Abstract
Pharmacological and genetic evidence reveals that GABAA receptor (GABAA-R) expression and localization are modulated in response to acute and chronic ethanol (EtOH) exposure. To determine molecular mechanisms of GABAA-R plasticity in response to in vivo acute EtOH, we measured early time changes in GABAA-R subunit localization. Single doses of EtOH (3 g/kg via i.p. injection in rats) produced decreases in surface levels of GABAA-R α4 and δ subunits at 5–15 min post-EtOH in hippocampus CA1 and dentate gyrus, verifying our earlier report (Liang et al., 2007). Here we also examined the β3 subunit and its phosphorylation state during internalization. β3 also was internalized during 5–15 min after EtOH exposure, while phosphorylation of β3 was increased, then decreased at later times, ruling out β3 dephosphorylation-dependent endocytosis. As early as 5 min post-EtOH, there is an initial increase in association between the δ subunits with clathrin adaptor proteins AP2-μ2 revealed by coimmunoprecipitation, followed by a decrease in association 15 min post-EtOH. In vitro studies using glutathione S-transferase fused to the δ subunit intracellular domain (ICD) show that two regions, one containing a classical YxxΦ motif and the other an atypical R/K-rich motif, directly and differentially bind to AP2-μ2, with the former YRSV exhibiting higher affinity. Mutating both regions in the δ-ICD abolishes μ2 binding, providing a possible mechanism that can explain the rapid downregulation of extrasynaptic α4βδ-GABAA-R following in vivo EtOH administration, in which the δ-ICD increases in affinity for clathrin AP2-μ2 leading to endocytosis.
Introduction
Ionotropic GABAA receptors (GABAA-Rs) mediate the majority of phasic and tonic neural inhibition in the brain and are critical for the regulation of neural activity. GABAA-Rs are members of the Cys-loop, heteropentameric ligand-gated ion channel superfamily. A large variety of functional GABAA-R isoforms are assembled from 19 related subunits, usually in a combination of two α subunits (α1–6), two β subunits (β1–3), and one γ(1–3), δ, ε, π, θ, or ρ(1–3) subunit. These individual subunits exhibit distinct, yet overlapping, expression patterns, as well as functional and pharmacological properties. Each subunit contains a large extracellular N-terminal ligand-binding domain, four membrane-spanning domains (M1–M4), a large intracellular domain (ICD), and a short extracellular C terminus (Olsen and Sieghart, 2008).
Studies show the ICD of β(1–3) and γ2 GABAA-R subunits interact with various scaffolding and signaling proteins, thereby regulating the trafficking and plasticity of GABAA-R (for review, see Chen and Olsen, 2007; Jacob et al., 2008; Luscher et al., 2011). Less is known about the scarcer δ subunit and its ICD, but trafficking of δ subunits may be distinct from the mechanisms that govern trafficking of other subunits (Joshi and Kapur, 2009). The δ subunit exhibits unique properties: δ-GABAA-Rs are located exclusively extrasynaptically where they tend to assemble with α4, α6, and α1 subunits only (Nusser et al., 1998; Wei et al., 2003; Liang et al., 2006; Glykys et al., 2007). With these α subunits, δ-GABAA-Rs mediate tonic currents (Itonic) that are sensitive to activation by the low concentrations of GABA found at extrasynaptic membrane compartments (Mody and Pearce, 2004; Meera et al., 2011), and to modulation by low concentrations of neurosteroids, some anesthetics, and EtOH (Mihalek et al., 1999; Sundstrom-Poromaa et al., 2002; Stell et al., 2003; Wallner et al., 2003; Belelli et al., 2009).
A single intoxicating dose of EtOH produced a rapid decrease in function and surface levels of α4/δ-containing GABAA-R in rat hippocampus, and a slightly later upregulation of α4/γ2-containing GABAA-R, both in vivo (Liang et al., 2007; Olsen and Spigelman, 2012), and in cultured hippocampal neurons (Shen et al., 2011); the receptor subunit decrease was demonstrated as increased endocytosis rather than decreased surface insertion. These acute EtOH-induced GABAA-R changes were reversible in naive rats, but virtually irreversible in chronic intermittent ethanol (CIE) rats (Cagetti et al., 2003; Liang et al., 2006). Plasticity of GABAA-Rs may involve regulated trafficking via protein–protein interactions. Direct interactions between membrane receptors and the clathrin adaptor complex (AP2), which drives clathrin coat formation and mediates endocytosis, often alter surface amounts in β and γ2 subunit-containing GABAA-R (Kittler et al., 2000, 2005; Herring et al., 2003; Smith et al., 2008). The present study begins to identify previously uncharacterized δ interactions and their contribution to the mechanism(s) involved in the early decrease of GABAA-R following EtOH exposure (Liang et al., 2007; Shen et al., 2011). How EtOH regulates restructuring of extrasynaptic GABAA-R remains to be fully resolved, but the mechanisms are probably crucial to alcohol dependence and potential new approaches to treat alcohol use disorders.
Materials and Methods
Animals and EtOH treatment.
