SUMMARY
Signaling via the Akt serine/threonine protein kinase plays critical roles in the self-renewal of embryonic stem cells and their malignant counterpart, embryonal carcinoma cells (ECCs). Here we show that in ECCs, Akt phosphorylated the master pluripotency factor Oct4 at threonine 235, and that the levels of phosphorylated Oct4 in ECCs correlated with resistance to apoptosis and tumorigenic potential. Phosphorylation of Oct4 increased its stability, and facilitated its nuclear localization and its interaction with Sox2, which promoted the transcription of the core stemness genes POU5F1 and NANOG. Furthermore, in ECCs, unphosphorylated Oct4 bound to the AKT1 promoter and repressed its transcription. Phosphorylation of Oct4 by Akt resulted in dissociation of Oct4 from the AKT1 promoter, which activated AKT1 transcription and promoted cell survival. Therefore, a site-specific, post-translational modification of the Oct4 protein orchestrates the regulation of its stability, subcellular localization and transcriptional activities, which collectively promotes the survival and tumorigenicity of ECCs.
INTRODUCTION
The similarities between tumorigenesis and embryonic development have been noticed for some time, and are receiving increasing attention now that the cancer stem cell hypothesis has been postulated (Gupta et al., 2009; Pardal et al., 2003; Reya et al., 2001). The hypothesis proposes that only small subsets of cells within tumors, termed cancer stem cells (CSCs), are responsible for tumor growth, maintenance and recurrence due to their stem-cell-like self-renewal capacity and unlimited proliferative potential. CSCs are experimentally defined by their ability to recapitulate the heterogeneity of the original tumor when transplanted into immuno-compromised mice. Embryonal carcinoma cells (ECCs) are the stem cells of teratocarcinomas and the malignant counterpart of embryonic stem cells (ESCs) that are derived from the inner cell mass of blastocyst-stage embryos, and are now considered to be a useful model for CSCs at the embryonic stage (Sharif et al., 2011; Silvan et al., 2009). Proteomic and genomic profiling revealed there is differential expression of Notch, TGFβ, and PI3K/Akt signaling molecules between ESCs and ECCs (Chaerkady et al., 2010; Liu et al., 2006; Sperger et al., 2003) and also highlighted a general up-regulation of anti-apoptosis and cell survival genes in ECCs (Liu et al., 2006; Sperger et al., 2003). Of interest, both ESCs and ECCs exhibit a plasticity that allows them to convert to their counterparts under certain conditions. For instance, ECCs can lose their malignant phenotype and participate in normal embryonic development when transplanted into blastocysts, whereas ESCs can acquire karyotypic changes and malignancy with prolonged culture in vitro (Andrews et al., 2005). Therefore, it is not only important to identify the common mechanisms of self-renewal and pluripotency that ESCs and ECCs share, we also need to determine their critical differences and define the mechanisms that control the functional switches between them. This knowledge will not only provide important clues about cancer occurrence and treatment, but also help to minimize the risk of tumorigenesis occurring when employing stem cells for regenerative medicine.
Thus far, only a few transcription factors are known to play essential roles in the maintenance of self-renewal and pluripotency of both ESCs (Young, 2011) and ECCs (Jung et al., 2010), and they achieve this by regulating their own or each other’s transcription via combinatorial interactions (Boiani and Scholer, 2005). Among them, Oct4 (encoded by POU5F1, also known as Oct3/4) has been convincingly established as a master regulator (Ng and Surani, 2011; Pardo et al., 2010; van den Berg et al., 2010). A precise amount of Oct4 in a narrow concentration range is critical both for sustaining stem cell self-renewal and turning on divergent differentiation programs (Niwa et al., 2000). Moreover, accumulating evidence indicates that Oct4 is expressed in somatic tumors such as bladder carcinoma (Chang et al., 2008), ovarian carcinoma (Peng et al., 2010) and lung adenocarcinoma (Chiou et al., 2010), and Oct4 overexpression can lead to epithelial dysplasias by blocking the differentiation of progenitor cells (Hochedlinger et al., 2005). These findings suggest a role for Oct4 in tumorigenesis and call for in-depth studies of the regulation and activity of Oct4 in various cell types including ECCs and ESCs. It is proposed that post-translational modifications (PTMs) including sumoylation (Wei et al., 2007), ubiquitination (Xu et al., 2009) and phosphorylation (Brumbaugh et al., 2012; Saxe et al., 2009) regulate Oct4 protein function. However, it is unclear which specific signaling pathways mediate the PTM of Oct4 and how their modifications affect specific gene transcription profiles. Here we show that in ECCs, the Akt serine/threonine protein kinase phosphorylated Oct4 at threonine 235 (T235) and that this Oct4 modification enhanced transcription of core stemness genes (including POU5F1, NANOG) and cell survival genes (including AKT1), which tipped the fate of the pluripotent stem cells towards tumorigenesis.
RESULTS
Oct4 is phosphorylated by Akt in ECCs
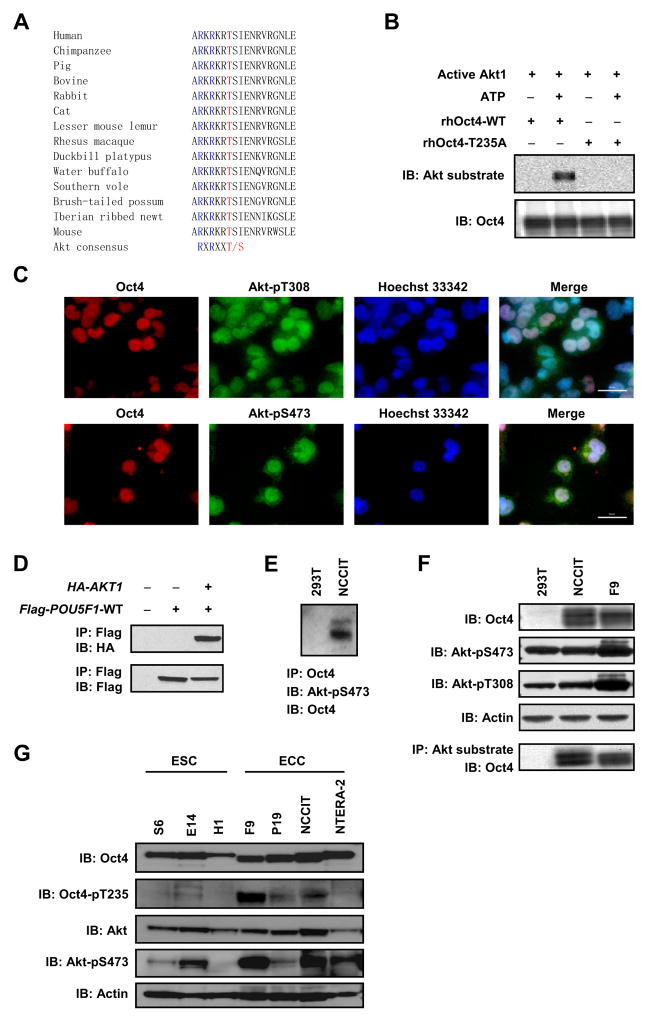
We performed bioinformatic analysis of human Oct4 and identified a putative substrate motif between residues 230 to 235 (RKRKRT) for the Akt serine/threonine kinase [the consensus motif for Akt substrates is R-X-R-X-X-pS/pT, where X represents any amino acid residue and pS or pT represents the phosphorylated serine or threonine (Manning and Cantley, 2007)]. The motif is highly conserved in Oct4 proteins from all species examined, suggesting it is important for Oct4 function (Fig. 1A).
