Abstract
Objective
Cisplatin is a DNA-damaging antitumor agent that is highly effective in treating ovarian cancer. It activates the p53/p21 pathway for its cytotoxic mode of action, but it does not induce p21-dependent cell cycle arrest in G1. Therefore, we investigated this paradox, and used the model analog DAP as a positive control for p21-dependent G1-arrest.
Methods
Studies were conducted in p53-proficient ovarian A2780 tumor cells to examine Cdk activity, cell cycle distribution and DNA damage signaling after cisplatin or DAP in combination with the mitotic inhibitor nocodazole.
Results
Cisplatin consistently induced transient S-phase arrest by inhibiting Cdk2/cyclin A complex in S-phase at 12 h and then a durable G2/M-arrest by inhibiting Cdc2/cyclin B complex at 12–18 h. These inhibitions were associated with Chk1 and Chk2 activation and resultant increase in inhibitory tyrosine phosphorylation of Cdk2 and Cdc2. Cisplatin also potently inhibited G1-phase Cdk4/cyclin D1 and Cdk2/cyclin E activities at ~18 h. In agreement, exposure of cisplatin-treated A2780, HCT-116p53−/− and HCT-116p21−/− tumor cells to nocodazole revealed limited G1-arrest that was dependent on p53 and p21. In contrast, the durable G1-arrest by DAP, which failed to activate Chk1 and Chk2, was unaffected by nocodazole.
Conclusions
Cisplatin induced G1-arrest, but at an attenuated level. This was primarily due to orchestration of Cdk inhibition in S-phase first, then in G2, and finally in G1 that effectively blocked cells in G2 and prevented cells from progressing and arresting in G1. These studies demonstrate that cisplatin unequivocally activates G1-checkpoint response, but the fidelity of G1-arrest is compromised by Chk1/2 activation and checkpoint response in S- and G2/M-phase.
Keywords: Cisplatin, DNA damage, Checkpoint response, Cell cycle arrest, Cdk activity
Introduction
Cisplatin is highly effective in the treatment of ovarian cancer. Its mechanism of action is ascribed to intrastrand binding to DNA, with potent cellular signaling events that eventually lead to apoptosis. A general critical event associated with DNA damage is activation of cell cycle checkpoints, which ultimately result in inhibition of cyclin-dependent kinase (Cdk) complexes. Upstream events in checkpoint response involve activation of ATR and ATM kinases, with resultant increase in Chk1 and/or Chk2 phosphorylation to regulate cell cycle progression [1,2]. More specifically, S- and G2-phase checkpoint responses are manifested by Chk1/2-dependent downregulation of Cdc25 phosphatase, with the consequence that Cdk within the Cdk2/cyclin A and Cdc2/cyclin B complexes remain in the inhibitory tyrosine phosphorylated state. Although all three isoforms of Cdc25 (Cdc25A, Cdc25B and Cdc25C) may dephosphorylate Cdk2 and Cdc2, recent knock-out studies indicate that Cdc25A is the more critical [3]. G1-checkpoint response is also dependent on upstream kinases, which stabilize p53 to transactivate p21 and inhibit G1-phase Cdk4/cyclin D and Cdk2/cyclin E complexes [1,2].
Cdk inhibition in G1, S and/or G2 phases normally leads to cell cycle retardation or arrest, which allows DNA repair and prevents DNA replication or mitosis in the presence of genomic damage. When repair fails, DNA damage leads to apoptosis. Therefore, cell cycle arrest and cell fate are intimately linked, and understanding their inter-relationship has the potential for clinical benefits [1,4,5]. In this regard, platinum-based agents demonstrate potent activity only against tumor cells proficient in G1-arrest [6], which is consistent with the development of cisplatin resistance when p21 cannot be upregulated [7,8]. Since the experimental non-cross-resistant analog 1R,2R-diaminocyclohexane (trans-diacetato)(dichloro)platinum(IV) (DAP) is a potent inducer of G1-arrest, and largely devoid of S- and G2/M-arrest [9], it supports the notion that activation of G1-checkpoint response is important for platinum-mediated antitumor effects.
Although G1-checkpoint response or arrest correlates with platinum drug activity, it is paradoxical that cisplatin is not associated with G1-arrest; instead, it predominantly induces a transient S-phase arrest that is followed by a robust G2/M-arrest, irrespective of the p53 status [7,10–14]. Indeed, there has been no definitive study to examine the effect of cisplatin on G1-phase Cdk complexes, particularly in parallel with S- and G2/M-checkpoint responses. Where G1-arrest by cisplatin has been reported, these are largely observed in p53-defective cells (e.g., HeLa cells [15]) and/or at high cisplatin concentrations (e.g., N5 μM [13,15]), the mechanism for which is not understood. Therefore, to examine the potential of G1-checkpoint response with cisplatin, we have undertaken a systematic biochemical and molecular analysis in ovarian p53-proficient A2780 cells, and used DAP as a positive control for G1-arrest and a negative control for S- and G2/M-arrest in this tumor model system [9,16].
Materials and methods
Cell culture and drug treatment
A2780, wild-type HCT-116wt, p53-deficient HCT-116p53−/− and p21-deficient HCT-116p21−/− cells were maintained as previously described [16]. Cells were plated in 100 mm dishes and incubated for at least 24 h before being exposed to freshly prepared cisplatin or DAP. The cells were further cultured and collected at selected times. Drug-treated cultures also received nocodazole, where required. Fluorescence activated cell sorting (FACS) and extraction of total proteins for biochemical assays were conducted as previously described, utilizing cells at <40% confluence to avoid contact inhibition [9].
