Abstract
Objectives
Male vulnerability in health and growth outcomes has often been reported in very low birth weight (VLBW) preterm neonates. On the basis of gender-difference theories, possible associations were explored between the levels of postnatal salivary testosterone/cortisol and the outcomes of neonatal health/growth.
Methods
This study used an exploratory and comparative research design. One-hundred-one mother–VLBW preterm neonate pairs were recruited from the neonatal intensive care unit (NICU) of a tertiary medical center in the Southeastern, US. Demographic information, health and growth variables of neonates, and pregnancy and labor variables of mothers were obtained from the medical record reviews and interviews of mothers. Saliva samples from each pair were collected between 9 and 60 days of age. The levels of testosterone and cortisol were determined by using an enzyme immunoassay methodology.
Results
Linear regression analysis showed that neonatal health problems were positively associated with the levels of postnatal salivary testosterone and cortisol, while growth delays were positively associated with the levels of postnatal salivary testosterone after adjusting for the characteristics of neonates and mothers and day of saliva sampling. The salivary levels of testosterone and cortisol were higher in neonates than in mothers. A positive correlation between the levels of testosterone and cortisol was found in neonates and in mothers.
Conclusions
The level of postnatal salivary testosterone is a more reliable marker in assessing neonatal health and growth outcomes compared to salivary cortisol. Further research on both testosterone and cortisol measurements at various stages during the neonatal period may elucidate further these associations.
Keywords: Salivary testosterone, Salivary cortisol, Neonatal health and growth
1. Introduction
Very low birth weight (VLBW) preterm male neonates experience more health and growth problems than female VLBW preterm neonates do [1]. It is unclear which factors explain the vulnerability of male VLBW preterm neonates to these problems and whether these factors are physiological in origin. Although causal relationships have not been established, well accepted theories that gender impacts health outcomes in neonates support the possibility that elevated levels of testosterone function as a biological risk factor [2-5].
According to gender-difference theories [2,6], elevated levels of testosterone diminish the size of the developing thymus gland [2], delay pulmonary maturation, and inhibit synthesis of lung surfactant [7]; hence, preterm infants with high levels of testosterone may experience health problems related to respiratory function. Testosterone increases susceptibility to neurological insults and decreases recovery from brain damage [8,9]. Whether testosterone levels contribute to the significantly higher rates of Grades III and IV intraventricular hemorrhage, neurodevelopmental impairment, and death in VLBW preterm males remains unknown. Elevated levels of testosterone are also associated with intrauterine growth retardation [10] and with slower catch-up growth in humans and other mammals [11].
The role of testosterone in neonatal health and growth has been implicated in findings from many studies on cortisol. Exposure to prenatal stressors, such as maternal stress and anxiety, stimulates fetal cortisol secretion [12]. Furthermore, fetal stress, determined by an elevated level of cortisol in human fetal blood and amniotic fluid, is further increased by an elevated level of testosterone. A positive association between the levels of cortisol and testosterone has been found in amniotic fluid, fetal blood, and cord blood [13-15]. Interestingly, these findings are opposite that for adult men, for whom a negative association between cortisol and testosterone concentrations has been reported [16].
The role of testosterone in various cognitive and behavioral outcomes has been extensively investigated [17-20], however, little is known about its role in health and growth outcomes of VLBW preterm neonates. This study attempts to explore possible associations, rather than causal relationships, between the neonatal outcomes and the levels of postnatal salivary testosterone and cortisol because causal effects have not been firmly established.
We addressed two research questions in this study: (a) are the levels of postnatal salivary testosterone and cortisol associated with neonatal health after adjusting for the characteristics of neonate and mother and days of saliva sampling? (b) Are the levels of postnatal salivary testosterone and cortisol associated with neonatal physical growth after adjusting for the characteristics of neonate and mother and days of saliva sampling? On the basis of previous observations, we hypothesized that elevated levels of postnatal salivary testosterone and cortisol are positively associated with health problems and delayed physical growth and that the levels of testosterone and cortisol in VLBW preterm neonates are positively correlated.
2. Method
We used an exploratory, comparative research design to answer the research questions.
