Abstract
Ischemic postconditioning has been established for its protective effects against stroke in animal models. It is performed after post-stroke reperfusion and refers to a series of induced ischemia or a single brief one. This review article addresses major hurdles in clinical translation of ischemic postconditioning to stroke patients, including potential hazards, the lack of well-defined protective paradigms, and the paucity of deeply-understood protective mechanisms. A hormetic model, often used in toxicology to describe a dose-dependent response to a toxic agent, is suggested to study both beneficial and detrimental effects of ischemic postconditioning. Experimental strategies are discussed, including how to define the hazards of ischemic (homologous) postconditioning and the possibility of employing non-ischemic (heterologous) postconditioning to facilitate clinical translation. This review concludes that a more detailed assessment of ischemic postconditioning and studies of a broad range of heterologous postconditioning models are warranted for future clinical translation.
Keywords: ischemic postconditioning, preconditioning, stroke, hormesis, clinical translation
Introduction
Ischemic postconditioning (IPostC) was recently defined to contrast with ischemic preconditioning (IPreC) [1,2]. While IPreC is a sublethal ischemia performed in advance of severe brain ischemia [3]—an induced minor stroke before a major stroke—IPostC conventionally refers to a single brief or series of brief occlusions/reperfusions that are performed after ischemia/reperfusion—a minor stroke or a series of minor strokes after a major stroke. Both IPostC and IPreC confer neuroprotective effects on brain ischemia. Note that both pre- and post-conditioning may include a broad range of sublethal insults, from ischemia, neurotoxic agents and pharmacological agents to physical exercise[4–7]. However, in this article IPreC and IPostC refer specifically to pre- and post-conditioning that is induced by ischemia. The research history, experimental models, protective mechanisms and clinical relevance of preconditioning have been previously reviewed [6–8]. My colleagues and I have reviewed most of these topics associated with postconditioning [4,9,10] and will not repeat them here. This review focuses instead on the issues of IPostC in animal stroke models most relevant to clinical translation.
Many researchers agree that clinical translation of basic research in pre- and postconditioning is the foremost goal. Although IPreC has not reached the clinical translation stage after more than 20 years of stroke research [11,12], recent advancements in clinical trials for remote preconditioning against both cardiovascular and cerebral vascular diseases have shed light on the potential clinical success of preconditioning [13–18]. Despite these potential applications, a major roadblock is that IPreC requires a stroke onset prediction for most patients. In contrast, IPostC has a therapeutic time-window advantage over IPreC as it follows post-stroke reperfusion. Thus, in principle, the predictability of stroke onset is not a prerequisite for clinical translation of IPostC. IPostC nonetheless has many other critical hurdles to clear before clinical translation. I review these hurdles as well as possible remedies, including the potential hazards of IPostC, the lack of well-defined protective paradigms and the paucity of known protective mechanisms. Hopefully this will provoke further study of innovative experimental strategies and alternative models that advance clinical translation of postconditioning including, but not limited to, IPostC.
Factors that shape decisions for clinical trials of IPostC in stroke treatment
Whether or not IPostC can be successfully translated to the clinic, even as a pilot study, is not simply an issue of pure science; there are also social, psychological and medical ethics implications. In advancing basic science to the clinic, the potential of IPostC is guided by the principles of medical ethics—justice, respect for autonomy, non-maleficence, and beneficence [19]. The latter two principles, non-maleficence and beneficence, form the core of medical ethics [20]. Non-maleficence refers to “do no harm” to patients, while beneficence represents any benefit brought to patients. In IPostC clinical trials the most important criteria are whether or not IPostC itself is detrimental and whether or not stroke patients benefit from such treatment.
Therefore, the following factors must be assessed before a decision is made to commence IPostC clinical trials. First, the scientific evidence for protective effects of IPostC in experimental stroke models must be presented. These include optimal IPostC paradigms, stroke models that reflect clinical stroke and mechanisms that underlie its protective effects. Second, concerns of patients and medical doctors must be addressed regarding the risk of additional injury when one or more minor strokes are induced after a major stroke. Persistent fears will influence both patients and doctors on their acceptance of IPostC.
Experimental hormetic strategies to assess risk and treatment efficacy of IPostC
To ensure that performance of IPostC does not endanger the brain, a safety zone of IPostC must be defined. The concept of hormesis can help identify such a safety zone, not only because postconditioning is considered a form of hormesis but also because hormetic theory provides a clear strategy to identify beneficial and detrimental effects of a stress such as ischemia [21–24]. This idea was inspired by Dr. Rajiv Ratan, professor of neurology and neuroscience at Weill Cornell Medical College, who proposed that preconditioning is a form of hormesis during the Translational Preconditioning Workshop in Miami on December 8, 2011.