Immunoblotting, Cdk immunoprecipitation and kinase assays
These experiments were performed as previously described [9,16]. The description and source of antibodies for immunoblotting and immunoprecipitation are provided in Table S1. The kinase activities of Cdk immunoprecipitates were assayed using as substrate the Rb fusion protein (Santa Cruz; for Cdk4 activity) or histone H1 (Boehringer Mannheim; for Cdk2 or Cdc2 activity). To specifically assess S-phase Cdk2/cyclin A activity, G1-phase Cdk2/cyclin E was first removed by cyclin E immunodepletion before the Cdk2 was immunoprecipitated.
RNA interference
siRNA duplexes for ATM (cat. ID: 214707; sense, 5′GCAACAUUUGCCUAUAUCAdTdT3′; antisense, 5′UGAUAUAGGCAAAUGUUGCdTdT3′), ATR (cat. ID: P-002090-01-05; sense, 5′CCUCCGUGAUGUUGCUUGA3′; antisense, 5′UCAAGCAACAUCACGGAGGdTdT3′), Chk1 (cat. ID: P-002076-01-05; sense, 5′GCGUGCCGUAGACUGUCCAdTdT3′; antisense, 5′UGGACAGUCUACGGCACGCdTdT3′), Chk2 (cat. ID: 118299; sense, 5′GAACAGAUAAAUACCGAACdTdT3′; antisense 5′GUUCGGUAUUUAUCUGUUCdTdT3′), and control siRNA against luciferase GL3 (cat. ID: 4611; sense, 5′CUUACGCUGAGUACUUCGAdTdT3′; antisense, 5′UCGAAGUACUCAGCGUAAGdTdT3′) were purchased from either Ambion (Austin, TX) or Dharmacon (Lafayette, CO). A2780 cells were transfected at 37 °C with 100 nM siRNA for 5 h using LipofectAMINE 2000 (LF2000), as described previously [17]. Cells were then washed, re-incubated with fresh RPMI 1640 complete media for the next 19 h, treated with cisplatin or DAP, and finally collected after 24-h drug exposure to process for immunoblot analysis.
DNA damage recognition by HMGB proteins
Recognition of DNA adducts by HMGB was determined by the damaged-DNA affinity precipitation assay [18–20]. However, to compensate for the lower reaction rate of DAP than cisplatin with DNA in a cell-free system [21], DNA-cellulose (7 mg DNA/g cellulose) was reacted with the platinum complex at an rf molar drug:DNA ratio of 0.03 for cisplatin (30 μM) and 0.3 for DAP (300 μM). After 24 h, the DNA-cellulose was washed and re-incubated for a further 24 h to maximize formation of bifunctional adducts. The targeted rb molar ratio for Pt:DNA of ~0.02 for each drug was confirmed by flameless atomic absorption spectrophotometry. For damage recognition, protein (20 μg) from nuclear extracts, prepared as described previously [22,23], was used with platinated or control DNA-cellulose to pull down HMGB1 and HMGB2, which were released and resolved on 12.5% SDS-polyacrylamide gels, and then visualized by silver staining.
Results
Dose-response and temporal effects of cisplatin on cell cycle progression
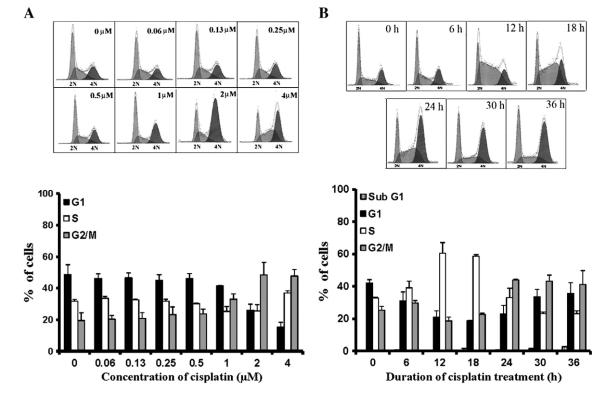
A2780 cells exposed to cisplatin accumulated in G2/M, and this was initially seen at 1.0 μM drug concentration, and becoming maximal at 2.0 μM (Fig. 1A). Concomitant decreases in S- and G1-phase populations were observed, but higher concentrations increased relative distribution of cells in S-phase and decreased it in G2/M. G1 Accumulation was not observed at any concentration. Based on these data, a cisplatin concentration of 1.0 μM (~5×IC50 in this model [24]) was selected for all remaining studies.
Fig. 1.
Variation in cell cycle distribution and kinetics with cisplatin. A, A2780 cells were treated with the indicated concentrations of the drug, and analyzed 36 h later. B, Cells were exposed to 1.0 μM cisplatin and analyzed at 6 h intervals for up to 36 h. In each case, analysis was by FACS, with the results presented as percent distribution of cells in different phases of the cell cycle. The data in the bar graphs are shown as mean±SD of 3–4 independent studies.
Accumulation of A2780 cells exposed to cisplatin was initially observed at 6 h in S-phase, became prominent by 12 h (Fig. 1B) and, thereafter, declined progressively. Cell distribution in G1- and G2/M-phase, on the other hand, concomitantly decreased at 12 h and then subsequently increased, beginning at 18 h in G2/M and 30 h in G1. In the case of G1, distribution of cells never exceeded control levels. It is noteworthy that changes in cell cycle distribution were not due to loss of cells from apoptosis since sub-G1 population remained minimal (≤2% at 36 h) by FACS analysis (Fig. 1B) or trypan blue dye exclusion (data not shown).