2.1. Participants
We recruited 101 VLBW preterm neonate–mother pairs, including three neonates from three twins, from the NICU of a major academic medical center in the southeast region of the United States. Neonates were recruited if they were (a) 6–10 days old, (b) less than 34 weeks of gestational age (GA), and (c) less than 1500 g at birth. Neonates were excluded if they (a) were going to be discharged from the hospital before the 1st week or (b) had history or symptoms of substance exposure. Neonates were also excluded if their mother was (a) less than 15 years (b) did not speak English, (c) dependent on narcotics or drugs, (d) HIV positive, or (e) had a documented serious medical or psychological problem such as cancer or postpartum psychosis.
2.2. Measures
The data were obtained through medical record reviews, interviews of mothers, and biochemical measurements. The medical record for the neonate contained demographic information, neonatal history, and concurrent health problems during the sampling period. For the mother, data collected included demographic information, pregnancy history, delivery record, and concurrent health problems during the sampling period.
2.2.1. Neonatal health
As shown in Table 1, variables on neonatal health included GA, 1- and 5-minute Apgar scores, cardiopulmonary resuscitation (CPR) at birth (0–6), presence of medical complications (0–16), days of hospitalization, technology dependence at discharge (0–6), and neurobiologic risk score (NBRS; [21]). The summed and un-weighted (0 = absence, 1 = presence) variables were created based on the studies conducted by one of the co-authors. The variable of CPR consisted of six items: use of oxygen, bagging and mask, continuous positive airway pressure (CPAP), intubation, chest compression, and epinephrine. The total CPR score was the sum of the scores for all six items and ranged from 0 to 6, with higher scores indicating more advanced resuscitation at birth. The variable of medical complications was related to 16 binary (0 = no, 1 = yes) questions asking whether the neonate was treated with any of the five means (surfactant, antenatal steroid, indomethacin, ventilation/CPAP, and surgery) and whether the neonate had been diagnosed as having any of the 11 conditions (patent ductus arteriosus, seizure, IVH, PVL, brain cyst, sepsis, meningitis, parenteral hyperalimentation, necrotizing enterocolitis, gastrointestinal perforation, and retinopathy of prematurity). The total complication score reported here is the sum of the scores for all 16 categories and ranged from 0 to 16, with a higher score indicating more medical/health problems. The variable of technology dependence at discharge from the hospital consisted of six items: use of oxygen, G-tube and/or tube feeding, ventilator/CPAP, tracheostomy, apnea monitor, and medications. The total technology dependence score, the sum of the scores for all six categories, ranged from 0 to 6, with a higher score reflecting the prevalence of medical/health problems at discharge.
Table 1.
Summary of measures used in the study.
| Construct | Measure | Variable | Items |
|---|---|---|---|
| Neonatal health | Chart review | Gestational age | – |
| 1-and 5-min Apgar scores | – | ||
| CPR at birth (0–6) | Oxygen, bagging/mask, CPAP, intubation, chest compression, and epinephrine | ||
| Medical complications (0–16) | Surfactant, antenatal steroid, indomethacin, ventilation/CPAP, PDA, surgery, seizure, IVH, PVL, brain cyst, sepsis, meningitis, parenteral hyperalimentation, NEC, GI perforation, and ROP | ||
| Days of hospitalization | – | ||
| Technology dependence (0–6) | Oxygen, G-tube/tube feeding, ventilator/CPAP, tracheostomy, apnea monitor, and medications | ||
| NBRS | Degree of neurological insults | – | |
| Neonatal growth | Chart review | Physical measurement | Weight, length, and head circumference at birth and at the 1st week |
| Maternal health | Chart review | Pregnancy complications (0–6) | Diabetes, use of insulin, current HBP, previous HBP, antepartum hemorrhage, and chorioamnionitis |
| Labor complications (0–9) | Rupture of membrane, antenatal steroid, antibiotics, and numbers of antibiotics used (0–6) |
Note: CPR = cardiopulmonary resuscitation, CPAP = continuous positive airway pressure, PDA = patent ductus arteriosus; IVH = intraventricular hemorrhage, PVL = periventricular leucomalacia, NEC = necrotizing enterocolitis, GI = gastrointestinal, ROP = retinopathy of prematurity, NBRS = neurobiologic risk score, HBP = high blood pressure.
The total NBRS is the sum of seven possible neurological insults and ranges from 0 to 28, with a higher score indicating a higher prevalence of insults. This measure has good psychometric properties and has correlations of −.37 and −.65 with the Bayley Mental and Psychomotor Indexes at 6 and 24 months’ corrected age, respectively [21]. Internal consistency of the NBRS was reported as 0.72 for premature infants [22].
