusion criteria were as follows: younger than 18 years of age; allergies to mannitol, local anesthetics, or sulfites; pregnancy; history of significant medical conditions (American Association of Anesthesiologists Class II or higher); taking any medications (over-the-counter pain relieving medications, narcotics, sedatives, antianxiety or antidepressant medications) that may affect pain assessment; active pathosis at the site of injection; and inability to give informed consent. The Ohio State University Human Subjects Review Committee approved the study, and written informed consent was obtained from each subject.
Using a crossover design, 40 adult subjects received 2 IAN blocks consisting of a 3.18 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine and a 5 mL formulation of 127.2 mg lidocaine with 50 μg epinephrine (3.18 mL) plus 0.5 M mannitol (1.82 mL) in 2 separate appointments spaced at least 1 week apart.
Equal numbers of mandibular right and left sides were tested, with the first and second molars, first and second premolars, and lateral and central incisors chosen as the test teeth.
With the crossover design, 80 IAN blocks were administered and each subject served as his or her own control. The same side chosen for the first IAN block was used again for the second IAN block. The mandibular contralateral canine was used as the control to ensure that the pulp tester was operating properly and that the subject was responding appropriately. A visual and clinical examination was conducted to ensure that all teeth were free of caries, large restorations, crowns, periodontal disease, and that none had a history of trauma or sensitivity.
Before the injection at each of the 2 appointments, the experimental tooth and the contralateral canine (control) were tested 3 times with the electric pulp tester (Kerr, Analytic Technology Corp, Redmond, Wash) to ensure tooth vitality and obtain baseline information. The teeth were isolated with cotton rolls and dried with an air syringe. Toothpaste was applied to the probe tip, which was placed in the middle third of the buccal or labial surface of the tooth being tested. The value at the initial sensation was recorded. The current rate was set at 25 seconds to increase from no output (0) to the maximum output (80). Trained personnel, who were blinded to the anesthetic formulations, administered all preinjection and postinjection tests.
Before the experiment, the 2 anesthetic formulations were randomly assigned 5-digit numbers from a random number table. Each subject was randomly assigned to each of the 2 anesthetic formulations to determine which formulation was to be administered at each appointment. Only the random numbers were recorded on the data collection sheets to further blind the experiment.
The anesthetic formulations were prepared as follows. Under sterile conditions, 3.18 mL of 4% lidocaine (Astra Pharmaceuticals Products Inc, Westborough, Mass) was drawn into a 5 mL Luer-Lok disposable syringe (Becton, Dickinson, and Company, NJ). Added to this solution was 0.05 mL of 1 : 1000 epinephrine (American Regent Laboratories Inc, Shirley, NY) drawn from a 1 mL ampule using a 1 mL tuberculin syringe (Becton-Dickinson). All solutions used were checked to ensure that they had not expired. The 3.18 mL formulation contained 127.2 mg of lidocaine with 50 μg of epinephrine. For the second formulation, 3.18 mL of 4% lidocaine with 50 μg epinephrine was prepared as described above. Using a 1 mL tuberculin syringe (Becton-Dickinson), 1.82 mL of 0.5 M mannitol was added to the 5 mL syringe. The syringe was then inverted 20 times to mix the solution. The 1.82 mL of mannitol was withdrawn from a 50 mL vial of a 25% (12.5 g/50 mL) supersaturated mannitol solution (American Regent Laboratories). Each vial was used only once. Before the mannitol was added to the syringe containing the lidocaine with epinephrine, the 50 mL vial was heated in a water bath (Teledyne Hanau, Buffalo, NY) to 80°C for 15 minutes to dissolve any crystals present in the supersaturated solution. The vial was then allowed to cool to room temperature before use. The 5 mL formulation contained 127.2 mg of lidocaine with 50 μg of epinephrine (3.18 mL) plus 0.5 M mannitol (1.82 mL). Selected components and selected final anesthetic formulations had their pH values determined using a pH/millivolt meter (Orion Research Inc, Boston, Mass).
