Abstract
To better understand the roles of γδ T cells in mucosal infection, we utilized Salmonella enterica serovar Typhimurium (Salmonella serovar Typhimurium) infection in cattle as it closely approximates Salmonella serovar Typhimurium-induced enterocolitis in humans. Protein and gene expression in αβ and γδ T cells derived from lymphatic ducts draining the gut mucosa in Salmonella serovar Typhimurium infected calves were analyzed. In calves with enterocolitis, general gene expression trends in γδ T cells suggested subtle activation and innate response, whereas αβ T cells were relatively quiescent following Salmonella serovar Typhimurium infection. An increase in IL-2Rα expression on γδ T cells from infected calves and results from in vitro assays suggested that γδ T cells were primed by Salmonella serovar Typhimurium LPS to better respond to IL-2 and IL-15. Together with gene expression trends in vivo, these data support early priming activation of target tissue γδ T cells during Salmonella serovar Typhimurium infection.
Keywords: γδ T cells, mucosal, IL-2Rα, cell proliferation, Salmonella serovar Typhimurium, gene expression, lymphatic, priming
Introduction
Depending on the serotype and host, Salmonella can cause bacteremia, typhoid fever or enterocolitis. Bacteremia induced by infection with Salmonella enterica serovars Choleraesuis (swine adapted) and Dublin (bovine adapted) is relatively rare in humans [1]. Typhoid fever caused by Salmonella enterica serovar Typhi in humans is virtually eradicated in the United States, but remains a problem in regions of the developing world with poor sanitation practices [1]. In contrast, Salmonella-induced enterocolitis in humans, primarily caused by Salmonella enterica serovar Typhimurium (Salmonella serovar Typhimurium), is extremely common and is the most common cause of death by food-borne illness in the U.S. [1,2]. The role of T cells in infection with Salmonella species has largely been investigated in mice [3]. However, while infection of mice with Salmonella serovar Typhimurium is an excellent model for human typhoid fever, it does not induce enterocolitis [1,3]. In contrast, Salmonella serovar Typhimurium infection in calves induces enterocolitis that closely mirrors disease in humans and is also the most common Salmonella strain associated with ill cattle in the United States [1].
γδ T cells localize to the gut mucosa in all animals, including humans, express the γδ TCR of limited diversity, recognize unprocessed antigen, and respond rapidly to infection, in part by rapid recruitment to infected sites [4,5]. Whereas αβ T cells appear to be more important than γδ T cells in the clearance of Salmonella serovar Typhimurium in the typhoid fever model in mice [1,3], the roles of γδ T cells in the gut mucosa of a highly relevant model of enterocolitis have not been investigated. Since γδ T cells localize to the gut mucosa and are thought to participate in innate immune responses, they are likely involved in early protective responses to Salmonella serovar Typhimurium infection [6]. The phenotype of T cell populations in blood, peritoneal fluid, or lymphoid tissues, (the sources of lymphocytes in several murine and human investigations [3,7,8]), does not necessarily reflect that of those responding to initial infection in the target tissue. We hypothesized that γδ T cells derived from the gut mucosal lymphatic ducts provide an early innate response during Salmonella serovar Typhimurium enterocolitis. To address this hypothesis, cells in lymphatic fluid draining from the intestine were collected at early intervals during Salmonella serovar Typhimurium-induced enterocolitis in naïve bovine calves. T cells were analyzed by FACS and microarray to determine their protein and gene expression phenotypes. Among other indicators of activation, IL-2 receptor α (IL-2Rα transcripts and protein increased in expression on γδ T cells during Salmonella serovar Typhimurium infection in vivo whereas αβ T cells appeared minimally stimulated. In an in vitro functional assay, highly purified γδ T cells stimulated with Salmonella serovar Typhimurium LPS had enhanced proliferation in response to IL-2 and IL-15. Our results indicate an early priming activation of γδ T cells, relative to a less dramatic early role of αβ T cells in an infrequently studied but highly relevant model of Salmonella serovar Typhimurium induced enterocolitis.
Materials and Methods
Surgery and experimental infection
Four to six week old calves with no evidence of prior Salmonella infection were used for the surgery and infection. Catheters were inserted into the mesenteric efferent lymphatic vessel following standard surgical procedures. Calves recovered from surgery for approximately 20 hours, then the time 0 lymphatic fluid and blood were collected just prior to infection with 4.7 × 107 to 4 × 108 CFUs of a natural calf isolate of Salmonella serovar Typhimurium (Type O, Group B) or mock infection with an equivalent volume of sterile Luria broth (LB) media. Prior to infection, 100 μl of Salmonella serovar Typhimurium stock was added to 5ml of LB media and shaken for 6 hours at 37°. Bacterial cell growth was determined by absorbance reading and comparison to a standard growth curve for the same strain. CFU counts were verified by plating serial dilutions of bacterial suspensions on LB agar plates. Blood and lymphatic fluid were collected at intervals post infection during which the calves were closely monitored. Enterocolitis was defined as fever (>105° F) and diarrhea between 24 and 48 hours post infection. At this time fecal samples were submitted to the Montana State Veterinary Diagnostic Service and those from experimentally infected calves tested positive for the input strain of Salmonella serovar Typhimurium and negative for other infectious agents. All animal protocols were reviewed and approved by the MSU Institutional Animal Care and Use Committee.
