Abstract
PURPOSE
One hypothesis by which exercise-based cardiac rehabilitation (CR) reduces mortality and cardiac events in patients with coronary artery disease (CAD) invokes a beneficial effect of exercise on autonomic modulation. This study aimed to evaluate the autonomic effects of CR in patients with CAD.
METHODS
Participants referred to Phase 2 CR underwent 4 bicycle stress tests, 2 prior to starting CR, and 2 after. On visits 1 and 3, a symptom-limited bicycle stress test was performed. On visits 2 and 4, the subject exercised to the same workload, but atropine was administered during maximal exercise to achieve parasympathetic blockade. Parasympathetic effect in exercise recovery was computed before and after CR. Heart rate variability for each segment was also quantified. Plasma catecholamine levels were obtained at baseline, peak exercise, and during recovery.
RESULTS
Seventeen subjects (age 56±10 years; 4 female) were enrolled. Six completed the post-CR testing. There was a significant increase in parasympathetic effect during exercise recovery post-CR (P<.001). There was also a significant increase in heart rate variability during exercise recovery post-CR (P<.001). Resting catecholamine levels were not different pre- and post-CR (P=NS). Post-CR, there was a blunted increase in peak exercise plasma catecholamine levels vs. pre-CR but this was not statistically significant.
CONCLUSIONS
We demonstrated a shift towards increased parasympathetic and possibly, blunted sympathetic effect in this cohort after completion of an exercise-based CR program. Our findings provide insight into the mechanism for the observed changes in exercise parameters following exercise training, and the improved outcomes seen after CR.
Key words or phrases: Heart rate recovery, Cardiac rehabilitation, Exercise, Parasympathetic modulation, Sudden cardiac death
Exercise-based cardiac rehabilitation (CR) has been shown to reduce mortality and cardiac events in patients with coronary artery disease (CAD).1,2 One hypothesis invokes a beneficial effect of exercise on autonomic tone.3,4 Although exercise training has been suggested to modify sympathovagal control of heart rate (HR) towards an increase in parasympathetic effect,3,4 there has been little direct evaluation of parasympathetic effects related to CR. This study aimed to prospectively evaluate the exercise-related autonomic effects of CR.
METHODS
Patients age 18–80 years, with CAD, referred to a Phase 2 CR program, were screened. Inclusion criteria included recent angioplasty/stent placement for a myocardial infarction or unstable angina. Exclusion criteria included recent coronary artery bypass grafting, diabetes, known autonomic disorders, ejection fraction ≤35%, significant arrhythmias such as atrial fibrillation or frequent ectopy, decompensated congestive heart failure, and implanted pacemakers. Subjects had to be on stable medical regimens and medications were not altered during the study period. The study protocol was approved by the Institutional Review Board of Northwestern University.
Procedures
The protocol required participants to complete 4 bicycle stress tests separated by 72 hours, 2 prior to starting CR, and 2 after completing the program. On visit 1, a symptom-limited standard bicycle stress test was performed. A 12-lead ECG was obtained and continuous Frank-lead ECG (Predictor I, Arrhythmia Research Technology, Fitchburg, MA) was recorded for 5 minutes at rest. Subjects were then instructed to exercise, keeping the pedal speed at 80 rpm. Workload was maintained at 50 Watts for 4 minutes, and then increased by 25 Watts every 2 minutes, depending on patient symptoms. At the maximum workload that could be tolerated, the subject was asked to exercise at that level for another 3 minutes. At the end of exercise, peak HR was recorded. A 12-lead ECG was obtained immediately postexercise, and every minute thereafter for 10 minutes. Continuous Frank-lead ECG was recorded for 5 minutes following cessation of exercise. Blood was drawn for plasma catecholamine levels at baseline, peak exercise, and 1, 2, 5, and 10 minutes into recovery.
On visit 2, an identical exercise protocol was performed, except that intravenous atropine (0.04 mg/kg) was administered during maximal exercise, in divided doses (0.01 mg/kg every 30 seconds) to achieve parasympathetic blockade.5 The subject was asked to exercise in the same manner as Visit 1, staying on the bicycle for the same duration with the same maximal workload achieved. In the last exercise stage, atropine was given at 0.01 mg/kg every 30 seconds. Hence, the last 2 minutes represent exercise during complete parasympathetic blockade.
The subject then began Phase 2 CR as per protocol at Northwestern Memorial Hospital. Target HR goals and workload increases were determined by the CR staff. Within 2 weeks after the last session of Phase 2 CR, followup bicycle stress tests were performed using the same protocol as Visits 1 and 2. Subjects were asked to exercise on the bicycle to the same workload (as the baseline tests) for Visits 3 and 4. During Visit 4, atropine was similarly administered during the maximum workload stage of the test.
