Abstract
Metabolic sensing neurons are conserved across multiple animal species and allow the organism to monitor nutrient availability to maintain energy homeostasis. Miyamoto et al. (2012) describe fly neurons that are highly tuned to fructose availability and are critical determinants of ingestive behavior on a diet of simple sugars.
Organisms require a host of sensors in order to respond appropriately to perturbations in their external environment. Given the importance of ingestive behavior for survival, virtually all species have specialized taste receptors that allow them to differentiate essential qualities in potential fuels: for example, friend versus foe, essential nutrient versus toxin. However, once consumed, a mechanism is required to monitor the internal processing of ingested substrates for regulation of overall energy homeostasis, the balance among intake, expenditure, and storage of energy. This role is served by specialized metabolic sensing neurons (MSN) in mammals, which respond in a graded fashion to ambient metabolic substrate levels such as glucose and fatty and amino acids—and hormones such as insulin and leptin by altering their activity (Levin et al., 2011) (Figure 1). Such neurons are localized in clusters throughout the brain. Individual MSN regulate their activity by integrating nutrient and hormonal signals and afferent neural inputs from peripheral metabolic sensors (Levin et al., 2011). Their polysynaptic efferent pathways then relay this information to the brain and peripheral systems to regulate behavior, as well as metabolic and physiological activities involved in energy homeostasis. Fructose is the most abundant dietary carbohydrate in many fruits consumed by fruit flies. In a recent issue of Cell, Miyamoto et al. (2012) demonstrate that, although flies can taste fructose via peripheral taste receptors, the main function of fly MSN neurons in the brain is to monitor internal levels of fructose as a means of regulating food intake. This discovery provides a superb example of the preservation of MSN across the animal kingdom.
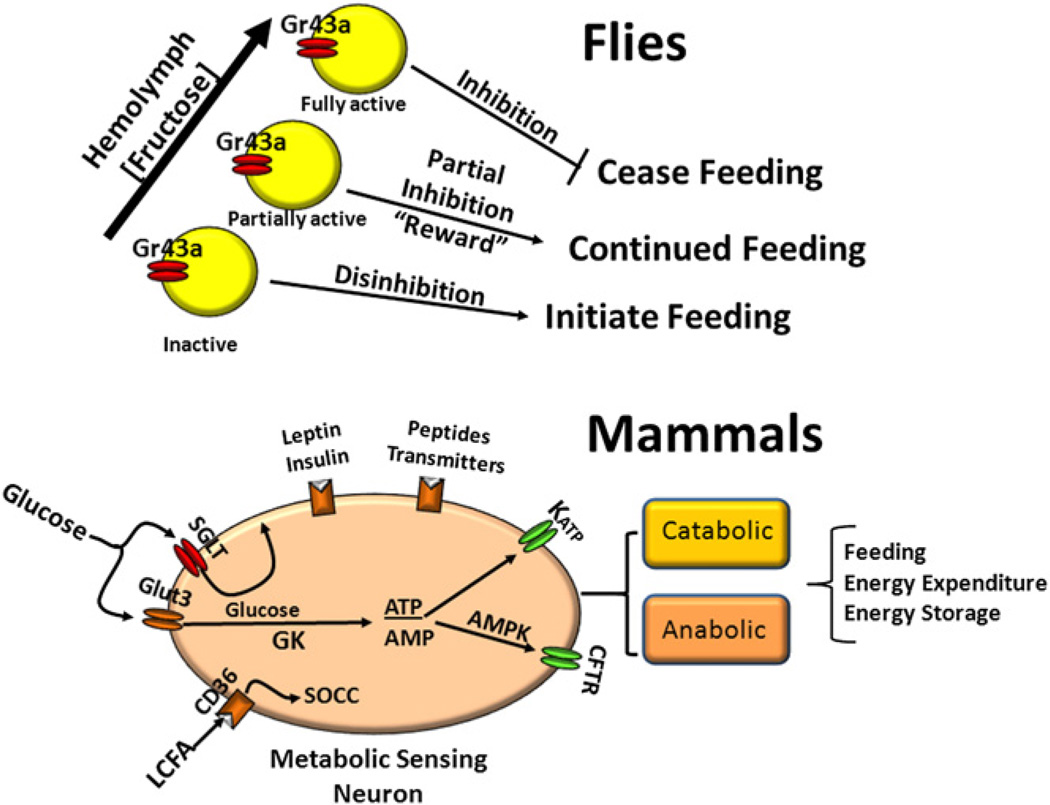
Figure 1. Hypothetical Comparison of Feeding and Energy Homeostasis Systems in Flies and Mammals.