Flow Cytometry and FACS
RBCs in whole blood were lysed in ACK (0.15M ammonium chloride, 1mM potassium carbonate, 0.1mM EDTA disodium salt) buffer and lymphatic fluid cells were washed twice with PBS with 2% horse serum. A small volume of cells from blood and lymphatic fluid was stained with the following mouse anti-bovine antibodies (specificity): GD3.8 (pan γδ T cell), ILA29 (WC1, γδ T cell subset), CC21 (B cells), CC42 (CD2, αβ T cells and γδ T cell subset), BN180 (monocytes), BN115 (neutrophils), CACT116A (IL-2Rα; VMRD Inc., Pullman, WA). Flow cytometry was performed following standard protocols as previously described [9]. Because the changes in cell percentages between 0 and 6 hours post infection were highly variable, and were potentially residual fluctuations that occurred post surgery, cell percentages at these two time points were averaged to arrive at a new time 0 post infection value represented on the graphs.
For one mock infection (calf 156) and two experimental Salmonella serovar Typhimurium infections (calves 112 and 162), the lymphatic cells were stained with GD3.8 directly conjugated to FITC, washed, and sorted on a Vantage SE cell sorter (BD Immunocytometry Systems) as previously described [9]. Percent purities of the sorted cells were as follows: calf 112; 0 hour αβ T cell 81%, 0 hour γδ T cell 97%, 6 hour αβ T cell 85%, 6 hour γδ T cell 93%, 24 hour αβ T cell 78%, 24 hour γδ T cell 96%, 48 hour αβ T cell 89%, 48 hour γδ T cell 97%, 72 hour αβ T cell 95%, 72 hour γδ T cell 91%, calf 156; 0 hour αβ T cell 98%, 0 hour γδ T cell 89%, 48 hour αβ T cell 82%, 48 hour γδ T cell 89%, calf 162; 0 hour αβ T cell 87%, 0 hour γδ T cell 88%, 48 hour αβ T cell 81%, 48 hour γδ T cell 86%. The αβ T cell populations were contaminated by a mixture of cells, mainly B cells and a few γδ T cells, whereas γδ T cells were mainly contaminated with αβ T cells. Sorted γδ and αβ T cells were directly lysed in TRIzol reagent (Invitrogen; calf 112) or in Buffer RLT (Qiagen; calves 156 and 162) and genomic DNA sheared using Qiashredder columns, then frozen at −80°C.
RNA extraction, amplification, and microarrays
RNA was extracted following the manufacturer's protocol for TRIzol (Invitrogen) extraction, or RNeasy (Qiagen) column purification, assessed on a Bioanalyzer 2100 (Agilent Technologies), and amplified either using Affymetrix Two-cycle (calf 112) target labeling protocol with 100 ng total RNA or the One-cycle protocol (calves 156 and 162) with approximately 1.6μg of total RNA as described in the GeneChip® Expression Analysis Technical Manual (June 2004). Hybridizations to Genechip® Bovine Genome Arrays (Affymetrix) were performed with 15 μg biotin labeled cRNA. Washing and staining was performed in the GeneChip® Fluidics Station 450 using the Midi_euk2v3 protocol. Chip scans were performed on the Affymetrix GeneChip® Scanner 3000. GeneChip® Operating Software (GCOS v.1.1, Affymetrix) [10,11] was used for data collection and analysis of gene lists using GCOS assigned detection calls and significance values of signal log ratios of >1 or <−1 (2 fold changes). Annotation of these lists was improved by approximately 50% by blasting the bovine genome dataset against the mouse and human datasets. Other analyses were done with Genespring (Agilent Technologies) software, applying RMA preprocessing to the Affymetrix CEL files. All data was normalized to time 0 post infection values and only genes with a raw value of greater than 100 expression level and that changed by at least 2 or 2.5 fold during the time course were considered significant.
Priming Assay
Vantage sorted bovine peripheral blood γδ T cells (>97% purity) were washed with HANKS buffer and loaded with 0.25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) in the dark for 5 minutes. Cells were assayed in duplicate or triplicate wells, depending on the sort yield and primed with PBS, or phenol-extracted LPS from E. coli O111:B4 (Sigma L2630) or Salmonella serovar Typhimurium (Sigma, L6511, 10μg/ml) in XVIVO (Cambrex, Walkersville, MD) media for 48 hours at 37°C. Priming medium was removed and replaced with medium containing IL-2 (1 ng/ml) or IL-15 (10 ng/ml) then cultured another 72 hours at 37°C. Cells were then read on a FACS Calibur HTS using high-throughput settings. Gates were placed on lymphocyte populations and GD3.8 positive cells, for analysis of CFSE staining.
Statistical analyses
Anova (Two factor with replication) analysis was used to calculate the significance of the increase in IL-2Rα expression in γδ T cells as compared to non-γδ T cells. Statistical analyses of priming assay data were performed with a two-tailed paired t test. Mean values of percent of gated cells were calculated with 1 to 3 experimental replicates (sample wells) from each of at least 3 individual calves (biological replicates). Extensive experience with this assay in a high throughput screen setting indicates that the percent of gated cells follows an approximately normal distribution. Mean values of purified γδ T cells treated with PBS were compared to those treated with E. coli LPS or Salmonella serovar Typhimurium LPS. The n values are as follows: for the set that proliferated in IL-2; PBS, 8; E. coli LPS, 4 and Salmonella serovar Typhimurium LPS, 9 for set that proliferated in IL-15; PBS, 8; E. coli LPS, 6 and Salmonella serovar Typhimurium LPS, 8.