ECG Analysis
The 5 minute continuous Frank-lead ECG recordings at rest and exercise recovery were analyzed with custom software developed with MATLAB (Mathworks, Natick, MA). QRS complexes were detected using a template matching algorithm from which RR intervals were computed. The QRS markings and RR intervals were manually overread and premature beats were identified. The RR interval preceding and the 2 RR intervals following the premature beat were excluded from analysis.
Each of the 5 minute recordings of RR intervals were divided into ten consecutive 30-second segments. The mean of the RR intervals was computed for each segment. Parasympathetic effect for each segment of exercise recovery was computed before and after CR as the difference between the mean RR interval with or without parasympathetic blockade.6,7 Heart rate variability (HRV) for each segment was quantified using the root mean square of successive differences (rMSSD) and the root mean square (RMS) residuals of the linear regression of RR intervals versus time. Both measures have been shown to reflect parasympathetic reactivation during recovery from exercise.6
Statistical Analysis
Results are reported as mean ± standard deviation. Exercise parameters and catecholamine levels were compared using the Student’s t-test or paired t-test for parametric data and the Mann Whitney Rank Sum Test or Signed Rank test for nonparametric data. Multiple groups were compared using the analysis of variance (ANOVA) for parametric data and ANOVA on Ranks for nonparametric data, with multiple comparison performed using the Dunn’s Method. A P value <.05 was considered significant. All analyses were performed using Sigma Stat software (Systat Software Inc., Point Richmond, CA).
RESULTS
Seventeen patients (mean age 56±10 years; 4 female) were enrolled in the study. Most (88%) had a recent myocardial infarction. Nearly half had hypertension and/or hyperlipidemia, and none were current smokers. Mean ejection fraction was 54±9%.
Precardiac Rehabilitation
Baseline HR did not differ between visits 1 and 2 (66.4±12.3 versus 65.9±11.7 bpm). Mean peak workload was 85.7±28.9 Watts (8.6±3.2 minutes). Peak exercise HR on visit 1 was 135.4±23.4 bpm and was significantly higher after atropine administration (145.9±19.3 bpm; P<.001). HR in recovery was higher on visit 2 than visit 1 (P<.001). The 1 and 2 minute HRR on visit 1 were 30.9±25.5 and 34.8±12.9 bpm, respectively, which were significantly higher than those on visit 2 [14.2±8.9 (P<.001) and 26.5±15.8 bpm (P=.038), respectively)]. There was a progressive increase in parasympathetic effect from early recovery (69.7±32.1 ms) that plateaued by 4 minutes into exercise recovery (range=121.9–127.2 ms).
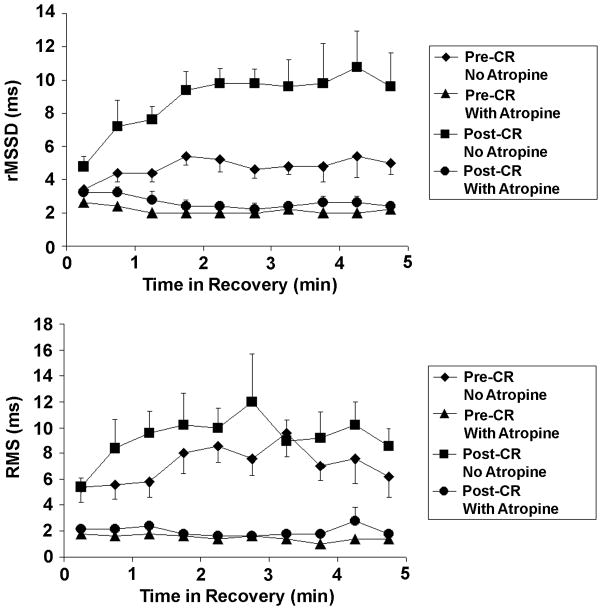
There were no differences between visits 1 and 2 rest rMSSD (27.5±2.7 and 24.5±3.1 ms) and rest RMS (25.1±2.9 and 22.9±2.5 ms). On visit 1, both the rMSSD and RMS increased progressively from 4.2±1.9 ms and 5.5±4.0 ms in early recovery, reaching a plateau at 4 to 5 minutes into recovery, with peak values of 10.3±17.8 ms (P=.017) and 9.7±10.5 ms (NS). On visit 2, after parasympathetic blockade, both rMSSD and RMS were markedly attenuated with no change over the course of exercise recovery (range: rMSSD=2.3–3.7 ms, RMS=1.7–2.5 ms).