In flies, hemolymph concentrations of fructose are detected in the brain by fructose-sensing neurons expressing Gr43a (yellow). The neurons are inactive at low and activated at high fructose concentrations. This negative feedback system promotes feeding when neurons are inactive and halts feeding above a critical fructose concentration. At intermediate concentrations, activation of Gr43a neurons also provides a reward function that promotes continued feeding. In mammals, there is a complex set of nutrient (glucose, long-chain fatty acid [LCFA]), hormonal (leptin, insulin) and neural inputs from the periphery, which are integrated at the level of membrane potential and alter neuronal activity in different sets of catabolic and anabolic neurons. Glucose is sensed by transport through the Glut3 transporter, phosphorylation by glucokinase (GK) followed by production of ATP, which acts on the ATP-sensitive K+ (KATP) channel to activate glucose excited neurons. When glucose levels fall, AMP is produced, activating neuronal AMP-activated kinase (AMPK) and the cystic fibrosis transmembrane receptor (CFTR), which then stimulates glucose-inhibited neurons. The sodium-glucose cotransporter (SGLT) generates an electrogenic potential that activates glucose excited neurons independently of glucose metabolism. LCFA interact with the fatty-acid translocator/CD36 receptor (CD36) to activate store-operated calcium channels (SOCC), leading to inhibition or excitation of subsets of metabolic sensing neurons (MSN).
Glucose and its disaccharide, trehalose, are the major sugars present in hemolymph (fly ‘‘blood’’). Miyamoto et al. (2012) show, however, that, during feeding, hemolymph levels of glucose and trehalose remain relatively unchanged while fructose levels remain low but increase 3- to 10-fold. This makes fructose an ideal nutrient for signaling changes in internal metabolic status. Using a GAL4 knock-in allele to label neurons expressing the gustatory receptor 43a (Gr43aGAL4), the authors demonstrated that flies express this gene in both peripheral taste organs and a set of ~6 brain neurons. Using calcium imaging as a surrogate for changes in neuronal activity, they showed that, while the peripheral taste receptors coexpress the broader sweet taste GR64f receptor, which also responds to sucrose, glucose, and maltose, the brain GR43a neurons are selectively responsive to changes in fructose concentrations. Mutation of the Gr43a gene significantly attenuated, while transgene rescue reinstated, the fructose sensing of these neurons in vitro. The mutation and transgenes similarly modulated the flies’ preferences for specific sugars and their ingestive behavior. By mutating all Gr43a genes and reinstating function only in brain neurons, the authors show that the regulatory effects of fructose sensing on ingestion are dependent upon the central fructose MSN. Importantly, loss of function of these central Gr43a neurons resulted in overconsumption of sucrose and glucose, both of which are metabolized to fructose in the fly.