Inhibition of Cdk activities in S and G2/M phase
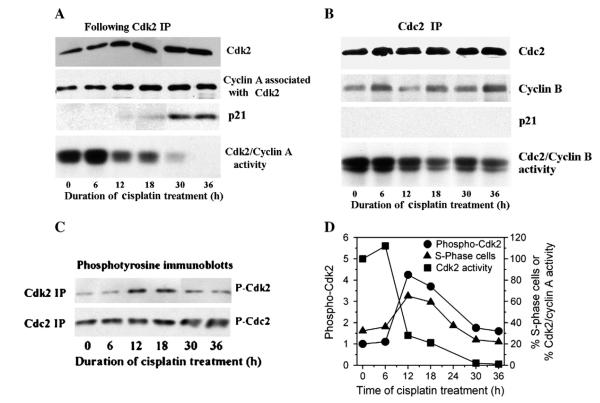
We examined inhibition of Cdk2/cyclin A and Cdc2/cyclin B activities for congruence with cisplatin-induced S- and G2/M-arrests in our specific tumor model. Since Cdk2/cyclin E would confound conclusions, it was first removed from lysates by cyclin E immunodepletion. In Cdk2 immunoprecipitates, levels of cyclin A were not grossly changed (Fig. 2A). However, Cdk2/cyclin A activity was reduced ~80% by 12 h compared to initial levels (Fig. 2A and D) and correlated with the onset of S-phase arrest (see Fig. 1B). This activity declined further to about 1% of initial level by 36 h that coincided with significant p21 accumulation. Interestingly, cells did not remain arrested in S-phase; instead, cell population in S-phase decreased progressively beginning at 18 h (Figs. 1B and 2D).
Fig. 2.
Effects of cisplatin on Cdk2/cyclin A and Cdc2/cyclin B kinases. A, A2780 cells were treated with 1.0 μM cisplatin for up to 36 h and lysates prepared at timed intervals. Lysates were immunodepleted with cyclin E antibodies to remove Cdk2/cyclin E complex and then Cdk2 immunoprecipitates isolated and immunoblotted for the indicated proteins and assessed for Cdk2 activity using histone H1 as substrate. B, Cdc2 immunoprecipitates from cells treated with cisplatin as in A were immunoblotted for proteins and assessed for Cdc2 kinase activity using histone H1 as substrate. C, Immunoprecipitates of Cdk2 and Cdc2 isolated from cells treated as in A were immunoblotted with the PY99 antibody to assess tyrosine-15 phosphorylation of Cdk2, or with antibodies that specifically recognize Cdc2 phosphorylated at tyrosine-15. D, Phosphotyrosine-Cdk2 bands in immunoblots from C were quantified by densitometry and plotted against time to show kinetic relationships with Cdk2/cyclin A activity from A and percentage of cells in S-phase from Fig. 1B.
In Cdc2 immunoprecipitates from cisplatin-treated cells, levels of cyclin B fluctuated slightly during the 36-h period, and p21 was undetectable (Fig. 2B). Nevertheless, the Cdc2 kinase activity decreased to ~50% of initial levels by 12 h and declined further to ~40% during 18–36 h. However, it is noteworthy that cells in G2/M first decreased at 12 h, and then increased at ~18 h (Fig. 1B). This is likely due to inhibition of Cdc2/cyclin B activity lagging behind Cdk2/cyclin A activity, which at the earlier time points prevented cells from progressing through S-phase and enter G2, but allowed cells to exit G2.
Tyrosine phosphorylation of Cdk by cisplatin
The lack of involvement of p21 in the early onset of cisplatin-dependent Cdk2 inhibition in S-phase and of Cdc2 inhibition in G2/M-phase necessitated examination of tyrosine phosphorylation in Cdk2 and Cdc2 immunoprecipitates as the mechanism for kinase inhibition. As shown in Fig. 2C, phosphotyrosine-Cdk2 levels increased transiently by cisplatin, peaking at 12–18 h. It is notable that the temporal profile of S-phase arrest correlated with inhibitory tyrosine phosphorylation of Cdk2, but not with inhibition of Cdk2/cyclin A activity (Fig. 2D).
Phosphotyrosine-Cdc2 was also increased by cisplatin, but this was not observed until 30–36 h (Fig. 2C), which occurs much later than the inhibition of Cdc2 activity (see Fig. 2B). However, this inconsistency is not unexpected since most of the Cdc2 in the Cdc2/cyclin B complex exists in the inhibited phosphotyrosine form, and its activation by dephosphorylation occurs within a very narrow window of time to allow cells to enter mitosis [25,26]. Therefore, G2/M-arrest by cisplatin (Fig. 1C) is due to a mechanism that inhibits Cdc2 dephosphorylation, as previously suggested [27], and accumulation of cells in G2/M is likely responsible for the increase in phosphotyrosine-Cdc2 levels.
Inhibition of Cdk4 and Cdk2 activities in G1 phase
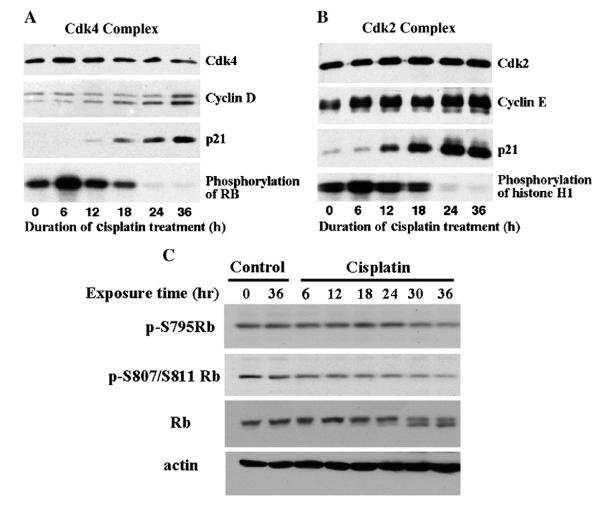
G1 arrest by DNA-damaging agents requires p21 to efficiently bind to and inhibit Cdk4/cyclin D and Cdk2/cyclin E complexes [28], and this has been observed with DAP [16]. Since cisplatin potently activates the p53/p21 pathway in A2780 cells [8], the lack of G1-arrest could be due to absence of p21 in the G1-phase Cdk complex. However, p21 was demonstrated to be present in immunoprecipitates of these Cdk complexes, beginning at 12 h and then increasing over the next 24 h (Fig. 3A and B). No p21 was immunoprecipitated with control IgG antibody (data not shown). Cyclin D1 bound to Cdk4 and cyclin E bound to Cdk2 also increased (Fig. 3A and B), and this may relate to the additional role of p21 as an assembly factor for the Cdk complex [29]. Both Cdk4 and Cdk2 activity declined to below initial levels by 18 h as p21 levels in the complex increased. As a result, Rb as the downstream target was hypophosphorylated at Ser795 and Ser807/811, beginning at 12–18 h; this is better appreciated in immunoblots of total Rb, which demonstrates both hyper- (upper band) and hypo-phosphorylated form (lower band) (Fig. 3C). It is notable that the temporal profiles for inhibition of G1-phase Cdk4 and Cdk2 activity with cisplatin are essentially similar to those obtained with DAP [16].