The Institutional Animal Care and Use Committee approved all animal experiments. Male Sprague Dawley rats (200–350 g) were housed in the vivarium under a 12 h light/dark cycle and had ad libitum access to food and water. Rats were administered a single dose of EtOH (3 g/kg, as a 25% w/v solution in distilled water) or distilled water (vehicle) via intraperitoneal injection and decapitated 5, 10, 15, 30, or 60 min later.
Microdissection of rat brain.
Following decapitation, rat brains were bathed with ice-cold artificial CSF (ACSF), removed, and quickly sliced with a tissue chopper to obtain 500 μm coronal slices. Slices containing hippocampus regions were immediately placed into ice-cold ACSF, and hippocampus was microdissected for dentate gyrus (DG) and CA1 regions for normal and cross-linked Western blotting analyses (Liang et al., 2007). All slices for biochemical experiments using rat brain regions were allowed to recover in fresh ACSF at 35°C for 20–60 min.
For coimmunoprecipitation analyses, only dissection of DG region was performed since DG and CA1 showed similar patterns following cross-linking experiments, and DG is richer in α4 and δ subunits, and can be obtained much faster and cleaner if it is the only region collected. The DG was allowed to recover in fresh ACSF, 35°C for 20 min, and prepared for coimmunoprecipitation experiments. Membranes were prepared for total GABAA-R subunit level analyses as discussed previously (Cagetti et al., 2003). GABAA-R β3 subunit phosphorylation was estimated with substrate-specific antibody anti-phospho-β3 (Terunuma et al., 2008).
Cross-linking to measure surface GABAA-R subunits.
Following the recovery period, brain regions were incubated with or without bis(sulfosuccinimidyl)suberate (BS3) in ice-cold ACSF (Grosshans et al., 2002). Slices were homogenized in 1:4 buffer A/B; where A is 1% SDS, 1 mm EDTA, 50 mm NaF, 10 mm Tris-HCl, pH 8.0, 100 mm phenylmethylsulfonyl fluoride (PMSF), and Complete Protease Inhibitor Cocktail tablet (Roche), and B is 20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 100 mm PMSF. Protein concentration was determined by BCA Protein Assay Kit, and samples were prepared as standard for SDS-PAGE/Western blot. Relative signal intensities from each band (absorbance) were measured using ImageJ software. Samples were analyzed relative to one another on the same membrane and within the same experiment. Group differences were evaluated by two-way ANOVA, followed by post hoc Holm-Sidak test.
Membrane preparation for coimmunoprecipitation.
Following the slice recovery period, brain regions were homogenized in ice-cold, freshly prepared lysis buffer (50 mm Tris, pH 8.0, 0.5 mm EDTA, 2 mm EGTA, 1% Triton X, 50 mm NaF, 100 mm PMSF) and centrifuged at 15,000 × g for 30 min at 4°C. Cell lysate was diluted to ∼1 mg/ml total protein, precleared with 50 μl of Sepharose A beads at 4°C for 30–60 min, and immediately used for coimmunoprecipitation (co-IP).
Coimmunoprecipitation.
Sepharose A beads (30–50 μl) were incubated with 3–10 μg of antibody for 1 h, 4°C before incubating with 400 μl of precleared lysate, at 4°C for 4–18 h. IP complexes were washed with lysis buffer, twice with TBS containing 100 mm NaCl, and once more with lysis buffer. The washed complex was eluted with 2× SDS sample buffer and boiled, and loaded on a 4–15% gradient SDS polyacrylamide gel for protein separation, followed by transfer and antibody blotting. The secondary antibody was replaced with Clean-Blot IP HRP detection reagent (Thermo Fisher Scientific) when blotting with a same-species primary antibody as the antibody used for immunoprecipitation. Rabbit IgG (3–10 μg) was used as control.
Antibodies.
Dr. W. Sieghart (Medical University, Vienna, Austria) generously provided the following polyclonal GABAA-R antibodies: anti-α4 subunit (1–14) N-terminal antibody, anti-α4 subunit (379–421), anti-β3 subunit (345–408), anti-GABAA-R γ2 subunit (319–366), anti-GABAA-R δ subunit (N1–44), and anti-GABAA-R δ subunit (1–44). The anti-GABAA-R phospho-β3 subunit (p408/p409) was prepared by the Moss laboratory (Terunuma et al., 2008). The following commercial antibodies were used for this study: polyclonal anti-GABAA-R δ subunit (331–430) from Santa Cruz Biotechnology, Inc, monoclonal mouse anti-GABAA-R β2/3 (clone 62-3G1) from Millipore, monoclonal mouse anti-β-actin (clone AC-15) from Sigma, polyclonal anti-β-actin from Anaspec, and monoclonal mouse anti-AP2 μ2 subunit (110–230) from BD Biosciences.
Glutathione S-transferase-δ ICD cDNA.