2.2.2. Neonatal growth
Data on physical measurement (weight, length, and head circumference) at birth and the 1st week were obtained through the medical record. The neonate was weighed on a battery-operated, electronic scale with a capacity of 20 kg and accuracy within 10 g. Length was measured on a collapsible board with increments to the nearest 0.25 cm. Head circumference was determined with a disposable tape that measured to the nearest 0.25 cm. All growth parameters were measured by NICU nurses between 4 and 6 am.
2.2.3. Covariates
Characteristics of neonates (gender) and mothers (age, race, body mass index [BMI], education, marital status, pregnancy complications, and labor complications); as well as, day of saliva sampling (9 to 60 days) were modeled as potential confounders or effect modifiers when we assessed the association between neonatal health and growth outcomes and the level of testosterone and cortisol. As shown in Table 1, the variable of pregnancy complications consisted of six items (0–6) and the variable of labor complications consisted of four items (0–9), with higher scores indicating more medical problems. These variables were selected from the NICU mother’s data form that was designed to assess neonates’ birth conditions that could affect neonatal health and growth outcomes.
2.2.4. Testosterone
Perinatal salivary testosterone levels were determined by enzyme immunoassay (EIA; Salimetrics, LLC, State College, PA). The intra- and inter-assay coefficients of variation were 2.5% and 5.6%, respectively. Saliva samples from neonates were collected by a research nurse by using a low-pressure suction with a 1-cc plastic syringe. All samples were collected between 9 and 60 days of age because relatively high and stable testosterone levels are found from the 1st week through 3 months [23,24]. Sampling a week after birth minimizes the effects of maternal antenatal steroid, analgesia, and length of labor on the testosterone level of neonates. In teenage and adult males, testosterone levels are higher in the morning than in the evening [16]; however, whether this fluctuation occurs in neonates remains uncertain. To control for any potential circadian rhythm of testosterone, we obtained the samples between 8 am and noon. Date and time of the mother’s sampling were matched with those of the neonate’s sampling.
2.2.5. Cortisol
The salivary samples used for testosterone measurement were also used for cortisol determination. Salivary cortisol levels were determined by EIA (Salimetrics, LLC, State College, PA). The intra- and inter-assay coefficients of variation were 3.3% and 3.7%, respectively. Cortisol levels in adults vary diurnally and are higher in the morning than in the evening. This variation might or might not exist in neonates and has not been manipulated for the study purpose.
2.3. Procedure
The study was approved by the Institutional Review Boards for Human Use. Potential neonate participants were identified on a daily basis through the NICU admission log. A research nurse in the NICU discussed the study with the mothers, and the informed-consent form was available for their review.
Saliva from the neonates was obtained 1 h before or after feeding. Three samples (0.1 ml each) were collected within a 2-h period because of the episodic secreting pattern of steroid hormones [25]. Neonates’ saliva was collected by using a low-pressure suction with a 1-cc plastic syringe with a blunt tip. Mothers’ saliva was collected by having them use a straw to expectorate into a small tube. The samples were obtained 1 h before or after any oral intake. Three samples of maternal saliva (0.1 ml each) were collected within a 2-h period to minimize the influence of the episodic pattern of steroid hormones. All saliva samples were placed on ice and stored in a −80 °C freezer.
2.4. Data analysis
Two-sample t tests and chi-square tests for contingency tables were used to compare demographic and health characteristics of neonates and mothers by neonates’ gender. The correlation between the level of postnatal salivary testosterone and that of cortisol was computed separately for neonates and mothers. Linear regression was the main analytic tool for assessing the association between neonatal health and growth outcomes and the levels of postnatal salivary testosterone and cortisol after adjusting for characteristics of neonates and of mothers and day of saliva sampling.
3. Results
3.1. Demographic and health characteristics of mothers and neonates by gender of neonates
The levels of mothers’ salivary testosterone and cortisol did not differ by gender of neonates (Table 2). In comparison with the levels of testosterone and cortisol in mothers, those levels in neonates were approximately 5.4 times (281 vs. 53 pg/ml) and 1.5 times (0.3 vs. 0.2 μg/dl) higher, respectively (Tables 2 and 3). t Tests and chi-square tests showed that none of maternal characteristics differed by neonates’ gender, except that mothers of female neonates had a greater number of labor complications than mothers of male neonates.
Table 2.