Figure 1. Akt phosphorylates Oct4 in ECCs.
(A) Amino acid sequence alignment of Oct4. (B) Purified recombinant human Oct4-WT (rhOct4-WT) or Oct4-T235A (rhOct4-T235A) protein was incubated with active Akt1 protein in the absence or presence of 200 μM ATP, and immunoblotted with the indicated antibodies. (C) NCCIT cells were co-immunostained with anti-Oct4/anti-Akt-pT308 (upper) or anti-Oct4/anti-Akt-pS473 (lower), and counterstained with Hoechst 33342. Bars, 50 μm. (D) Flag-POU5F1-WT and HA-AKT1 were co-transfected into 293T cells. Cell lysates were immunoprecipitated with anti-Flag M2 beads and immunoblotted with the indicated antibodies. (E) Cell lysates were immunoprecipitated with mouse anti-Oct4. Immuno-complexes were sequentially immunoblotted with rabbit anti-Akt-pS473 (upper band) and rabbit anti-Oct4 (lower band). (F) Whole cell lysates were immunoprecipitated with anti-phospho-Akt substrate rabbit mAb. Immuno-complexes were immunoblotted with mouse monoclonal anti-Oct4 (bottom panel). Upper panels are whole cell lysate immunoblots (IB) probed with the indicated antibodies. (G) Whole cell lysates were resolved by PAGE and immunoblotted with the indicated antibodies.
We first tested if Oct4 could be phosphorylated by Akt in vitro. Recombinant proteins of human Oct4 (rhOct4) with either the wild type (WT) sequence (rhOct4-WT) or a sequence with a mutation of the threonine at 235 to alanine (rhOct4-T235A), were incubated with active Akt1 protein in the presence or absence of ATP. We observed that rhOct4-WT protein was phosphorylated by Akt in vitro in the presence of ATP, as detected by the Akt substrate antibody but the T235A mutation abolished Oct4 phosphorylation, indicating that the antibody recognition was specific and that T235 is the primary Akt phosphorylation site in human Oct4 (Fig. 1B).
To look for evidence of phosphorylation of Oct4 by Akt in vivo, we examined the human ECC line NCCIT and found that endogenous Oct4 co-localized with two activated forms of Akt (Akt-pT308 and Akt-pS473) in the nucleus (Fig. 1C). HA-Akt1 co-immunoprecipitated with Flag-Oct4 when they were co-expressed in 293T cells (Fig. 1D), and endogenous active Akt was co-immunoprecipitated with Oct4 in NCCIT cells (Fig. 1E), indicating an interaction between the two proteins. Furthermore, Oct4 was detected in immunoprecipitates (IP) from NCCIT cells and a mouse ECC line (F9) using the phospho-specific Akt substrate antibody, but not detected in IPs from 293T cells (Fig. 1F). We raised a phospho-specific antibody against an Oct4 peptide that spans the phosphorylated T235 site (229ARKRKRpTSIENRV241-C). When tested, our antibody reacted ~250 fold higher with the phospho-peptide immunogen than the non-phosphorylated counterpart in ELISA (Fig. S1A). It recognized a phosphorylated Oct4 (Oct4-pT235) band that was diminished by pre-incubation of the antibody with the phospho-peptide immunogen (Fig. S1B), and diminished in cells treated with Akti-1/2, a specific Akt inhibitor (Fig. S1C). In general, the band was stronger in ECC lines than in ESC lines (Fig. 1G and Fig. S1D), suggesting that Oct4 is a bona fide substrate of Akt in ECCs.
Akt-mediated phosphorylation stabilizes Oct4 in ECCs
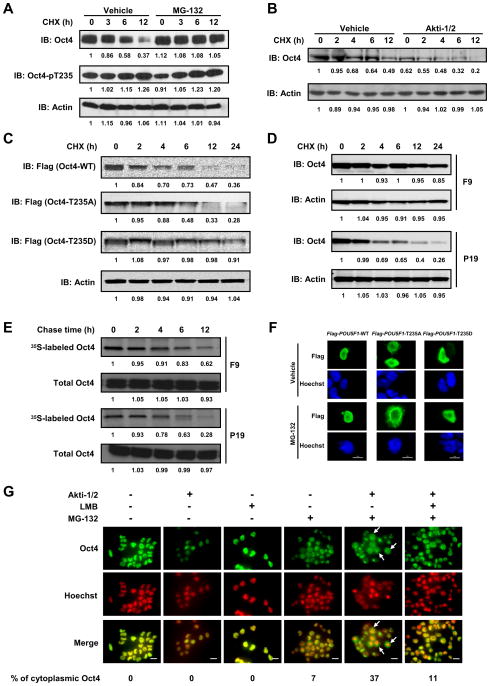
Mounting evidence suggests that finely-tuned Oct4 protein levels are crucial for maintaining ESC or ECC pluripotency and self-renewal (Kang et al., 2009; Niwa et al., 2000) and that an active, proteasome-dependent mechanism plays a key role in removing excess Oct4 (Saxe et al., 2009; Xu et al., 2009). Because the expression of a constitutively active Akt mutant (HA-AKT1-CA), but not its kinase-dead counterpart (HA-AKT1-KD), dramatically increased the level of endogenous Oct4 in ECCs (Fig. S2A), we asked if the Akt-mediated phosphorylation of Oct4 regulated its intracellular stability. NCCIT cells were treated with cycloheximide (CHX) to block synthesis of new proteins, and endogenous total Oct4 was observed to degrade with a half-life of 6–12 h (Fig. 2A and Fig. S2B). In contrast, phosphorylated Oct4 had a much slower rate of degradation (Fig. 2A). To determine if Oct4 degradation occurred via a proteasome-dependent pathway, NCCIT cells were pre-treated with MG-132, a specific and cell-permeable proteasome inhibitor, for 2 h prior to CHX treatment. This pre-treatment significantly decreased Oct4 degradation, indicating that it requires proteasome activity (Fig. 2A, Fig. S2B). In contrast, inhibition of the Akt kinase by Akti-1/2 markedly accelerated the degradation of endogenous Oct4, suggesting that Akt-mediated phosphorylation stabilizes intracellular Oct4 (Fig. 2B, Fig. S2C). To examine the relationship between Akt-mediated phosphorylation at T235 and the stability of Oct4, Flag-tagged Oct4-WT, Oct4-T235A, and Oct4-T235D (a residue substitution which mimics constitutive phosphorylation at T235) were each expressed in NCCIT cells, and then the cells treated with CHX for up to 24 h. Immunoblotting showed that Oct4-T235A protein degraded faster than Oct4-WT and Oct4-T235D degraded much more slowly with a longer half-life than Oct4-WT (Fig. 2C). Because we found that mouse ECC F9 cells contain much higher levels of Oct4-pT235 than mouse ECC P19 cells (Fig. 1G, Fig. S1D), we compared the turnover rate of endogenous Oct4 in these two cell lines. After CHX treatment, Oct4 protein degraded in F9 cells much more slowly than in P19 cells (Fig. 2D). Moreover, in the absence of CHX, 35S-labeled Oct4 also degraded more slowly in F9 cells (Fig. 2E), further supporting our hypothesis that Akt-mediated phosphorylation stabilizes Oct4 and slows its degradation. However, Akt-mediated phosphorylation of Oct4 did not seem to significantly affect its ubiquitination (Fig. S2D), indicating it may affect a step downstream of Oct4 ubiquitination such as the unfolding or translocation of ubiquitinated Oct4 prior to proteolysis. Collectively, our results suggest that an active proteasome targets Oct4 for degradation in ECCs, and Akt-mediated phosphorylation prevents the proteolysis thereby stabilizing Oct4 protein levels.
