Abstract
A high-throughput screen identified adamantane dipeptide 1 as an inhibitor of Ebola virus (EboV) infection. Hit-to-lead optimization to determine the structure–activity relationship (SAR) identified the more potent EboV inhibitor 2 and a photoaffinity labeling agent 3. These antiviral compounds were employed to identify the target as Niemann-Pick C1 (NPC1), a host protein that binds the EboV glycoprotein and is essential for infection. These studies establish NPC1 as a promising target for antiviral therapy.
Keywords: Ebola virus, small molecule inhibitor, viral entry, photoaffinity label, target identification, Niemann-Pick C1
Transmission of Ebola virus (EboV) from infected primates to humans causes outbreaks of hemorrhagic fever. The massive production of proinflammatory cytokines in response to infection causes capillary leak and hypovolemic shock. Mortality exceeds 50% in many outbreaks.1,2
Recent studies indicate how EboV enters cells: virus particles adhere to lectins on the surface of susceptible cells and are taken up and transported to late endosomes/lysosomes (LE/LY) containing host proteins cathepsin B and Niemann-Pick C1 (NPC1),3−10 which are essential for infection. These studies show that EboV glycoprotein (GP) is cleaved by cathepsin protease and that cleaved GP is a ligand for NPC1.8,9
We recently reported the identification of small molecules that inhibit EboV entry.8 The adamantane dipeptide 1 was identified in a high-throughput screen for compounds that specifically inhibit infection by vesicular stomatitis virus (VSV) particles pseudotyped with EboV Zaire GP but not VSV G or Lassa fever virus GP (Figure 1). Because 1 is specific and nontoxic, the structure–activity relationship (SAR) was determined to increase potency and identify position(s) to attach functional groups for target identification. Herein, we describe the hit-to-lead optimization of 1 leading to 2 as well as derivatization of 2 into a photoaffinity labeling agent 3 that established NPC1 as the molecular target. Compounds 1–3 prevent infection by targeting NPC1 and interfering with binding of NPC1 to protease-cleaved EboV GP.8
Figure 1.
Structures of compounds 1–3.
The strategy to obtain SAR was based on dividing 1 into three parts, adamantanacetyl group, glycine linker, and benzylpiperazine tail, and testing derivatives using the cell-based infection assay to assess potency, specificity, and cytotoxicity. At the outset, the effect of replacing the adamantanacetyl group with other hydrophobic moieties was tested. Several analogues were synthesized in a straightforward manner as shown in Scheme 1.
Scheme 1. Synthesis of 4a–g.
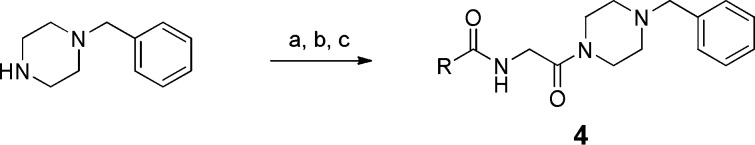
Reagents and conditions: (a) BocNCH2CO2H, BOP, (i-Pr)2NEt, DMF. (b) TFA, DCM. (c) Adamantyl-CH2COCl, (i-Pr)2NEt, DCM.
Thus, coupling of 1-benzylpiperazine with Boc-glycine followed by deprotection generated 4-benzylpiperazine glycinamide, and the terminal amine was acylated to give the product 4. We found that the replacement of the adamantane group abolished antiviral activity (Table 1). Also, we noted that the presence of the methylene spacer linking the adamantane to the central glycine was also essential (1 vs 4g).