The following calculation was utilized to determine the molarity for the final volume of the lidocaine/mannitol formulation. Molarity (M), or molar concentration, is defined as a ratio between the number of moles of a solute per liter of solution. The mole of a compound is the amount of the compound in grams equal to its molecular weight. Therefore, the number of moles in 12.5 g of mannitol would be: moles of mannitol = 12.5 g (per 50 mL vial) × 1 mol/182.17 g (molecular weight of mannitol) = 0.0686 moles. The molarity of the mannitol solution used in this study would be: molarity = 0.0686 moles mannitol/0.05 L solution = 1.372 M. Because the solutions were diluted by the lidocaine with epinephrine solution, the final molarity must be calculated from the molarity after dilution using the following formula: (Mi)(Vi) = (Mf)(Vf) where initial molarity (Mi) multiplied by the initial volume (Vi) is equal to the final molarity (Mf) multiplied by the final volume (Vf). Therefore, for the mannitol formulation the calculated molarity of 0.5 M mannitol would require the following volumes: (1.372 M)(X) = (0.5 M)(5.00 mL) where X = 1.82 mL of mannitol. For the total volume of 5 mL of a 0.5 M mannitol solution, 3.18 mL of lidocaine was combined with 1.82 mL of mannitol.
A standard IAN block14 was administered with a 27-gauge 1½-inch needle (Monoject; Sherwood Medical, St Louis, Mo) using the 2 anesthetic formulations. Following needle penetration, and as the needle was advanced during placement, 0.2 mL of solution was deposited. After the target area was reached and aspiration was performed, 1 minute was used to deposit the anesthetic solution, and the subject was asked to rate the pain of solution deposition. The pain scale was from 0 to 3. Zero indicated no pain. One indicated mild pain, pain that was recognizable but not discomforting. Two indicated moderate pain, pain that was discomforting but bearable. Three indicated severe pain, pain that caused considerable discomfort and was difficult to bear. The principal investigator (S.S.) performed all IAN block injections.
At 1 minute after the IAN block, the first and second molars were pulp tested. At 2 minutes, the first and second premolars were tested. At 3 minutes, the central and lateral incisors were tested. At 4 minutes, the control canine was tested. This cycle of testing was repeated every 4 minutes for 60 minutes. At every fourth cycle, the control tooth (the contralateral canine) was tested with a pulp tester without batteries to test the reliability of the subject. If subjects responded positively to an inactivated pulp tester then they were not reliable and could not be used in the study. Subjects were asked if their lips/tongues were numb every minute for 5 minutes and at every fourth minute during pulp testing. If profound lip numbness was not recorded within 5 minutes following the IAN block, the block was considered unsuccessful and the subject was then reappointed. Two of 80 (2%) IAN blocks were unsuccessful in this study, and these subjects required an additional appointment. All testing was stopped at 60 minutes postinjection.
All subjects completed postinjection surveys after each IAN block administered. The subjects rated pain in the injection area, using the previous pain scale (none, mild, moderate, severe), immediately after the numbness wore off, and again each morning upon arising for 3 days. The subjects were also asked to record subjectively any additional comments or side effects not relating to pain.
No response from the subject at the maximum output (80 reading) of the pulp tester was used as the criterion for pulpal anesthesia. Total percent pulpal anesthesia was defined as the total of all the times of pulpal anesthesia (80 readings) over the 60 minutes. With a nondirectional alpha risk of 0.05 and assuming a total pulpal anesthesia rate of 10% for the central incisor, a sample size of 40 subjects was required to demonstrate a difference in anesthetic success of ±10 percentage points with a power of 0.99.
Comparisons between the 2 formulations regarding total percent pulpal anesthesia were assessed using multiple exact McNemar tests adjusted using the step-down Bonferroni method of Holm. Comparisons between the 2 formulations regarding solution deposition pain and postinjection pain were made using multiple Wilcoxon matched pairs, signed rank tests adjusted using the step-down Bonferroni method of Holm. Comparisons were considered significant at P < .05.
RESULTS
Twenty-three women and 17 men, ranging in age from 19 to 31 years, with an average age of 24 years, participated in this study.
One hundred percent of the subjects used for the data analysis had subjective lip and tongue anesthesia with the IAN blocks. The discomfort ratings of solution deposition for the 2 formulations are presented in Table 1. There was no significant difference (P > .05) between the formulations. The pH of the solutions were: 7 for the mannitol; 6.68 for the 4% lidocaine; 3.37 for the 1 : 1000 epinephrine; 6.60 for the 3.18 mL formulation of lidocaine with epinephrine; and 6.72 for the 5 mL volume of lidocaine/mannitol.
Table 1.
