Abstract
Fluorescent microbubbles have been fabricated with the capacity to have their emission modulated by ultrasound. These contrast agent particles could potentially be used in the future to extract fluorescence modulation from a strong light background to increase imaging depth and resolution in scattering media. Fluorescence intensity modulation was demonstrated at the ultrasound driving frequency.
Despite over half a century of research validating the link between tissue microenvironment and cancer malignancy, we have yet to develop a practical screening method to detect these distinct biochemical states1, 2. Types of tissue have specific optical absorption and scattering properties which can modify the spectrum of an optical signal. Additionally, contrast agents can be tuned to provide information about local tissue properties by the inclusion of fluorophores whose emission wavelength and intensity are sensitive to the environment3. The spectrum of the detected fluorescence can inform about relative tissue oxygenation, pH, and other properties which can be disease indicators4. Although fluorescent probes have been developed to efficiently sense hypoxia, optical tissue scattering has been a barrier to their clinical translation5–9. A hybrid modality of ultrasound (US) and fluorescence imaging can break this barrier by exploiting their individual strengths of spatial resolution and sensitivity, with the added benefits of being non-ionizing, low cost, and real-time. While traditional acousto-optic imaging has these benefits and an optical readout, it lacks the selectivity to modulate fluorophores independently of the autofluorescent tissue background10, 11.
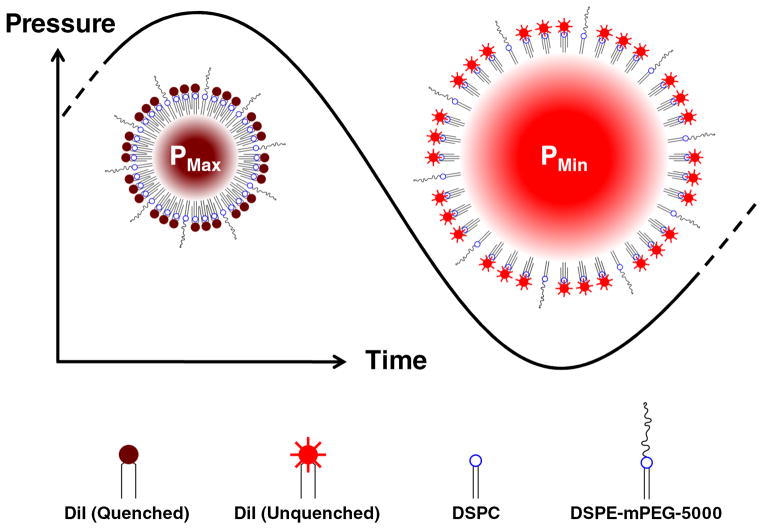
An US-modulated beacon of light has the potential to enable optical detection at the spatial resolution of US (~1 mm), beyond sub-millimeter depths12. The sensitivity of fluorescence will allow the discrimination of a small signal of interest from a large background. Since random noise and autofluorescence photons will not fluctuate with any characteristic frequency, they can be removed by filtering or spectral analysis. The underlying technology developed in this work is a microbubble (MB), surface-loaded with a fluorophore at sufficiently high concentration to exhibit self-quenching. The MB oscillates radially in response to US insonation, altering the intermolecular spacing on the surface (Fig. 1). In the high pressure portion of the wave, the bubble compresses, increasing the surface density of fluorophore, and the degree of fluorescence quenching. In the low pressure portion of the wave, the bubble expands, decreasing the surface density of the fluorophore, and also decreasing the amount of quenching. Thus, the degree of quenching is modulated at the frequency of the applied US.
Fig. 1.
Schematic of Principle. A microbubble (MB) experiences radial oscillations in response to acoustic (pressure) waves. The surface concentration of the dye (DiI) is inversely proportional to the square of the bubble radius. Due to the non-linear distance dependence of dye quenching efficiency, a small increase in intermolecular distance can produce a large increase in the fluorescence output.
