Abstract
Directed catalytic asymmetric hydroborations of 1,1-disubstituted alkenes afford γ-dioxaborato amides and esters in high enantiomeric purity (90–95% ee).
Chiral organoboronates are useful synthetic intermediates for a growing number of stereospecific transformations.1 As such, there is renewed interest in enantioselective methods for their preparation.2,3,4,5,6,7,8,9 We reported advances in the carbonyl-directed catalytic asymmetric hydroboration (CAHB) of (E)- and (Z)-disubstituted and trisubstituted alkenes contained within a β,γ-unsaturated amide framework.10,11 Their rhodium-catalysed reactions employ simple chiral monophosphite ligands to produce β-borylated products regio- and enantioselectively. For example, directed-CAHB of 1 by pinacolborane (pinBH) or 4,4,6-trimethyl-1,3,2-dioxaborinane (tmdBH, 5)12 using Rh(nbd)2BF4 in conjunction with TADDOL-derived phosphite (R,R)-6 gives β-dioxaborato amide (S)-2 in high enantiomeric purity (tmdBH: 79% (96% ee); pinBH: 77% (95% ee), Figure 1). Only trace amounts of the regioisomeric γ-substituted product are formed (<3%).
Figure 1.

Regio- and enantioselective carbonyl-directed CAHB of 1,2- and 1,1-disubstituted alkenes (ee determined after oxidation).
As highlighted in several recent reports,13 1,1-disubstituted alkenes (i.e., methylidene substrates) are particularly challenging substrates for asymmetric hydroboration.14 Directed-CAHB of the methylidene substrate, β,γ-unsaturated amide 3, affords predominantly (R)-4 (tmdBH: 72% (95% ee); pinBH: 68% (60% ee); Figure 1). In contrast to unsaturated amide 1, the isomeric substrate 3 affords the γ-borylated, rather than β-borylated, product predominantly. Equally unexpected, using the same chiral ligand and catalyst, tmdBH adds to opposite faces of the alkene in the isomeric substrates.
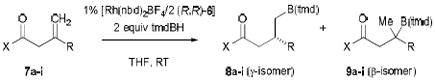
The results obtained for a series of methylidene substrates are summarized in Table 1. Amides 7a–e bearing a primary or secondary alkyl substituent and the phenyl-substituted amide 7f each give their respective γ-borylated product predominantly (i.e., 8a–f, 90–94% ee). Our previous reports of carbonyl-directed CAHB used amide directing groups exclusively (i.e., -C(O)N(H)Ph and -C(O)N(Me)OMe). Here, we find the β,γ-unsaturated tert-butyl esters serve equally well; 7g–i afford γ-borylated esters 8g–i (91–94% ee). Competing alkene reduction and formation of the β-borylated regioisomer 9 account for the remainder of products formed; regiocontrol is most problematic for amide substrates bearing relatively small substituents (i.e., 7a-b, R = Me or Et).
Table 1.
Enantioselective CAHB of 1,1-Disubstituted Alkenes 7a–7i.a
| |||||
|---|---|---|---|---|---|
| 7 | X | R | γ-isomer | % yld (% ee)a | % yld 9b |
| a | NHPh | Me | (S)-8a | 53 (94) | 11 |
| b | NHPh | Et | (R)-8b | 60 (92) | 10 |
| cc | NHPh | (CH2)2Ph | (R)-8c | 73 (94) | 3 |
| dc | NHPh | (CH2)3Ph | (R)-8d | 70 (92) | 3 |
| ec | NHPh | c-C6H11 | (S)-8e | 72 (90) | 2 |
| fc | NHPh | C6H5 | (S)-8f | 71 (93) | 4 |
| g | O-tBu | Me | (S)-8g | 62 (94) | 6 |
| h | O-tBu | Et | (R)-8h | 65 (91) | 5 |
| i | O-tBu | i-Bu | (R)-8i | 78 (91) | 4 |
Isolated yield and (% ee) of 8; enantiomeric purity determined by chiral HPLC analysis after oxidation and for 7g–i subsequent amidation.
Isolated yield of β-isomer 9.
Reaction run at 40 °C.
Trifluoroborate salts are excellent reagents for Suzuki-Miyaura cross-coupling reactions.15 Using Molander’s conditions,8 the γ-borylated amide (S)-8a is cleanly converted to γ-trifluoroborato amide (S)-10a (65%). The latter readily undergoes palladium-catalyzed cross-coupling with several representative aryl halides (70–81%, Table 2 entries 1–3) complementing the cross-couplings of β-borylated carbonyl derivatives.16 The tert-butyl ester derivatives (S)-10g and (R)-10i react similarly (80–94%, entries 4–9). Heteroaromatic cross-couplings are also promising (entries 10–12). For example, trifluoroborate (R)-10h couples to 3-chlorothiophene (80%, entry 13) giving the precursor to a chiral antispasmodic compound previously reported only as the racemate.17
Table 2.