Table 1. Anti-EboV Activity of Amide Analogues of 4.
| entry | R | IC50 (μM) |
|---|---|---|
| 1 | adamantyl-CH2– | 1.3 |
| 4a | c-Hex– | 27 |
| 4b | Ph– | 31 |
| 4c | c-HexCH2– | >40 |
| 4d | PhCH2– | >40 |
| 4e | (4-Cl-Ph)CH2– | 19 |
| 4f | (CH3)3CCH2– | >40 |
| 4g | adamantyl– | 29 |
To determine whether the glycine linker was critical to the function of 1, several analogues that harbor a different amino acid linker were prepared and tested (Scheme 2 and Table 2). The addition of either functionalized or unfunctionalized side chains as small as a methyl group (5a) on the α-carbon of the glycine linker was highly deleterious. Of note, when side chains are present, increasing their mass and hydrophobicity improved antiviral activity slightly (5i > 5d > 5a). Homologation (5b) and conformational restriction (5k) of the linker also rendered the compound significantly less active, as did N-methylation (5j), thus suggesting that 1 binds to the target in an extended conformation that is perturbed by modification of the adamantaneacetyl or the glycine moieties.
Scheme 2. Synthesis of 5a–k.
Reagents and conditions: (a) BocN-X-CO2H, BOP, (i-Pr)2NEt, DMF. (b) TFA, DCM. (c) Adamantyl-CH2COCl, (i-Pr)2NEt, DCM.
Table 2. Anti-EboV Activity of Linker Variants 5.
| entry | R | X | IC50 (μM) |
|---|---|---|---|
| 1 | H | –CH2– | 1.3 |
| 5a | H | –CH(CH3)– | >40 |
| 5b | H | –CH2CH2– | 17 |
| 5c | H | –CH(i-Pr)– | >40 |
| 5d | H | –CH(i-Bu)– | 29 |
| 5e | H | –CH(CH2CN)– | 22 |
| 5f | H | –CH(CH(OH)CH3)– | >40 |
| 5g | H | –CH(CH(OBn)CH3)– | 29 |
| 5h | H | –CH(CH2CH2CO2H)– | >40 |
| 5i | H | –CH(CH2CH2CO2Bn)– | 8.7 |
| 5j | CH3 | –CH2– | 6.7 |
| 5k | –CH2CH2CH2– | 22 |
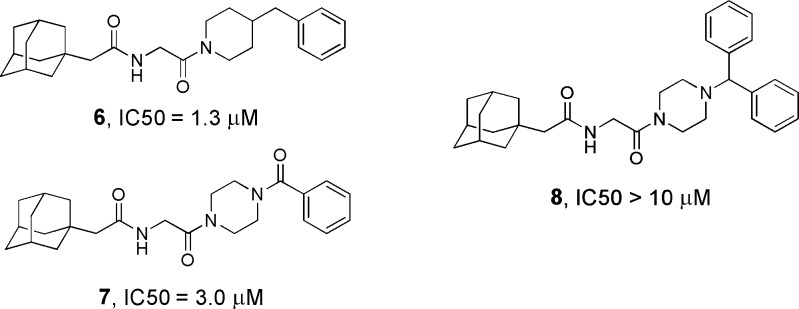
Having found that the left-hand side of 1 is sensitive to modification, we focused our attention to derivatization of the benzylpiperazine group. We found that the piperidine analogue 6 and the amide 7 were essentially equipotent to 1 (Figure 2), indicating that the distal basic nitrogen is not necessary for anti-EboV activity. These findings strongly suggest that antiviral activity is not dependent on lysosomal trapping conferred by protonation of the tertiary amine. A bulky substituent such as a phenyl group (8) at the benzylic position was not tolerated. The piperazine ring was retained in the ensuing SAR study because of the ease of derivatization and superior solubility of the products.
Figure 2.
Analogues with variation in the benzyl piperazine moiety.
The synthesis was carried out in reverse order (Scheme 3). After Boc-glycine was coupled with Cbz-protected piperazine, the Boc group was removed before the free amine was acylated by adamantanacetyl chloride. The Cbz group was then cleaved by hydrogenolysis, and the resulting amine 9 was reductively alkylated using various benzaldehydes. The final step employed parallel synthesis and purification involving solid-phase extraction (SPE) workup on strong cation exchange (SCX) cartridges.11 The library containing the first round of simple benzylpiperazine amides exhibited dynamic SAR (Table 3).