B. Yuan has provided a theoretical description of a similar system using a fluorophore-quencher pair13, 14, predicting a possible 100% shift fluorescence intensity. The MB structure presented in this work is achieved by incorporating a single dye component into a stabilizing phospholipid monolayer, to instead operate by a self-quenching mechanism. While the concept of this particle has been previously modeled theoretically in the literature, we present the first experimental evidence of dye-loaded MBs whose fluorescence emission can be modulated in response to US.
MBs coated with the lipophilic carbocyanine fluorophore, DiI and biodegradable phospholipids were fabricated using variations on published formulations15, 16 similar to those used in Definity®, an FDA approved US contrast agent. As micron-scale agents, MBs are cleared rapidly and are too large to extravasate, so they are used to highlight the blood pool. A detailed preparation procedure is described in the Supplementary Information. A gas mixture containing perfluorocarbon was used as the MB core due to the perfluorocarbon’s inertness and low solubility in blood, which helps prolong MB in vivo biological half-life17. MBs were easily purified from excess lipid and dye by centrifugation due to their buoyancy. Multiple washings yielded a very pure sample while preserving the MBs (Fig. 2a,b). The fabricated MBs ranged between 1–5 μm in diameter, optimal to achieve resonance with medical US frequencies of 1–10 MHz18. The MBs were strongly echogenic, as verified by a clinical ultrasound scanner, which was able to observe a strong contrast enhancement at a 1:1000 dilution (Fig. 2d).
Fig. 2.
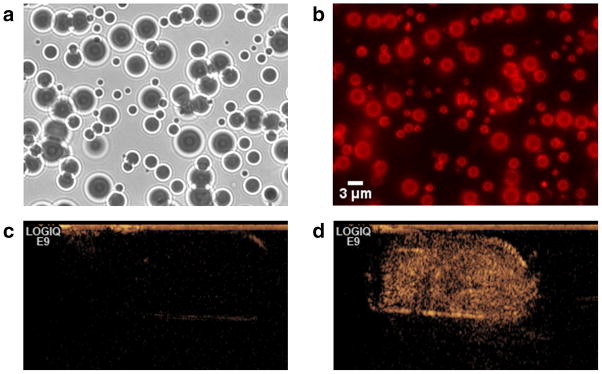
Microbubbles surface-loaded with a high concentration of DiI (2 mol %), imaged in (a) brightfield, (b) fluorescence, and (d) ultrasound contrast-mode. (a) Brightfield microscope images show microbubbles with a 1-5 μm distribution. (b) Although fluorescence quenching is occurring to an extent, residual fluorescence can still be seen. Even unloaded MBs (not shown here) will appear as a bright ring in a fluorescent medium, due to reflection off the gas/liquid boundary, but fluorescence from the center of the bubble is evidence of surface-loaded dye. (c) A saline buffer control shows a faint outline of the transfer pipette container with no contrast enhancement. (d) A 1:1000 dilution of microbubbles in saline buffer generated a strong contrast enhancement.
MBs were fabricated with varying concentrations of DiI and relative fluorescence intensity of individual MBs was quantified by fluorescence microscopy (Fig. 3). The average fluorescence emitted from a molecule of DiI decreases with increased surface concentrations, following a trend characteristic of self-quenching. The self-quenching of DiI is a well-documented dynamic quenching mechanism, evidenced by a change in fluorescence lifetime at increasing concentrations19. For the relationship between quenching efficiency and intermolecular distance, there was good agreement between experimental results and a theoretical curve developed from a FRET efficiency model. Many similar carbocyanine dyes exist at longer wavelengths, but the excitation and emission spectra of DiI were ideally matched with light sources and detectors available to us.
Fig. 3.