Glutathione S-transferase-δ (GST-δ) cDNA containing the entire GABAA-R δ subunit ICD was discussed previously (Kittler et al., 2005). Truncated and mutated forms of GST-δ were made by standard PCR using Platinum Taq Polymerase High Fidelity (Invitrogen) with a 50 μl total reaction volume in a standard PCR cycler and the following primers, as necessary: attatcttGGATCCCACTTCAATGCCGACTACAGGAAG and attatcttGAATTCctaAGCCAACTCCTGACTGACCCC to create δ ICD residues 316–359 (called δ 316–359, or δR interchangeably); attatcttGGATCCATCTCCCGTCGTCAAGGCC attatcttGAATTCctaGATGTCGATGGTGTCTGCATCGA to create δ ICD residues 360–410 (called δ 360–410, or δY interchangeably); attatcttGAACCTCATGGGTTCCGCTAGGTCTGTG along with primers 1 and 4 from above to create δ ICD (375Y→A) mutant (also called δY→A interchangeably); attatcttGCCGACTACGGGGCGGCAGGGGCAGCCAAGGTC was used along with primers 1 and 4 to create δ ICD (322RKKRK326→GAAGA) (also called δR→G interchangeably). The cDNA of the 375Y→A mutant (Y→A mutant) was used as template along with mutation primer 322RKKRK326→GAAGA) to create the δ ICD double μ2-motif mutant, also called δ2 mutant. Sequencing of transformed plasmid DNA verified desired constructs to use for expression of proteins.
Expression of GST fusion proteins.
GST construct DNAs were transformed into Escherichia coli strain BL21, and a standard protocol for expressing and purifying GST proteins was followed (Einarson et al., 2007). Protein concentrations were determined by BCA assay. GST pull-down assays were performed as described by Kittler et al. (2005).
Concentration-dependent binding of GST-constructs to μ2.
GST-affinity beads bound to GST-fusion proteins (as in pull-down experiments) were incubated with the varying concentrations of purified AP2 ([1 nm, 0.1 μm, 0.1 mm] plus lysate), at 4°C for 2–18 h, washed, and subjected to SDS-PAGE followed by Western blot or Coomassie stain. GST fusion mutants' binding to μ2 was normalized to its respective GST amount, and then compared with GST-δ full-length ICD (wild type). Each full-length mutant GST was then compared with each of the others.
Statistical analysis.
Normalized optical density measurements were used to calculate percentage changes, normalized according to experiment: For total protein level and surface level analysis, optical density of each band was normalized to its corresponding β-actin loading control. For coimmunoprecipitation analysis, optical density of each coimmunoprecipitated band was normalized to the original immunoprecipitated protein (the one pulled down by its specific antibody). All quantifications are expressed as the mean ± SEM. Using the GraphPad Prism 4.0 program and SigmaStat, statistical analysis are as follows.
For Figures 1 and 2, a two-way ANOVA followed by Holm-Sidak post hoc test was used to determine significant differences among treatment time and among brain regions and between control and treated samples at a given time and region.
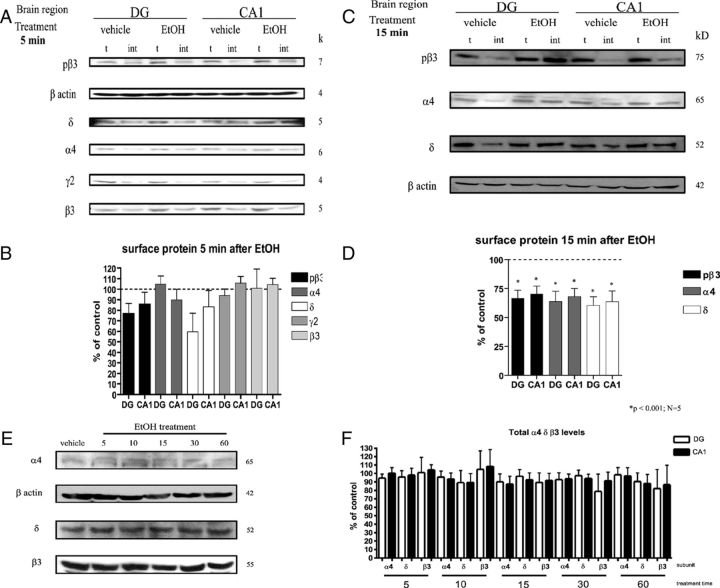
Figure 1.
A single in vivo EtOH dose causes decreases in extrasynaptic surface GABAA-R in hippocampal DG and CA1 within 15 min. A–D, Crosslinking experiments show a trending decrease in surface levels of α4, pβ3, and δ subunits as early as 5 min following EtOH dose (A, B), with significant decreases measured 15 min post-EtOH (C, D). t, Total protein (untreated slices); int, internal protein (slices treated with BS3). β3 and γ2 showed no significant changes. Pictures show representative blots of the average changes; graphs (B, D) plot the average of several experiments, N = 5. E, F, GABAA-R subunit levels at early time points after EtOH treatment. Total GABAA-R subunit changes in DG and CA1 at times 5–60 min following EtOH (3 g/kg) treatment do not change significantly (N = 4–5).
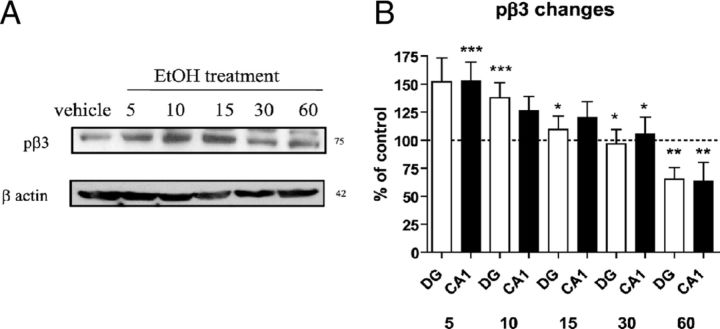
Figure 2.