Efficient Cross-Coupling of γ-Borylated Esters and Amides.

| |||
|---|---|---|---|
| Entry | Trifluoroboratea | Aryl halide | % Yield |
| 1 | (S)-10a | chlorobenzene | 81 |
| 2b | (S)-10a | 3-bromoanisole | 71 |
| 3 | (S)-10a | methyl-4-bromobenzoate | 70 |
| 4 | (S)-10g | chlorobenzene | 82 |
| 5 | (S)-10g | 3-bromoanisole | 82 |
| 6 | (S)-10g | methyl-4-bromobenzoate | 88 |
| 7 | (R)-10i | chlorobenzene | 80 |
| 8 | (R)-10i | 3-bromoanisole | 92 |
| 9 | (R)-10i | methyl-4-bromobenzoate | 94 |
| 10 | (R)-10i | 3-chlorothiophene | 98 |
| 11 | (R)-10i | 5-chloro-2-furaldehyde | 84 |
| 12 | (R)-10i | 5-chloro-2-fluoropyridine | 51 |
| 13 | (R)-10h | 3-chlorothiophene | 80 |
Enantiomeric purity of 8 is given in Table 1.
Reaction was run with SPhos in place of RuPhos.
Directed CAHB can also be used to set the stage for intramolecular cross-couplings. CAHB of tert-butyl ester 12 produces (R)-13 (74%, 90% ee, Scheme 1). Subsequent conversion to the corresponding trifluoroborate (76%) followed by palladium-catalyzed intramolecular cross-coupling affords (S)-14 in excellent yield (91%). Alternatively, hydroboration followed by mild oxidation with NaBO3 produces γ-hydroxyester (R)-15 (73%, 90% ee). The latter undergoes palladium-catalyzed C-O cross-coupling18 to afford the novel seven-membered ring ether (R)-16 (75%).
Scheme 1.

(a) 2% Rh(nbd)2BF4, 4.1% (R,R)-6, 2 equiv tmdBH, THF, 40 °C (74%, 90% ee). (b) KHF2, MeCN/H2O (76%). (c) 5% Pd(OAc)2, 10% RuPhos, K2CO3, PhMe/H2O, 85 °C, 24 h (91%). (d) NaBO3, THF/H2O (98%). (e) 5% Pd(OAc)2, 10% RuPhos, K3PO4, PhMe, 85 °C, 24 h (75%).
Directed CAHB of amides 3 and 7c using (R,R)-6 followed by oxidative work-up with basic H2O2 gives the respective γ-hydroxyamides (R)-17 and (R)-18 (71% yield for each, 95 and 94% ee, respectively) (Figure 2). Similarly, CAHB of tert-butyl ester 7i followed by mild oxidation with NaBO3 affords the labile γ-hydroxyester (R)-19 (77%, 91% ee). However, under basic H2O2 work-up conditions, the intermediate γ-hydroxyester spontaneously lactonizes to afford a chiral β-substituted γ-lactone. For example, CAHB of tert-butyl ester 7i using ligand (S,S)-6 affords lactone (S)-20 (78%, 91% ee); the latter has been used as a precursor to the anticonvulsant drug pregabalin.19 Similarly, tert-butyl ester 21a gives (R)-22a (80%, 95% ee) and 21b gives (R)-22b (79%, 95% ee) using the catalyst with (R,R)-6. With the enantiomeric ligand (i.e., (S,S)-6),CAHB-oxidation of 21c affords (S)-22c (75%, 92% ee). β-Substituted butyrolactones undergo diastereoselective alkylation and have been used in syntheses of the lignan natural products (-)-enterolactone and (+)-arctigenin.20
Figure 2.

Preparation of chiral γ-hydroxy amides and esters and β-substituted γ-lactones via CAHB-oxidation.