Microarray Data
The microarray data and the supplemental Table 1 discussed in this publication have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE3439.
Results
Blood and lymphatic fluid cell changes during Salmonella infection
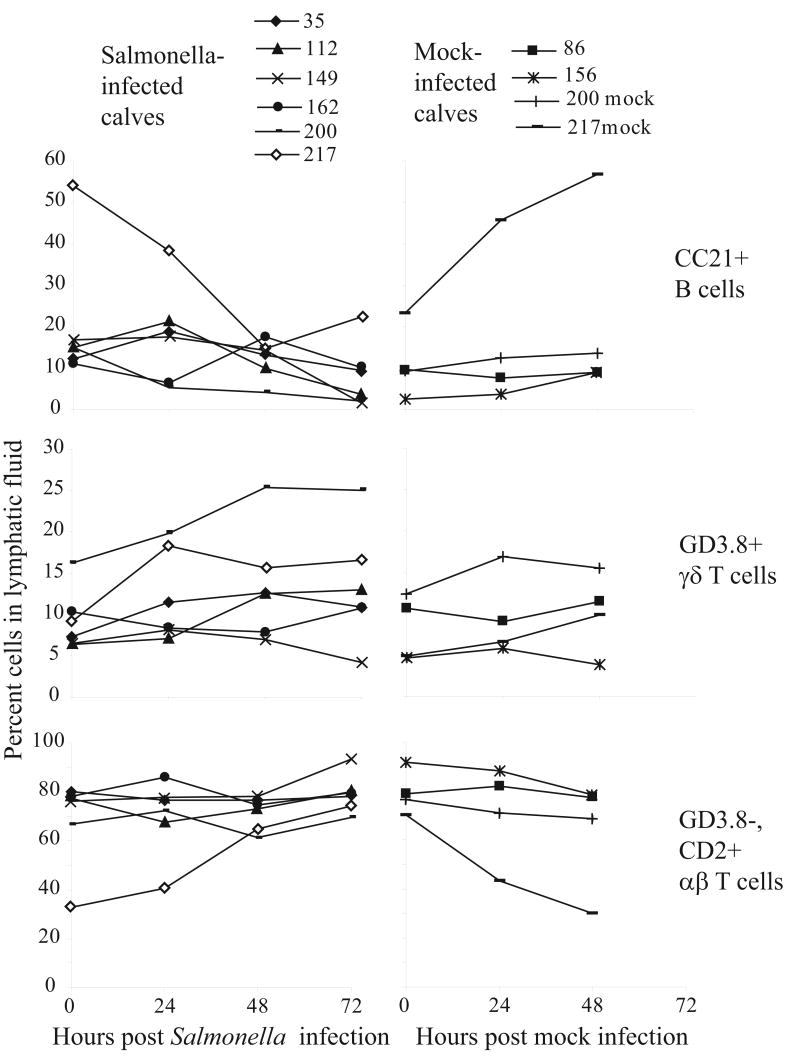
The mesenteric lymphatic ducts of four different calves (35, 112, 149, and 162) were cannulated. The calves were then orally infected with Salmonella serovar Typhimurium and monitored for 72 hours. Four additional calves (86, 156, 200, and 217) were treated identically, mock infected and monitored for 48 hours. Calves 156, 200 and 217 were cannulated and monitored for a 48 hour mock infection, and then infected with Salmonella, providing both a mock and experimental infection in the same animal. All infected calves developed enterocolitis by 24 to 48 hours post infection whereas mock infected calves had no signs of infection.
Lymphocyte populations were monitored in the blood and lymphatic fluids of Salmonella- and mock- infected calves. Whereas there were no major changes in lymphocyte populations in the blood, changes in frequency of monocytes and neutrophils were detected, but followed no clear patterns (data not shown). In the lymphatic fluid, γδ T cell frequency seemed to increase compared to αβ T cells (Figure 1) but results were variable and not statistically significant. Percentages of the inflammatory WC1+, CD8− γδ T cell subset normally found in circulation [4,5] were also measured in the lymphatic fluid of Salmonella serovar Typhimurium- and mock-infected calves to determine the extent of recruitment during infection (data not shown). The increases in this subset followed similar trends as the total γδ T cell population, and suggested some recruitment or induction of the WC1 antigen along with representation of the tissue resident subset in lymphatic fluid. Others have similarly observed an increase in gut mucosal resident CD8+ γδ T cells (equivalent of many WC1− γδ T cells in cattle) in the intraepithelial lymphocytes at 3 to 10 days post infection in the murine typhoid fever model [12]. Lacking a definitive increase in γδ T cells in the lymphatic fluid as an indication of their early response, we sought to better characterize their response early in Salmonella serovar Typhimurium infection.
Figure 1.
Analysis of cell populations in lymphatic fluid. Mutli-color FACS analysis of leukocyte populations in lymphatic fluid during Salmonella serovar Typhimurium-induced enterocolitis (calves 39, 112, 149, 162, 200, 217, black lines) or mock infection (calves 86, 156, 200mock, 217mock, grey lines).