EtOH-induced changes in GABAA-R β3 phosphorylation at early time points. A, Phosphorylation of β3 as identified with sequence-specific phospho-peptide antibody. Initial increases at 5 and 10 min in phosphorylation of β3 subunit compared with control treated at 5 and 10 min (153.10 ± 40.14%, 138.08 ± 29.20%, respectively; N = 4–5). B, Plot of quantified pβ3 level changes following various EtOH time treatments and brain regions. *p < 0.05; **p < 0.001 between 5 min treatment and stated treatment times in each brain region; ***p < 0.05 between control and EtOH-treated rats from same time point treatment; two-way ANOVA.
For each experiment in Figure 3, a two-way ANOVA followed by Bonferroni post hoc test was used to determine significant differences among treatment time and subunits.
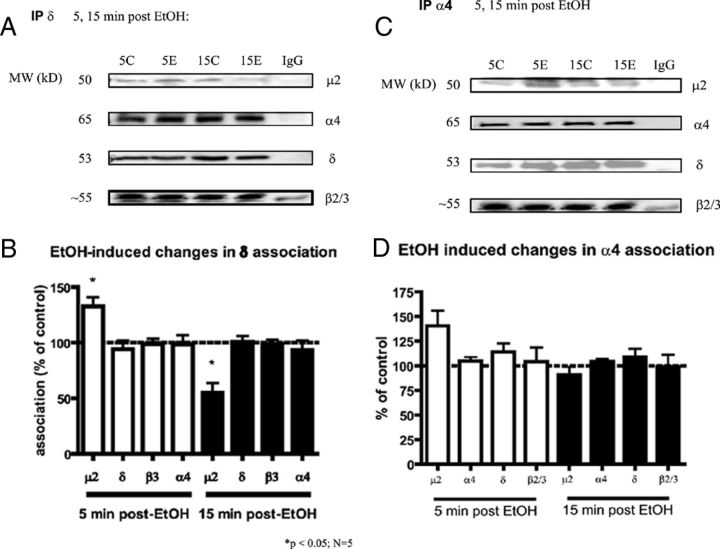
Figure 3.
GABAA-R δ and α4 subunit association with μ2 changes following EtOH exposures. A, B, EtOH caused a 32.7% increase in δ association with μ2 at 5 min, and a 55.1% decrease in δ association with μ2 15 min following EtOH treatment. The EtOH-induced changes in association of δ with μ2 are statistically significant from each other (**p < 0.0001), and statistically significant from control treated δ-μ2 associations (two-way ANOVA, Bonferroni post hoc test; *p < 0.05; N = 5). C, D, GABAA-R α4 subunit associations following EtOH exposure. EtOH caused a 38% increase in α4 association with μ2 at 5 min (not significant, p = 0.05, N = 4; but was significantly different from association of α4 and μ2 at 15 min, p < 0.001). This suggests that, at least at an early time point, these subunits are internalizing with δ-containing GABAA-R.
For Figure 4C, a one-way ANOVA followed by Bonferroni post hoc test was used to determine significant differences among the constructs and binding efficacy. p < 0.001 was considered significant that the 2x mutant differed in μ2 binding versus full-length wild-type δ-μ2 binding. p < 0.05 was considered significant for the double mutant (2X mutant) binding to μ2 across all other GST-δ constructs. For Figure 4D, a one-way ANOVA followed by a Holm-Sidak post hoc test found a significant difference between the δY and δRs ability to bind to μ2 (using means as comparisons), with p < 0.05; N = 3 (experiment done on three batches of proteins, repeated at least twice per experiment).
Figure 4.

Characterization of δ intracellular domain. A, Putative μ2 binding domains within the GABAA-R δ subunit ICD. The entire rat δ ICD (aa 316–410) is shown. A classical YxxΦ motif (375YRSV) is located at residues 375–378, while an atypical basic rich motif similar to those seen in GABAA-R β(1–3) is found at residues 322–334. Both are highlighted. B, Diagram of GST δ ICD constructs in this study: 1, GST only; 2, GST+ δICD full-length residues 316–410 (δ); 3, GST + first half of δ ICD residues 316–359 (δR); 4, GST + second half δ ICD residues 360–410 (δY); 5, GST + δ full-length R/K piece mutated residues 322–326 (δ322RKKRK→GAAGA); 6, GST + δ full-length Y375 mutated to A (δ375Y→A); 7, GST + δ full-length, where both the 322R/K piece and 375Y are mutated (δ2X mutant). C, Each μ2 binding domain of the δ ICD binds to μ2. GST-only (control) does not bind to μ2, but constructs 2–6 all bind to μ2. This includes all δ ICD constructs containing at least one complete μ2-binding domain (322R/K region only shown as δR; 375YRSV-only shown as δY). Only the construct lacking both μ2-binding domains shows no binding to μ2. Therefore, each of the two μ2 binding domains directly bind to μ2. Quantification of binding shows there are no significant differences between mutants and δ ICD full-length, except for the double mutant, which significantly lost all μ2 binding (*p < 0.001; one-way ANOVA). All other constructs are significantly different from the double mutant (p < 0.05; one-way ANOVA). D, The 375YRSV-motif of the δ ICD detects lower [μ2]. The 1 nm, 0.1 μm, and 0.1 mm AP2 fractions were incubated with the GST-δ truncation proteins to see if one region binds at a lower μ2 concentration than the other. The procedure and analysis were similar to those done with the pull-down experiments, with representative blot and cumulative quantified μ2 binding plot shown here. The 1 nm concentration showed no binding, but 0.1 μm showed μ2 binding more to the construct lacking the basic rich region, suggesting this lower-concentration binding is more influenced by the 375YRSV-motif. The difference between binding seemed to be undetectable at higher μ2 concentrations, likely because the basic-rich 322R/K motif is now participating in its own μ2 binding (p < 0.05; one-way ANOVA; N = 3).