The relative energies of a series of octahedral intermediates formed upon two-point binding of amide substrates followed by oxidative addition of borane were evaluated by DFT (Figure 3, 23A/C and 24B/D).21 The modelled structures employ the symmetric borane pinBH and the caged phosphite P(OCH2)3CH to simplify the calculations. In line with experiment, the overall lowest energy structure for the model methylidene substrate is consistent with favored formation of the γ-borylated product upon alkene insertion into the Rh-H bond (i.e., A); the lowest energy structure leading to β-borylation (i.e., B) is calculated to be about 1.9 kcal higher in energy and arises from complexation to the opposite face of the π-system. Structures C and D model a simple 1,2-disubstituted alkene with with the (E)-geometry (i.e., a model for amide 1). Consistent with the experimental observations, the β-leading isomer D is favored for this substitution pattern.
Figure 3.

Relative energies of model octahedral intermediates leading to γ- and β-borylation for 1,1- and (E) 1,2-disubstituted substrates (A/B and C/D, respectively; only the vinyl C-H and Rh-H included for clarity.
In contrast to tri- and other disubstitution patterns, CAHBs of β,γ-unsaturated methylidene amides and esters afford the γ-borylated product and proceed with the opposite sense of asymmetric induction. Chiral γ-borylated derivatives are intermediates for inter- and intramolecular cross-couplings, the formation of chiral γ-hydroxy carbonyl derivatives, and β-substituted-γ-lactones. Preliminary computational studies suggest that the preferred conformation of the chelated substrate relative to the Rh-H bond may explain the observed regio- and π-facial selectivity. Further studies are in progress.
Supplementary Material
Acknowledgments
Financial support from the NSF (CHE-0809637) and NIH (GM100101) is gratefully acknowledged. We thank D. Boadwine for optimizing the reactions leading to 22.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/.
Notes and references
- 1.a) Scott HK, Aggarwal VK. Chem Eur J. 2011;17:13124. doi: 10.1002/chem.201102581. [DOI] [PubMed] [Google Scholar]; b) Crudden CM, Glasspoole BW, Lata CJ. Chem Commun. 2009:6704. doi: 10.1039/b911537d. [DOI] [PubMed] [Google Scholar]
- 2.Catalytic asymmetric diboration of dienes and alkenes: Kliman LT, Mlynarski SN, Ferris GE, Morken JP. Angew Chem Int Ed. 2012;51:521. doi: 10.1002/anie.201105716.; Schuster CH, Li B, Morken JP. Angew Chem Int Ed. 2011;50:7906. doi: 10.1002/anie.201102404.; Hong K, Morken JP. J Org Chem. 2011;76:9102. doi: 10.1021/jo201321k.; Burks HE, Morken JP. Chem Commun. 2007:4717. doi: 10.1039/b707779c.; and references cited therein.
- 3.Catalytic asymmetric hydroboration of dienes and vinyl arenes: Ely RJ, Morken JP. J Am Chem Soc. 2010;132:2534. doi: 10.1021/ja910750b.; Sasaki Y, Zhong C, Sawamura M, Ito H. J Am Chem Soc. 2010;132:1226. doi: 10.1021/ja909640b.; Noh D, Chea J, Ju J, Yun J. Angew Chem Int Ed. 2009;48:6062. doi: 10.1002/anie.200902015.; Moteki SA, Toyama K, Liu Z, Ma J, Holmes AE, Takacs JM. Chem Commun. 2012;48:263. doi: 10.1039/c1cc16146f.; Moteki SA, Takacs JM. Angew Chem Int Ed. 2008;47:894. doi: 10.1002/anie.200703127.; Moteki SA, Wu D, Chandra KL, Reddy DS, Takacs JM. Org Lett. 2006;8:3097. doi: 10.1021/ol061117g.
- 4.Catalytic asymmetric conjugate boration of α,β-unsaturated systems: Cid J, Gulyás H, Carbó JJ, Fernández E. Chem Soc Rev. 2012;41:3558. doi: 10.1039/c2cs15291f.
- 5.Catalytic asymmetric conjugate additions to vinyl boronates: Jung H–Y, Feng X, Kin H, Yun J. Tetrahedron. 2012;68:3444.; Lee JCH, Hall DG. J Am Chem Soc. 2010;132:5544. doi: 10.1021/ja9104057.; Sasaki K, Hayashi T. Angew Chem Int Ed. 2010;49:8145. doi: 10.1002/anie.201004980.
- 6.Catalytic asymmetric hydrogenation of vinyl boronates: Smilović IG, Casas-Arcé E, Roseblade SJ, Nettekoven U, Zanotti-Gerosa A, Kovačevič M, Časar Z. Angew Chem Int Ed. 2012;51:1014. doi: 10.1002/anie.201106262.; Paptchikkine A, Cheruku P, Engman M, Andersson PG. Chem Commun. 2009:5996. doi: 10.1039/b912590f.; Moran WJ, Morken JP. Org Lett. 2006;8:2413. doi: 10.1021/ol060735u.