Demographic characteristics of mothers.
| Variables | Overallb (n=101) | Maleb (n=45) | Femaleb (n=56) | p valuec |
|---|---|---|---|---|
| Salivary testosterone (pg/ml) | 52.9 (22.1) | 49.7 (18.8) | 55.5 (24.4) | 0.19 |
| Salivary cortisol (μg/dl) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.18 |
| Age (years) | 24.7 (5.4) | 24.2 (5.2) | 25.1 (5.5) | 0.41 |
| Education (1–5)a | 2.4 (1.0) | 2.3 (1.0) | 2.4 (1.0) | 0.68 |
| Married (%) | 36.6 | 46.7 | 28.6 | 0.10d |
| Race: white (%) | 48.5 | 42.2 | 53.6 | 0.35d |
| Weight (kg) | 89.1 (25.9) | 87.1 (25.8) | 90.7 (26.1) | 0.48 |
| Height (cm) | 163.2 (8.0) | 163.0 (6.5) | 163.4 (9.0) | 0.78 |
| BMI (kg/m2) | 33.3 (8.7) | 32.6 (8.2) | 33.9 (9.2) | 0.46 |
| Gravida | 2.3 (1.6) | 2.2 (1.4) | 2.3 (1.8) | 0.90 |
| Parity | 1.9 (1.3) | 1.8 (1.3) | 1.9 (1.4) | 0.63 |
| Pregnancy (0–6) | 1.4 (0.9) | 1.3 (0.9) | 1.4 (0.9) | 0.36 |
| Labor (0–9) | 3.8 (1.4) | 3.5 (1.2) | 4.1 (1.5) | 0.04 |
| Delivery: C/S (%) | 57.4 | 60.0 | 55.4 | 0.79d |
Note: Pregnancy = number of complications during pregnancy, Labor = number of complications at labor, C/S = caesarian section.
Education: 1, <12th grade; 2, high school degree; 3, partial college; 4, college degree; and 5, graduate degree.
Values are expressed as mean (SD) unless otherwise indicated.
P values are for the t-test except those for marital status, race, and delivery that are for the chi-square test.
P values are for the chi-square test.
Table 3.
Demographic characteristics of neonates.
| Variables | Overalla (n=101) | Malea (n=45) | Femalea (n=56) | p valueb |
|---|---|---|---|---|
| Salivary testosterone (pg/ml) | 281 (112) | 271 (92) | 290 (126) | 0.39 |
| Salivary cortisol (μg/dl) | 0.3 (0.3) | 0.2 (0.1) | 0.3 (0.4) | 0.05 |
| Gestational age (weeks) | 29 (2) | 29 (2) | 29 (2) | 0.12 |
| 1-minute Apgar scorec | 4 (5) | 5 (5) | 4 (5) | 0.21 |
| 5-minute Apgar scorec | 7 (1) | 8 (1) | 7 (1) | 0.73 |
| CPR (0–6) | 2.0 (1.0) | 1.8 (1.1) | 2.0 (2.4) | 0.26 |
| Complication (0–16) | 2.7 (1.7) | 2.5 (1.7) | 2.8 (1.7) | 0.38 |
| NBRS (0–28) | 1.9 (2.5) | 1.8 (2.7) | 1.9 (2.7) | 0.70 |
| Days of hospitalization | 62 (25) | 60 (27) | 64 (24) | 0.50 |
| Technology dependence (0–6) | 0.4 (0.8) | 0.3 (0.8) | 0.4 (0.9) | 0.53 |
| Weight at birth (g) | 1094 (300) | 1159 (291) | 1043 (300) | 0.06 |
| Length at birth (cm) | 37 (4) | 37 (3) | 36 (4) | 0.16 |
| Head circumference at birth (cm) | 26 (2) | 26 (2) | 25 (2) | 0.02 |
| Weight at week 1 (g) | 1012 (282) | 1074 (272) | 963 (282) | 0.05 |
| Length at week 1 (cm) | 37 (4) | 37 (3) | 37 (4) | 0.25 |
| Head circumference at week 1 (cm) | 26 (3) | 26 (4) | 25 (3) | 0.07 |
| Weight change (g) | −82 (117) | −121 (89) | −43 (79) | 0.87 |
| Length change (cm) | 0.4 (2.2) | 1.2 (1.2) | 0.8 (0.9) | 0.69 |
| Head circumference change (cm) | 0.2 (2.1) | −0.1 (1.1) | 0.3 (0.7) | 0.76 |
Note: CPR = cardiopulmonary resuscitation, NBRS = neurobiolgic risk score.
Values are expressed as mean (SD) unless otherwise indicated.