Microarray analysis of γδ and αβ T cells in lymphatic fluid
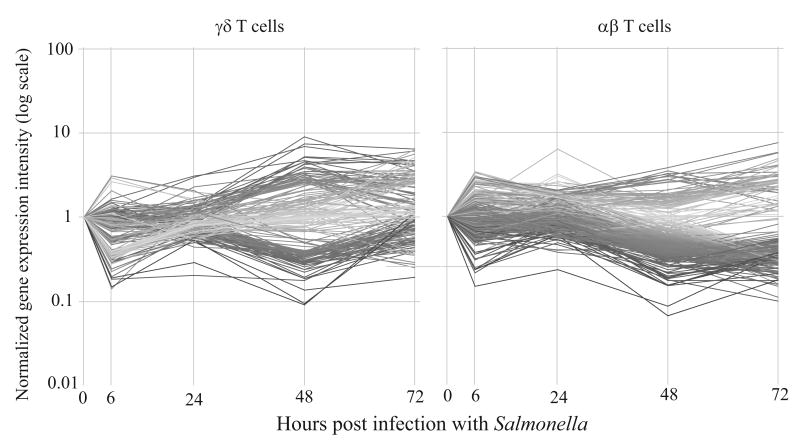
γδ and αβ T cells were sorted from the mucosal lymphatic fluid at 0, 6, 24, 48 and 72 hours post infection from calf 112. The cells were sorted and relative mRNA expression was analyzed by microarray. Figure 2 illustrates the genes that changed in expression level by at least 2.5 fold at one or more time points compared to expression level at time 0. Both T cell subsets had an initial response by 6 hours post infection that may reflect an ongoing response to surgery. At 48 hours after infection, after the animal responded clinically, the majority of gene changes occurred and differences in gene expression between the subsets emerged. Specifically, in γδ T cells similar numbers of genes increased as decreased in expression, whereas in αβ T cells genes more than twice as many genes decreased in expression than increased.
Figure 2.
Gene expression in γδ and αβ T cells during Salmonella serovar Typhimurium infection. Gene expression changes within γδ and αβ T cells in calf 112 were greatest 48 hours after infection. Genes with raw expression >100 and at least a 2.5 fold difference in at least one time point compared to time 0 are shown. The graphs are colored by expression levels at 48 hours post infection, greater changes in expression are indicated by a darker gray shade.
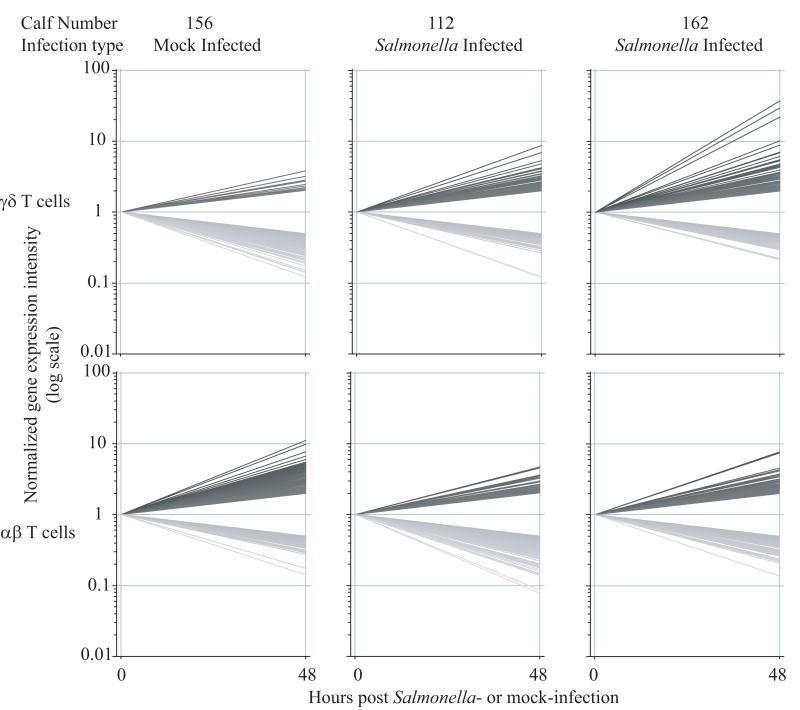
Given the apparent significance of 48 hours post Salmonella serovar Typhimurium infection, efforts were focused on the 0 and 48 hour post infection times in another infected calf (calf 162) and a mock infection (calf 156). The samples representing αβ and γδ T cells at 0 and 48 hour post infection were hybridized to microarrays. Figure 3 graphically illustrates the genes that changed by greater than 2 fold between 0 and 48 hours post Salmonella serovar Typhimurium (calves 112 and 162) or mock (calf 156) infection in the two major T cell subsets. There was a striking difference in the numbers of genes that changed between the mock and experimental infections. Specifically, in Salmonella infected calves, γδ T cells responded by increasing expression (by at least 2 fold) of 220 and 224 genes and decreasing expression of 196 and 252 genes in calves 112 and 162 respectively. In contrast, in γδ T cells in the mock infected calf, expression increased in only 57 genes, whereas 288 genes decreased in expression. An opposite effect was observed in αβ T cells. In this case, mock infection or the surgery alone induced an increase in expression of 267 genes in αβ T cells in calf 156 and a decrease in expression in 129 genes. Whereas Salmonella serovar Typhimurium infection induced increases in only 162 and 135 genes and far greater decreases; 368 and 375 genes in αβ T cells in calves 112 and 162 respectively. Thus, in terms of the numbers of genes changing, αβ and γδ T cells seemed to respond very differently to Salmonella serovar Typhimurium infection.
Figure 3.