Results
EtOH administration to rats induces rapid internalization of extrasynaptic hippocampal GABAA-R subunits
EtOH administered via intraperitoneal injection in rats is detectable in the brain within 2–3 min (Liang et al., 2007), and tolerance/desensitization to some EtOH-induced behavioral effects, such as rotarod performance, occurs within 3–5 min in mice (LeBlanc et al., 1975). This suggests that alterations in the function and/or expression of some GABAA-Rs occur immediately after EtOH exposure. Figure 1A–D shows receptor cross-linking assays using hippocampal slices in vitro, after EtOH administration in vivo. Rats were treated with 3 g/kg EtOH (intraperitoneally), or vehicle, and killed at early time points (5–60 min) to determine the time course for the EtOH-induced GABAA-R internalization of surface receptors, as seen in Liang et al., 2007. We simultaneously examined the phosphorylation state of the β3 subunit over the same time course, because dephosphorylation of β3 subunits has been shown to regulate clathrin-mediated internalization of receptors (Kittler et al., 2005; Jacob et al., 2008; Terunuma et al., 2008). Here, surface levels in α4, δ, and the phosphorylated form of β3 GABAA-R subunits trended to a decrease as early as 5 min after EtOH exposure, although none was significantly different (Fig. 1A,B). Statistically significant changes were seen 15 min after the EtOH dose, at which time surface α4 levels decreased to 63.9 ± 8.8% in the DG and 68.1 ± 6.9% in CA1; pβ3 decreased to 66.8 ± 6.5% and 70.5 ± 6.7%; and δ decreased to 60.4 ± 7.4% and 63.8 ± 9.2% compared with vehicle-treated rats (Fig. 1C,D, p < 0.001, compared with control of same subunit; N = 5). These results suggest that EtOH stimulates the internalization of receptors composed of α4β3δ subunits. Figure 1, E and F, shows there were no changes in total GABAA-R subunits tested at early time points 5–60 min, as expected (there is no detectable degradation nor synthesis at early time points).
Dephosphorylation of β3 subunit does not correlate in time with EtOH-induced internalization
The phosphorylated state of GABAA-R β3 subunit was detected by Western blot with a substrate-specific antibody in control animals untreated with EtOH. Significant increases were observed for phosphorylated β3 (pβ3) at early time points 5 and 10 min following EtOH treatment, times at which protein internalization was observed. The levels of pβ3 decreased at later time points, 30 and 60 min after EtOH, as shown in Figure 2. Thus dephosphorylation does not appear to induce internalization of α4, β3, and δ subunits of GABAA-R.
EtOH treatment in rats increases association of hippocampal extrasynaptic GABAA-R subunits with clathrin adaptor protein AP2 μ2 subunit observed by coimmunoprecipitation
Since α4, pβ3, and δ subunit surface levels significantly decreased at 15 min following EtOH administration (Fig. 1C,D), the effects that this treatment had on association with the clathrin μ2 subunit were measured. Coimmunoprecipitation experiments of δ subunits showed an initial significant increase in association with AP2-μ2 (132.7 ± 8.2% of vehicle) as early as 5 min following EtOH. There was a subsequent decrease in δ association with μ2 (55.1 ± 8.7% of vehicle) 15 min after EtOH treatment (Fig. 3A,B). These results suggest that the increase in GABAA-R internalization observed in Figure 1 (via cross-linking) is, in part, due to an increased association of pentameric receptors containing δ with μ2 at 5 min following EtOH treatment, resulting in an initiation of endocytosis of the δ-containing receptor. The decreased δ-μ2 association 15 min after EtOH, in return, supports the observation that a significant amount of δ-containing GABAA-R has already internalized—there is less δ available for interaction. Co-IP shows the δ subunit interacts with α4- and β2/β3-GABAA-R; there were no other changes seen in association between the δ subunit with other GABAA-R subunits.
Because α4 subunits internalized at early time points after EtOH (Fig. 1), and since α4 is commonly found with δ subunits in the dentate gyrus, which shows increased co-IP with clathrin μ2, association between the extrasynaptic α4 subunits and μ2 was also measured. Coimmunoprecipitation of α4 subunit containing GABAA-R showed a trend to increase in α4 association with μ2 (138.1 ± 15.6%) at 5 min following EtOH (Fig. 3C,D); no significant changes were seen at 15 min. These results are consistent with the suggestion that, at least at early time points, the α4 subunits are internalizing with δ-containing GABAA-R, through the same clathrin-mediated mechanism, likely as a complete functional heteropentameric complex along with β subunits. The pβ3 subunit did not appreciably pull-down with the μ2 subunit, as previously shown (Terunuma et al., 2008). Various GABAA-R subunit ICDs interact directly with the μ2 subunit of the AP2 complex as cited before, and changes in these interactions are important in regulating the synaptic strength and plasticity of GABAA-R (Kittler et al., 2005).