Scheme 3. Synthesis of 10a–ii.
Reagents and conditions: (a) 1-Cbz-piperazine, BOP, (i-Pr)2NEt, DMF. (b) TFA, DCM. (c) Adamantyl-CH2COCl, (i-Pr)2NEt, DCM. (d) H2, Pd/C, MeOH. (e) ArCHO, NaBH(OAc)3, DCM.
Table 3. Anti-EboV Activity of Variously Substituted Benzylpiperazines 10.
| entry | Ar | IC50 (μM) | entry | Ar | IC50 (μM) |
|---|---|---|---|---|---|
| 10a | 2-MeO-Ph | 2.1 | 1 | Ph | 1.3 |
| 10b | 2-BnO-Ph | 2.0 | |||
| 10c | 2-Cl-Ph | 3.2 | 10t | 4-MeO-Ph | 5.4 |
| 10d | 2-Br-Ph | 3.4 | 10u | 4-Cl-Ph | 2.5 |
| 10e | 2-CN-Ph | 3.9 | 10v | 4-Br-Ph | 3.2 |
| 10f | 2-HO-Ph | 2.9 | 10w | 4-CN-Ph | 8.0 |
| 10g | 2-CF3-Ph | 1.8 | 10x | 4-HO-Ph | 7.0 |
| 10h | 2-(CO2Me)-Ph | 8.0 | 10y | 4-Me-Ph | 1.1 |
| 10i | 2-(CO2H)-Ph | >40 | 10z | 4-(CO2Me)-Ph | 15 |
| 10j | 2-pyridine | 8.5 | 10aa | 4-(CO2H)-Ph | >40 |
| 10k | 3-MeO-Ph | 2.0 | 10bb | 4-(NHAc)-Ph | 7.5 |
| 10l | 3-BnO-Ph | 4.0 | 10cc | 4-pyridine | 14.5 |
| 10m | 3-Br-Ph | 1.0 | |||
| 10n | 3-CN-Ph | 1.2 | 10dd | 2-HO-5-Br-Ph | 0.5 |
| 10o | 3-Me-Ph | 1.1 | 10ee | 2,4-Cl-Ph | 6.6 |
| 10p | 3-CF3-Ph | 2.6 | 10ff | 3,4-Cl-Ph | 0.75 |
| 10q | 3-(CO2Me)-Ph | 14 | 10gg | 4-HO-2,6-Me-Ph | 8.0 |
| 10r | 3-(CO2H)-Ph | >40 | 10hh | 1-naphthyl | 3.4 |
| 10s | 3-pyridine | 6.8 | 10ii | 2-naphthyl | 3.0 |
Many substitutions at a single position did not markedly alter activity. Hydrophilic groups at the para-position were poorly tolerated (10w, 10x, 10aa, 10bb, and 10cc) and carboxylic acids (10i, 10r, and 10aa) were completely inactive, which may be due to poor cellular uptake of the resulting zwitterion. Remarkably, the combination of ortho- and meta- substitutions in the 2,5-disubstituted form was synergistic (10dd as compared to 10f and 10m), and this finding was exploited later to design the target probe. While meta-substituted benzylpiperazines were slightly more potent than corresponding ortho-analogues, the ortho-position was found to accommodate a larger group such as benzyloxy (10b vs 10l). Therefore, we decided to explore the 2-benzyloxy group further. Substituted 2-(aryloxy)benzylpiperazine derivatives were prepared according to Scheme 4. Thus, alkylation of salicylaldehyde by variously substituted benzyl bromide was followed by reductive amination (Scheme 4). The majority of the analogues did not differ significantly in potency with the notable exception of the 4′-carboxylic ester 2, which exhibited dramatically enhanced antiviral activity (IC50 = 0.02 μM, Table 4).
Scheme 4. Synthesis of 11a–k.
Reagents and conditions: (a) ArCH2Br, K2CO3, DMF. (b) Compound 9, NaBH(OAc)3, DCM.