The authors conclude that central Gr43a fructose sensing neurons are inhibitory to feeding. In the food-deprived, low-fructose state, they are inactive, and the fly will seek and ingest food. When these neurons are activated by rising hemolymph fructose levels during feeding, their increasing activity progressively inhibits feeding. In an additional wrinkle, using nonnutritive sugars, Miyamoto et al. (2012) demonstrate that activation of central Gr43a fructose neurons also provides a rewarding property to ingested nutrients, independent of feeding state. Thus, this increasing fructose-driven ‘‘reward’’ activity may enhance intake early on to be overridden by the inhibitory effects of rising fructose levels as the meal progresses (Figure 1). Finally, the authors utilized neuroanatomical studies to show that brain Gr43a fructose-sensing neurons send axons to other brain areas, while others extend into the gut musculature, providing a pathway for regulation of postingestive nutrient processing. In addition, dendrites from these neurons ramify widely into the foregut lumen providing them with a potential mechanism for monitoring gut contents.
Mammals have a similar but more complex system for guaranteeing a steady supply of glucose, an essential fuel for cellar function. During states of glucoprivation, glucosensors in the periphery and glucosensing neurons in the brain detect these low levels. This initiates a complex sequence of events that drive feeding and provoke a neurohumoral response that stimulates glucose production and mobilization (Page et al., 2011; Sherwin, 2008). However, aside from extreme glucose deprivation, physiological changes in blood glucose levels are not the primary regulator of ingestion (Dunn-Meynell et al., 2009). Instead, mammals have evolved a far more complex system by which food deprivation leads to decreased production of the anorectic hormones leptin and insulin. Their withdrawal stimulates orexigenic/anabolic pathways and inhibits anorectic/catabolic pathways in the brain by acting on MSN that integrate their signals, along with those of the contemporaneous levels of glucose and fatty and amino acids through receptors, transporters, ion channels, metabolic gatekeepers, and sensors (Figure 1) (Gonzalez et al., 2008; Levin et al., 2011; Migrenne et al., 2011) (Begg and Woods, 2012; Blouet and Schwartz, 2010; Routh, 2010). Such a complex system befits the increased complexity and omnivorous dietary habits of many mammals. For the fly it is sufficient to have a simpler method for controlling intake by monitoring fructose, a major dietary constituent. This, of course, does not exclude the possibility of other leptin- or insulin-like hormones in the fly from influencing the activity of Gr43a neurons.
In conclusion, the fly has evolved a simple but elegant set of central neurons that monitor and regulate the intake of fructose, a primary sugar in its diet. Whereas glucose and trehalose can be utilized for cellular metabolism throughout the body, fructose appears to serve predominantly as a marker for overall nutrient sufficiency that allows the finely tuned brain Gr43a-expressing neurons to respond to the gradient of hemolymph fructose levels that occur from depletion to repletion. This system is highly appropriate to the relatively simple diet of the fruit fly. While convincing, some important pieces of the story remain to be filled in. First, neurophysiological studies must demonstrate that alterations in intracellular calcium evoked by changing fructose levels are a surrogate for neuronal activity. Second, there are likely to be other sugar sensors and hormones that also play into this relatively simple system. Finally, and most importantly, the neuroanatomical and physiological links between presumptive changes in Gr43a neuronal activity and the complex regulation of feeding behavior will have to be further defined. Nevertheless, Miyamoto et al. (2012) have demonstrated that flies, like mammals, have evolved MSN that allow them to monitor their metabolic status as a means of regulating bodily energy homeostasis.
REFERENCES
- Begg DP, Woods SC. Handb. Ex. Pharmacol. 2012;209:111–129. doi: 10.1007/978-3-642-24716-3_5. [DOI] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Behav. Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. J Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Reimann F, Burdakov D. J. Physiol. 2008;587:41–48. doi: 10.1113/jphysiol.2008.163410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Endocrinology. 2011;152:2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migrenne S, Le Foll C, Levin BE, Magnan C. Diabetes Metab. 2011;37:83–88. doi: 10.1016/j.diabet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. Cell. 2012;141:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. J. Clin. Invest. 2011;121:4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH. Sensors (Basel) 2010;10:9002–9025. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin RS. Diabetes. 2008;57:2259–2268. doi: 10.2337/db08-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]