Fig. 3.
Effects of cisplatin on the composition, activities and downstream effects of Cdk4/cyclin D1 and Cdk2/cyclin E complexes. A and B, A2780 cells were treated with 1.0 μM cisplatin for up to 36 h and cell lysates from timed samples were immunoprecipitated with either anti-Cdk4 (A) or anti-Cdk2 (B) antibodies. The immunoprecipitates were examined by immunoblot for the indicated proteins and assessed for kinase activity using either Rb (A) or H1 (B) protein as substrate. C, Cells were treated with 1.0 μM cisplatin for up to 36 h and cell lysates from timed samples were immunoblotted for total and phospho-specific Rb antibodies. The total Rb antibody recognizes both hyper- (upper band) and hypophosphorylated (lower band) forms.
Demonstration of G1-arrest by cisplatin
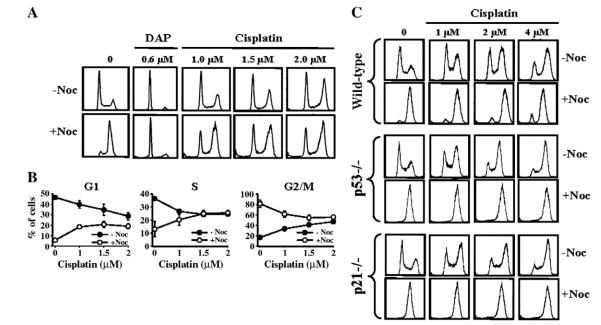
We have previously observed that the platinum analog DAP induces G1-arrest, but not S- or G2/M-arrest [9]. Since cisplatin inhibits G1-phase Cdk but does not induce G1-arrest, it was possible that S- and G2/M-arrests restrict accumulation of cells in G1, and that some of the cells present in low numbers in G1 may actually be in an arrested state. To examine this, we used nocodazole to allow unarrested cells in G1 and S to progress and arrest in M-phase. Thus, N90% of A2780 tumor cells treated with nocodazole alone accumulated in M-phase (Fig. 4A). In contrast, DAP-treated cells as positive control remain in G1 when exposed to nocodazole and, thereby, verify the strong G1-arrest. Following cisplatin exposure, cells accumulated in G2/M in a concentration-dependent manner, with concomitant concentration-dependent decreases in G1 cells. Exposure to nocodazole unequivocally demonstrated that over a half of these G1 cells do not progress and were indeed arrested in this phase (Fig. 4A and B). Similar treatment of wild-type and p53- or p21-deficient HCT-116 cells with cisplatin and nocodazole confirmed G1-arrest in wild-type cells that was p53- and p21-dependent (Fig. 4C).
Fig. 4.
G1-arrest by cisplatin and its dependence on p53 and p21. A and B, A2780 cells were incubated with the indicated concentrations of cisplatin or DAP for 30–36 h and then nocodazole was added 8–10 h later. Cells were collected and the cell cycle distribution quantified by FACS (A) and percentage of cells in different phases of the cell cycle was plotted against cisplatin concentration (B). The data in B are shown as mean±SD of 3–4 independent studies. C, Wild-type, p53−/− and p21−/− HCT-116 cells were treated with cisplatin±nocodazole and processed for cell cycle distribution by FACS as described for A2780 cells in A.
Differential effects of cisplatin and DAP on the Chk/Cdc25 pathway
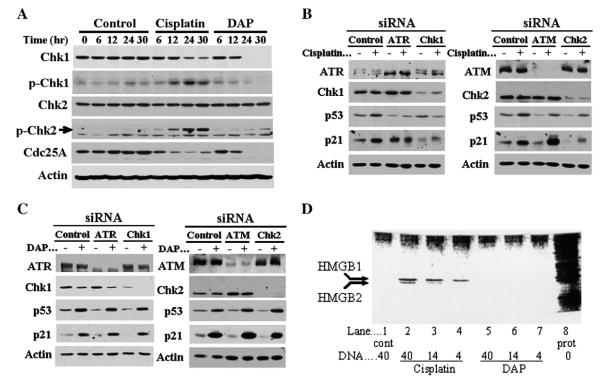
To provide an explanation for differential profile of cell cycle arrest by cisplatin and DAP, we examined activation of Chk1 and Chk2. Control A2780 cells demonstrated no significant change in any protein levels over 0–30 h (Fig. 5A). Similarly, total Chk2 levels were not altered following treatment with either drug at the 5×IC50 drug concentrations. On the other hand, cisplatin significantly increased phosphorylation of Chk1 and Chk2 during the 6–30 h period, with Chk1 phosphorylation preceding decreases in total Chk1 levels at 24–30 h. In contrast, DAP only marginally increased phosphorylation of Chk2 at the 24 and 36 h time points, and actually decreased phosphorylation of Chk1, which may be due to depletion of total Chk1 by DAP at 24–30 h. Both cisplatin and DAP reduced levels of the downstream Chk1 target Cdc25A, with cisplatin reducing levels as early as 6 h, whereas the effect of DAP was not observed until 12 h. At 24–30 h, Cdc25A levels were further reduced, with DAP demonstrating a substantially greater effect than cisplatin. These results highlight seminal differences between cisplatin and DAP in the activation of Chk1 and Chk2.