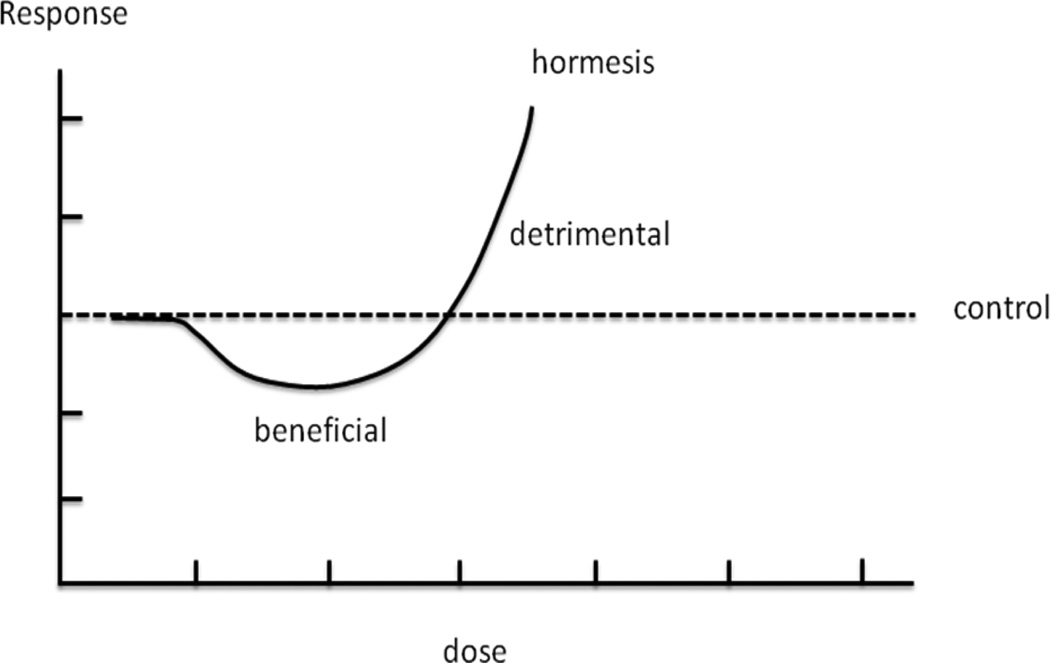
Hormesis is a term originally defined in toxicology [25] and refers to a phenomenon where a low dose of a toxic agent is stimulatory and beneficial to a biological system whereas a high dose is inhibitory and detrimental[25]. These dose-dependent effects have been shown in many chemicals [26–30] as manifested as J-shaped or U-shaped profiles [20,31](Fig. 1). Hormesis appears to be universal, existing not only in toxicology but also in immunological response, psychological disease and ecological problems. Interested readers are referred to the following comprehensive reviews on this subject [20,30–34]. As IPreC and IPostC represent minor ischemia before and after stroke and as ischemia of different durations corresponds to varying ranges of toxicant doses, the concepts of IPreC and IPostC are consistent with hormesis in toxicology. IPreC and IPostC have been thus rephrased as pre- and postconditioning hormesis in the research field of hormesis [20,31]. Extensive discussion of hormesis, its relationship with IPreC and IPostC, along with its applicability and feasibility to stroke research, is beyond of the scope of this review. Nevertheless, the concept of hormesis is useful to address the double-edged implications of IPostC for stroke, that is, its potential beneficial and detrimental effects.
Fig. 1.
The “J” shaped curve shows the hormetic dose response of a toxic agent on a biological system or an organism (modified from [20]). Lower dose ranges of the toxic agent are beneficial while doses above a threshold are inhibitive or detrimental.