Table 4. Anti-EboV Activity of Variously Substituted (2-Benzyloxy) Benzyl-Piperazines 11.
| entry | Ar | IC50 (μM) |
|---|---|---|
| 10b | Ph | 2.0 |
| 11a | 2-NO2-Ph | 1.6 |
| 11b | 3-Me-Ph | 0.35 |
| 11c | 3-Br-Ph | 2.4 |
| 11d | 3-MeO-Ph | 1.9 |
| 11e | 3-(CO2Me)-Ph | 2.4 |
| 11f | 3-pyridine | 2.1 |
| 11g | 4-NO2-Ph | 1.7 |
| 11h | 4-CN-Ph | 4.4 |
| 11i | 4-(CONHMe)-Ph | 2.1 |
| 11j | 4-(CONMe2)-Ph | 3.3 |
| 11k | 4-pyridine | 0.8 |
| 11l | 4-(CO2H)-Ph | 4.0 |
| 2 | 4-(CO2Me)-Ph | 0.02 |
Importantly, 2 did not inhibit VSV G or Lassa fever virus GP-dependent infection or reduce the growth or ATP content of target cells. The marked increase in activity of 2 was a surprise as other closely related analogues such as 3′-ester (11e) and 4′-amides (11i and 11j) were >100-fold less active. The activity of 11l was also <1% of 2 in the cell-free assay for antiviral activity, thus discounting the possibility that the product of esterase-mediated hydrolysis of 2 is the active species in the cell. The addition of similarly sized ethynyl group in the same position as the 4′-carboxylic acid methyl ester (3, see below) also enhanced antiviral activity, suggesting that hydrophobic and/or van der Waals interactions of the methyl ester group with the target are beneficial. The antiviral activities of 1 and 2 were confirmed using an EboV growth assay on Vero cells.8
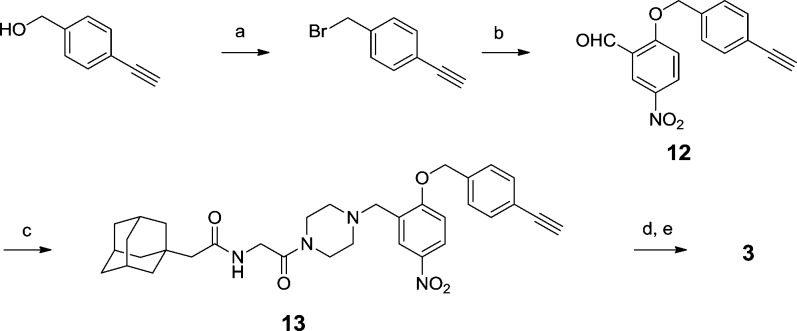
Functional studies strongly suggest that NPC1 is the target of the antiviral compounds.8 We developed derivatives of 2 suitable to test this hypothesis. On the basis of the SAR described above, we chose the 5-position of the (2-aryloxy) benzylpiperazine for attachment of an azide for photoactivation by ultraviolet light and the 4′-position acetylene for an alkyne for conjugation to Alexa Fluor 488 azide by click chemistry. Toward these goals, compound 3 was prepared according to Scheme 5. First, 5-nitrosalicylaldehyde was alkylated by 4-ethynylbenzyl bromide, prepared from the corresponding alcohol, to give the benzyl ether 12.12 The piperazine diamide intermediate 9 was then reductively alkylated by 12 to give 13. Reduction of the nitro group was followed by diazotization in the presence of sodium azide to obtain the desired bait 3.13,14 Gratifyingly, 3 retained potent anti-EboV activity (IC50 < 0.5 μM) and was not cytotoxic.
Scheme 5. Synthesis of 3.
Reagents and conditions: (a) CBr4, Ph3P, 2,6-lutidine, DCM. (b) 5-NO2-salicylaldehyde, K2CO3, DMF. (c) Compound 9, NaBH(OAc)3, DCM. (d) SnCl2-2H2O, MeOH. (e) NaNO2, HCl, then NaN3, NaOAc.