Gene expression changes at 48 hours post-infection. Graphical representation of the genes that changed by at least 2 fold in γδ and αβ T cells between 0 and 48 hours post Salmonella serovar Typhimurium-infection (calves 112 and 162) or mock-infection (calf 156). Genes with increased expression are darkly shaded, and those with decreased expression are lightly shaded.
As to be expected in genetically dissimilar individuals with varied backgrounds, the genes that changed in response to Salmonella infection were not exactly the same. However, some general trends emerged from these experiments. A number of genes consistent with subtle activation and innate response to infection increased in expression in γδ T cells whereas gene changes specific to αβ T cells were largely decreases in expression or were indicative of quiescence (Table 1, supplemental data, http://www.ncbi.nlm.nih.gov/geo/). Specific gene changes in response to Salmonella serovar Typhimurium infection in lymphocytes derived from the mucosal lymphatic ducts suggested distinct responses to infection for αβ and γδ T cells.
Increase in IL-2Rα expression on γδ T cells
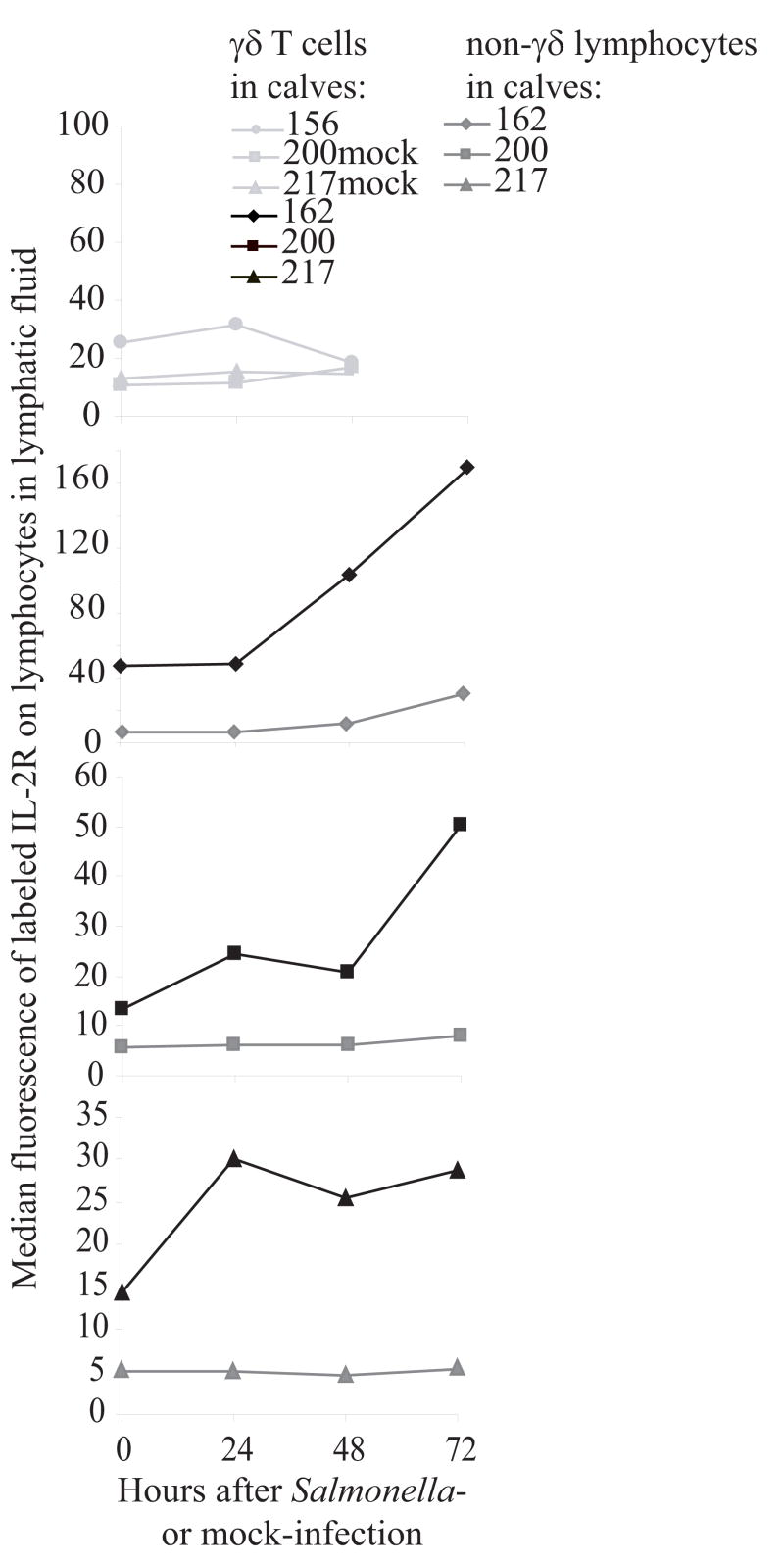
Included in the genes that were increased in expression in γδ T cells during Salmonella serovar Typhimurium infection was a marker for activation on γδ T cells, transcripts encoding the IL-2Rα, the private receptor for IL-2. To further investigate this change and determine if changes in gene expression were reflected in the phenotype, an IL-2Rα-specific antibody was used to detect changes in protein expression on cells from experimental (subsequent to calf 112) and mock infections. Fluorescent intensity of labeled IL-2Rα increased significantly (p=0.008) on γδ T cells in lymphatic fluid during experimental Salmonella serovar Typhimurium enterocolitis compared to non-γδ T cells (Figure 4). IL-2Rα remained at low level expression on γδ T cells in the negative control calves and on non-γδ T cell lymphocytes in all calves. In addition to increases in fluorescence, the percent of cells expressing IL-2Rα also increased following infection in most calves (data not shown). These data indicate that, consistent with the gene expression data, expression of IL-2Rα increased on γδ T cells in the mucosal lymphatic duct during early Salmonella infection.