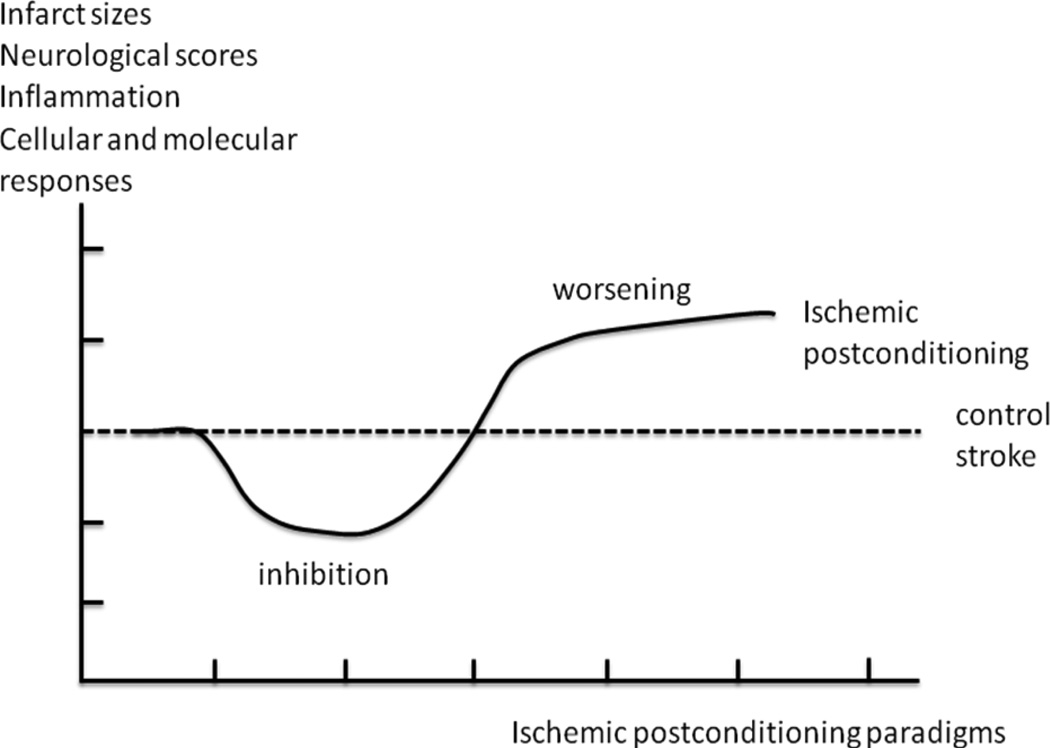
The theory of hormesis states that many toxic agents, stresses and insults execute two opposite effects on a biological system in a dose-dependent pattern, usually portrayed as a “J” or “U” shape[20,31]. This reviewer instead uses a horizontal “scoop” to represent a possible pathological outcome in response to IPostC in stroke (Fig. 2). As unlimited doses of a toxic agent to an organism will eventually result in death, a “J” shape may best represent the hormetic response (Fig. 1). Nevertheless, as the duration or number of IPostC cycles increases, prolonged ischemia will eventually lead to maximally permanent ischemia-like stroke and potentially cause a maximal, but not unlimited, infarction as seen in permanent ischemia. Therefore, the shape of a horizontal scoop may best represent the pathology in animal subjects treated with various paradigms of IPostC from brief ischemia, prolonged ischemia, to unlimited cycles of repeated ischemia (Fig. 2).
Fig. 2.
Hormetic hypothesis for IPostC. A horizontal scoop shape is adopted to reflect a possible dose-dependent response of IPostC. Like a typical biphasic response of hormesis, IPostC with certain paradigms generates protective effects while it may exacerbate brain injury with other paradigms. This model is used as a guide for exploring a safety range of performing IPostC.
IPostC can be performed and assessed in intact animals to help determine the possible negative effects in experimental stroke. However, the detrimental effects of IPostC on intact animals versus animals with previous stroke differ. Experiments in both stroke-induced and intact animals must therefore be performed to assess the hazards of IPostC.
According to this hormetic model of IPostC (Fig. 2), two zones or ranges of IPostC “doses” can be identified. Within the first range, IPostC may generate from slight to clear beneficial effects. IPostC performed in the second range may cause detrimental effects. Appropriate temporal end points must be selected to evaluate the effects of IPostC. Animal survival or mortality, infarct size, neurological scores or behavioral tests, and cellular/molecular cascades may be used to evaluate the protective or detrimental effects of IPostC.
Exploration of the multifaceted protective mechanisms of IPostC
Once the safety zone of IPostC is resolved, understanding the underlying protective mechanisms of IPostC will help to refine its clinical applications and develop alternative strategies, which will assist with better clinical trial design and safer strategies. We have previously reviewed the potential protective mechanisms of IPostC [4,9,10], including its attenuation of free radical production[1], inhibition of inflammatory responses and apoptosis[1,35] along with promotion of Akt survival pathways[36–41]. However, these so-called mechanisms may only reflect correlations between protective effects and pathological changes as well as cellular and molecular signaling pathways. To what extent these studies reveal the protective mechanisms of IPostC is unknown. A comparison of mechanisms between IPostC and IPreC, has found that both share “similar mechanisms”[4]. This conclusion however requires further study.
Despite some similar “protective mechanisms” of IPostC and IPreC, there are fundamental differences between the two neuroprotectants. First, IPreC exerts its effects on a non-stroke brain, which adapts the naïve brain to a second lethal stroke. In contrast, IPostC is conducted after ischemic pathogenesis has initiated. IPostC therefore never has a chance to affect the brain in its naïve state. This situation makes it difficult to investigate the cause-and-effect relationship between IPostC and stroke. The measured final pathological outcomes in animals receiving both stroke and IPostC at the cellular and molecular levels cannot be simply considered as the causation of the protective effects of IPostC. Second, the effects of IPreC alone in animals without a subsequent stroke can be measured, which may provide meaningful data for understanding the underlying mechanisms of “causation” of IPreC. Nevertheless, it may not be meaningful to investigate the effects of IPostC alone on the brain in the absence of previous stroke except to clarify if IPostC alone is detrimental to naïve brains. Thus, although studies have attempted to compare the protective mechanisms of IPostC and IPreC, we must be cautious when we conclude that they share similar protective mechanisms.