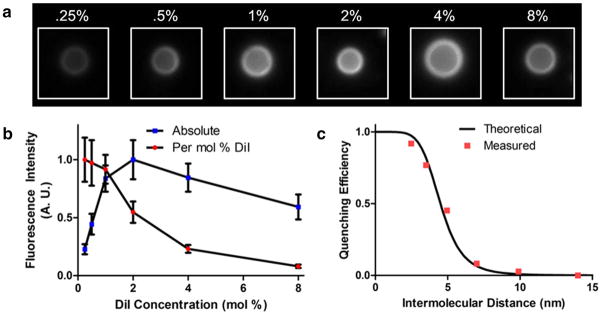
Self-quenching of DiI loaded on the surface of MBs (0.25-8 mol%). (a) Representative MBs loaded with increasing dye concentrations were imaged, and the fluorescence intensity per unit area was calculated. (b) The normalized fluorescence intensity is plotted against DiI concentration, both in absolute intensity, and adjusted for DiI concentration. (c) Quenching efficiency was plotted, assuming the complete absence of quenching for the lowest concentration, and shown with a theoretical quenching curve. DiI intermolecular distance was calculated based on molar concentration. See the Supplementary Information for a more detailed description of the theoretical calculation and measured values.
It has been shown that in response to US the radius of a lipid-stabilized MB can change by a factor of 5 or more from its most compressed state to its most expanded state20. The efficiency of dynamic quenching has a nonlinear dependence on distance, which would suggest that even a much smaller oscillation could result in a large change in fluorescence emission21. The results shown in Figure 3b predict a concentration of 2 mol % to be in the optimal range, since it is there that the slope of fluorescence intensity vs. dye concentration is highest.
An in-house designed high-throughput MB characterization setup22 was modified to detect US-modulated fluorescence as described in the Supplementary Information. In brief, a Verdi-V5 continuous-wave 532nm laser source (Coherent Inc., Santa Clara, CA, USA) was focused into a 2 liter black water bath through a transparent optical window. A 2.25 MHz focused single-element Panametrics V306 ultrasound transducer (Olympus NDT Inc., Waltham, MA, USA) was aligned with its focus confocal with the laser focus downstream of the sample infusion tube. The US was pulsed at a 1Hz repetition rate, with 15 cycles per pulse at 2.25MHz, which was within the resonance frequency range of the 1–5 μm MBs. Fluorescence from oscillating microbubbles was detected by a R6095 PMT optical detector (Hamamatsu Corp., Bridgewater, NJ, USA) operated at high gain.
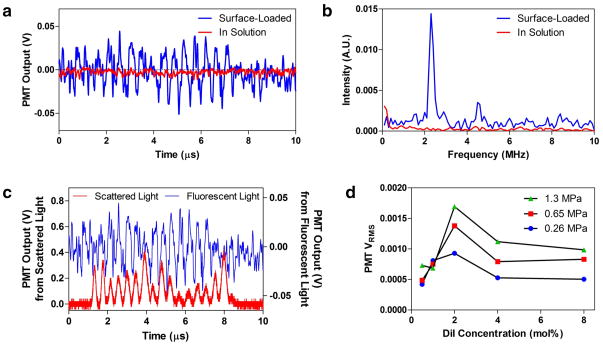
Experiments were conducted in deionized water and no additional scattering media was added. A representative voltage output of the photomultiplier tube (PMT) detecting MB fluorescence emission is shown in Figure 4a as a function of time. There is a very clear time domain oscillation with the corresponding Fourier transform (Fig. 4b) revealing a strong component at the US driving frequency of 2.25 MHz. Second harmonic frequency signatures at 4.5 MHz were also detected on occasion, indicating non-linear effects stemming from known asymmetric oscillations of lipid MBs23, 24. Peak-to-peak voltage was approximately 65mV, yielding a signal-to-noise ratio (SNR) of 3.68 for the single 15-cycle pulse.
Fig. 4.