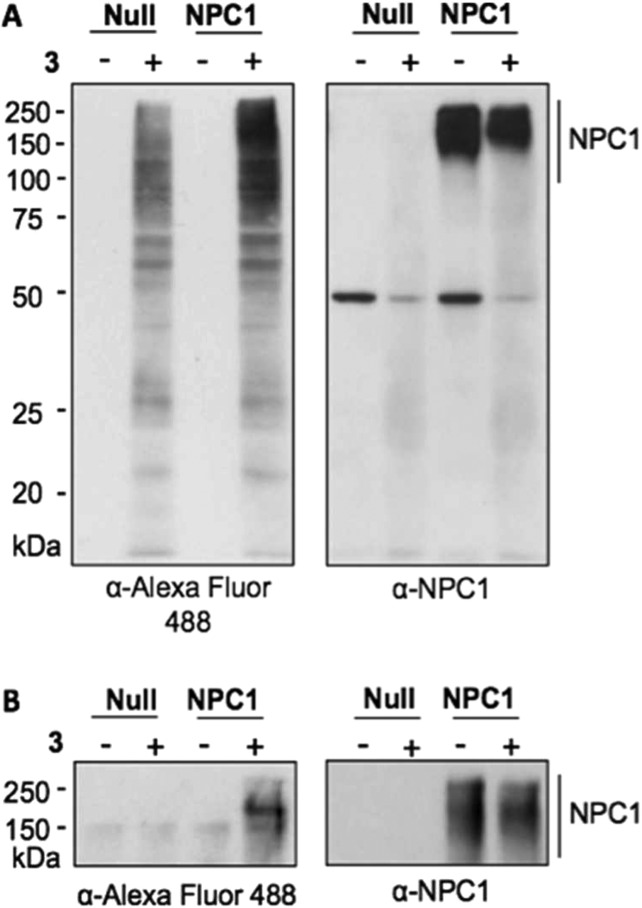
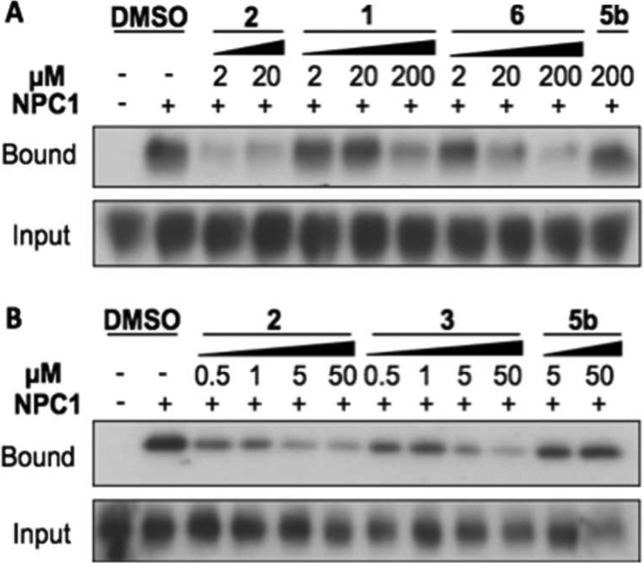
Late endosomal/lysosomal membranes from Chinese hamster ovary fibroblasts lacking NPC1 (CHONull) and Chinese hamster ovary fibroblasts expressing NPC1 (CHONPC1) cells incubated with 3 were exposed to UV light, and proteins cross-linked to 3 were labeled with Alexa Fluor 488 azide by click chemistry and analyzed by immunoblot probed with Alexa Fluor 488 antibody. A number of proteins covalently linked to 3 were identified in both CHONull and CHONPC1 membranes (Figure 3A, left). The marked increase in labeling of >150 kDa species in CHONPC1 correlated with expression of NPC1 (Figure 3A, right), and immunoprecipitation using NPC1 antibodies (Figure 3B, right) pulled down a single protein corresponding to NPC1 that was detected using Alexa Fluor 388 antibodies (Figure 3B, left). Because cleaved EboV GP is a ligand for NPC1 and NPC1 is required for infection,8,9 we reasoned that the antiviral compounds interfere with NPC1 receptor function. Consistent with this hypothesis, we found that these compounds inhibit binding of cleaved EboV GP to membranes expressing NPC1 in a concentration-dependent manner that is closely correlated with antiviral activity (2 > 6 > 1, Figure 4A, and 2 > 3 > 5b, Figure 4B). Taken together, these findings strongly suggest that the antiviral compounds target NPC1 and interfere with NPC1 binding to cleaved EboV GP that is required for infection.