Figure 4.
Increase in IL-2Rα expression. Consistent with the gene expression data, in the lymphatic fluid of every Salmonella serovar Typhimurium infected calf (black lines), fluorescence of the anti-IL-2Rα staining increased on γδ T cells at 48 hours post infection. Expression did not increase in non-γδ lymphocytes (dark grey lines) or in the mock-infected calves (light grey lines).
In vitro priming
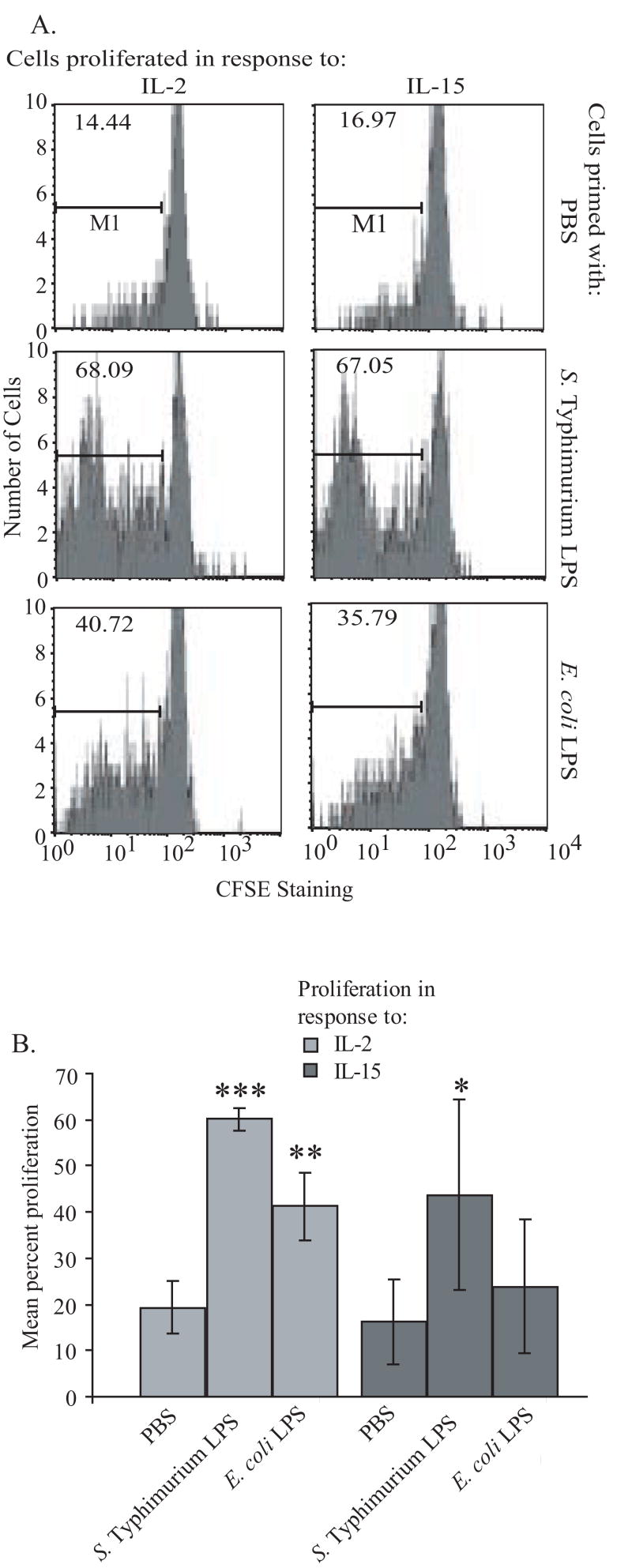
The increase in IL-2Rα expression in vivo suggested that γδ T cells could be rendered more sensitive to further activation by IL-2. To determine if this response could be due to a direct response of γδ T cells to Salmonella serovar Typhimurium molecular patterns, a functional proliferation assay was performed in which purified (>97% pure) bovine γδ T cells were pre-treated with PBS, E. coli LPS or Salmonella serovar Typhimurium LPS and then stimulated with IL-2 and allowed to proliferate. Even though the response to IL-15 is unrelated to IL-2Rα, it is known to promote γδ T cell proliferation [13] and was also included. Salmonella LPS alone had no impact on γδ T cell proliferation (data not shown); however as shown in Figure 5, it was particularly effective in priming a major proportion of the γδ T cells to proliferate in response to both IL-2 and IL-15. Despite great variation between individual calf donors, the proliferation response was statistically significant. These data underscore the functional relevance of the increase in IL-2Rα expression following Salmonella infection, suggest a functional increase in the IL-15 receptor, and support priming of γδ T cells in the target tissue early in infection by Salmonella serovar Typhimurium molecular patterns.
Figure 5.