The ICD of the GABAA-R δ subunit binds to AP2 subunit μ2 demonstrated by GST-protein pull-downs
The δ ICD contains two possible μ2 binding motifs (Fig. 4A): a region at residues 322–334, similar to the atypical basic-rich residues μ2 binding motifs found in synaptotagmin1, GABAA-R β(1–3), γ2, and GluR3, and a region containing a classical Yxxϕ motif in residues 375–378 (375YRSV). The full-length δ ICD has been previously shown to bind to μ2 (Kittler et al., 2005).
To determine which region(s) of the ICD directly bind μ2, GST protein fused to the ICD region of δ GABAA-R were made: GST-δ ICD full length (316–410); two truncations of the δ ICD, each of which contains one of the putative μ2 binding domains; two GST-δ ICD full-length constructs, each with one of the two μ2 binding domains mutated; and a GST-δ ICD full length with both μ2 binding domains mutated (Fig. 4B).
A GST pull-down assay confirmed that the GST-only (control) did not bind μ2, while the full-length δ ICD did bind μ2. Interestingly, both δ ICD truncations maintained partial ability to bind to μ2 (Fig. 4C): mutation of full-length δ ICD residue 375Y to A (to abolish the tyrosine-μ2 binding motif) still bound μ2, suggesting that the binding to μ2 was maintained by the basic-rich μ2 binding motif. Similarly, mutation of a piece of the basic-rich region 322RKKRK to GAAGA also still bound μ2, meaning μ2 binding was maintained due to the tyrosine-μ2 binding motif. A GST-δ ICD containing both mutations 375Y→A and 322RKKRK→GAAGA finally showed no binding to μ2. These results suggest that each μ2 binding region of the δ ICD is sufficient for binding to μ2, and at least one or the other motif is necessary for the δ ICD to interact with μ2.
Because each of the δ ICD regions on its own was sufficient to bind to μ2, we examined whether there was a detectable difference between the affinities for μ2. Pull-down assays using varying concentrations of purified μ2 were performed. Concentration-dependent binding studies showed (Fig. 4D) that the 375YRSV motif is more sensitive to μ2 at a relative concentration of ∼0.1 μM, while the 322R/K motif is much less sensitive to the same concentration of μ2. The differences in binding are significant only at the lower [μ2]. These results suggest that the 375YRSV motif may be more important in the EtOH-induced increased μ2 binding, which then initiates endocytosis of δ subunit-containing receptors.
Discussion
This study provides additional evidence that EtOH administration to rats causes rapid internalization of α4βδ GABAA-R following a single EtOH dose (Liang et al., 2007, Shen et al., 2011). Demonstration of increased association between α4βδ GABAA-R and the μ2 clathrin adaptor proteins mediating endocytosis sheds light on the possible mechanisms for regulating extrasynaptic GABAA-R, by changing interactions between GABAA-R subunit δ and μ2-AP2. Characterization of the two putative μ2-binding domains within the δ ICD (one YxxΦ motif and the other an R/K rich motif) reveals that each can bind μ2-AP2 on its own. We show that the two domains exhibit differential affinity: the 375Y-motif is capable of binding lower concentrations of μ2 than the 322R/K-rich motif of the δ subunit. Altogether, these results suggest that clathrin-mediated endocytosis directs the early EtOH-induced internalization of extrasynaptic GABAA-R primarily via δ subunit interactions with μ2.
EtOH-dependent downregulation of neurotransmission and tolerance resulting from ligand-induced receptor reduction
Activity-dependent changes in neurotransmission are widespread mediators of various brain activities including learning, memory, and pathological states of neuronal excitability, often by altering the number and composition of receptors available to respond to released neurotransmitters (Collingridge et al., 2004; Arancibia-Carcamo et al., 2009). Chronic benzodiazepine exposure leads to GABAA receptor downregulation (Gallager et al., 1984; Tehrani and Barnes, 1997; Ali and Olsen, 2001; Tan et al., 2010), as does chronic EtOH treatment (Cagetti et al., 2003; Kumar et al., 2010). Such a mechanism is key to diminishing the (hyper-inhibitory) response in the face of continuous stimulus, and an initial downregulation also occurs following single doses of EtOH (Liang et al., 2007), also seen in this study. These results are consistent with a model in which agonists and positive modulators induce a conformation of the receptor that is a substrate for the biochemical events producing downregulation, possibly by cell internalization. The overstimulation of EtOH-sensitive receptors in the presence of moderate to high doses of EtOH begins the first step of downregulation of the EtOH-sensitive GABAA receptors. During the course of this study, we (Shen et al.,2011) showed that comparable 20 min effects were reproduced in primary cultured neurons as well, and also provided evidence with cleavable biotinylation that these extrasynaptic δ-GABAA-Rs directly underwent endocytosis (rather than decreased membrane insertion). This is consistent with δ-GABAA-Rs as initial responders.