Figure 2. Akt-mediated phosphorylation stabilizes Oct4 and facilitates its nuclear localization in ECCs.
(A) NCCIT cells were pretreated with MG-132 or vehicle for 2 h, followed by addition of CHX and further incubation of 0 to 12 h still in the presence of MG-132. Whole cell lysates were immunoblotted with the indicated antibodies. (B) NCCIT cells were pretreated with vehicle or Akti-1/2 for 72 h, followed by addition of CHX and further incubation of 0 to 12 h. (C) NCCIT cells were transfected with Flag-POU5F1-variants, treated with CHX for the indicated time periods, and whole cell lysates were immunoblotted with the indicated antibodies. (D) F9 or P19 cells were treated with CHX for the indicated time periods, and whole cell lysates were immunoblotted with the indicated antibodies. (E) Slower turnover of endogenous Oct4 in F9 cells vs. P19 cells detected by 35S pulse-chase assay. (F) NCCIT cells were transfected with Flag-POU5F1-variants, and treated with vehicle or MG-132 for 6 h before being fixed and examined for the expressed Flag-Oct4 variants. Bars, 20 μm. (G) F9 cells were pre-treated with Akti-1/2 for 2 days, followed by treatment with MG-132 for 2 h, and then with leptomycin B (LMB) for another 6 h in the presence of both Akti-1/2 and MG-132. Bars, 20 μm.
Akt-mediated phosphorylation facilitates the nuclear localization of Oct4
We observed that T235 is positioned adjacent to the putative nuclear localization signal RKRKR in the Oct4 protein (Fig. S2E) (Pan et al., 2004) so we examined cells to determine if Akt-mediated phosphorylation affected the nuclear localization of Oct4. When overexpressed in NCCIT cells, Flag-tagged Oct4-WT, -T235A and -T235D proteins all exhibited predominant nuclear localization, although Oct4-T235A had the weakest fluorescent intensity (Fig. S2F). Remarkably, MG-132 treatment dramatically increased the amount of Flag-Oct4-T235A (from 0 to 83%), and to a lesser extent, that of Flag-Oct4-WT (from 0 to 21%) or Oct4-T235D (from 0 to 19%), in the cytoplasm indicating that Flag-Oct4-T235A was actively redistributed (but degraded) into the cytoplasm (Fig. 2F, Fig. S2G). MG-132 treatment caused a similar accumulation of endogenous Oct4 protein in the cytoplasm of both F9 cells (Fig. 2G) and P19 cells (Fig. S2H) that was dramatically increased by Akti-1/2 pre-treatment, suggesting that Akt-mediated phosphorylation can facilitate the nuclear localization of Oct4. The nuclear export inhibitor leptomycin B (LMB) partially reversed the effect of Akti-1/2, indicating that the translocation of nuclear Oct4 to the cytoplasm is at least partially dependent on the CRM1-mediated nuclear export pathway.
Akt-mediated phosphorylation promotes the interaction of Oct4 with other stemness factors
Oct4 is known to associate with Sox2 (Ambrosetti et al., 1997) and Klf4 can directly interact with Oct4 and Sox2 to promote somatic cell reprogramming (Wei et al., 2009). Therefore, we tested if Akt-mediated Oct4 phosphorylation could regulate its interaction with these two reprogramming factors. We detected binding between endogenous Oct4 and Sox2 in F9 cells (Fig. 3A) and NCCIT cells (Fig. S3A and S3B), but it was significantly attenuated by pre-treatment of cells with Akti-1/2, indicating that Akt-mediated Oct4 phosphorylation is required to maintain their interaction. In support of this observation, the Oct4-T235A mutant protein showed reduced interaction with Sox2 whereas the Oct4-T235D phosphorylation mimic mutant showed an increased association (Fig. 3B). Moreover, substantially more recombinant human Klf4 and human Sox2 co-immunoprecipitated with human Oct4 when active Akt1 and ATP were present (Fig. 3C), suggesting that Akt-phosphorylated Oct4 has a significantly higher affinity for Klf4 and Sox2 than the non-phosphorylated protein. Further supporting this hypothesis, Oct4-pT235 was significantly enriched in anti-Sox2 immunoprecipitates compared to whole cell lysates of P19 (Fig. S3C). In addition to forming heterodimers with Sox2 and other transcription factors, Oct4 can also form homodimers in several conformations (Kang et al., 2009). Therefore, we tested if Akt-mediated phosphorylation affected the homodimerization of Oct4 in our experimental system. However, when overexpressed in NCCIT cells, neither Oct4 mutant showed an altered dimerization pattern compared to Oct4-WT (Fig. S3D and S3E), indicating that the phosphorylation of T235 does not affect the homodimerization of Oct4.
Figure 3. Akt-mediated phosphorylation of Oct4 promotes its interaction with Sox2 and differentially regulates the transcription of key stemness genes containing Oct4-Sox2 binding motifs.
(A) F9 cells were treated with vehicle or Akti-1/2 for 2 days, cross-linked with formaldehyde, and immunoprecipitated with anti-Oct4. The immunocomplexes were subjected to immunoblotting with anti-Oct4 (upper) or anti-Sox2 (lower). (B) Enhanced interaction of ectopically expressed Flag-Oct4-T235D with endogenous Sox2 in NCCIT cells. (C) Akt-mediated phosphorylation of Oct4 enhances its binding with Sox2 and Klf4 in vitro. (D) Akt-mediated phosphorylation of Oct4 enhances its binding to the promoters of three key stemness genes in vitro. The arrow indicates the Oct4-Sox2-probe complexes. (E) Akt-mediated phosphorylation enhances Oct4 transcriptional activity in a reporter assay. (F) NCCIT cells were treated with vehicle or Akti-1/2 for 3 days and subjected to ChIP-based analysis. (G) NCCIT cells were treated with vehicle or Akti-1/2 for 3 days, and the transcription levels of POU5F1, NANOG and SOX2 determined by qRT-PCR. (H) NCCIT cells were treated in the same manner as (G) and samples subjected to immunoblotting. The error bars in the (E), (F) and (G) represent mean ± SD from three independent experiments. * and *** denotes P < 0.05 and P < 0.001, respectively.