γδ T cells are primed by Salmonella serovar Typhimurium LPS. (A) Representative CSFE staining after priming of purified γδ T cells with PBS, Salmonella serovar Typhimurium, or E. coli LPS proliferation in the presence of IL-2 or IL-15 is shown. (B) Mean value of percent of gated cells for multiple experimental replicates with cells from at least 3 individual calves, error bar denotes standard deviation. ***p=0.0001, **p=0.0095, *p=.0015.
Discussion
In this investigation the early transcriptional activities of mucosal lymphatic T lymphocyte subsets in Salmonella serovar Typhimurium-induced enterocolits have begun to be elucidated, revealing substantial differences in the responses of naive αβ T cells and γδ T cells to this infection. The bovine model of Salmonella serovar Typhimurium-induced enterocolitis directly reflects the pathogenesis and progression of human Salmonella serovar Typhimurium-induced disease. Others have detected a γδ T cell response to Salmonella infections in mice [6,12], but the relevance of this response to enterocolitis is unclear. Analysis of peripheral blood γδ T cell subsets in chickens 14 days after immunization with Salmonella serovar Enteritidis also identified increases in IL-2Rα transcripts, but this was not confirmed on a protein expression or functional level [14]. Our data suggests that similar, very early protein changes in mucosal lymphatic-derived bovine γδ T cells in response to Salmonella infection occur and the responses likely have functional significance. Specifically, we found that γδ T cells were subtly activated, or primed, early in Salmonella serovar Typhimurium-infected calves, as evidenced by the increase in IL-2Rα on cells derived from intestinal lymphatic ducts. Minimal changes in γδ T cells were observed in the blood of infected calves, consistent with responses seen in human Salmonella serovar Typhimurium induced enterocolitis [8]. The functional significance of increased expression of the IL-2Rα receptor using purified γδ T cells in vitro was demonstrated. Our data suggests that in this enterocolitis model, which is highly relevant to human disease, γδ T cells are primed early in infection to later expand in response to secondary signals.
The difference in gene expression between γδ and αβ T cells suggested priming of γδ T cells and a minimal response of αβ T cells. Infection did not increase gene expression in αβ T cells over that of the negative control, rather, Salmonella serovar Typhimurium infection appeared to have a dampening effect on this T cell subset. Considering that B cells express PAMP receptors, this contaminating cell population in the sorted αβ T cells might have skewed gene expression toward activation, but this was not detected. αβ T cells were the main contaminating cell in the γδ T cell populations, but they clearly had little to contribute to changes in gene expression. The collection of lymphatic fluid during Salmonella infection is a technically difficult feat with inherent variability, especially considering individual calf's genetics and experience. Thus, although lack of sufficient biological repetition preclude discussion of the significance of changes in individual genes in vivo in this study, these analyses indicated distinct responses to early Salmonella serovar Typhimurium infection by mucosal lymphatic-derived γδ and αβ T cells that were critical in directing, and are supported by, subsequent investigations.
Our working model is that the rapid in vivo response of γδ T cells early in infection with Salmonella is a response to pathogen associated molecular patterns (PAMPs), such as LPS or flagella, generated by bacterial infection in the gut. Earlier microarray and real time RT-PCR analysis of purified human and bovine γδ T cells stimulated in vitro with E. coli LPS documented increases in mRNAs encoding IL-2Rα and other genes indicative of priming [15], similar to those upregulated in vivo after Salmonella infection. Here we show that IL-2Rα protein upregulation on γδ T cells could be reproduced in vitro with Salmonella serovar Typhimurium LPS alone. Using highly purified and largely naïve (derived from neonatal animals) γδ T cells we demonstrated, in part, the functional significance of this priming event, namely, enhanced expansion in response to IL-2 or IL-15. This response is similar, in part, to an antigen driven response, except it occurs with a large fraction of the naive γδ T cell population. Also, though it is widely accepted that γδ T cells recognize microbial nonpeptidic phosphorylated molecules [16-18] and alkylamines [19,20] in a TCR-dependent manner, LPS has not, to our knowledge, been described as a TCR ligand. While we have not precisely determined the mechanism of LPS detection, transcripts encoding many TLRs and other pattern recognition proteins are readily detected in γδ T cells [15,21-25]. Other groups have shown that TLR agonists have co-stimulatory effects in combination with TCR engagement on γδ T cells, similar in many respects to the priming effect described here [21,25]. A similar costimulatory effect of the TLR2 agonist with TCR engagement that is enhanced by IL-2 on regulatory T cells in mice, and lack of effect of LPS on non-γδ T lymphocytes [26] is also consistent with our results. An additive response of Salmonella serovar Typhimurium LPS and IL-2 in the absence of TCR engagement on regulatory T cells has also been observed [27]. Finally, a similar priming event has been well defined for macrophages, where prior exposure to LPS dramatically increases subsequent responses to secondary signals [28].
The priming event described here is subtle and in some instances many primarily be reflected at only the transcriptional level in PAMP-exposed γδ T cells [15]. Priming alone does not lead to proliferation unless IL-2 or IL-15 is present, which may vary widely between animals, potentially explaining the inconsistent changes in γδ T cells in the lymphatic fluid within 48-72 hours of Salmonella infection. Priming also leads to a rather restrictive pattern of cytokine production by γδ T cells, reflected mainly in secretion of select chemokines, such as MIP1-α, GM-CSF and RANTES [15]. We have no evidence that prototypic cytokines of inflammatory γδ T cells, such as IFN-γ or TNF-α, are induced by this mechanism in bovine cells (data not shown), although this may be a downstream effect following secondary signals [29]. Nonetheless, downstream proliferation is likely, as we have demonstrated in vitro. Increased lymphatic γδ T cells in calves correlated with resistance to Salmonella infection (data not shown), and would contribute to dendritic cell maturation [30], potentially present antigen [31] and may interact with monocytes to resolve inflammation [32].