The results of this study are consistent with previous studies investigating how quickly tolerance to some acute EtOH effects occur in both rats and humans. Such effects are surprisingly fast, varying from minutes to 1 d, measured both by in vivo behavior and by in vitro recording (LeBlanc et al., 1975; Pearson et al., 1997; Ludvig et al., 2001; Liang et al., 2007). Importantly, these studies show that repetitive administration (intermittent cycles) of EtOH accelerates tolerance to EtOH. The GABAergic trafficking mechanisms behind acute tolerance may provide clues to how the highly complex chronic EtOH tolerance develops.
Clathrin-mediated endocytosis and phosphoregulation
Previous studies show that GABAA-Rs undergo both constitutive and activity-dependent endocytosis primarily via clathrin-dependent mechanisms (Kittler et al., 2000; Herring et al., 2003; Kanematsu et al., 2006). Much research on endocytosis and GABAA-R shows β3 and γ2 regulate clathrin-AP2 endocytosis via phosphoregulation (Kittler et al., 2005; Smith et al., 2008). Such studies involve synaptic GABAA-R, but neither endocytosis nor phosphoregulation of extrasynaptic GABAA-R have been studied. Upon detecting internalization of phospho-β3 in this study, we considered its possible role in regulating endocytosis. However, together with results of EtOH exposure in cultured hippocampal neurons (Shen et al.,2011), we find that EtOH exposure stimulates endocytosis of α4β3δ GABAA-R with no significant changes in β2/3 association with AP2 machinery. This rules out the role of phosphoregulation increasing GABAA-R activity, as seen in previous β3 studies. Phosphorylation of α4 is not likely responsible for endocytosis we see here, since Abramian et al. (2010) show α4 phosphorylation dictates insertion into the membrane, rather than endocytosis. This leads to the final possibility: primary interactions involving the δ subunit. We find evidence for δ subunit interactions with the μ2 subunit of the AP2 complex, similar to other GABAA-R subunits. The basic AP2 binding motif seen in the δ subunit is similar to those found in GABAA-R β subunits, while the 375YRSV δ motif is a classical Y motif similar to those seen in several membrane receptors, including the GABAA-R γ2 subunit (Kittler et al., 2005; Smith et al., 2008).
Each of the two μ2 binding domains in the δ ICD was shown to bind to μ2 in vitro, as also seen with the γ2 subunit. Interactions between γ2-μ2 and β3-μ2 produced additive effects on function inferring separate binding and enhancement of αβ3γ2 GABAA-R endocytosis (Smith et al., 2008). Because the β3 subunit is predominantly phosphorylated in the present study, it is not likely contributing to the μ2 interaction), so its effects would not be additive for electrophysiological studies. Instead, it is likely that the loss of interaction between μ2 and β3 is compensated by the double δ-μ2 interaction, reinforcing the notion that dephosphorylation of phospho-β3 is not directing the early EtOH-internalization of GABAA-R. Obviously competition between different regulatory mechanisms including phosphoregulation of different subunits in the pentamer could complicate matters in a cell; however, some are almost certainly able to dominate over others, and anyway, most β3 subunits and δ subunits are not in the same pentamers. The increased clathrin interaction of GABAA-R δ subunit after EtOH exposure seen at 5 min but changing to a reduction at 15 min suggests complex kinetics for endocytosis which we suggest is consistent with the loss of substrate after the process is initiated, but might reflect other factors, including possible non-clathrin-mediated endocytosis, and/or possible heterogeneity of the δ-GABAA-R protein pools. Examining the electrophysiology of αβδ GABAA-R while blocking μ2 interactions would provide a clearer answer. Further analysis on the two motifs showed differences between their apparent affinity for μ2, with the 375YRSV motif showing greater sensitivity to μ2. It is possible that the EtOH-induced increase in endocytosis is mediated primarily by the 375YRSV motif ability to bind to μ2, or both motifs are involved, with similar or different sensitivity to EtOH-induced regulation. Phosphorylation of either or both μ2 regions in δ may help regulate the binding to μ2, with subsequent results on endocytosis. To date the ICD has not been shown to be directly phosphorylated by PKC or PKA as are other GABAA Rs. Several studies do, however, show that EtOH activates several kinases, and standard PKC isoforms (α, β, γ) may be activated within minutes in the nucleus accumbens and hippocampus following EtOH administration in rats (e.g., Choi et al., 2008), who suggest that PKC-δ phosphorylates δ-containing GABAA-R, since PKC-δ increases the presence of δ GABAA-R-mediated tonic currents. This PKC isoform may phosphorylate the δ-GABAA-R and regulate endocytosis, possibly by direct or indirect effects on μ2 binding, as in the case of PRIP proteins (Kanematsu et al., 2006).