P values are for the t-test unless otherwise indicated.
Values are expressed as inmedian (interquartile range; IQR) unless otherwise indicated.
As shown in Table 3, the level of postnatal salivary testosterone did not differ by gender of neonates whereas that of postnatal salivary cortisol was significantly higher in female neonates than male neonates. The variables of neonatal health did not differ by neonates’ gender. As for neonatal growth female neonates had smaller head circumference at birth and weighed less at the 1st week than male neonates.
3.2. Correlations between testosterone and cortisol
The levels of postnatal salivary testosterone and cortisol in neonates were positively related, r=.43, p=<.0001 (Fig. 1). This correlation remained positive after removing one outlier on the upper rightmost corner of the plot, r=.38, p=<.0001. Similarly, the levels of testosterone and cortisol in mothers were also positively related, r=.23, p=.02. No significant correlation was found between the levels of neonates’ and mothers’ salivary testosterone or between salivary cortisol in the mothers and neonates.
Fig. 1.
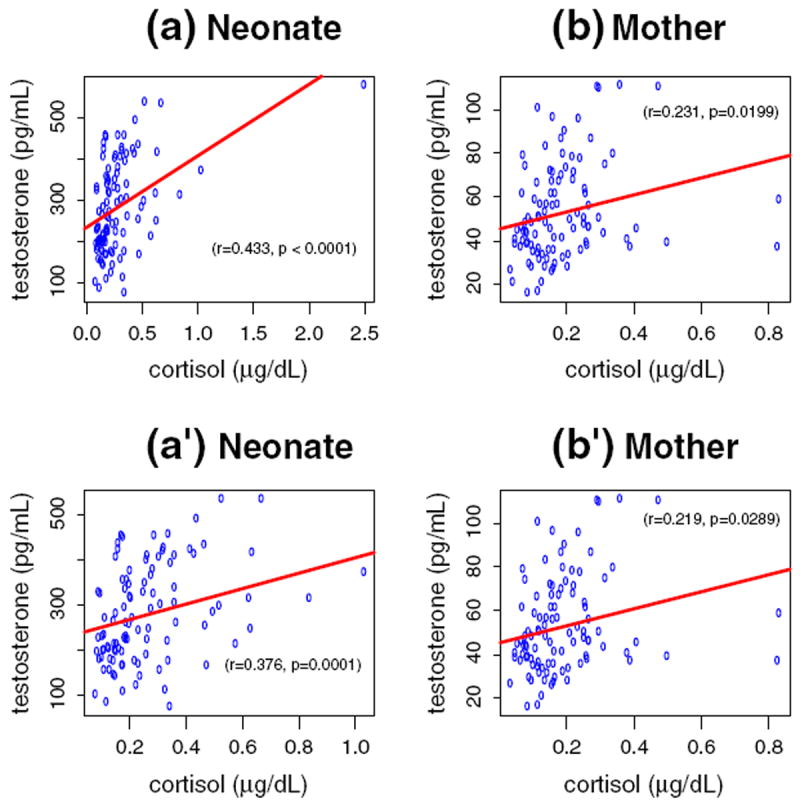
Relationship between testosterone and cortisol in neonates (a and a′ — left columns) and inmothers (b and b′ — right columns) with (a and b — upper rows) orwithout (a′ and b′ — lower rows) one outlier. Note that the scale on the X axes changes from one to another figures (a–a′).
3.3. Association between testosterone and neonatal health and physical growth
A higher level of postnatal salivary testosterone was positively associated with a number of neonatal health and growth outcomes (Table 4). In comparison with neonates with a lower postnatal salivary testosterone level, neonates with a higher testosterone level had a shorter GA, a higher rate of receiving CPR at birth, more medical complications, a longer hospitalization, and more technology-dependence at discharge. Neonates with a higher testosterone level weighed less, were shorter, and had smaller head circumference at birth; as well as, weighed less and were shorter at the 1st week of age.
Table 4.