Akt-mediated phosphorylation of Oct4 differentially regulates the transcription of stemness genes containing Oct4-Sox2 binding motifs
The enhancer or promoter regions of the POU5F1, SOX2 (Chew et al., 2005) and NANOG genes (Kuroda et al., 2005) all contain Oct4-Sox2 binding motifs and the transcription of these three key stemness genes is under the combinatorial control of Oct4 and Sox2 (Fig. S3F). We asked what the effect of Akt-mediated Oct4 phosphorylation had on Oct4 binding to these promoter and enhancer sequences in in vitro electrophoretic mobility shift assays (EMSA). We found that when complexed with Sox2, phosphorylated Oct4 exhibited substantially higher binding affinity than non-phosphorylated Oct4 for the promoters of all three genes (Fig. 3D). To determine if Akt-mediated phosphorylation of Oct4 could affect its transcriptional activity, POU5F1-WT, POU5F1-T235A, and POU5F1-T235D genes were transfected into 293T cells together with Oct4 promoter-luciferase and Renilla reporter plasmids. Transcriptional activity of Oct4 was measured by the relative luciferase activity. Compared to Oct4-WT, Oct4-T235D enhanced the luciferase activity by 200% whereas Oct4-T235A reduced it by 50% (Fig. 3E), suggesting that T235 phosphorylation by Akt promotes the transcriptional activity of Oct4. To determine the intracellular binding and transcription, we conducted the ChIP analysis with Akti-1/2 or vehicle-treated NCCIT cells. The binding of Oct4 to the promoters of all the three genes was significantly reduced by Akti-1/2 treatment (Fig. 3F). Interestingly, the transcription of POU5F1 and NANOG was reduced, but transcription of SOX2 was enhanced by the Akti-1/2 treatment (Fig. 3G). In agreement, we showed the inhibition of Oct4 phosphorylation by Akti-1/2 reduced the protein levels of Oct4 and Nanog, but not Sox2 (Fig. 3H). Therefore, we conclude that Akt-mediated phosphorylation of Oct4 differentially controls the transcription levels of POU5F1, NANOG and SOX2, most likely by regulating its interaction with Sox2 and/or its binding affinity to the target promoter regions.
Akt-mediated phosphorylation of Oct4 promotes the self-renewal of ECCs and specifically suppresses their differentiation toward ectoderm
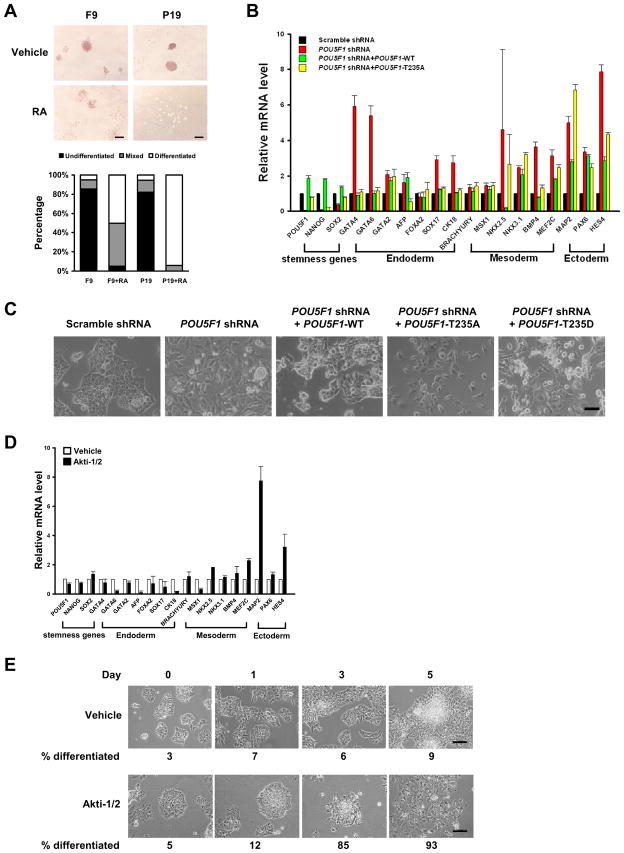
We found that F9 and P19 cells have similar levels of total Oct4 but remarkably different levels of Akt-phosphorylated Oct4 (Fig. 1G, Fig. S1D). To compare the self-renewal capacities of these cells, retinoic acid (RA) was employed to induce cell differentiation. Alkaline phosphatase (ALP) staining assay showed that F9 is more resistant to RA than P19 cells (Fig. 4A) suggestive of the higher self-renewal capacity of the former cell.
Figure 4. Akt-mediated phosphorylation of Oct4 promotes the self-renewal of ECCs and specifically suppresses their differentiation toward ectoderm.
(A) F9 and P19 cells were treated with vehicle or 1 μM retinoic acid (RA) for 4 days before being stained for Alkaline Phosphatase. Bars, 200 μm. 50 to 65 colonies from triplicate wells (i.e., a total of 150 to 200 colonies) in each group were scored and the percentages of cells of each category were plotted. (B) Knock down of endogenous Oct4 and overexpressing Flag-Oct4-T235A in NCCIT cells leads to down-regulation of stemness markers and up-regulation of ectodermal lineage markers. (C) Morphological changes of NCCIT cells knocked down of the endogenous Oct4 and overexpressing Flag-POU5F1 variants. Bar, 100 μm. (D) NCCIT cells were treated with vehicle or Akti-1/2 for 5 days, and the expression levels of lineage-specific markers were determined by qRT-PCR. (E) Morphological changes of NCCIT cells treated with Akti-1/2. The average percentages of differentiated cells were given at the bottom. Bars, 200 μm. The error bars in the (B) and (D) represent mean ± SD from three independent experiments.
To define the role of Oct4 phosphorylation at T235 on ECC self-renewal and differentiation, lentiviruses harboring a single POU5F1 shRNA or both the POU5F1 shRNA and one of the Flag-POU5F1 variants were generated and used to infect NCCIT cells to functionally replace the endogenous Oct4 with expressed Oct4-T235 mutants. The POU5F1 shRNA that targets the 3′-UTR of POU5F1 efficiently decreased both Oct4 mRNA (Fig. 4B) and protein (Fig. S4A) levels by >95%. It also dramatically reduced the mRNA level of NANOG, and to a lesser extent, that of SOX2 (Fig. 4B). Under such conditions, most lineage-specific markers of the three germ layers were up-regulated (red bars, Fig. 4B) and this was accompanied by flattened cell morphology indicative of differentiation (Fig. 4C). Because a >50% increase or decrease of POU5F1 mRNA level in ESCs resulted in their differentiation toward primitive endoderm/mesoderm or trophectoderm, respectively (Niwa et al., 2000), lentiviral infection conditions were optimized to allow a <50% increase or decrease of the expressed Flag-POU5F1-T235A mRNA level over endogenous POU5F1 level. The replacement of endogenous Oct4 with ectopically expressed Oct4-T235A significantly up-regulated the expression of ectodermal markers, and to a lesser extent, that of mesodermal markers, but not that of endodermal markers (yellow bars, Fig. 4B). Unexpectedly, replacing endogenous Oct4 with Oct4-T235D resulted in a global up-regulation of almost all of the examined lineage-specific markers (Fig. S4B).