Demonstration of fluorescence modulation with US. (a-c) Microbubbles loaded with 2 mol% DiI were excited with 15 cycles at 2.25 MHz while a CW laser excited their fluorescence. (a) Voltage output from the PMT which is detecting the fluorescence. (b) FFT of the time-domain signal showing a strong peak at the fundamental and a smaller peak at the second harmonic. Non-fluorescent microbubbles were diluted into a fluorescent medium as a control, which showed no fluorescence modulation. (c) Simultaneous acquisition of fluorescent and scattered light from a sample of MBs. Two PMTs were used to independently detect the signals, which were observed to be in phase. This demonstrates that the fluorescent signal is directly correlated with the contraction and expansion of the MBs. The US peak-to-peak pressure used was 1.3 MPa. (d) Mean modulation intensity at different dye concentrations and US intensities. MBs were fabricated with varying surface concentrations of fluorophore. For a population of detection events, the mean modulation intensity was taken as a measure of the signal strength. With too little fluorophore, there is not much quenching. With too much fluorophore, there is too much quenching. An optimal concentration was determined to be around 2 mol% of the stabilizing lipid shell.
The proposed mechanism could be confounded by the ability of MBs to amplify the acousto-optic modulation of fluorescence, as has been previously shown in the literature25. To control for this effect, fluorescent liposomes were added to a sample of MBs prepared without the fluorophore coating, to achieve the same bulk fluorescence intensity as in the surface-loaded MBs. Under the same conditions, no fluorescence oscillations were seen in the control samples. This supports that the observed fluorescence modulation is due to the hypothesized self-quenching effect as opposed to modulation of the excitation beam or a change in the optical properties of the MB. MBs without any additional fluorescence also failed to produce any modulation of the detected light.
The experimental setup described in the Supplementary Information was also capable of monitoring US-induced size oscillations of MBs through the detection of scattered light26. Since the light was collected orthogonal to the incident laser, unfiltered light was representative of the side scatter intensity, which has a strong dependence on particle size. By observing the radial oscillations of individual MBs, this method verified that oscillating MBs were present in the control samples.
A second detector was used to observe the oscillations of the scattered light at the same time as the fluorescence oscillations by inserting a beamsplitter, and filtering only one path (Fig. A, Supplementary Information). Peaks in fluorescence were in phase with peaks in the scatter signal (Fig. 4c). This was expected since in the rarefaction phase, when the bubble diameter is the greatest, the bubble will scatter more light and the fluorophore molecules should be most separated, giving the greatest fluorescence intensity. Although the amount of scattered light increases and decreases with the MB oscillations, the increase is far greater than the decrease due to the nonlinear dependence of scatter intensity on object size. This is a result of Mie scattering, which shows that as a MB increases in size, the cross section and scatter intensity increase approximately with the square of the diameter27, 28. The scattered light modulation was seen consistently and prevalently in all samples containing microbubbles. However, the fluorescence modulation signal was only observed for a subset of these microbubbles, all of which were fluorescent (Fig. 2b), and was not always present for those bubbles which generated the largest scattered light modulations. These observations suggest that the fluorescence modulations are produced by the ability of a distinct population of microbubbles to undergo the above described fluorescence quenching of surface dyes.
As the MB dye concentration was varied, it was shown that the signal intensity was dependent on the surface concentration of the fluorophore (Figure 4d). An optimal concentration was found near 2 mol% where the transition of the MB from fully contracted to fully expanded corresponds with the greatest change in degree of quenching. Insufficient fluorophore concentration resulted in weakly quenched MBs, even when fully contracted. Similarly, MBs containing very high concentrations of fluorophore were always overly quenched. The signal strength also increased with US intensity, even to a peak negative pressure of 0.5 MPa (1.3 MPa peak-to-peak) where the pressure was sufficiently large to destroy some of the MBs in the first 2 cycles. Still, most MBs were able to stably oscillate for more than 10 cycles at this pressure.