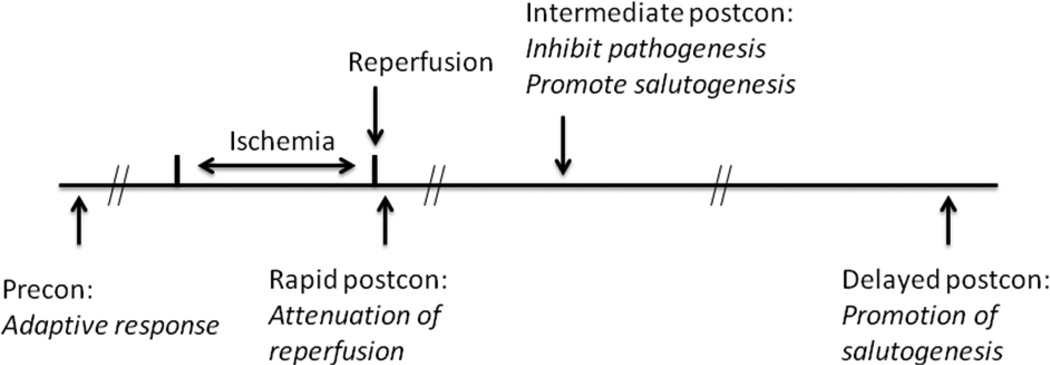
The protective mechanisms of IPostC may differ depending on when it is applied. Three therapeutic time windows are approximately: (1) immediately or within a few minutes after reperfusion[1,41,42]; (2) from a few to 12 hours after reperfusion[35,43]; and (3) from 24 hours to a few days after reperfusion[44–46]. The pathological status at these three stages can differ greatly. Therefore I define these three therapeutic time windows as “rapid,” “intermediate” and “delayed” even though the latter two windows have previously been combined under the term “delayed.” The rapid time window includes a sudden increase in cerebral blood flow (CBF)[47], a drastic recovery of anoxic depolarization[48,49], production of ROS[47], glutamate release/uptake[50] and intracellular/extracelluar Ca2+ oscillation[51]. During the intermediate time window the initial hyperemic response subsides and hypotension evolves, eventually leading to the no-reflow phenomenon[52–54]. Within a few hours, necrotic and apoptotic pathways are triggered[55–57] and ischemic core tissues may die, leading to initiation of a secondary inflammatory response[58–61]. During the delayed time window, from 24 hours to a few days post-reperfusion, the infarction matures, inflammatory cells infiltrate the ischemic brain, apoptosis and necrosis are largely complete, and the ischemic brain has begun to reorganize for recovery[62–64].
This review proposes that IPostC has three distinct protective mechanisms that correspond to the three therapeutic time windows, with possibility of both temporal and mechanistic overlaps (Fig. 3). The response of the ischemic brain to IPostC at each therapeutic time window is determined by the current status of the ischemic brain. Correspondingly, the protective mechanisms of IPostC differ at each stage.
Fig. 3.
Hypothesis for the protective mechanisms of rapid, intermediate and delayed postconditioning, and comparison with that of IPreC. IPreC mainly generates an adaptive response; rapid IPostC interrupts the early reperfusion, thus attenuates reperfusion-induced injury; intermediate IPostC may inhibit pathogenesis while promoting salutogenesis; delayed IPostC mainly promotes salutogenesis.
The protective effects of rapid IPostC may result mainly from its attenuation of side effects induced by sudden reperfusion. At stroke onset, the cutoff of blood supply to the brain causes ATP depletion, immediate loss of cerebral ion homeostasis, sudden anoxic depolarization in the ischemic core and spreading depression (SD)-like depolarization in the ischemic penumbra or peri-infarct regions[65,66]. Large amounts of glutamate released from intracellular spaces into extracellular spaces increases intracellular Ca2+ and Ca2+-dependent protease activity[51]. Although restoration of CBF after stroke leads to ATP regeneration and recovery, homeostatic disturbance in the preceding ischemic period already initiated severe pathogenesis, including irreversible cascades that will lead to the death of certain neurons, especially those in the ischemic core. The sudden subsequent onset of reperfusion results in over-production of reactive oxygen species (ROS) and reactive nitrogen species (RNS)[67]. Due to their highly reactive nature, ROS can attack critical cell components, including proteins, DNA and lipids, resulting in cell dysfunction[67]. RNS derived from nitric oxide also play modulatory roles in ischemia/reperfusion injury, including modification of macromolecules, attacks on endothelial cells and promotion of inflammatory products. ROS and RNS activity during reperfusion further tilt the existing homeostatic imbalance caused by ischemia toward necrosis and apoptosis. Rapid IPostC executed at early reperfusion interrupts this sudden reperfusion, plausibly blocking production of ROS and RNS and thus attenuating reperfusion-induced brain injury[1,4].