Percentages (Ratio) and Discomfort Ratings of Solution Deposition symbols, in order * †
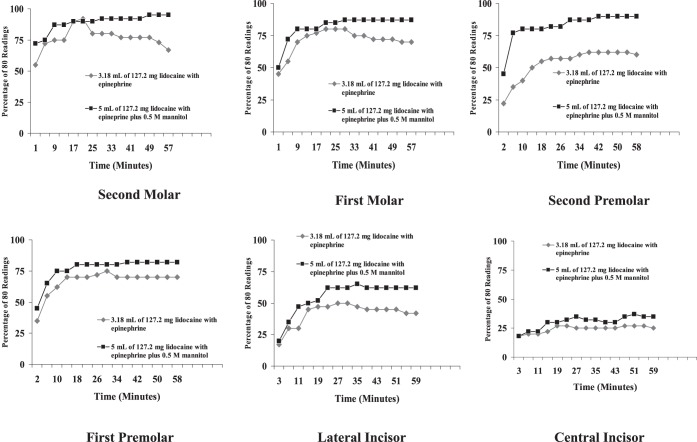
Percent total pulpal anesthesia is presented in Table 2. The posterior teeth had higher values of total pulpal anesthesia than the anterior teeth. The 5 mL solution of 127.2 mg lidocaine with 50 μg epinephrine (3.18 mL) plus 0.5 M mannitol (1.82 mL) was statistically better than the 3.18 mL solution of 127.2 mg lidocaine with 50 μg epinephrine for all teeth. The incidence of pulpal anesthesia for the 2 formulations is presented in the Figure.
Table 2.
Percent Total Pulpal Anesthesia
Incidence of pulpal anesthesia for the molars, premolars, and lateral and central incisors as determined by lack of response to electric pulp testing at the maximum setting (percentage of 80/80), at each postinjection time interval, for the 2 anesthetic formulations.
The postoperative pain ratings are summarized in Table 3. There were no significant differences (P > .05) between the formulations. Table 4 summarizes the number of subjects reporting postinjection trismus for the 2 formulations.
Table 3.
Percentages (Ratio) and Discomfort Ratings for Postinjection Survey

Table 4.
Percentage and Number of Subjects Reporting Postinjection Trismus
DISCUSSION
The use of the 80 reading (signaling maximum output) as a criterion for pulpal anesthesia was based on the studies of Dreven et al15 and Certosimo and Archer.16 These studies15,16 showed that no patient response to an 80 reading with the electric pulp tester ensured pulpal anesthesia in vital asymptomatic teeth. Additionally, Certosimo and Archer16 demonstrated that electric pulp test readings less than 80 resulted in pain during operative procedures in asymptomatic teeth.
Percent total pulpal anesthesia with 3.18 mL of 127.2 mg of lidocaine with 50 μg of epinephrine for the IAN block occurred from 25 to 77% of the time (Table 2; Figure). Previous studies by Nusstein et al1 and Wali et al6 found that increasing the volume of 2% lidocaine with epinephrine to 3.6 mL (2 cartridges) or increasing the epinephrine concentration in a lidocaine formulation did not increase the incidence of pulpal anesthesia with the IAN block. However, neither study used as high an amount of lidocaine or epinephrine as was used in the current study. In the study by Wolf et al,7 total pulpal anesthesia for 1.8 mL of 36 mg lidocaine with 18 μg epinephrine ranged from 12 to 75% for the IAN block. In the current study, increasing the formulation to 3.18 mL of 127.2 mg lidocaine with 50 μg epinephrine resulted in total pulpal anesthesia of 25 to 77%. The difference between the two studies of 2 to 23% is better with the 3.18 mL formulation but total pulpal anesthesia was still not 100%. Additionally, based on the amount of lidocaine with epinephrine used in the current study, increasing the lidocaine concentration to 5% (50 mg/mL) using a 1.8 mL cartridge (90 mg/1.8 mL) as shown in several studies10–13 would probably still not provide complete pulpal anesthesia. Therefore, the 3.18 mL formulation would not provide complete pulpal anesthesia for the mandibular teeth (Table 2; Figure), which could present meaningful clinical problems since the teeth may not be anesthetized for procedures requiring complete pulpal anesthesia. Practitioners should consider supplemental techniques, such as intraosseous,17–20 mandibular infiltrations of 4% articaine with 1 : 100,000 epinephrine,21,22 or periodontal ligament injections23 when anesthetic formulations fail to provide pulpal anesthesia for a particular tooth. Because we studied a young adult population, the results of this study may not apply to children or the elderly.