Figure 3.
NPC1 is a target of the EboV inhibitor 3. (A) Membrane targets of adamantane dipeptide 3. Left: purified LE/LY membranes from CHONull and CHO cells overexpressing human NPC1 (CHOhNPC1) were incubated with 3 (25 μM) and exposed to UV light. Treated membranes were dissolved in NP40/Triton X-100, and solubilized proteins cross-linked to 3 were labeled with Alexa Fluor 488-Azide using click chemistry and identified by immunoblot using Alexa-fluor 488-specific antibodies. Right: NPC1 expression in membranes analyzed in the left panel was assessed by immunoblot probed with NPC1 antibodies. (B) NPC1 is a target of 3. Left: LE/LY membranes were prepared as in panel A, and NPC1 was recovered from lysates by immunoprecipitation using NPC1-specific antibodies. The presence of proteins cross-linked to 3 was assessed by probing an immunoblot using Alexa Fluor 488 antibodies. Right: Recovery of NPC1 by immunoprecipitation from membrane lysates was assessed by immunoblot using NPC1 antibodies.
Figure 4.
EboV inhibitors block binding of EboV GP to NPC1 membranes. Purified LE/LY membranes from CHONull or CHONull cells stably expressing mouse NPC1 (CHONPC1) were immobilized on ELISA plates and incubated with protease-cleaved EboV GP in the presence of specified concentrations of 1, 2, 5b, and 6 (A) and 2, 3, and 5b (B). Membranes were washed to remove unbound GP, solubilized in NP40, and analyzed for the presence of bound EboV GP by immunoblot probed with GP1 antibodies. The results are representative of findings in six experiments.
In summary, we have identified adamantane dipeptides by high-throughput screening and hit-to-lead optimization that target the lysosome membrane protein NPC1 and interfere with its function as a receptor for EboV GP. In this regard, the chemical basis of the anti-EboV activity of the adamantane dipeptides may be analogous to that of the Food and Drug Administration (FDA)-approved drug maraviroc, which inhibits human immunodeficiency virus (HIV) infection by targeting the gp120 receptor CCR5.15 Adamantane dipeptides are a new class of antiviral compounds.
Acknowledgments
We thank Daniel Ory for CHOnull and CHONPC1 cell lines, Bryden Considine for technical support, and Su Chiang for thoughtful review of the manuscript.
Glossary
Abbreviations
- CHONull
Chinese hamster ovary fibroblasts lacking NPC1
- CHONPC1
Chinese hamster ovary fibroblasts expressing NPC1
- EboV
Ebola virus
- FDA
Food and Drug Administration
- GP
glycoprotein
- HIV
human immunodeficiency virus
- IC50
half-maximal inhibitory concentration
- LE/LY
late endosome/lysosome
- NPC1
Neimann-Pick C1
- SAR
structure–activity relationship
- VSV
vesicular stomatitis virus
Supporting Information Available
Experimental protocols for synthesis and characterization of compounds, cell culture, virus infection, cell membrane purification, virus GP binding, and photoactivation and click chemistry. This material is available free of charge via the Internet at http://pubs.acs.org.