During the intermediate therapeutic time window[43], from a few hours to 12 hours after reperfusion, a mixture of both pro-death and pro-survival signals is triggered by the aforementioned products of ROS and RNS in the early ischemia/reperfusion period. At this stage, various cell signaling pathways involved in necrosis and apoptosis have been activated. The mitochondrial permeability transition (MPT) pore is open, ATP is further depleted, ROS activity is exacerbated, and organelles are swollen and ruptured [67]. The necrotic pathway also activates TNFα and the DNA repair enzyme poly (ADP-ribose) polymerase-1 (PARP1) [67]. Meanwhile, the following are also triggered: apoptotic pathways implicated with cytochrome c and apoptosis inducing factor (AIF) release[57,68], pro-death members of the Bcl-2 protein family (e.g., Bax, Bak) [67], σPKC cleavage [69] and caspase-9 and -3 [67]. It is well known that neuronal survival is determined by a balance between cell-death and cell-survival signaling pathways. Amid pro-death cell-signaling pathways, pro-survival cell-signaling pathways such as the PI3K/Akt pathway[70], anti-apoptotic Bcl-2 family proteins—such as Bcl-Xl and Bcl-2[71]— and ε PKC[72] are also initiated. IPostC applied at this intermediate stage may block death pathways while it enhances pro-survival signals. On one hand, IPostC may protect against injury by interrupting detrimental responses, but it is unknown how it achieves such effects. In addition, at this intermediate stage, cell death signals have promoted inflammatory responses involved in recruitment of inflammatory cells and secretion of chemokines and cytokines [67], which may be also be attenuated by IPostC. On the other hand, neurons in the ischemic tissue subjected to the same period of ischemia are not affected to the same extent, as some ischemic tissues in the penumbra could still be viable and some peri-infarct areas could be relatively “intact” with little damage. Intermediate IPostC may stimulate these relatively-intact tissues and promote survival signals, producing salutogenic effects. In other words, if any neurons in these areas are relatively intact but destined to suffer from second or third waves of injury, IPostC may function through mechanisms similar to IPreC that stimulate adaptive responses in relatively intact brain tissues and prevent the subsequent waves of injury. Even in a single neuron that is destined to die, both cell death and survival signals may co-exist, the balance of which may be shifted by IPostC. Thus, intermediate IPostC may both block pathogenesis and promote salutogenesis.
In the delayed therapeutic time window from 24 hours to a few days post-stroke, the physiological and pathological status of the ischemic brain differ greatly from the rapid and intermediate stages. At this last stage, infarction, necrosis and apoptosis have largely matured, and leukocytes including neutrophils [73], monocytes[74], and T cells have infiltrated the ischemic core and penumbra[58]. Pathology progresses gradually in the peri-infarct and remote areas to the ischemic core and spontaneous recovery of brain function begins upon survival of the initial injury. Delayed IPostC therefore does not target ischemic tissues that are destined to die or are already dead. It may instead affect homeostatic mechanisms that promote the remodeling of active structures and functions already underway as well as stimulate rewiring, synaptogenesis, sprouting of new axons, angiogenesis, and neurogenesis[75]. Although delayed postconditioning also blocks some very delayed ischemic neuronal death, its major function may be to promote salutogenesis. We must stress that the application of this delayed postconditioning is most feasible for most stroke patients, yet little is known for the pathological and protective mechanisms, which should be investigated in much more detail.
A difficult situation exists regarding how to categorize the therapeutic time window of postconditioning performed between 12 and 24 hours, which may belong to either intermediate or delayed postconditioning, for few studies have tested postconditioning within this time period. Nevertheless, if any postconditioning performed during this period executes protective effects, its mechanisms may overlap with either intermediate or delayed postconditioning.
In brief, this section of the review defines a sequence of three therapeutic time windows for IPostC and proposes three distinct therapeutic mechanisms. Rapid IPostC may block ischemic injury mainly by attenuating the early reperfusion injury; intermediate IPosC may simultaneously inhibit the detrimental effects and promote neuronal survival signals; and delayed IPosC may mainly promote brain recovery through generating salutogenesis. As evidence to support these hypotheses has only begun to accumulate, further studies and future experiments are warranted.