Consistent with the results of Oct4-T235A replacement above, in NCCIT cells treated with Akti-1/2, most ectodermal markers were up-regulated while endodermal markers were down-regulated (Fig. 4D), resulting in differentiation toward a neuronal morphology (Fig. 4E). Taken together, our results indicate that the phosphorylation of Oct4-T235 promotes the self-renewal of ECCs and specifically suppresses their differentiation toward ectoderm by directing their differentiation toward mesendodermal lineages.
Oct4 regulates the transcription of AKT1 gene
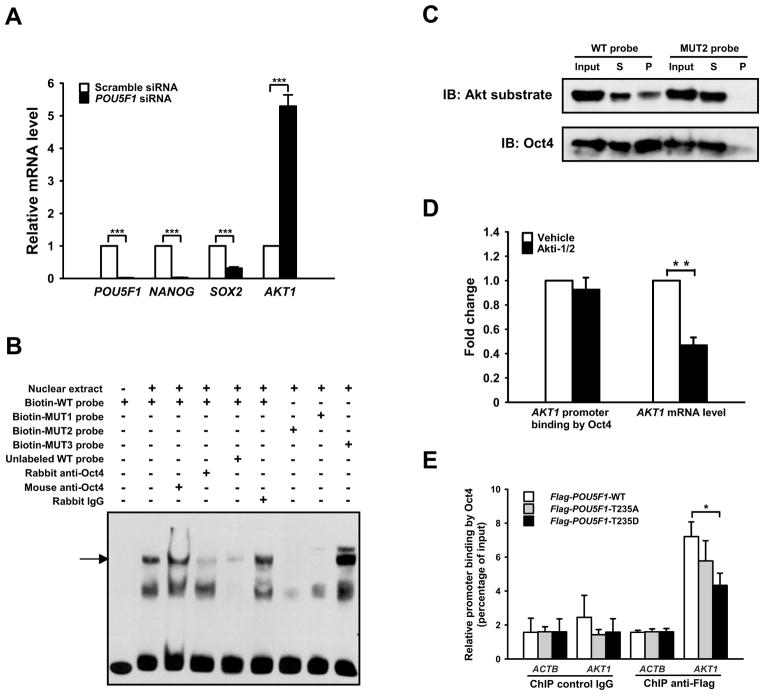
The expression of Oct4 in various human cancers (Chiou et al., 2010; Mathieu et al., 2011; Peng et al., 2010) and its roles in the self-renewal of epithelial progenitor cells (Hochedlinger et al., 2005) and adult stem cells (Tai et al., 2005) support the CSC hypothesis that proposes cancers arise in stem cells or early committed progenitors due to their acquired abilities to self-renew and differentiate (Sell and Pierce, 1994). A global gene expression profiling study revealed that apoptosis-repressing genes were positively correlated to pou5f1 transcription while apoptosis-inducing genes were negatively correlated (Campbell et al., 2007). These results suggest Oct4 may directly regulate the transcription of apoptosis-repressing genes such as AKT1. To test if reducing Oct4 protein levels affected the transcription of AKT1, specific siRNAs against POU5F1 that decreased the total Oct4 mRNA and protein levels by >90% were introduced into NCCIT cells (Fig. 5A, Fig. S5A and S5B). Under these conditions, the transcription of AKT1 was dramatically enhanced (Fig. 5A) and was accompanied by an increase in both phosphorylated forms of Akt (Fig. S5A).
Figure 5. Oct4 regulates the transcription of AKT1 gene.
(A) NCCIT cells were transfected with scramble siRNA or POU5F1 siRNAs, and the mRNA levels of POU5F1, NANOG, SOX2, and AKT1 were determined by qRT-PCR. (B) Electrophoretic mobility shift assay of biotinylated AKT1 probes. The arrow indicates Oct4-bound probe. (C) Biotinylated DNA pull-down assay. Biotinylated WT or MUT2 probe was incubated with NCCIT nuclear extract, followed by addition of streptavidin conjugated agarose beads. The bead-DNA-protein complexes were collected and immunoblotted with anti-Oct4. The membrane was then stripped and re-probed with anti-phospho-Akt substrate (RXXpS/pT) antibody. (D) NCCIT cells were treated with vehicle or Akti-1/2 for 3 days. ChIP-based analysis was carried out with anti-Oct4, and the mRNA level of AKT1 was determined by qRT-PCR. The derived values of Akti-1/2-treated samples were then normalized against the vehicle-treated samples. (E) NCCIT cells were infected with lentiviruses harboring Flag-POU5F1 variants, and ChIP-based analysis was carried out with anti-Flag M2 beads. The error bars in the (A), (D) and (E) represent mean ± SD from three independent experiments. *, ** and *** denotes P < 0.05, P < 0.01, and P < 0.001 respectively.
To test if Oct4 regulates AKT1 transcription by binding directly to its promoter region, −700 bp to +299 bp of DNA relative to transcription start site of the AKT1 gene was analyzed for potential Oct4 binding sites. Three candidate Oct4 binding sites were identified (Fig. S5C) and an AKT1 promoter probe spanning each site (Fig. S5D) was incubated with NCCIT nuclear extracts then analyzed by EMSA. One probe (−388 bp to −354 bp relative to transcription start site) spanning the octamer GTAATTAT was associated with NCCIT nuclear proteins (Fig. S5E). Mutations introduced into the middle of the octamer (MUT2) resulted in a significantly reduced association between the probe and the nuclear proteins, whereas mutations in the flanking regions had a lesser effect (Fig. 5B). When we added anti-Oct4 antibody to the biotin-probe/nuclear extract mixture, it caused the specific disappearance of the protein-associated probe band, indicating that the probe was binding to Oct4 in vitro (Fig. 5B). Furthermore, when streptavidin beads were added to the mixture and used to pull down the biotin-probes and their binding partners, Oct4 was detected in the pull-down complex. In contrast, considerably less Oct4 was pulled down by the MUT2 probe, indicating that the binding between Oct4 and AKT1 octamer is specific (Fig. 5C). The membrane was stripped and re-probed with the phospho-Akt substrate antibody and a band overlapping the Oct4 band was observed. However, the ratio of Akt-phosphorylated Oct4 to total Oct4 was much lower in pull-down sample than in whole nuclear fraction (input), indicating that it is T235-non-phosphorylated form of Oct4 (which could have phosphorylated residues at other sites) that preferentially binds to the AKT1 promoter in vitro (Fig. 5C).
We confirmed intracellular binding of Oct4 to the AKT1 promoter using ChIP assays (Fig. 5D, Fig. 5E). NCCIT cells were treated with Akti-1/2 for three days, after which time both total and Akt-phosphorylated Oct4 protein levels were reduced, with reduction of the latter more prominent (Fig. 3H, Fig. S1C). Under such conditions, no significant change in the binding of Oct4 to the AKT1 promoter was observed (Fig. 5D, Fig. S5F). Unlike POU5F1 siRNA treatment, however, Akti-1/2 treatment significantly decreased the AKT1 mRNA level (Fig. 5D, Fig. S5G), indicating that Akt-mediated Oct4 phosphorylation is required for the transcription of AKT1 and additional Akt-dependent factors may also contribute to the process. When ectopically overexpressed in NCCIT cells, Flag-Oct4-T235D showed reduced binding to the AKT1 promoter compared to its WT counterpart (Fig. 5E). Therefore, the data suggest that Oct4 binds to the AKT1 promoter to repress AKT1 transcription and that phosphorylation of Oct4 at T235 causes its disassociation from the AKT1 promoter, thereby activating AKT1 transcription and promoting cell survival.