Resting plasma epinephrine and norepinephrine levels on visits 1 and 2 were similar (NS). At peak exercise, there was a large increase in catecholamine levels (P<.001). There was no significant difference in catecholamine levels between peak exercise and 1 minute into exercise recovery (NS). By 2 minutes into exercise recovery, epinephrine and norepinephrine levels had decreased by 38% for epinephrine and 18% for norepinephrine. By 5 minutes into exercise recovery, there was a further large decrement to 63% for epinephrine and 49% for norepinephrine. There was no significant difference in plasma catecholamine levels with or without atropine (NS).
Postcardiac Rehabilitation
All patients completed Phase 2 CR; however, only 6 patients (mean age 51±9 years, p=NS versus entire cohort; 2 female) completed the postrehabilitation testing. Reasons for noncompletion were lack of interest and conflict with work schedules. Baseline exercise parameters of the subjects who completed the protocol were not different from those who did not.
Resting HR tended to be lower after CR (66.2±7.0 bpm versus 69.0±8.2 bpm; P<.055). Peak exercise HR also tended to be lower after CR (127.0±20.5 bpm versus 140.5±13.1 bpm; P<.052). However, the 1 minute and 2 minute HRR values were not different pre- and post-CR (1 minute HRR=26.2±9.2 bpm versus 25.0±8.4 bpm, [NS]; 2 minute HRR=34.8±12.9 bpm versus 35.7±9.5 bpm, [NS]). Figure 1 demonstrates the parasympathetic effect before and after CR. There was a significant increase in parasympathetic effect during exercise recovery after completion of an exercise-based CR program (P<.001).
Figure 1.
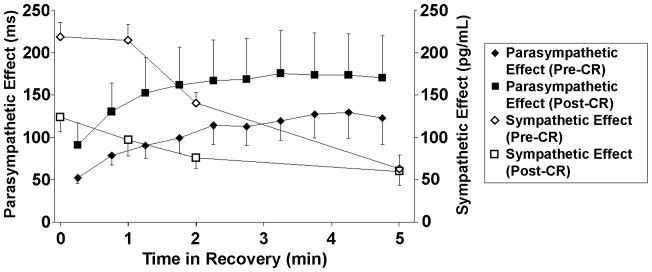
Sympathetic and parasympathetic effects in the exercise recovery period pre- and postcardiac rehabilitation (CR) (mean±SE). Parasympathetic effect was defined as the difference in mean RR interval for each 30 second segment in exercise recovery with and without atropine. Sympathetic effect was reflected by mean plasma epinephrine levels in exercise recovery.
Resting HRV after CR was similar between visits 3 and 4 (NS). Resting HRV did not differ post-CR compared to pre-CR (NS). Figure 2 depicts HRV in exercise recovery pre- and post-CR. There was a significant increase in HRV during exercise recovery post-CR (P<.001 for rMSSD and P=.002 for RMS versus pre-CR). Post-CR, both rMSSD and RMS increased rapidly in exercise recovery, and reached a higher peak with a steeper slope in the first two minutes of exercise recovery (Figure 2). At 3–4 minutes into exercise recovery, peak rMSSD was 10.8±4.9 ms (P=.024 versus initial 30 seconds) and peak RMS was 12.0±8.2 ms (NS versus initial 30 seconds). On visit 4, both rMSSD and RMS were markedly attenuated with no change over the course of exercise recovery (NS).
Figure 2.
Heart rate variability in exercise recovery pre- and postcardiac rehabilitation (CR) (mean±SE). Heart rate variability represented as the root mean square of successive differences (rMSSD) and the root mean square (RMS) residuals of the linear regression of RR intervals versus time.
Resting epinephrine and norepinephrine levels were not different pre and post-CR (NS). At peak exercise, there was a large increase in plasma epinephrine and norepinephrine levels (P<.05 for both versus resting levels), but less so compared to pre-CR (Figure 1). Plasma epinephrine and norepinephrine levels remained lower in exercise recovery after CR, but these were not significantly different.
DISCUSSION
This prospective cohort study demonstrated a significant increase in parasympathetic effect in patients with CAD after completion of an exercise-based CR program. Patients were able to exercise to the same workload after CR with improved parasympathetic reactivation during recovery and, possibly, reduced sympathetic activation. These findings were consistent with the changes noted in measures of HRV during exercise recovery, which peaked earlier and attained higher values compared to pre-CR. Interestingly however, the net effect of these changes had only mild effects on the resting HR and no significant effect on resting measures of HRV. Thus, exercise parameters, rather than resting parameters, may be more sensitive for detection of salutary autonomic changes associated with CR.