Heterologous postconditioning may overcome disadvantages of homologous postconditioning and clear hurdles to clinical trials
IPostC has evolved [76] to employ a broad range of postconditioning triggers such as hypoxic[46], hyperoxic[77], ischemic remote[78–80], pharmacologic[44] and anesthetic methods[81]. For convenience, this review uses homologous versus heterologous postconditioning to describe ischemic conditioning versus other patterns of postconditioning[21]. IPostC is defined as homologous because its trigger is ischemia, similar to the original stroke. The remaining triggers are heterologous because their triggers differ from traditional ischemia. IPostC includes some basic components, such as a lack of oxygen to the brain, insufficient energy (glucose) supply, and brief disturbances of ion homeostasis due to brief ischemia. It is likely that heterologous postconditioning, as long as it contains any of these basic components of IPostC, may serve as a form of postconditioning. Because the core principle of medical ethics is to “do no harm,” development of heterologous postconditioning strategies, especially those least hazardous, is crucial to successful clinical translation. For example, remote limb preconditioning has been recently tested in stroke patients largely due its safety[82]. The same strategy also applies to postconditioning.
Advocates of heterologous postconditioning, however, recognize the importance of laboratory research on homologous IPostC. The protective effects of homologous IPostC should serve as a standard to compare against heterologous postconditioning. In addition, the underlying protective mechanisms of heterologous and homologous postconditioning should be explored and compared with the hope of providing strong evidence, rationale and strategies for clinical translation of heterologous postconditioning.
Summary, conclusion and future directions
Difficult hurdles—risks, efficacy, therapeutic time windows and underlying protective mechanisms—must be assessed and cleared before clinical translation of IPostC. To reduce its potential risks, heterologous postconditioning may prove to be more effective for clinical translation in most cases. Thus, more detailed assessments along with studies of a broad range of heterologous postconditioning models are warranted for successful clinical translation. Along with the major hurdles discussed above, future studies must also address other important issues in successful clinical translation, including type of stroke, age of onset, sex and pre-existing pathologies, as recommended in the STAIR guidelines for preclinical stroke research[83]. Postconditioning efficacies in stroke models with reperfusion, with partial reperfusion and without any reperfusion should be studied and compared. Potential differences between postconditioning in ischemic and hemorrhagic stroke should also be investigated. Furthermore, age and sex are critical factors that affect pathological outcomes [84,85] yet current studies mostly include relatively-young adult male animals. Moreover, stroke in clinical patients is usually complicated by concomitant pathology, such as hypertension[86], diabetes[87] and hyperlipidemia[88]. How postconditioning could similarly protect animals with these diseases requires further study. Last but not least, since t-PA is the only FDA-approved thrombolytic agent for stroke treatment, several important issues related with concomitant use of t-PA with postconditioning should be investigated, including whether postconditioning is effective in ischemic models in which reperfusion is successfully achieved by t-PA application, whether there are any synergistic effects between postconditioning and t-PA application, and whether postconditioning can attenuate implications or toxic effects induced by t-PA application.
Acknowledgements
The authors thank Ms. Cindy H. Samos for manuscript assistance. This study was supported by AHA grant in aid and NIH grant 1R01NS 064136-01 (HZ).
Footnotes
Statement for conflict of interest: The author declares that he has no conflict of interest.
References
- 1.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26(9):1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29(5):873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, et al. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol (Berl) 2005;109(3):237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8(4):398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26(5):248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 8.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2(4):313–318. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13(2):173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, et al. 'Ischemic tolerance' phenomenon detected in various brain regions. Brain Res. 1991;561(2):203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528(1):21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SR, Tang T, Sadat U, Dutka DP, Gaunt ME. Cardioprotection by remote ischaemic preconditioning. Br J Anaesth. 2007;99(5):611–616. doi: 10.1093/bja/aem273. [DOI] [PubMed] [Google Scholar]
- 14.Walsh SR, Tang TY, Sadat U, Gaunt ME. Remote ischemic preconditioning in major vascular surgery. J Vasc Surg. 2009;49(1):240–243. doi: 10.1016/j.jvs.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer R, Lorenzen J, Kabbani M, Herold C, Busche M, Vogt PM, et al. Acute effects of remote ischemic preconditioning on cutaneous microcirculation--a controlled prospective cohort study. BMC Surg. 2011;11:32. doi: 10.1186/1471-2482-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol. 2012;24(2):42–48. [PubMed] [Google Scholar]