The addition of 0.5 M mannitol to 127.2 mg of lidocaine with 50 μg of epinephrine resulted in a statistically higher percentage of total pulpal anesthesia for all teeth when compared to 127.2 mg of lidocaine with 50 μg epinephrine (Table 2). In these teeth, the increase in mean percent total pulpal anesthesia, over the 127.2 mg of lidocaine with 50 μg of epinephrine, ranged from 4 to 17% (Table 2). This would seem to confirm the study by Antonijevic et al8 who found that a 0.5 M solution of mannitol opened the perineurial membrane allowing for an enhanced penetrability of lipophilic compounds or, based on the findings of Matsuka and Spigelman,9 the mannitol possibly delayed or blocked action potential propagation in select neurons. If complete pulpal anesthesia had been obtained in the majority of the teeth with the lidocaine/mannitol formulation, increasing the amount of lidocaine with epinephrine would have been justified. Wolf et al7 found 5 mL of 63.6 mg lidocaine with 36 μg epinephrine plus 0.5 M mannitol resulted in total pulpal anesthesia ranging from 20 to 93%. While there was a higher incidence of total pulpal anesthesia for most teeth in the current study than recorded by Wolf et al,7 the differences in percentages ranged from 2 to 13%. Therefore, increasing the amount of lidocaine in combination with mannitol still did not result in total pupal anesthesia.
In the mannitol formulation, both the lidocaine and epinephrine concentrations were diluted. While we could have added additional formulations to the study by increasing the total injected volume of lidocaine with epinephrine without mannitol (by adding normal saline or a similar solution), we felt that dilution would not increase the potential success of the IAN block. Overall, combining mannitol with the lidocaine with epinephrine formulation did statistically increase success. Therefore, diluting the formulations with mannitol increased the efficacy of the lidocaine with epinephrine. Wolf et al7 found similar results when diluting mannitol/lidocaine formulations.
We chose a hyperosmolar solution of mannitol since studies8,24 have shown it is inert and it has been used extensively in medicine.25 Antonijevic et al8 demonstrated the injection of 0.5 M mannitol into rat plantar subcutaneous paw tissue was without effect. The hypertonic solution of mannitol did not induce an inflammatory cell infiltrate when the tissues were examined histologically.8
In order to keep the mannitol concentration at 0.5 M, the final volume of the solution has to be calculated based on the formula for molarity (see Materials and Methods). The result is a higher volume of solution than would be used if only the lidocaine solution was administered. The 25% (12.5 g/50 mL) solution of mannitol was chosen for this study because it was the highest concentration available commercially. Therefore, when combining the mannitol with lidocaine, the final volume of the solution could still be kept to a clinically acceptable amount. When mannitol was combined with the lidocaine solution, no precipitate formed. Because mannitol is inert,8,24 we would not expect it to chemically combine or react with the lidocaine or epinephrine. The pH values of the lidocaine and lidocaine/mannitol formulations were similar, 6.60 and 6.72, respectively. It is unlikely that pH caused differences in success rates of the IAN blocks.
Moderate-to-severe solution deposition pain ranged from 7 to 14% for the two solutions (Table 1). Other studies2,4,5,7 of the IAN block, using 2% lidocaine with 1 : 100,000 epinephrine, have reported a 20 to 25% incidence of moderate/severe pain. Therefore, the IAN block has the potential to be painful even though the anesthetic formulation was deposited slowly over 1 minute. There was no significant difference between the formulations. Therefore, the addition of 0.5 M mannitol was not found to be any more painful on injection than the formulation without mannitol (Table 1).
Moderate postinjection pain, at the time subjective numbness wore off, was 15% for the lidocaine with epinephrine formulation (Table 3). The percentage was similar for the lidocaine with epinephrine solution in other studies,2,4,5,7 using 2% lidocaine with 1 : 100,000 epinephrine, in which a 14 to 17% incidence of moderate/severe pain was reported at the time subjective numbness wore off. Judging from this study and others,2–5,7 the IAN block has the potential to result in moderate postinjection pain. For the lidocaine formulation, moderate pain decreased to 2% by day 1 and 2, with no reports of moderate pain for day 3 (Table 3). Similar results have been reported in other studies2,4,5,7 of postinjection pain for the IAN block.