Fig. 5.
Differential activation of checkpoint kinase pathways by cisplatin and DAP, and recognition of their DNA adducts by HMG proteins. A, A2780 cells were exposed to 1.0 μM cisplatin or 0.6 μM DAP and harvested at timed intervals over a 30 h period to assess cell lysates for levels of total Chk1, Chk2 and Cdc25A, and phospho-Ser345-Chk1 and phospho-Thr68-Chk2. β-Actin is shown as loading control. B, Cells were transfected with control siRNA oligos or gene-specific duplex siRNA oligos targeting ATR, Chk1, ATM and Chk2 for 5 h and then treated 19 h later with 1.0 μM cisplatin. After a 24-h drug exposure, cell lysates were prepared and immunoblotted to examine the specified proteins. Note that in the ATR immunoblot, the upper band is ATR and the lower bands are non-specific. C, Experiments in B were repeated, but using 0.6 μM DAP. D, Nuclear proteins binding to DNA-cellulose adducts of cisplatin or DAP were separated by SDS-PAGE and silver-stained to visualize HMGB1 and HMGB2 proteins. Lane 1, nuclear proteins bound to 40 μg control (cont) untreated DNA-cellulose; lanes 2–7, nuclear proteins bound to 4–40 μg of DNA-cellulose following treatment with either cisplatin (lanes 2–4) or DAP (lanes 5–7); lane 8, total nuclear proteins (prot).
Relative dependence of cisplatin and DAP on upstream DNA damage kinases
To consolidate evidence for differential dependence of cisplatin and DAP on Chk1 and Chk2 for DNA damage signaling, and expand the investigation to include ATM and ATR, we employed siRNA to knockdown each kinase and used p53 and p21 expression as readout. A2780 tumor cells transfected with control siRNA did not prevent induction of p53 and p21 by either cisplatin (Fig. 5B) or DAP (Fig. 5C). On the other hand, gene-specific siRNA oligos were highly effective in reducing levels of targeted ATR, ATM, Chk1 and Chk2 proteins, but induction of p53 and/or p21 by cisplatin was substantially inhibited in cells depleted of ATR, Chk1 or Chk2, but not in cells following ATM knockdown. In contrast, knockdown of any of the four genes by siRNA did not prevent activation of the p53/p21 pathway by DAP.
Independent recognition of DNA adducts of cisplatin and DAP by HMG proteins
To reconcile with independent DNA damage signaling transduced by cisplatin and DAP, we examined the possibility of differential recognition of their DNA adducts. HMGB1 and HMGB2 proteins are reported to recognize cisplatin-induced DNA adducts [19], and this was confirmed in the present study, with band intensities of these proteins dependent on the level of damaged DNA in the reaction mix (lanes 2–4) (Fig. 5D). In contrast, HMGB1 and HMGB2 did not interact with undamaged control DNA (lane 1) or DNA adducts of DAP (lanes 5–7), and this clearly demonstrates that adducts of cisplatin and DAP are recognized independently.
Discussion
Cell cycle arrest in a given phase of the cell cycle is indicated when cell numbers in that phase increase above control levels. Based on this limited criterion, G1-arrest is not observed with cisplatin. However, cisplatin in our detailed investigation induced not only the expected S- and G2/M-arrests, but also G1-arrest, which was consistent with inhibition of G1-phase Cdk4/cyclin D1 and Cdk2/cyclin E complexes and the dependence on p53 and p21. The low level of G1-arrest by cisplatin was due to attenuated progression of cells in S-phase and subsequent robust retention of cells at G2/M, and this was supported with DAP as a positive control for G1-arrest by virtue of its inability to activate S- and G2/M-checkpoint responses.
Several reports have previously described that cisplatin induces S- and G2/M-phase arrests in a sequential manner [10,30]. As with cisplatin, a predominant G2/M-phase arrest has also been noted with a variety of antitumor agents, such as nitrogen mustard, and in congruence with cisplatin, G2/M-arrest is always preceded by transient S-phase arrest [31–33]. These events in S- and G2/M-phase are associated with Chk1 and Chk2 activation and resultant phosphorylation and proteosomal degradation of Cdc25A [1,2,34–36], and our data from the present study are consistent with these literature observations. On the other hand, some antitumor agents, such as ionizing radiation and adriamycin, have a stronger propensity for G1-phase arrest [37]. Interestingly, G1-arrest is the predominant cell cycle effect of DAP, and this is most likely associated with its DNA damage signaling being independent of Chk1 and Chk2. At the same time, it was surprising that Chk1 and Cdc25A levels were decreased by DAP, but such reductions have also been observed in other systems and ascribed to p53/p21-dependent gene repression [38–41]. Nevertheless, it is apparent that the phase in which cells arrest is strongly dependent on which pathways are activated by the individual DNA damaging agent. However, the underlying mechanisms inducing the specific signal transduction pathways have not been clearly defined in the literature, but it appears logical that this is related to recognition of distinct DNA damage elicited by each agent. This premise is supported by our finding that DNA damage recognition proteins HMGB1/2 can distinguish between cisplatin- and DAP-DNA adducts, which are distinct by virtue of structural differences between the two platinum compounds [24].