Associations between postnatal salivary testosterone/cortisol and neonatal health/growth outcomes with and without adjustment for characteristics of neonates and of mothers and day of saliva sampling.
| Variables | Without covariate adjustment
|
With covariate adjustment
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testosterone
|
Cortisol
|
Testosterone
|
Cortisol
|
|||||||||
| β | F(1,99) | p value | β | F(1,99) | p value | β | F(1,88) | p value | β | F(1,88) | p value | |
| Health | ||||||||||||
| GA | −0.008 | 22.99 | 0.00 | −1.871 | 5.97 | 0.02 | −0.007 | 14.33 | 0.00 | −0.871 | 1.15 | 0.29 |
| Apgar 1 | −0.003 | 2.16 | 0.15 | −0.163 | 0.03 | 0.85 | −0.004 | 2.55 | 0.11 | 0.756 | 0.50 | 0.48 |
| Apgar 5 | −0.001 | 0.74 | 0.39 | 0.401 | 0.34 | 0.56 | −0.002 | 1.43 | 0.23 | 1.048 | 1.65 | 0.20 |
| CPR | 0.002 | 7.23 | 0.01 | 0.176 | 0.23 | 0.63 | 0.003 | 9.56 | 0.00 | −0.322 | 0.59 | 0.44 |
| Complication | 0.004 | 7.33 | 0.01 | 1.478 | 5.97 | 0.02 | 0.003 | 3.92 | 0.06 | 0.952 | 1.74 | 0.19 |
| NBRS | 0.003 | 1.43 | 0.24 | 0.974 | 1.14 | 0.29 | 0.002 | 0.86 | 0.36 | 1.104 | 1.09 | 0.30 |
| DOH | 0.066 | 8.97 | 0.00 | 25.382 | 7.91 | 0.01 | 0.047 | 4.03 | 0.05 | 19.583 | 3.72 | 0.06 |
| TD | 0.002 | 7.77 | 0.01 | 1.091 | 13.91 | 0.00 | 0.001 | 1.36 | 0.25 | 0.834 | 6.57 | 0.02 |
| Growth | ||||||||||||
| Wt. at birth | −0.655 | 6.25 | 0.02 | −273.870 | 6.81 | 0.02 | −0.388 | 2.17 | 0.14 | −195.761 | 2.92 | 0.09 |
| Lt. at birth | −0.009 | 7.90 | 0.01 | −3.155 | 6.38 | 0.02 | −0.006 | 3.45 | 0.07 | −2.020 | 2.04 | 0.16 |
| HC at birth | −0.005 | 7.07 | 0.01 | −1.709 | 4.64 | 0.04 | −0.004 | 3.51 | 0.07 | −0.914 | 1.09 | 0.30 |
| Wt. at week 1 | −0.776 | 10.33 | 0.00 | −248.242 | 6.31 | 0.02 | −0.572 | 5.24 | 0.03 | −142.061 | 1.71 | 0.19 |
| Lt. at week 1 | −0.012 | 16.39 | 0.00 | −3.460 | 7.77 | 0.01 | −0.009 | 7.46 | 0.01 | −1.830 | 1.61 | 0.21 |
| HC at week 1 | −0.003 | 0.76 | 0.39 | −1.676 | 2.06 | 0.15 | −0.001 | 0.06 | 0.80 | −1.483 | 1.23 | 0.27 |
| Wt. change | −0.121 | 1.33 | 0.25 | 25.628 | 0.37 | 0.55 | −0.184 | 2.26 | 0.14 | 53.700 | 1.02 | 0.32 |
| Lt. change | −0.003 | 2.92 | 0.09 | −0.306 | 0.15 | 0.70 | −0.003 | 1.94 | 0.17 | 0.190 | 0.04 | 0.84 |
| HC change | 0.003 | 2.02 | 0.16 | 0.032 | 0.00 | 0.97 | 0.003 | 1.86 | 0.18 | −0.569 | 0.36 | 0.55 |
Note: β = estimated parameter, GA = gestational age, Apgar 1 = 1-min Apgar score, Apgar 5 = 5-min Apgar score, CPR = cardiopulmonary resuscitation, complication = medical complications, NBRS = neurobiolgic risk score, TD = technology dependence at discharge, Wt. = weight, Lt. = length, HC = head circumference.
A higher level of postnatal salivary cortisol was also positively associated with neonatal health problems and growth delays. In comparison with neonates with a lower postnatal salivary cortisol level, neonates with a higher cortisol level had a shorter GA, more medical complications, a longer hospitalization, and more technology-dependence at discharge. Neonates with a higher cortisol level weighed less, were shorter, and had smaller head circumference at birth; likewise, weighed less and were shorter at the 1st week of age.