The working principle of the MBs studied here relies on the relatively uniform and elastic expansion of the MB shell. If shell “cracking” occurs, where the lipid monolayer splits open in a region instead of stretching uniformly, a much less pronounced effect would be expected, since most of the fluorophores will remain closely packed. This is likely to happen for MBs stabilized with lipids forming domains, segregated regions of certain lipid molecules29. Therefore, solubility of the dye in the lipid monolayer is key to maintaining a homogeneous distribution. Also, phase segregation in stabilizing lipid monolayers is more present in formulations containing a significant amount of lipopolymer30, 31. For this reason, the amount of lipopolymer in our formulation was limited to the minimum required for stability. For neutrally charged MBs, a certain amount of polymer (typically polyethylene glycol) is often used for steric repulsion, in order to prevent coalescence and gas dissolution and maintain a stable MB suspension32.
The insonation frequency also has a role in the design of a fluorescence-modulating MB, since it has an impact on the velocity of molecular separation. Physically separated lipid molecules in water are not in a thermodynamically stable state. If given time, they will rearrange to pack closely and minimize the exposure of their hydrophobic regions. For this reason, a MB or emulsion suspension made with limited surfactant is unlikely to have sparsely-coated droplets. Instead, there will either be fewer number of large droplets, or more likely, the particles will coalesce to reduce the surface-to-volume ratio until there is a small enough surface area which can be completely coated. By using MHz frequencies, the lipid molecules are forced to separate and come back together in under a microsecond. This study indicates that this time is insufficiently long for them to rearrange, since the basic mechanism of modulation is dependent on molecular separation.
Conclusion
Stable MBs were fabricated and surface-loaded with a lipophilic self-quenching dye. These MBs were demonstrated to modulate their fluorescence emission in response to applied US. While many known methods use acoustic waves to modulate the paths of existing photons, to our knowledge this is the first experimental demonstration of US modulation of the number of emitted fluorescent photons. Therefore, the acoustic insonation is changing the ratio of excitation energy converted into radiative vs. non-radiative energy.
The US-modulated fluorescent emission of the MB will enable the extraction of optical information at the higher spatial resolution possible with US. A promising potential application of this technique is to use the optical spectral information detected to determine biochemical properties of the tissue being probed. A clinically relevant future approach will be to design modulating MBs utilizing near-infrared fluorophores to enhance signal-to-background ratios and increase light penetration depth through the body. The system described in this work has the potential to be used in conjunction with the above-mentioned environmental biochemical sensors, and to allow spatially precise measurements of deep tissue properties.
Supplementary Material
Acknowledgments
This study was supported in part by NCI-5U54CA119335-05, ICMIC P50-CA128346, and NIH R25-CA153915, DOD Army PCRP IDA (W81XWH-07-1-0125). The authors thank Emilia Olson for help with ultrasound imaging of microbubbles.
Notes and references
- 1.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The British journal of radiology. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 2.Seton-Rogers S. Nature reviews Cancer. 2012;12:320–321. doi: 10.1038/nrc3267. [DOI] [PubMed] [Google Scholar]
- 3.Niu CG, Gui XQ, Zeng GM, Yuan XZ. Analyst. 2005;130:1551–1556. doi: 10.1039/b504882f. [DOI] [PubMed] [Google Scholar]