Akt-mediated phosphorylation of Oct4 promotes the survival and tumorigenicity of ECCs
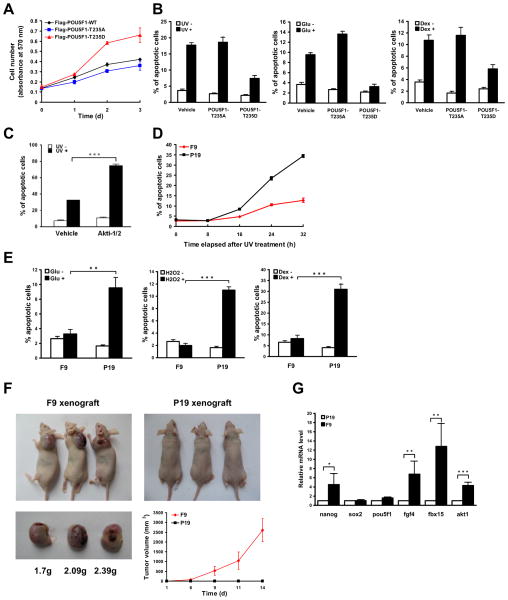
Because the total replacement of endogenous Oct4 with either the Flag-Oct4-T235A or -T235D mutant led to the differentiation of NCCIT cells (Fig. 4B, Fig. 4C, Fig. S4B), we reasoned that some amount of wild type Oct4 is required to maintain the stemness of ECC cells. Therefore, Flag-POU5F1-T235A, -T235D and -WT (as a control) were individually expressed at low levels in NCCIT cells (Fig. S6A and S6B), to partially displace the endogenous Oct4. Under these conditions, the growth of the cells expressing Flag-POU5F1-T235D was approximately 2 times faster than those expressing the -T235A or -WT constructs (Fig. 6A). The T235D cells were also more resistant to a variety of apoptotic stimuli including UV irradiation, high glucose and dexamethasone treatment (Fig. 6B). The results indicate that site-specific phosphorylation of T235 in Oct4 promotes the proliferation and survival of ECCs. Furthermore, in Akti-1/2-treated NCCIT cells, UV-induced apoptosis was greatly augmented (Fig. 6C, Fig. S6C) whereas cell proliferation was largely inhibited (Fig. S5H), consistent with a regulatory role for Akt-mediated T235 phosphorylation in those processes.
Figure 6. Akt-mediated phosphorylation of Oct4 promotes the survival and tumorigenicity of ECCs.
(A) NCCIT cells were infected with lentiviruses harboring Flag-POU5F1 variants at an MOI of 1. Cell proliferation was determined with the MTT assay at various time points. (B) The above NCCIT cells were stresses with 100 J/m2 of ultraviolet (UV) light, 300 μM glucose (Glu), or 600 μM dexamethasone (Dex), and analyzed by flow cytometry to determine the percentage of apoptotic cells. (C) NCCIT cells pre-treated with vehicle or Akti-1/2 for 48 h were subjected to 100 J/m2 of UV, and analyzed for the percentage of apoptotic cells 16 h after UV exposure. (D) F9 cells and P19 cells were subjected to 20 J/m2 of UV light, and analyzed for the percentages of apoptotic cells at various time points after UV exposure. (E) F9 and P19 cells were stresses with 300 μM glucose (Glu), 1.5 mM H2O2 or 600 μM dexamethasone (Dex), and analyzed for the percentage of apoptotic cells. (F) 2.5×106 of F9 and P19 cells were inoculated subcutaneously into each female athymic nude (nu/nu) mouse and inoculation was conducted in triplicate. The external diameter of the tumors was measured at various time points up to 2 weeks, and the volume of the tumors was calculated and plotted. The mice were killed and tumors were weighed 2 weeks after inoculation. (G) The total mRNAs in F9 and P19 cells were isolated and the mRNA levels of fgf4, akt1 and four stemness genes were determined by qRT-PCR, and normalized to that of gapdh. The derived values of F9 cells were then normalized to those of P19 cells. The error bars in all panels represent mean ± SD from three independent experiments. *, ** and *** denotes P < 0.05, P < 0.01, and P < 0.001 respectively.
As previously noted, in spite of having similar levels of total Oct4, F9 cells contain a dramatically higher level of Oct4-pT235 than P19 cells (Fig. 1G, Fig. S1D). Therefore, comparing the anti-apoptotic capability and tumorigenicity of these two cell lines is of particular interest and relevance. First, the two cell lines were exposed to 20 J/m2 of UV irradiation followed by measuring the percent of apoptotic cells at various time points. F9 cells were more resistant to UV-induced apoptosis than P19 cells (Fig. 6D, Fig. S6D); Second, P19 cells stressed with 300 μM glucose, 1.5 mM H2O2 or 600 μM dexamethasone displayed much higher apoptotic rates than F9 cells (Fig. 6E); Third, P19 cells overexpressing HA-AKT1-CA were more resistant to apoptotic stimuli than parental cells or cells transfected with HA-AKT1-KD (Fig. S6E and S6F); Finally, F9 cells were more tumorigenic than P19 cells in xenografting experiments (Fig. 6F), which is consistent with previous findings obtained from teratoma formation assays, nuclear cloning assays (Blelloch et al., 2004) and mouse embryo transplantation (Astigiano et al., 2005). To investigate the mechanism underlying the observed differences for P19 and F9 cells, the transcription levels of four stemness genes (nanog, pou5f1, sox2 and fbx15) and the cell growth gene fgf4, all of which contain an Oct4-Sox2 binding motif, were compared between F9 cells and P19 cells. With the exception of sox2, the expression of all these genes was found to be higher in F9 cells than in P19 cells (Fig. 6G); akt1, which is likely regulated by Oct4, was also present at higher levels in F9 cells than in P19 cells (Fig. 6G). Taken together, our data suggest Akt-mediated phosphorylation of Oct4 promotes the survival and tumorigenicity of ECCs, possibly by up-regulating the expression of certain stemness genes and cell survival genes.
DISCUSSION
In addition to being an established, major anti-apoptosis and cell survival factor in many types of cancer (Carnero, 2010; Vivanco and Sawyers, 2002), Akt has also been implicated as an important regulator of stemness (Armstrong et al., 2006; Singh et al., 2012; Watanabe et al., 2006). Moreover, emerging evidence indicates that the PI3K-Akt signaling pathway plays a key role in CSC biology (Bleau et al., 2009; Dubrovska et al., 2009; Li and Zhou, 2011), and could be an effective drug target for killing CSCs (Eyler et al., 2008; Korkaya et al., 2009; Martelli et al., 2011). However, it remains unclear if and how the two roles of Akt are interconnected in the context of CSCs. By directly linking Akt to the master pluripotency factor Oct4, we uncovered a fundamental mechanism underlying both the stemness and tumorigenicity of ECCs, which may also function in other CSCs.