The research was supported by NIH U54 AI057159 (NERCE/BEID). J.M. was supported by PIDS-Sanofi-Pasteur and NIH K08AI079381. B.G. was supported by Stena Foundation. M.C. was supported by Fonds de la Recherche en Santé du Québec.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Sullivan N.; Yang Z.-Y.; Nabel G. J. Ebola Virus Pathogenesis: implications for vaccines and therapies. J. Virol. 2003, 77189733–9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T. W.; Jahrling P. B. Exotic emerging viral diseases: progress and challenges. Nat. Med. 2004, 10, S110–S121. [DOI] [PubMed] [Google Scholar]
- Kondratowicz A. S.; Lennemann N. J.; Sinn P. L.; Davey R. A.; Hunt C. L.; Moller-Tank S.; Meyerholz D. K.; Rennert P.; Mullins R. F.; Brindley M.; Sandersfeld L. M.; Quinn K.; Weller M.; McCray. P. B. Jr.; Chiorini J.; Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. U.S.A. 2011, 108208426–8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbo A.; Imai M.; Watanabe S.; Noda T.; Takahashi K.; Neumann G.; Halfmann P.; Kawaoka Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010, 69e1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M. F.; Kolokoltsov A. A.; Albrecht T.; Davey R. A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010, 69e1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K.; Sullivan N. J.; Felbor U.; Whelan S. P.; Cunningham J. M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005, 30857281643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornberg K.; Matsuyama S.; Kabsch K.; Delos S.; Bouton A.; White J. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 2006, 8084174–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M.; Misasi J.; Ren T.; Bruchez A.; Lee K.; Filone C. M.; Hensley L.; Li Q.; Ory D.; Chandran K.; Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 2011, 4777364344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette J. E.; Raaben M.; Wong A. C.; Herbert A. S.; Obernoster G.; Mulherkar N.; Kuehne A. I.; Kranzusch P. J.; Griffin A. M.; Rutherl G.; Dal Cin P.; Dye J. M.; Whelan S. P.; Chandran K.; Brummelkamp T. R. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011, 4777364340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. H.; Obernosterer G.; Raaben M.; Herbert A. S.; Deffieu M. S.; Krishnan A.; Ndungo E.; Sandesara R. G.; Carette J. E.; Kuehne A. I.; Rutherl G.; Pfeffer S. R.; Dye J. M.; Whelan S. P.; Brummelkamp T. R.; Chandran K. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012, 3181947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley C. W.; Zhao Z.; Leister W. H.; Strauss K. A. Fluorous-tethered amine bases for organic and parallel synthesis: Scope and limitations. Tetrahedron Lett. 2002, 43366319–6323. [Google Scholar]
- Vincent A.; Jose B.; Crowley J. D.; Goldup S. M.; Haennie K. D.; Leigh D. A.; Lusby P. J.; Ronaldson V. E.; Slawin A. M. Z.; Viterisi A.; Walker D. B. Catalytic “active-metal” template synthesis of [2]rotaxanes,[3]rotaxanes, and molecular shuttles, and some observations on the mechanism of the Cu(I)-catalyzed azide-alkyne 1,3-cycloaddition. J. Am. Chem. Soc. 2012, 1293911950–11963. [DOI] [PubMed] [Google Scholar]
- Chauhan J.; Fletcher S. One-pot synthesis of 2,1-benzisoxazoles (anthranils) by a stannous chloride-mediated tandem reduction-heterocyclization of 2-nitroacylbenzenes under neutral conditions. Tetrahedron Lett. 2012, 53374951–4954. [Google Scholar]
- More S. S.; Shanmughapriya D.; Lingam Y.; Patel N. B. First total synthesis of (Z)-11-(2-oxopropylidene)-2,3,11,11a-tetrahydro-1H-benzo[e]pyrrolo[1,2-a][1.4]diazepin-5(10H)-one. Synth. Commun. 2009, 39112058–2066. [Google Scholar]
- Kondru R.; Zhang J.; Ji C.; Mirzadegan T.; Rotstein D.; Sankuratri S.; Dioszegi M. Molecular interactions of CCR5 with major class of small molecule anti-HIV CCR5 antagonists. Mol. Pharmacol. 2008, 73, 789–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.