Cell cycle arrest in the specific phase of the cell cycle is expected to reflect inhibition of the corresponding Cdk activity, and this holds true for cisplatin in G1, S and G2/M. Moreover, our systematic study indicates that the mechanisms for Cdk inhibition are orchestrated so that the onset of inhibition in each phase occurs in a sequential manner. In addition, these mechanisms are not identical. Two of the major mechanisms involve inhibitory binding of p21 to the Cdk via the p53/p21 pathway and the increase in inhibitory phosphotyrosine levels of Cdk [1,2,42]. Our study has demonstrated that the p53/p21 pathway is critical for cisplatin-induced G1-arrest, whereas both tyrosine phosphorylation (early event) and p21 (late event) are involved in S-arrest. This mechanism of S-phase arrest, however, appears to be more complex than previously recognized since our data indicate that the transient profile of cell accumulation in this phase correlates directly with inhibitory phosphotyrosine-Cdk2 levels, and not with actual kinase inhibition. Thus, it was unexpected that S-phase progression resumed in the face of robust inhibition of Cdk2/cyclin A activity. The exact mechanism underlying this seminal observation and the role of phosphotyrosine in the process are presently unknown. In contrast to G1- and S-phase Cdk, p21 did not bind to Cdc2, which was inhibited primarily by mechanisms inducing the increase in phosphotyrosine levels. This was surprising since p21 has been reported to inhibit Cdc2 kinase [43]. On the other hand, Cdc2 inhibition by p21 requires supraphysiological levels of the inhibitor, which may not be possible in cells following DNA damage [2]. The absence of S- and G2/M-arrests with DAP as a potent activator of the p53/p21 pathway [9] confirms that p21 has a minimal role in G2/M-arrest, and perhaps also in S-phase arrest.
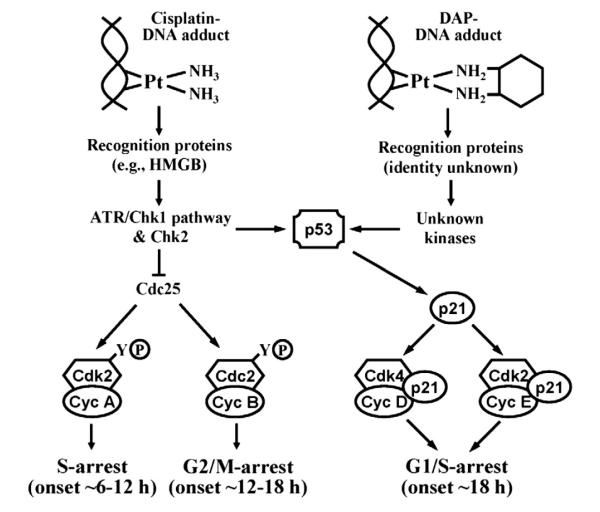
Based on signaling pathways activated and temporal relationships established for Cdk inhibitions in our study, we can begin to appreciate underlying mechanisms that lead to divergent cell cycle arrests by cisplatin and DAP, and this is schematically presented in Fig. 6. Essentially, differential recognition of DNA adducts of cisplatin and DAP and resultant downstream effects on independent DNA damage pathways induce only G1-arrest with DAP, but the orchestrated early S- and G2/M-arrest with cisplatin impact the fidelity of G1-arrest even though G1 Cdks are inhibited. These results provide a seminal understanding that inhibition of G1-phase Cdk is a more reliable index of cisplatin-induced G1-checkpoint and cytotoxic response in ovarian cancer that is now fully consistent with the reported correlation between the cellular integrity of G1-arresting machinery and platinum sensitivity in the NCI 60-cell line tumor panel [6].
Fig. 6.
A proposed model for primary cell cycle effects of cisplatin and DAP. Based on their chemical structures, cisplatin and DAP form qualitatively different intrastrand adducts, which are shown in the Pt(II) form although the Pt(IV) state for the DAP adduct is possible [50]. The damaged DNA after cisplatin and DAP is differentially recognized by independent proteins to activate downstream kinases that subsequently induce p53, which then transactivates p21 to inhibit G1-phase Cdk. In addition, the ATR, Chk1 and/or Chk2 activated by only cisplatin downregulate Cdc25, which then inhibits both Cdk2 in S-phase and Cdc2 in G2/M by increasing phosphorylation at Y-15. The approximate times at which cell cycle arrest is observed in each of the specific phases are shown to explain how the sequence of S- and G2/M-arrests by cisplatin obscures G1-arrest.
Our investigations in this study have focused on cisplatin, but it is important to note that conclusions on cell cycle checkpoint response will also apply to carboplatin, particular since this analog, like cisplatin, forms identical DNA adducts [44,45], activates similar cytotoxic response pathways, including phosphorylation of Chk1 and upregulation of p21 in a p53-dependent manner [6,46,47], and produces a similar cell cycle arrest profile [47]. This is important since carboplatin is a critical drug in the treatment of ovarian cancer, and it is clear that its DNA damage response characteristics are consistent with the findings from our present and previously reported study[7,24] and those of Vekris et al. [6] that an intact p53-dependent checkpoint response or cell cycle arrest in G1 is essential for greater chemosensitivity of tumor cells to platinum drugs. Since platinum drug-induced G1-checkpoint response is fundamentally dependent on p21 and, therefore, on an intact p53-p21 pathway, failure of platinum-based therapy to upregulate p21 will likely result in chemoresistance. Indeed, about 50% of patients with ovarian cancers harboring wild-type p53 fail to respond to cisplatin or carboplatin therapy [48,49], and this is associated with loss of p21 expression [7,8]. In such cases, restoration of the p53-p21 pathway, through independent upstream DNA damage signaling activated by novel agents represented by DAP [8,24], provides a rational option to reverse chemoresistance and, thereby, increase therapeutic response.