3.4. Association between testosterone and neonatal health and physical growth after adjusting for characteristics of neonates and mothers and day of saliva sampling
With covariate adjustment, a higher level of postnatal salivary testosterone was positively associated with neonatal health problems and delays in physical growth after adjustment was done for characteristics of neonates (gender) and of mothers (age, race, BMI, education, marital status, pregnancy complications, and labor complications); as well as, day of saliva sampling (Table 4). In comparison with neonates with a lower postnatal salivary testosterone level, neonates with a higher testosterone level had a shorter GA, a higher rate of receiving CPR at birth, and a longer hospitalization. Neonates with higher testosterone levels weighed less and were shorter at the 1st week of age.
A higher level of postnatal salivary cortisol was positively associated with neonatal health problems, but not with growth delays, after adjustment was made for characteristics of neonates and mothers and day of saliva sampling. In comparison with neonates with a lower postnatal salivary cortisol level, neonates with a higher cortisol level had more technology-dependence at discharge.
4. Discussion
We examined the potential associations between the levels of postnatal salivary testosterone and cortisol and neonatal health and growth outcomes among VLBW preterm neonates. Because free sex steroids and glucocorticoids, unbound to proteins, are more physiologically relevant, we measured saliva hormones instead of serum or plasma for both neonates and mothers. The relevance of salivary testosterone and cortisol measurement has been well recognized in research and in clinical practice. These measurements are convenient, non-invasive, and safely handled [26,27].
Gender differences were found in the levels of postnatal salivary cortisol but not testosterone in VLBW preterm neonates. This finding is possibly explained by prematurity of the enrolled neonates [23]. Testosterone levels overlap for males and females at birth, after which the levels increase in males through 3 months of age while remaining low in females throughout life [24,28]. All participating neonates were younger than 36 weeks of GA at the period of the saliva sample collection; consequently, their overlapping testosterone levels are not unexpected.
We assumed that outcomes of neonatal health and physical growth might be affected by the characteristics of neonates and of mothers; as well as, day of saliva sampling. Thus, we adjusted for these variables as covariates. The statistical associations were attenuated after the covariates were included. Regardless, elevated levels of postnatal salivary testosterone were associated with neonatal health problems (shorter GA, higher rate of receiving CPR at birth, and longer hospitalization) and delays in physical growth (smaller weight and shorter length at the 1st week of age) while elevated levels of postnatal salivary cortisol were only associated with one neonatal health problem (more technology-dependence at discharge).
It is important to note that, in comparison with postnatal salivary cortisol levels, postnatal salivary testosterone levels were more closely associated with health (GA) and growth (BW) outcomes in VLBW preterm neonates (Fig. 2), especially after adjusting for the covariates and removing the outlier (Table 5). High levels of salivary cortisol have been reported to be inversely related to health and growth outcomes [29]. In the present study high levels of salivary cortisol were negatively associated with health but not with growth outcomes after we adjusted for the covariates. Testosterone levels ought to be higher in males in post-term period, and thus, multiple determinations of testosterone and cortisol levels at various stages in the neonatal period are necessary for a more comprehensive approach to assessing male vulnerability in neonatal health and growth outcomes.
Fig. 2.
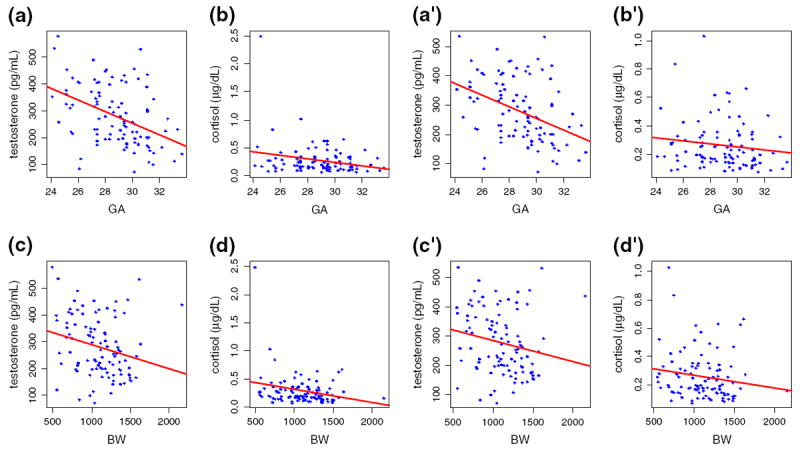
Association between the levels of postnatal salivary testosterone/cortisol and gestational age (a and b, a′ and b′; upper two rows) or birth weight (c and d, c′ and d′; lower two rows) with (a–d, left two columns) or without (a′–d′, right two columns) one outlier. Note that in all cases the Y-axis for testosterone data covers the same range while that for cortisol covers different ranges with and without the outlier (b and d vs. b′ and d′).