- 7.Stoichiometric asymmetric hydroboration: González JR, González AZ, Soderquist JA. J Am Chem Soc. 2009;131:9924. doi: 10.1021/ja9047202.; Muñoz-Hernández L, Soderquist JA. Org Lett. 2009;11:2571. doi: 10.1021/ol900865y.
- 8.Asymmetric alkylation: Molander GA, Shin I, Jean-Gérard L. Org Lett. 2010;12:4384. doi: 10.1021/ol101865e.
- 9.Enantioselective metalation: Webster MP, Aggarwal VK. In: Boronic Acids. Hall DG, editor. Vol. 2. Wiley-VCH Verlag GmbH and Co.; Weinheim: 2011. p. 479.
- 10.Smith SM, Uteuliyev M, Takacs JM. Chem Commun. 2011;47:7812. doi: 10.1039/c1cc11746g. [DOI] [PubMed] [Google Scholar]; Smith SM, Takacs JM. J Am Chem Soc. 2010;132:1740. doi: 10.1021/ja908257x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith SM, Takacs JM. Org Lett. 2010;12:4612. doi: 10.1021/ol101932q. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith SM, Thacker NC, Takacs JM. J Am Chem Soc. 2008;130:3734. doi: 10.1021/ja710492q. [DOI] [PubMed] [Google Scholar]
- 11.See also: Rubina M, Rubin M, Gevorgyan V. J Am Chem Soc. 2003;125:7198. doi: 10.1021/ja034210y.
- 12.The chirality of tmdBH was shown to be inconsequential, see ref 10.
- 13.Corberán R, Mszar NW, Hoveyda AH. Angew Chem Int Ed. 2011;50:7079. doi: 10.1002/anie.201102398. [DOI] [PubMed] [Google Scholar]; Mazet C, Gérard D. Chem Commun. 2011;47:298. doi: 10.1039/c0cc01547d. [DOI] [PubMed] [Google Scholar]; Gonzalez AZ, Román JG, Gonzalez E, Martinez J, Medina JR, Matos K, Soderquist JA. J Am Chem Soc. 2008;130:9218. doi: 10.1021/ja803119p. [DOI] [PubMed] [Google Scholar]
- 14.Thomas SP, Aggarwal VK. Angew Chem Int Ed. 2009;48:1896. doi: 10.1002/anie.200805604. [DOI] [PubMed] [Google Scholar]
- 15.Tobisu M, Chatani N. Angew Chem Int Ed. 2009;48:3565. doi: 10.1002/anie.200900465. [DOI] [PubMed] [Google Scholar]; Molander GA, Ellis N. Acc Chem Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; Darses S, Genet J–P. Eur J Org Chem. 2003:4313. [Google Scholar]
- 16.Sandrock DL, Jean-Gérard L, Chen C–y, Dreher SD, Molander GA. J Am Chem Soc. 2010;132:17108. doi: 10.1021/ja108949w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Awano T, Ohmura T, Suginome M. J Am Chem Soc. 2011;133:20738. doi: 10.1021/ja210025q. [DOI] [PubMed] [Google Scholar]; Ohmura T, Awano T, Suginome M. J Am Chem Soc. 2010;132:13191. doi: 10.1021/ja106632j. [DOI] [PubMed] [Google Scholar]
- 17.Blicke FF, Leonard F. J Am Chem Soc. 1946;68:1934. doi: 10.1021/ja01214a019. [DOI] [PubMed] [Google Scholar]
- 18.Kuwabe S–i, Torraca KE, Buchwald SL. J Am Chem Soc. 2001;123:12202. doi: 10.1021/ja012046d. [DOI] [PubMed] [Google Scholar]
- 19.Ok T, Jeon A, Lee J, Lim JH, Hong CS, Lee H–S. J Org Chem. 2007;72:7390–7393. doi: 10.1021/jo0709605. [DOI] [PubMed] [Google Scholar]
- 20.Bode JW, Doyle MP, Protopopova MN, Zhou W–L. J Org Chem. 1996;61:9146. [Google Scholar]
- 21.Computational studies were performed using density functional theory (DFT) implemented in the ab-initio package Gaussian09. B3LYP functional along with 6-31+G(d,p) (all non-metal atoms) and LANL2DZ (Rh) basis sets were employed. Frequency calculations were performed at the same level of theory for each of the optimized structures to check for the absence of any imaginary frequencies. See supporting information for more details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