For the lidocaine/mannitol formulation, 22% had moderate pain, with 1 report of severe pain at the time subjective numbness wore off (Table 3). Wolf et al7 reported an incidence of 25% moderate pain at the time subjective numbness wore off for both a 2.84 mL formulation of 36 mg lidocaine with 18 μg epinephrine plus 0.5 M mannitol and a 5 mL formulation of 63.6 mg lidocaine with 36 μg epinephrine plus 0.5 M mannitol. In the current study, the lidocaine/mannitol formulation had a 15% incidence of moderate pain at day 1, which decreased to 2% at day 2 and 0% at day 3. Wolf et al7 also reported decreasing pain (2 to 0% over 3 days) for the lidocaine/mannitol formulations used in their study. There were no significant differences between the formulations (Table 3). Therefore, the addition of 0.5 M mannitol was not found to result in any more postinjection pain than the formulation without mannitol. Because studies have found that 0.5 M mannitol is inert,8,24 we would not expect an increased incidence of moderate postinjection pain.
In medicine, treatment of elevated intracranial or intraocular pressure requires a 1.5 to 2 g/kg dose of mannitol intravenously.25 To treat oliguria, 50 to 100 g of mannitol is infused intravenously over 90 minutes.25 Using these large amounts of mannitol results in a number of precautions (renal dysfunction, heart failure, or pulmonary congestion) in its use.25 The total amount of grams administered for the IAN blocks in the current study was 0.45 g (1.82 mL of mannitol). Therefore, the small amount administered and the inertness of the 0.5 M mannitol would negate the precautions and contraindications associated with mannitol for medical treatment.
The number of patients experiencing trismus postinjection ranged from 10 to 12% at the time subjective numbness wore off (Table 4). These moderate ratings continued for day 1, decreased for day 2, and then increased for day 3 for the lidocaine formulation. Previous studies of the IAN block using 2% lidocaine with 100,000 epinephrine recorded a 3 to 9% incidence of trismus postinjection.4,5 Wolf et al7 using 36 mg lidocaine without mannitol and 36 mg lidocaine and 63.6 mg of lidocaine with mannitol found a 2 to 5% incidence of trismus. The higher incidence in the current study most likely is related to the increased amount of lidocaine (127.2 mg). Because the formulation containing mannitol had a similar number of subjects reporting trismus compared to the formulation of lidocaine with epinephrine, the mannitol did not contribute to an increased incidence of trismus. Again, since 0.5 M mannitol is inert,8,24 we would not expect more trismus postinjection.
We concluded that adding 0.5 M mannitol to 127.2 mg lidocaine with 50 μg epinephrine was significantly more effective in achieving a greater percentage of total pulpal anesthesia than a 127.2 mg lidocaine with 50 μg epinephrine formulation without mannitol. Injection pain and postinjection pain were not statistically different between the lidocaine/mannitol formulation and the lidocaine formulation without mannitol.
ACKNOWLEDGMENTS
This study was supported by Graduate Endodontic Research Funds and The Steven Goldberg Memorial Fund.
REFERENCES
- 1.Nusstein J, Reader A, Beck FM. Anesthetic efficacy of different volumes of lidocaine with epinephrine for inferior alveolar nerve blocks. Gen Dent. 2002;50:372–375. [PubMed] [Google Scholar]
- 2.Nist R, Reader A, Beck M, Meyers W. An evaluation of the incisive nerve block and combination inferior alveolar and incisive nerve blocks in mandibular anesthesia. J Endod. 1992;18:455–459. doi: 10.1016/S0099-2399(06)80849-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Reader A, Beck M, Meyers WJ. Anesthetic efficacy of the mylohyoid nerve block and combination inferior alveolar nerve block/mylohyoid nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:557–563. doi: 10.1016/s1079-2104(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 4.Mikesell P, Nusstein J, Reader A, Beck M, Weaver J. A comparison of articaine and lidocaine for inferior alveolar nerve blocks. J Endod. 2005;31:265–270. doi: 10.1097/01.don.0000140576.36513.cb. [DOI] [PubMed] [Google Scholar]
- 5.Ridenour S, Reader A, Beck M, Weaver J. Anesthetic efficacy of a combination of hyaluronidase and lidocaine with epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2001;48:9–15. [PMC free article] [PubMed] [Google Scholar]