We define a unique, rapid response by bovine γδ T cells to Salmonella serovar Typhimurium infection that is defined as a priming event leading to increased responsiveness to secondary signals. The two step process leads to expansion of γδ T cells which is likely essential for induction of their full anti-microbial potential that results in resolution of and immunity to Salmonella enterocolitis. These studies are critical to our current efforts to develop therapeutic and/or prophylactic interventions that prime γδ T cells to enhance protection from mucosal disease.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200400009/N01-AI40009 and grant AI-41123; and supported by Initiative for Future Agricultural and Food Systems Grant no. 00-52100-9612 from the USDA Cooperative State Research, Education, and Extension Service; and in part by USDA formula funds and Montana Agricultural Experiment Station. Funding from NIH COBRE grant P20 RR020185, which supported the FACS analyses, is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J, Nunes J, Tsolis RM, Adams LG, Baumler AJ. Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea. Infect Immun. 2003;71:1–12. doi: 10.1128/IAI.71.1.1-12.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravindran R, McSorley SJ. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology. 2005;114:450–458. doi: 10.1111/j.1365-2567.2005.02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 5.Wilson E, Aydintug MK, Jutila MA. A circulating bovine gamma delta T cell subset, which is found in large numbers in the spleen, accumulates inefficiently in an artificial site of inflammation: correlation with lack of expression of E-selectin ligands and L-selectin. J Immunol. 1999;162:4914–4919. [PubMed] [Google Scholar]
- 6.Mixter PF, Camerini V, Stone BJ, Miller VL, Kronenberg M. Mouse T lymphocytes that express a γδ T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect Immun. 1994;62:4618–4621. doi: 10.1128/iai.62.10.4618-4621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittrucker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 8.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y. Predominant activation and expansion of V gamma 9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedges JF, Cockrell D, Jackiw L, Meissner N, Jutila MA. Differential mRNA expression in circulating γδ T lymphocyte subsets defines unique tissue-specific functions. J Leukoc Biol. 2003;73:306–314. doi: 10.1189/jlb.0902453. [DOI] [PubMed] [Google Scholar]
- 10.Liu WM, Mei R, Di X, Ryder TB, Hubbell E, Dee S, Webster TA, Harrington CA, Ho MH, Baid J, Smeekens SP. Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics. 2002;18:1593–1599. doi: 10.1093/bioinformatics/18.12.1593. [DOI] [PubMed] [Google Scholar]
- 11.Hubbell E, Liu WM, Mei R. Robust estimators for expression analysis. Bioinformatics. 2002;18:1585–1592. doi: 10.1093/bioinformatics/18.12.1585. [DOI] [PubMed] [Google Scholar]
- 12.Davies A, Lopez-Briones S, Ong H, O'Neil-Marshall C, Lemonnier FA, Nagaraju K, Metcalf ES, Soloski MJ. Infection-induced expansion of a MHC Class Ib-dependent intestinal intraepithelial gammadelta T cell subset. J Immunol. 2004;172:6828–6837. doi: 10.4049/jimmunol.172.11.6828. [DOI] [PubMed] [Google Scholar]
- 13.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human gamma delta T cells to nonpeptide microbial antigens. J Immunol. 1998;160:4322–4329. [PubMed] [Google Scholar]
- 14.Berndt A, Pieper J, Methner U. Circulating {gamma}{delta} T Cells in Response to Salmonella enterica Serovar Enteritidis Exposure in Chickens. Infect Immun. 2006;74:3967–3978. doi: 10.1128/IAI.01128-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges JF, Lubick KJ, Jutila MA. γδ T cells respond directly to pathogen associated molecular patterns. J Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 16.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human [gamma][delta] T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 18.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human [gamma][delta] T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 19.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Das H, Kamath A, Bukowski JF. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 21.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human {gamma}{delta} T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 22.Lubick K, Jutila MA. LTA recognition by bovine {gamma}{delta} T cells involves CD36. J Leukoc Biol. 2006;79:1268–1270. doi: 10.1189/jlb.1005616. [DOI] [PubMed] [Google Scholar]
- 23.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 24.Kress E, Hedges JF, Jutila MA. Distinct gene expression in human V[delta]1 and V[delta]2 [gamma][delta] T cells following non-TCR agonist stimulation. Mol Immunol. 2006;43:2002–2011. doi: 10.1016/j.molimm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human V{gamma}2V{delta}2 T cells after stimulation with antibody against the T-cell receptor plus the toll-like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aderem AA, Cohen DS, Wright SD, Cohn ZA. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986;164:165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafont V, Loisel S, Liautard J, Dudal S, Sable-teychene M, Liautard JP, Favero J. Specific signaling pathways triggered by IL-2 in human V{gamma}9V{delta}2 T cells: An amalgamation of NK and {alpha}{beta} T cell signaling. J Immunol. 2003;171:5225–5232. doi: 10.4049/jimmunol.171.10.5225. [DOI] [PubMed] [Google Scholar]
- 30.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 32.Born WK, Reardon CL, O'Brien RL. The function of [gamma][delta] T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.