Because substantial levels of active Akt protein are detected in ESCs, and known to be critical for maintaining their self-renewal (Singh et al., 2012; Watanabe et al., 2006), it is puzzling but noteworthy that the levels of Oct4-pT235 in ESCs appear to be much lower than the levels in ECCs. A previous large-scale phosphoproteomic analysis failed to identify Oct4-T235 as a phosphorylated residue in human ESCs (Swaney et al., 2009). Very recently, Thomson’s laboratory (Brumbaugh et al., 2012) combined high accuracy mass spectrometry with multiple dissociation techniques to examine Oct4 in human ESCs. Although the corresponding kinases were not identified in their study, they identified 14 phosphorylation sites on Oct4, which included T235 (referred to as T234 in the cited reference), confirming that Oct4-T235 is a bona fide phosphorylation site. It seems likely that certain mechanisms may exist in ESCs that maintain the T235 phosphorylation of Oct4 at low levels. Therefore, it could be important to search for serine/threonine phosphatases that are differentially expressed in ECCs and ESCs that could potentially maintain different phosphorylation levels of Oct4-pT235 in these two cell types.
In addition, the phosphorylation status of T235 could also be affected by the phosphorylation or other PTMs of adjacent residue(s) in Oct4. For instance, the large-scale phosphoproteomic analysis by Swaney et al. identified another phosphorylation site on Oct4 at S236 (Swaney et al., 2009), which is directly next to the T235 Akt-phosphorylation site. S236 (referred to as S235 in the cited reference) was also confirmed as a phosphorylation site by the Brumbaugh et al. study (Brumbaugh et al., 2012). Because bioinformatic analyses has indicated that S236 is phosphorylated by protein kinase A (Saxe et al., 2009), these findings raise critical questions regarding if and how crosstalk occurs between different signaling pathways via PTMs at different sites on Oct4. Therefore, it will be of interest to determine if PTMs at other sites (in particular sites adjacent to T235, see Fig. S2E) on Oct4 can account for the different levels of T235 phosphorylation observed in ECCs and ESCs. Very recently, it was reported that the T228 in mouse Oct4, which is equivalent to T235 in human Oct4, is O-GlcNAcylated in mouse ESCs (Jang et al., 2012). This has raised the intriguing possibility that Oct4 is modulated antagonistically by Akt-mediated phosphorylation and O-GlcNAcylation, and warrants further studies to systematically compare the status of these two PTMs of Oct4-T235 in ESCs and ECCs from human and mouse origin.
In the current study, we provide evidence showing that the site-specific phosphorylation of Oct4 can have different effects on its binding to the cis- regulatory elements of different target genes. It enhanced Oct4 binding to genes that it transcribes, which contain Oct4-Sox2 binding motifs (NANOG, POU5F1 and SOX2) but promoted its disassociation from the AKT1, which it represses and which does not contain a typical Oct4-Sox2 binding motif. Depending on the specific sequence, Oct4 binds to cis- regulatory elements in various modes or forms, including as a heterodimer with Sox2, a MORE-type or PORE-type homodimer, or a monomer (Jung et al., 2010; Remenyi et al., 2004). Therefore, Akt-mediated phosphorylation of Oct4 at T235 could modulate transcriptional profiles by altering the binding affinity of Oct4 that is in a particular binding mode for its target sequences, or by switching Oct4 from one binding mode to another. Compared to the sox-oct binding motifs in the mouse nanog and pou5f1 genes (Chew et al., 2005), the Oct4-Sox2 binding motifs in the human NANOG and POU5F1 genes are in reverse orientation with respect to each other [(Jung et al., 2010; Kuroda et al., 2005), Fig. S3F]. In contrast, the orientation of the Sox2-Oct4 binding motifs in the human SOX2 gene is the same as the mouse sox2 gene (Fig. S3F). This reversed orientation of the binding of Oct4/Sox2 complex might result in opposite transcriptional effects and may account for the different regulation of NANOG and POU5F1 transcription (up-regulated) versus SOX2 transcription (down-regulated) mediated by Akt-phosphorylated Oct4-T235 in our study. Recent reports suggest that Sox2 expression in ESCs can repress mesendodermal differentiation and promote neural ectodermal differentiation (Thomson et al., 2011; Wang et al., 2012). Our results are consistent with the hypothesis that the phosphorylation of Oct4-T235 by Akt could specifically suppress the differentiation of ECCs toward ectoderm by down-regulating Sox2 transcription.
Based on our findings that Akt-phosphorylated Oct4 is ubiquitously present in ECCs and its levels positively correlate with resistance to apoptosis and tumorigenic potential, we propose that Akt-phosphorylated Oct4 may be a useful biomarker for the tumorigenicity of pluripotent stem cells. The difference between ECCs and ESCs in Oct4 and Akt crosstalk may underlie the adaptation of ECCs towards tumorigenic growth by promoting self-renewal and survival, together with a reduced dependency on external cues for self-maintenance. Of interest, concurrent elevated Oct4 expression and Akt activation has been reported in some somatic tumor cells, where they are responsible for chemotherapeutic drug resistance (Wang et al., 2010). Further study to decipher functional crosstalk between the Oct4 and Akt pathways in various cancer cells may identify targets for the development of more effective and safer therapeutic interventions for killing CSCs.
EXPERIMENTAL PROCEDURES
Cell culture, transfection and treatment
The 293T, F9, P19, NCCIT and NTERA-2 cells were obtained from ATCC and cultured in DMEM (Invitrogen 21063-029) supplemented with 10% fetal bovine serum (GIBCO 10099) and 1% Penicillin/Streptomycin (GIBCO 15140-148). The mESC lines D3 and E14 from ATCC and S6 from Dr. Lei Xiao’s laboratory were cultured on gelatin-coated tissue culture plates in DMEM supplemented with 15% fetal bovine serum, 0.1 mM 2-Mercaptoethanol, 2 mM L-glutamine, 1 × NEAA and 10 ng/ml LIF (Chemicon LIF2010). The source and culture conditions for the hESC line H1 (WA01) were as previously described (Wu et al., 2008). For transfection, cells were grown on 6-well plates to 30–50% confluence then transfected with 1–3 μg of DNA per well using GeneTran reagent (Biomiga GT1211) or SuperFectin II. Where appropriate, cells were treated with 5 μM Akti-1/2, 20 μg/ml Cycloheximide (CHX), 10 μM MG-132 or 20 ng/ml leptomycin B (LMB) (with DMSO as the vehicle) either individually or in combination for the indicated durations.
Statistical analysis
All quantitative data are presented as means ± SD of three independent experiments. The statistical significance of compared measurements was evaluated using the two-tailed unpaired Student’s t test, and p ≤ 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Oct4 is phosphorylated at T235 by Akt in embryonal carcinoma cells
Akt-mediated phosphorylation of Oct4 activates the transcription of AKT1
Phosphorylation of Oct4 increases its stability and regulates the transcription of stemness genes
Levels of Akt-phosphorylated Oct4 correlate with resistance to apoptosis
Acknowledgments
We thank Drs. Kun-Liang Guan and Xin-Hua Feng for critical reading of the manuscript; Dr. Jinrong Peng for sharing lab facilities; Dr. Zhiming Yu and Jie Cheng for technical assistance; Qucheng Wei for preparing the schematic diagram; Dan Xu and Ping Dong for administrative assistance; Dr. Margaret Morgan for expert editing of the manuscript. This work was funded by the “863 Projects” of Ministry of Science and Technology of PR China (Y.-J.W.; No. 2011AA020118), an internal grant from the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, China (Y.-J.W.; 2010ZZ02), the McCabe award from Perelman School of Medicine and the URF award from University of Pennsylvania (H.Z.), and an NIH grant (B.S.; R01CA085344).