- 6.Wali M, Drum M, Reader A, Nusstein J. Prospective, randomized single-blind study of the anesthetic efficacy of 1.8 and 3.6 milliliters of 2% lidocaine with 1 : 50,000 epinephrine for inferior alveolar nerve blocks. J Endod. 2010;36:1459–1462. doi: 10.1016/j.joen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Wolf R, Reader A, Drum M, Nusstein J, Beck M. Anesthetic efficacy of combinations of 0.5 M mannitol and lidocaine with epinephrine in inferior alveolar nerve blocks: a prospective randomized, single-blind study. Anesth Prog. 2011;58:157–165. doi: 10.2344/11-30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonijevic I, Mousa S, Schafer M, Stein C. Perineural defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuka Y, Spigelman I. Hyperosmolar solutions selectively block action potentials in rat myelinated sensory fibers: implications for diabetic neuropathy. J Neurophysiol. 2004;91:48–56. doi: 10.1152/jn.00689.2003. [DOI] [PubMed] [Google Scholar]
- 10.Rood JP. Inferior alveolar nerve blocks. The use of 5 per cent lignocaine. Br Dent J. 1976;140:413–414. doi: 10.1038/sj.bdj.4803774. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge DJ, Rood JP. A double-blind trial of 5 per cent lignocaine solution. Br Dent J. 1977;142:129–130. doi: 10.1038/sj.bdj.4803877. [DOI] [PubMed] [Google Scholar]
- 12.Rood JP, Sowray JH. Clinical experience with 5% lignocaine solution. J Dent. 1980;8:128–131. doi: 10.1016/0300-5712(80)90028-7. [DOI] [PubMed] [Google Scholar]
- 13.Sandy J, Rood JP. Five percent lignocaine solution in children's dentistry. J Dent. 1980;8:312–314. doi: 10.1016/0300-5712(80)90045-7. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen NB, Hayden J., Jr . Premedication, Local and General Anesthesia in Dentistry. 2nd ed. Philadelphia, Pa: Lea & Febiger;; 1967. [Google Scholar]
- 15.Dreven L, Reader A, Beck M, Meyers W, Weaver J. An evaluation of the electric pulp tester as a measure of analgesia in human vital teeth. J Endod. 1987;13:233–238. doi: 10.1016/s0099-2399(87)80097-3. [DOI] [PubMed] [Google Scholar]
- 16.Certosimo A, Archer R. A clinical evaluation of the electric pulp tester as an indicator of local anesthesia. Oper Dent. 1996;21:25–30. [PubMed] [Google Scholar]
- 17.Dunbar D, Reader A, Nist R, Beck M, Meyers W. Anesthetic efficacy of the intraosseous injection after an inferior alveolar nerve block. J Endod. 1996;22:481–486. doi: 10.1016/S0099-2399(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 18.Guglielmo A, Reader A, Nist R, Beck M, Weaver J. Anesthetic efficacy and heart rate effects of the supplemental intraosseous injection of 2% mepivacaine with 1 : 20,000 levonordefrin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:284–293. doi: 10.1016/s1079-2104(99)70210-6. [DOI] [PubMed] [Google Scholar]
- 19.Gallatin E, Stabile P, Reader A, Nist R, Beck M. Anesthetic efficacy and heart rate effects of the intraosseous injection of 3% mepivacaine after an inferior alveolar nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:83–87. doi: 10.1016/s1079-2104(00)80019-0. [DOI] [PubMed] [Google Scholar]
- 20.Stabile P, Reader A, Gallatin E, Beck M, Weaver J. Anesthetic efficacy and heart rate effects of the intraosseous injection of 1.5% etidocaine (1 : 200,000 epinephrine) after an inferior alveolar nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:407–411. doi: 10.1016/s1079-2104(00)70120-x. [DOI] [PubMed] [Google Scholar]
- 21.Haase A, Reader A, Nusstein J, Beck M, Drum M. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Am Dent Assoc. 2008;139:1228–1235. doi: 10.14219/jada.archive.2008.0338. [DOI] [PubMed] [Google Scholar]
- 22.Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42:238–246. doi: 10.1111/j.1365-2591.2008.01507.x. [DOI] [PubMed] [Google Scholar]
- 23.Childers M, Reader A, Nist R, Beck M, Meyers W. The anesthetic efficacy of the periodontal ligament injection after an inferior alveolar nerve block. J Endod. 1996;22:317–320. doi: 10.1016/S0099-2399(96)80267-6. [DOI] [PubMed] [Google Scholar]
- 24.Neuwelt EA, Dahlborg SA. Implications of the Blood-Brain Barrier and Its Manipulation. Vol 2. New York, NY: Plenum;; 1989. Blood-brain barrier disruption in the treatment of brain tumors. Clinical implications; pp. 195–261. In. [Google Scholar]
- 25.Gilman AG, Goodman LS, Gilman A. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York, NY: Macmillan Publishing Co;; 1980. [Google Scholar]