- 17.Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW. A systematic review and meta-analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J R Soc Med. 2012;105(10):436–445. doi: 10.1258/jrsm.2012.120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2012;42(10):2960–2962. doi: 10.1161/STROKEAHA.111.622340. [DOI] [PubMed] [Google Scholar]
- 19.Kennelly J. Medical ethics: four principles, two decisions, two roles and no reasons. J Prim Health Care. 2011;3(2):170–174. [PubMed] [Google Scholar]
- 20.Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009;7(1):1–51. doi: 10.2203/dose-response.08-023.Hoffmann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Wijk R, Wiegant FA. Postconditioning hormesis and the homeopathic Similia principle: molecular aspects. Hum Exp Toxicol. 2010;29(7):561–565. doi: 10.1177/0960327110369860. [DOI] [PubMed] [Google Scholar]
- 22.Van Wijk R, Wiegant FA. Postconditioning hormesis and the similia principle. Front Biosci (Elite Ed) 2011;3:1128–1138. doi: 10.2741/e316. [DOI] [PubMed] [Google Scholar]
- 23.Wiegant F, Van Wijk R. The similia principle: results obtained in a cellular model system. Homeopathy. 2010;99(1):3–14. doi: 10.1016/j.homp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Wiegant FA, Prins HA, Van Wijk R. Postconditioning hormesis put in perspective: an overview of experimental and clinical studies. Dose Response. 2011;9(2):209–224. doi: 10.2203/dose-response.10-004.Wiegant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese EJ. Hormesis: Toxicological foundations and role in aging research. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese EJ. Hormesis: a revolution in toxicology, risk assessment and medicine. EMBO Rep. 2004;5(Spec No):S37–S40. doi: 10.1038/sj.embor.7400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66(5):594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calabrese EJ. Neuroscience and hormesis: overview and general findings. Crit Rev Toxicol. 2008;38(4):249–252. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese EJ. Hormesis and mixtures. Toxicol Appl Pharmacol. 2008;229(2):262–263. doi: 10.1016/j.taap.2008.01.024. author reply 264. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese EJ, Baldwin LA. Hormesis: a generalizable and unifying hypothesis. Crit Rev Toxicol. 2001;31(4–5):353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27(7):1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- 33.Calabrese EJ, Baldwin LA. Chemical hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19(1):2–31. doi: 10.1191/096032700678815585. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21(2):91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 35.Feng R, Li S, Li F. Toll-like receptor 4 is involved in ischemic tolerance of postconditioning in hippocampus of tree shrews to thrombotic cerebral ischemia. Brain Res. 2011;1384:118–127. doi: 10.1016/j.brainres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, et al. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105(3):943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy? Neuropharmacology. 2008;55(4):509–516. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Wang JK, Yu LN, Zhang FJ, Yang MJ, Yu J, Yan M, et al. Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res. 2010;1357:142–151. doi: 10.1016/j.brainres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Prasad SS, Russell M, Nowakowska M. Neuroprotection induced in vitro by ischemic preconditioning and postconditioning: modulation of apoptosis and PI3K-Akt pathways. J Mol Neurosci. 2011;43(3):428–442. doi: 10.1007/s12031-010-9461-7. [DOI] [PubMed] [Google Scholar]
- 41.Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28(2):232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 42.Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39(8):2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- 43.Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS ONE. 2008;3(12):e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danielisova V, Gottlieb M, Nemethova M, Burda J. Effects of bradykinin postconditioning on endogenous antioxidant enzyme activity after transient forebrain ischemia in rat. Neurochem Res. 2008;33(6):1057–1064. doi: 10.1007/s11064-007-9550-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C, Tu J, Zhang Q, Lu D, Zhu Y, Zhang W, et al. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3beta signaling. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40(10):3349–3355. doi: 10.1161/STROKEAHA.109.557314. [DOI] [PubMed] [Google Scholar]
- 47.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Asai S, Kanematsu K, Kunimatsu T, Kohno T, Ishikawa K. Real-time monitoring of the effects of normothermia and hypothermia on extracellular glutamate re-uptake in the rat following global brain ischemia. Neuroreport. 1997;8(9–10):2389–2393. doi: 10.1097/00001756-199707070-00057. [DOI] [PubMed] [Google Scholar]
- 49.Zhao H, Asai S, Kohno T, Ishikawa K. Effects of brain temperature on CBF thresholds for extracellular glutamate release and reuptake in the striatum in a rat model of graded global ischemia. Neuroreport. 1998;9(14):3183–3188. doi: 10.1097/00001756-199810050-00011. [DOI] [PubMed] [Google Scholar]
- 50.Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20(7):904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- 51.Choi DW, Weiss JH, Koh JY, Christine CW, Kurth MC. Glutamate neurotoxicity, calcium, and zinc. Ann N Y Acad Sci. 1989;568:219–224. doi: 10.1111/j.1749-6632.1989.tb12511.x. [DOI] [PubMed] [Google Scholar]