Supplementary materials related to this article can be found online at doi:10.1016/j.ygyno.2011.04.034.
Supplementary Material
Acknowledgment
We are thankful to Mr. Long Nguyen for technical assistance in the HMGB studies.
Abbreviations
- DAP
1R,2R-diaminocyclohexane(trans-diacetato)(dichloro) platinum(IV)
- Cdk
cyclin-dependent kinase
- IC50
inhibitory concentration reducing tumor cell growth by 50%
- HMGB
high mobility group box
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and rad3-related
Footnotes
Conflict of interest statement The authors have no conflict of interest to declare.
Grant support: U.S. Public Health Service grants CA127263 to Z.H.S. and CA16672 to MD Anderson Cancer Center, awarded by the National Cancer Institute, and in part to the Megan McBride Franz Endowed Research Fund.
References
- [1].Eastman A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J Cell Biochem. 2004;91:223–31. doi: 10.1002/jcb.10699. [DOI] [PubMed] [Google Scholar]
- [2].Samuel T, Weber HO, Funk JO. Linking DNA damage to cell cycle checkpoints. Cell Cycle. 2002;1:162–8. [PubMed] [Google Scholar]
- [3].Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol. 2005;25:2853–60. doi: 10.1128/MCB.25.7.2853-2860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwartz GK. Development of cell cycle active drugs for the treatment of gastrointestinal cancers: a new approach to cancer therapy. J Clin Oncol. 2005;23:4499–508. doi: 10.1200/JCO.2005.18.341. [DOI] [PubMed] [Google Scholar]
- [5].Eastman A, Kohn EA, Brown MK, Rathman J, Livingstone M, Blank DH, Gribble GW. A novel indolocarbazole, ICP-1, abrogates DNA damage-induced cell cycle arrest and enhances cytotoxicity: similarities and differences to the cell cycle checkpoint abrogator UCN-01. Mol Cancer Ther. 2002;1:1067–78. [PubMed] [Google Scholar]
- [6].Vekris A, Meynard D, Haaz MC, Bayssas M, Bonnet J, Robert J. Molecular determinants of the cytotoxicity of platinum compounds: the contribution of in silico research. Cancer Res. 2004;64:356–62. doi: 10.1158/0008-5472.can-03-2258. [DOI] [PubMed] [Google Scholar]
- [7].Hagopian GS, Mills GB, Khokhar AR, Bast RC, Jr, Siddik ZH. Expression of p53 in cisplatin-resistant ovarian cancer cell lines: modulation with the novel platinumanalogue (1R, 2R-diaminocyclohexane)(trans-diacetato)(dichloro)-platinum(IV) Clin Cancer Res. 1999;5:655–63. [PubMed] [Google Scholar]
- [8].Mujoo K, Watanabe M, Nakamura J, Khokhar AR, Siddik ZH. Status of p53 phosphorylation and function in sensitive and resistant human cancer models exposed to platinum-based DNA damaging agents. J Cancer Res Clin Oncol. 2003;129:709–18. doi: 10.1007/s00432-003-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuang J, He G, Huang Z, Khokhar AR, Siddik ZH. Bimodal effects of 1R,2R-diaminocyclohexane(trans-diacetato)(dichloro)platinum(IV) on cell cycle checkpoints. Clin Cancer Res. 2001;7:3629–39. [PubMed] [Google Scholar]
- [10].Ormerod MG, Orr RM, Peacock JH. The role of apoptosis in cell killing by cisplatin: a flow cytometric study. Br J Cancer. 1994;69:93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988;48:4484–8. [PubMed] [Google Scholar]
- [12].Zamble DB, Jacks T, Lippard SJ. p53-Dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc Natl Acad Sci U S A. 1998;95:6163–8. doi: 10.1073/pnas.95.11.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wilkins DE, Ng CE, Raaphorst GP. Cell cycle perturbations in cisplatin-sensitive and resistant human ovarian carcinoma cells following treatment with cisplatin and low dose rate irradiation. Cancer Chemother Pharmacol. 1997;40:159–66. doi: 10.1007/s002800050641. [DOI] [PubMed] [Google Scholar]
- [14].Attardi LD, de Vries A, Jacks T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene. 2004;23:973–80. doi: 10.1038/sj.onc.1207026. [DOI] [PubMed] [Google Scholar]
- [15].Koprinarova M, Markovska P, Iliev I, Anachkova B, Russev G. Sodium butyrate enhances the cytotoxic effect of cisplatin by abrogating the cisplatin imposed cell cycle arrest. BMC Mol Biol. 2010;11:49. doi: 10.1186/1471-2199-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, et al. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene. 2005;24:2929–43. doi: 10.1038/sj.onc.1208474. [DOI] [PubMed] [Google Scholar]
- [17].He G, Kuang J, Huang Z, Koomen J, Kobayashi R, Khokhar AR, Siddik ZH. Upregulation of p27 and its inhibition of CDK2/cyclin E activity following DNA damage by a novel platinum agent are dependent on the expression of p21. Br J Cancer. 2006;95:1514–24. doi: 10.1038/sj.bjc.6603448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Billings PC, Davis RJ, Engelsberg BN, Skov KA, Hughes EN. Characterization of high mobility group protein binding to cisplatin-damaged DNA. Biochem Biophys Res Commun. 1992;188:1286–94. doi: 10.1016/0006-291x(92)91371-v. [DOI] [PubMed] [Google Scholar]
- [19].Hughes EN, Engelsberg BN, Billings PC. Purification of nuclear proteins that bind to cisplatin-damaged DNA. Identity with high mobility group proteins 1 and 2. J Biol Chem. 1992;267:13520–7. [PubMed] [Google Scholar]
- [20].Marples B, Adomat H, Billings PC, Farrell NP, Koch CJ, Skov KA. Recognition of platinum-induced DNA damage by nuclear proteins: screening for mechanisms. Anticancer Drug Des. 1994;9:389–99. [PubMed] [Google Scholar]