- 4.Helmlinger G, Yuan F, Dellian M, Jain RK. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy AKM, MacCraith CM, BD Analyst. 1996;121:785–788. [Google Scholar]
- 6.Zhong WU, Mycek PMA. Journal of Physics D: Applied Physics. 2003;36:1689–1695. [Google Scholar]
- 7.Kiyose K, Hanaoka K, Oushiki D, Nakamura T, Kajimura M, Suematsu M, Nishimatsu H, Yamane T, Terai T, Hirata Y, Nagano T. Journal of the American Chemical Society. 2010;132:15846–15848. doi: 10.1021/ja105937q. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Palmer GM, Dewhirst MW, Fraser CL. Nature materials. 2009;8:747–751. doi: 10.1038/nmat2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda K, Okabe Y, Kadonosono T, Ueno T, Youssif BG, Kizaka-Kondoh S, Nagasawa H. Bioconjugate chemistry. 2012;23:324–329. doi: 10.1021/bc2004704. [DOI] [PubMed] [Google Scholar]
- 10.Wang LV. Disease markers. 2003;19:123–138. doi: 10.1155/2004/478079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan B, Gamelin J, Zhu Q. J Appl Phys. 2008;104:103102. doi: 10.1063/1.3021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farahi S, Benoit E, Grabar AA, Huignard J-P, Ramaz F. Opt Lett. 2012;37:2754–2756. doi: 10.1364/OL.37.002754. [DOI] [PubMed] [Google Scholar]
- 13.Yuan B. J Biomed Opt. 2009;14:024043. doi: 10.1117/1.3120493. [DOI] [PubMed] [Google Scholar]
- 14.Yuan B, Uchiyama S, Liu Y, Nguyen KT, Alexandrakis G. Applied Physics Letters. 2012;101:033703-033703-033705. doi: 10.1063/1.4737211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabalnov A, Bradley J, Flaim S, Klein D, Pelura T, Peters B, Otto S, Reynolds J, Schutt E, Weers J. Ultrasound Med Biol. 1998;24:751–760. doi: 10.1016/s0301-5629(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 16.Sirsi S, Borden M. Bubble Sci Eng Technol. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutt EG, Klein DH, Mattrey RM, Riess JG. Angew Chem Int Ed Engl. 2003;42:3218–3235. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 18.Phelps ADL, TG Acta Acustica united with Acustica. 1997;83:59–66(58). [Google Scholar]
- 19.Packard BS, Wolf DE. Biochemistry. 1985;24:5176–5181. doi: 10.1021/bi00340a033. [DOI] [PubMed] [Google Scholar]
- 20.Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibaov AL, Ferrara KW. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 21.Haigh EA, Thulborn KR, Sawyer WH. Biochemistry. 1979;18:3525–3532. doi: 10.1021/bi00583a014. [DOI] [PubMed] [Google Scholar]
- 22.Hsu MJ, Eghtedari M, Goodwin AP, Hall DJ, Mattrey RF, Esener SC. J Biomed Opt. 16:067002. doi: 10.1117/1.3583575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crum LA, Prosperetti A. Journal of the Acoustical Society of America. 1983;73:121–127. [Google Scholar]
- 24.Shi WT, Forsberg F. Ultrasound Med Biol. 2000;26:93–104. doi: 10.1016/s0301-5629(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 25.Yuan B, Liu Y, Mehl PM, Vignola J. Applied Physics Letters. 2009:95. [Google Scholar]
- 26.Hall DJ, Hsu MJ, Esener SC, Mattrey RF. SPIE Proceedings. 2009:7177. [Google Scholar]
- 27.Dean CE, Marston PL. Appl Opt. 1991;30:4764–4776. doi: 10.1364/AO.30.004764. [DOI] [PubMed] [Google Scholar]
- 28.Guan J, Matula TJ. J Acoust Soc Am. 2004;116:2832–2842. doi: 10.1121/1.1795334. [DOI] [PubMed] [Google Scholar]
- 29.Borden MA, Kruse DE, Caskey CF, Zhao S, Dayton PA, Ferrara KW. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1992–2002. doi: 10.1109/tuffc.2005.1561668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borden MA, Martinez GV, Ricker J, Tsvetkova N, Longo M, Gillies RJ, Dayton PA, Ferrara KW. Langmuir. 2006;22:4291–4297. doi: 10.1021/la052841v. [DOI] [PubMed] [Google Scholar]
- 31.Borden MA, Pu G, Runner GJ, Longo ML. Colloids Surf B Biointerfaces. 2004;35:209–223. doi: 10.1016/j.colsurfb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Klibanov AL. Topics in Current Chemistry. 2002;222:73–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.