- 52.Halls SB. The no-reflow phenomenon and dense fibrillary gliosis, cause of dark T2-weighted MR signal in stroke. AJNR Am J Neuroradiol. 1996;17(2):394–395. [PMC free article] [PubMed] [Google Scholar]
- 53.Ito U, Ohno K, Yamaguchi T, Tomita H, Inaba Y, Kashima M. Transient appearance of "no-reflow" phenomenon in Mongolian gerbils. Stroke. 1980;11(5):517–521. doi: 10.1161/01.str.11.5.517. [DOI] [PubMed] [Google Scholar]
- 54.Mercuri M, Ciuffetti G, Lombardini R, Neri C, Robinson M. Do leukocytes have a role in the cerebral no-reflow phenomenon? J Neurol Neurosurg Psychiatry. 1990;53(6):536. doi: 10.1136/jnnp.53.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, et al. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2003;23(10):1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- 56.Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, et al. Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci. 2001;21(13):4678–4690. doi: 10.1523/JNEUROSCI.21-13-04678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85(4):1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 58.Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43(7):1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castillo J, Alvarez-Sabin J, Martinez-Vila E, Montaner J, Sobrino T, Vivancos J. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol. 2009;256(2):217–224. doi: 10.1007/s00415-009-0058-4. [DOI] [PubMed] [Google Scholar]
- 60.Chapman KZ, Dale VQ, Denes A, Bennett G, Rothwell NJ, Allan SM, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1764–1768. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- 61.Jordan J, Segura T, Brea D, Galindo MF, Castillo J. Inflammation as therapeutic objective in stroke. Curr Pharm Des. 2008;14(33):3549–3564. doi: 10.2174/138161208786848766. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–266. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- 63.Seil FJ. Recovery and repair issues after stroke from the scientific perspective. Curr Opin Neurol. 1997;10(1):49–51. [PubMed] [Google Scholar]
- 64.Teasell R, Bayona N, Salter K, Hellings C, Bitensky J. Progress in clinical neurosciences: stroke recovery and rehabilitation. Can J Neurol Sci. 2006;33(4):357–364. doi: 10.1017/s0317167100005308. [DOI] [PubMed] [Google Scholar]
- 65.Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63(6):720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Zhang RL, Zhang ZG, Chopp M. Measurement of neuronal activity of individual neurons after stroke in the rat using a microwire electrode array. J Neurosci Methods. 2007;162(1–2):91–100. doi: 10.1016/j.jneumeth.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24(6):681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- 69.Shimohata T, Zhao H, Steinberg GK. Hypothermia suppresses PKC activation in a model of permanent middle cerebral artery occlusion. Soc Neurosci Abs. 2005 221.11 2005. [Google Scholar]
- 70.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25(42):9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Sobel RA, Cheng D, Steinberg GK, Yenari MA. Mild hypothermia increases Bcl-2 protein expression following global cerebral ischemia. Brain Res Mol Brain Res. 2001;95(1–2):75–85. doi: 10.1016/s0169-328x(01)00247-9. [DOI] [PubMed] [Google Scholar]
- 72.Shimohata T, Zhao H, Steinberg GK. Epsilon PKC may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke. 2007;38(2):375–380. doi: 10.1161/01.STR.0000254616.78387.ee. [DOI] [PubMed] [Google Scholar]
- 73.Ritter L, Davidson L, Henry M, Davis-Gorman G, Morrison H, Frye JB, et al. Exaggerated neutrophil-mediated reperfusion injury after ischemic stroke in a rodent model of type 2 diabetes. Microcirculation. 2011;18(7):552–561. doi: 10.1111/j.1549-8719.2011.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71(6):743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- 75.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 76.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Weaver J, Liu KJ. Rapid conditioning with oxygen oscillation: neuroprotection by intermittent normobaric hyperoxia after transient focal cerebral ischemia in rats. Stroke. 2012;43(1):220–226. doi: 10.1161/STROKEAHA.111.625756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoda MN, Siddiqui S, Herberg S, Periyasamy-Thandavan S, Bhatia K, Hafez SS, et al. Remote Ischemic Perconditioning Is Effective Alone and in Combination With Intravenous Tissue-Type Plasminogen Activator in Murine Model of Embolic Stroke. Stroke. 2012 doi: 10.1161/STROKEAHA.112.660373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Fathali N, Lekic T, Ostrowski RP, Chen C, Martin RD, et al. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011;42(2):439–444. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, et al. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 2010;104(2):191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- 82.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42(5):1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31(5):1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- 86.Hisham NF, Bayraktutan U. Epidemiology, Pathophysiology, and Treatment of Hypertension in Ischaemic Stroke Patients. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Alter M, Lai SM, Friday G, Singh V, Kumar VM, Sobel E. Stroke recurrence in diabetics. Does control of blood glucose reduce risk? Stroke. 1997;28(6):1153–1157. doi: 10.1161/01.str.28.6.1153. [DOI] [PubMed] [Google Scholar]
- 88.Warsch JR, Wright CB. Stroke: hyperlipidemia and cerebral small-vessel disease. Nat Rev Neurol. 2010;6(6):307–308. doi: 10.1038/nrneurol.2010.70. [DOI] [PubMed] [Google Scholar]