Footnotes
Author contributions: Y.-J.W. and H.Z. conceived the study. Y.-J.W. designed and oversaw most experiments. Y.L., Y.Y., W.L. and Q.C. performed the experiments with critical assistance from J.L., X.P., L.Z., C.L., C.C., J.H., H.C., H.Y. and L.Z.. X.X., Z.X., J.R. and L.X. provided key cell lines and reagents. Y.-J.W., H.Z., B.S., Y.L. and Y.Y. analyzed the data. Y.-J.W. wrote the manuscript with the input from H.Z., B.S. and L.L.
Competing financial interests: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosetti DC, Basilico C, Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 1997;17:6321–6329. doi: 10.1128/mcb.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005;33:1526–1530. doi: 10.1042/BST0331526. [DOI] [PubMed] [Google Scholar]
- Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- Astigiano S, Damonte P, Fossati S, Boni L, Barbieri O. Fate of embryonal carcinoma cells injected into postimplantation mouse embryos. Differentiation. 2005;73:484–490. doi: 10.1111/j.1432-0436.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blelloch RH, Hochedlinger K, Yamada Y, Brennan C, Kim M, Mintz B, Chin L, Jaenisch R. Nuclear cloning of embryonal carcinoma cells. Proc Natl Acad Sci U S A. 2004;101:13985–13990. doi: 10.1073/pnas.0405015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- Brumbaugh J, Hou Z, Russell JD, Howden SE, Yu P, Ledvina AR, Coon JJ, Thomson JA. Phosphorylation regulates human OCT4. Proc Natl Acad Sci U S A. 2012;109:7162–7168. doi: 10.1073/pnas.1203874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PA, Perez-Iratxeta C, Andrade-Navarro MA, Rudnicki MA. Oct4 targets regulatory nodes to modulate stem cell function. PLoS One. 2007;2:e553. doi: 10.1371/journal.pone.0000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- Chaerkady R, Kerr CL, Kandasamy K, Marimuthu A, Gearhart JD, Pandey A. Comparative proteomics of human embryonic stem cells and embryonal carcinoma cells. Proteomics. 2010;10:1359–1373. doi: 10.1002/pmic.200900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL, Wu CL. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008;68:6281–6291. doi: 10.1158/0008-5472.CAN-08-0094. [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. O-GlcNAc Regulates Pluripotency and Reprogramming by Directly Acting on Core Components of the Pluripotency Network. Cell Stem Cell. 2012;11:62–74. doi: 10.1016/j.stem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Jung M, Peterson H, Chavez L, Kahlem P, Lehrach H, Vilo J, Adjaye J. A data integration approach to mapping OCT4 gene regulatory networks operative in embryonic stem cells and embryonal carcinoma cells. PLoS One. 2010;5:e10709. doi: 10.1371/journal.pone.0010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Shakya A, Tantin D. Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem Sci. 2009;34:491–499. doi: 10.1016/j.tibs.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shin S, Zeng X, Zhan M, Gonzalez R, Mueller FJ, Schwartz CM, Xue H, Li H, Baker SC, et al. Genome wide profiling of human embryonic stem cells (hESCs), their derivatives and embryonal carcinoma cells to develop base profiles of U.S. Federal government approved hESC lines. BMC Dev Biol. 2006;6:20. doi: 10.1186/1471-213X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Follo MY, Ramazzotti G, Fini M, Giardino R, Manzoli L, McCubrey JA, Cocco L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells. Curr Med Chem. 2011;18:2715–2726. doi: 10.2174/092986711796011201. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Surani MA. The transcriptional and signalling networks of pluripotency. Nat Cell Biol. 2011;13:490–496. doi: 10.1038/ncb0511-490. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Pan G, Qin B, Liu N, Scholer HR, Pei D. Identification of a nuclear localization signal in OCT4 and generation of a dominant negative mutant by its ablation. J Biol Chem. 2004;279:37013–37020. doi: 10.1074/jbc.M405117200. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Pardo M, Lang B, Yu L, Prosser H, Bradley A, Babu MM, Choudhary J. An expanded Oct4 interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell. 2010;6:382–395. doi: 10.1016/j.stem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- Remenyi A, Scholer HR, Wilmanns M. Combinatorial control of gene expression. Nat Struct Mol Biol. 2004;11:812–815. doi: 10.1038/nsmb820. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Saxe JP, Tomilin A, Scholer HR, Plath K, Huang J. Post-translational regulation of Oct4 transcriptional activity. PLoS One. 2009;4:e4467. doi: 10.1371/journal.pone.0004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70:6–22. [PubMed] [Google Scholar]
- Sharif T, Auger C, Bronner C, Alhosin M, Klein T, Etienne-Selloum N, Schini-Kerth VB, Fuhrmann G. Selective proapoptotic activity of polyphenols from red wine on teratocarcinoma cell, a model of cancer stem-like cell. Invest New Drugs. 2011;29:239–247. doi: 10.1007/s10637-009-9352-3. [DOI] [PubMed] [Google Scholar]
- Silvan U, Diez-Torre A, Arluzea J, Andrade R, Silio M, Arechaga J. Hypoxia and pluripotency in embryonic and embryonal carcinoma stem cell biology. Differentiation. 2009;78:159–168. doi: 10.1016/j.diff.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, Menendez L, Kulik M, Dalton S. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312–326. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney DL, Wenger CD, Thomson JA, Coon JJ. Human embryonic stem cell phosphoproteome revealed by electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A. 2009;106:995–1000. doi: 10.1073/pnas.0811964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RT, Fan ST. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- Wei F, Scholer HR, Atchison ML. Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. J Biol Chem. 2007;282:21551–21560. doi: 10.1074/jbc.M611041200. [DOI] [PubMed] [Google Scholar]
- Wei Z, Yang Y, Zhang P, Andrianakos R, Hasegawa K, Lyu J, Chen X, Bai G, Liu C, Pera M, et al. Klf4 interacts directly with Oct4 and Sox2 to promote reprogramming. Stem Cells. 2009;27:2969–2978. doi: 10.1002/stem.231. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zhang W, Chen G, Cheng L, Liao J, Jia N, Gao Y, Dai H, Yuan J, Xiao L. Combinatorial signals of activin/nodal and bone morphogenic protein regulate the early lineage segregation of human embryonic stem cells. J Biol Chem. 2008;283:24991–25002. doi: 10.1074/jbc.M803893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang W, Li C, Yu H, Yang A, Wang B, Jin Y. WWP2 promotes degradation of transcription factor OCT4 in human embryonic stem cells. Cell Res. 2009;19:561–573. doi: 10.1038/cr.2009.31. [DOI] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.