- [21].Kido Y, Khokhar AR, al Baker S, Siddik ZH. Modulation of cytotoxicity and cellular pharmacology of 1,2-diaminocyclohexane platinum (IV) complexes mediated by axial and equatorial ligands. Cancer Res. 1993;53:4567–72. [PubMed] [Google Scholar]
- [22].Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee KA, Bindereif A, Green MR. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988;5:22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- [24].Siddik ZH, Hagopian GS, Thai G, Tomisaki S, Toyomasu T, Khokhar AR. Role of p53 in the ability of 1,2-diaminocyclohexane-diacetato-dichloro-Pt(IV) to circumvent cisplatin resistance. J Inorg Biochem. 1999;77:65–70. doi: 10.1016/s0162-0134(99)00144-0. [DOI] [PubMed] [Google Scholar]
- [25].Dunphy WG. The decision to enter mitosis. Trends Cell Biol. 1994;4:202–7. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- [26].Berry LD, Gould KL. Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog Cell Cycle Res. 1996;2:99–105. doi: 10.1007/978-1-4615-5873-6_10. [DOI] [PubMed] [Google Scholar]
- [27].Nylen U, He Q, Welander I, Lewin F, Skog S. Cisplatin-induced inhibition of p34cdc2 is abolished by 5-fluorouracil. Acta Oncol. 1998;37:355–63. doi: 10.1080/028418698430575. [DOI] [PubMed] [Google Scholar]
- [28].Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- [29].LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–62. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- [30].Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, Young LS. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–28. [PubMed] [Google Scholar]
- [31].O’Connor PM, Ferris DK, White GA, Pines J, Hunter T, Longo DL, Kohn KW. Relationships between cdc2 kinase, DNA cross-linking, and cell cycle perturbations induced by nitrogen mustard. Cell Growth Differ. 1992;3:43–52. [PubMed] [Google Scholar]
- [32].Nagasawa H, Li CY, Maki CG, Imrich AC, Little JB. Relationship between radiation-induced G1 phase arrest and p53 function in human tumor cells. Cancer Res. 1995;55:1842–6. [PubMed] [Google Scholar]
- [33].Azuma A, Huang P, Matsuda A, Plunkett W. 2′-C-cyano-2′-deoxy-1-beta-d-arabino-pentofuranosylcytosine: a novel anticancer nucleoside analog that causes both DNA strand breaks and G(2) arrest. Mol Pharmacol. 2001;59:725–31. doi: 10.1124/mol.59.4.725. [DOI] [PubMed] [Google Scholar]
- [34].Agner J, Falck J, Lukas J, Bartek J. Differential impact of diverse anticancer chemotherapeutics on the Cdc25A-degradation checkpoint pathway. Exp Cell Res. 2005;302:162–9. doi: 10.1016/j.yexcr.2004.08.035. [DOI] [PubMed] [Google Scholar]
- [35].Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Damia G, Filiberti L, Vikhanskaya F, Carrassa L, Taya Y, D’Incalci M, Broggini M. Cisplatinum and taxol induce different patterns of p53 phosphorylation. Neoplasia. 2001;3:10–6. doi: 10.1038/sj.neo.7900122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- [38].Gottifredi V, Karni-Schmidt O, Shieh SS, Prives C. p53 down-regulates CHK1 through p21 and the retinoblastoma protein. Mol Cell Biol. 2001;21:1066–76. doi: 10.1128/MCB.21.4.1066-1076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lohr K, Moritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507–16. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- [40].Rother K, Kirschner R, Sanger K, Bohlig L, Mossner J, Engeland K. p53 downregulates expression of the G1/S cell cycle phosphatase Cdc25A. Oncogene. 2007;26:1949–53. doi: 10.1038/sj.onc.1209989. [DOI] [PubMed] [Google Scholar]
- [41].Vigneron A, Cherier J, Barre B, Gamelin E, Coqueret O. The cell cycle inhibitor p21waf1 binds to the myc and cdc25A promoters upon DNA damage and induces transcriptional repression. J Biol Chem. 2006;281:34742–50. doi: 10.1074/jbc.M602492200. [DOI] [PubMed] [Google Scholar]
- [42].Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–47. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- [43].Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- [44].Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- [45].Siddik ZH. DNA-interactive alkylating agents and antitumour platinum-based drugs. In: Alison MR, editor. The cancer handbook. Nature Publishing Group; London: 2002. pp. 1295–312. [Google Scholar]
- [46].Di Felice V, Lauricella M, Giuliano M, Emanuele S, Vento R, Tesoriere G. The apoptotic effects of cisplatin and carboplatin in retinoblastoma Y79 cells. Int J Oncol. 1998;13:225–32. doi: 10.3892/ijo.13.2.225. [DOI] [PubMed] [Google Scholar]
- [47].Cruet-Hennequart S, Villalan S, Kaczmarczyk A, O’Meara E, Sokol AM, Carty MP. Characterization of the effects of cisplatin and carboplatin on cell cycle progression and DNA damage response activation in DNA polymerase etadeficient human cells. Cell Cycle. 2009;8:3039–50. [PubMed] [Google Scholar]
- [48].Lavarino C, Pilotti S, Oggionni M, Gatti L, Perego P, Bresciani G, et al. p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol. 2000;18:3936–45. doi: 10.1200/JCO.2000.18.23.3936. [DOI] [PubMed] [Google Scholar]
- [49].Righetti SC, Della TG, Pilotti S, Menard S, Ottone F, Colnaghi MI, et al. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996;56:689–93. [PubMed] [Google Scholar]
- [50].Reedijk J. New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA. Proc Natl Acad Sci U S A. 2003;100:3611–6. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.