More functional studies needed
GABAA-R δ subunit knock-out mice show little behavioral changes in response to EtOH, likely due to compensation by other subunits and other neurotransmitter receptors and ion channels (Mihalek et al., 1999). We reported a decrease in δ-containing GABAA-R currents at 1 h after EtOH exposure in rats (Liang et al., 2007) and in cultured neurons (Shen et al., 2011), the rationale for this study. Detecting even earlier loss of function correlating in time with increased GABAA-R-μ2 interactions would strengthen the results of these studies. It may be possible to detect EtOH-induced rapid decreases in tonic current, Itonic, by altering Itonic. Itonic in the hippocampus is mainly mediated by mutually exclusive α5-GABAA-R and δ-GABAA-R (Mody and Pearce, 2004), activated by different GABA concentrations: δ by basal, low [GABA], and α5 by increased activity-induced increases in [GABA] (Scimemi et al., 2005; Bright et al., 2011). Since EtOH has also been shown to increase GABA release (Olsen and Spigelman, 2012), both δ-GABAA-R and α5-GABAA-R may be contributing to EtOH-induced Itonic changes in studies such as Liang et al., 2007. As such, it is possible that at early time points (i.e., 5–15 min) after EtOH, when δ-GABAA-R (directly enhanceable by EtOH) begin to internalize, α5-GABAA-R are being recruited/activated by the elevated amounts of GABA. Those newly active α5-GABAA-R are now contributing to Itonic and possibly covering the ability to detect the loss of Itonic that is mediated by δ-GABAA-R. Therefore, electrophysiological analysis of EtOH-induced tonic currents should include a pharmacological block of α5-GABAA-R, to decrease the heterogeneity of the receptors contributing to Itonic and focus mainly on δ GABAA-R Itonic changes.
It is important to test the functional significance of these μ2 binding regions within the δ ICD, as they are likely important in regulating the cell surface number of δ-containing GABAA-R. GABAA-R containing the δ subunit as already mentioned are unique in limited regional and subcellular localization exclusively at extrasynaptic sites, unique properties including high sensitivity to GABA and low desensitization, and a particularly important role in mediating tonic inhibition. Tonic currents are seen to be responsive to low concentrations of EtOH, and are highly sensitive to neurosteroids; therefore endocytosis of δ-GABAA-R during EtOH exposure reduces important endogenous modulation by neurosteroids, as well as basal tonic inhibition in some areas. The current results suggest that tonic inhibition is subject to use-dependent downregulation, important to many physiological conditions.
Footnotes
This work was supported by National Institutes of Health Grants NS35985 and AA07680, and by funds from the Department of Molecular and Medical Pharmacology, Geffen School of Medicine, UCLA, Michael Phelps, Chair. We thank Jing Liang, Kerstin Lindemeyer, and Igor Spigelman for helpful discussions.
References
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;53:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2001;79:1100–1108. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, Yan Z, Kittler JT, Moss SJ. Ubiquitin-dependent lysosomal targeting of GABAA receptors regulates neuronal inhibition. Proc Natl Acad Sci U S A. 2009;41:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of δ-Containing GABAA receptors during spillover. J Neurosci. 2011;31:753–763. doi: 10.1523/JNEUROSCI.2996-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HMB, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cδ regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Einarson MB, Pugacheva EN, Orlinick JR. Identification of protein-protein interactions with glutathionine-S-transferase (GST) fusion proteins. CSH protoc. 2007 doi: 10.1101/pdb.top11. Advance online publication. Retrieved November 20, 2012. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308:74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Science STKE. 2002;2002(137):p18. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the β2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABAA receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Moss SJ, Nabekura J, Hirata M. Modulation of GABAA receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol Chem. 2006;281:22180–22189. doi: 10.1074/jbc.M603118200. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Commerford CE, Lai MA, Ren Q, Morrow AL. Ethanol reduces GABAA α1 subunit receptor surface expression by a PKCγ dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol. 2010;77:793–803. doi: 10.1124/mol.109.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ. Acute tolerance to ethanol in the rat. Psychopharmacologia. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvig N, George MA, Tang HM, Gonzales RA, Bungay PM. Evidence for the ability of hippocampal neurons to develop acute tolerance to ethanol in behaving rats. Brain Res. 2001;900:252–260. doi: 10.1016/s0006-8993(01)02319-8. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in GABAA receptor δ subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Spigelman I. GABAA receptor plasticity in alcohol withdrawal. In: Noebels J, Avoli M, Rogawski M, Olsen RW, Delgado-Escueta AV, editors. Jasper's basic mechanisms of the epilepsies. Vol 4. Oxford, UK: Oxford UP; 2012. pp. 562–573. Chap 43. [Google Scholar]
- Pearson BJ, Donatelli DP, Freund RK, Palmer MR. Differential development and characterization of rapid acute neuronal tolerance to the depressant effects of ethanol on cerebellar Purkinje neurons of low-alcohol-sensitive and high-alcohol-sensitive rats. J Pharmacol Exp Ther. 1997;280:739–746. [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors following ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol. 2011;79:432–442. doi: 10.1124/mol.110.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McAinsh K, Chen G, Arancibia-Carcamo IL, Haucke V, Yan Z, Moss SJ, Kittler JT. Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABAA receptor [beta]- and [gamma]-subunits with the clathrin AP2 adaptor. Neuropharmacology. 2008;55:844–850. doi: 10.1016/j.neuropharm.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani MH, Barnes EM., Jr Sequestration of gamma-aminobutyric acidA receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J Pharmacol Exp Ther. 1997;283:384–390. [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]