Despite the large body of work demonstrating the association between markers of autonomic activity and increased mortality,8–10 the pathophysiologic link between autonomic modulation and subsequent death remains elusive. Several studies have suggested that heightened parasympathetic modulation is protective against ventricular fibrillation after a myocardial infarction.11–12 Smith et al11 induced myocardial ischemia in a canine model by acute occlusion of the left circumflex artery during the last minute of exercise. They demonstrated that HRR was significantly higher in dogs that were resistant to ventricular fibrillation than in those who developed malignant arrhythmias. Susceptible animals had attenuated parasympathetic reactivation after exercise compared to resistant animals and the differences between exercise parameters were eliminated by atropine pretreatment.
Exercise training appears to be an effective and applicable modality to alter autonomic control of HRR; to date, this has been predominantly attributed to enhancement of parasympathetic effects.3 Several investigators have demonstrated that an exercise-based CR program results in increased HRR.13–15 Our study did not demonstrate an increase in HRR, however this protocol was designed to exercise patients to the same workload pre- and post-CR, rather than using symptom limited testing. Focusing on the same workload allowed us to examine the autonomic changes associated with this workload, but subjects achieved lower peak HR after CR. In this study, we demonstrated improved parasympathetic reactivation after CR and, possibly, blunted sympathoexcitation. Our present study suggests that CR may have salutary effects on both adrenergic and parasympathetic activity.
The major limitation of this study is the small sample size on follow-up testing. Despite this, a significant increase in parasympathetic effect in early exercise recovery was still demonstrated in this cohort. All patients received standard medical therapy postmyocardial infarction including beta-adrenergic blockers and angiotensin-converting enzyme inhibitors which could have affected the autonomic markers measured. However, this is unlikely to be a source of major bias as each patient served as their own control, and no patient medical regimen was altered during the study period. In addition, it is possible that some of the changes observed may not be related to CR itself, but to other factors such as natural event recovery. Finally, while HRV is not a direct measure of parasympathetic activity, the findings presented are strongly supported by the parasympathetic effect directly measured with parasympathetic blockade.
In summary, we demonstrated a shift towards increased parasympathetic effect and possibly lower adrenergic activation in this cohort of patients with CAD after completion of an exercise-based CR program. Our findings provide insight into the mechanism for the observed changes in exercise parameters noted following exercise training, and may have implications for explaining the improved outcomes seen following CR.
Acknowledgments
This research is supported in part by Grant #M01 RR-00048 from the National Center for Research Resources, National Institutes of Health, to the General Clinical Research Center of Northwestern Memorial Hospital and by Grant 1 RO1 HL 70179-01A2 from the National Heart, Lung, and Blood Institute.
We would like to thank the entire staff of the Cardiac Rehabilitation Program at Northwestern Memorial Hospital for their invaluable help and support with this project.
References
- 1.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: Combined experience of randomized clinical trials. JAMA. 1988;260:945–950. [PubMed] [Google Scholar]
- 2.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Malfatto G, Blengino S, Annoni L, Branzi G, Bizzi C, Facchini M. Primary coronary angioplasty and subsequent cardiovascular rehabilitation are linked to favorable sympathovagal balance after a first anterior myocardial infarction. Ital Heart J. 2005;6:21–27. [PubMed] [Google Scholar]
- 4.Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 5.Jose AD, Taylor RR. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J Clin Invest. 1969;48:2019–2031. doi: 10.1172/JCI106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J, Kadish AH. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol. 2006;290:H2446–H2452. doi: 10.1152/ajpheart.01118.2005. [DOI] [PubMed] [Google Scholar]
- 7.Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med. 2004;52:394–401. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 8.Jouven X, Empana J, Schwartz P, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 9.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Eng J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 10.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI Investigators. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 11.Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: A predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;288:H1763–H1769. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- 12.Vanoli E, De Farrar GM, Stramba-Badiale M, Hull SS, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1417–1418. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 13.Tiukinhoy S, Beohar N, Hsie M. Improvement in heart rate recovery after cardiac rehabilitation. J Cardiopulm Rehabil. 2003;23:84–87. doi: 10.1097/00008483-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hao SC, Chai A, Kligfield P. Heart rate recovery response to symptom-limited treadmill exercise after cardiac rehabilitation in patients with coronary artery disease with and without recent events. Am J Cardiol. 2002;90:763–765. doi: 10.1016/s0002-9149(02)02607-3. [DOI] [PubMed] [Google Scholar]
- 15.Lucini D, Milani RV, Costantino G, Lavie CJ, Porta A, Pagani M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J. 2002;143:977–983. doi: 10.1067/mhj.2002.123117. [DOI] [PubMed] [Google Scholar]