Table 5.
Associations between postnatal salivary testosterone/cortisol and neonatal health/growth outcomes with and without adjustment for characteristics of neonates and of mothers and day of saliva sampling after removing an outlier.
| Variables | Without covariate adjustment
|
With covariate adjustment
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testosterone
|
Cortisol
|
Testosterone
|
Cortisol
|
|||||||||
| β | F(1,98) | p value | β | F(1, 98) | p value | β | F(1,87) | p value | β | F(1,87) | p value | |
| Health | ||||||||||||
| GA | −0.008 | 18.13 | 0.00 | −1.734 | 1.85 | 0.18 | −0.007 | 14.52 | 0.00 | −0.331 | 0.07 | 0.79 |
| Apgar 1 | −0.004 | 2.55 | 0.11 | −0.966 | 0.43 | 0.51 | −0.004 | 2.28 | 0.13 | 0.038 | 0.00 | 0.98 |
| Apgar 5 | −0.002 | 1.12 | 0.29 | 0.201 | 0.03 | 0.86 | −0.002 | 1.29 | 0.26 | 0.691 | 0.30 | 0.59 |
| CPR | 0.003 | 7.67 | 0.01 | 0.446 | 0.54 | 0.47 | 0.003 | 9.03 | 0.00 | −0.122 | 0.03 | 0.85 |
| Complication | 0.003 | 5.10 | 0.03 | 1.425 | 1.98 | 0.16 | 0.003 | 3.83 | 0.06 | 0.918 | 0.67 | 0.42 |
| NBRS | 0.002 | 0.81 | 0.37 | 0.235 | 0.02 | 0.88 | 0.002 | 0.92 | 0.34 | 0.674 | 0.17 | 0.68 |
| DOH | 0.058 | 6.40 | 0.02 | 30.056 | 3.63 | 0.06 | 0.046 | 3.73 | 0.06 | 23.098 | 1.96 | 0.17 |
| TD | 0.002 | 4.17 | 0.05 | 0.874 | 2.93 | 0.09 | 0.001 | 1.75 | 0.19 | 0.367 | 0.49 | 0.49 |
| Growth | ||||||||||||
| Wt. at birth | −0.549 | 4.12 | 0.05 | −273.740 | 2.45 | 0.12 | −0.386 | 2.09 | 0.15 | −203.622 | 1.30 | 0.26 |
| Lt. at birth | −0.008 | 5.55 | 0.03 | −3.088 | 2.20 | 0.14 | −0.006 | 3.37 | 0.07 | −1.974 | 0.80 | 0.37 |
| HC at birth | −0.004 | 4.53 | 0.04 | −0.675 | 0.26 | 0.61 | −0.004 | 4.07 | 0.05 | 0.363 | 0.07 | 0.79 |
| Wt. at week 1 | −0.693 | 7.67 | 0.01 | −244.613 | 2.21 | 0.14 | −0.576 | 5.18 | 0.03 | −126.274 | 0.56 | 0.46 |
| Lt. at week 1 | −0.011 | 13.37 | 0.00 | −4.430 | 4.60 | 0.04 | −0.009 | 6.99 | 0.01 | −2.612 | 1.35 | 0.25 |
| HC at week 1 | −0.001 | 0.17 | 0.68 | −0.034 | 0.00 | 0.99 | −0.001 | 0.14 | 0.71 | 0.117 | 0.00 | 0.96 |
| Wt. change | −0.144 | 1.75 | 0.19 | 29.128 | 0.17 | 0.68 | −0.190 | 2.34 | 0.13 | 77.348 | 0.87 | 0.35 |
| Lt. change | −0.004 | 3.43 | 0.07 | −1.342 | 1.03 | 0.31 | −0.003 | 1.66 | 0.20 | −0.638 | 0.19 | 0.66 |
| HC change | 0.003 | 2.44 | 0.12 | 0.641 | 0.26 | 0.61 | 0.003 | 1.72 | 0.19 | −0.246 | 0.03 | 0.87 |
Note: β = estimated parameter, GA = gestational age, Apgar 1 = 1-min Apgar score, Apgar 5 = 5-min Apgar score, CPR = cardiopulmonary resuscitation, complication = medical complications, NBRS = neurobiolgic risk score, TD = technology dependence at discharge, Wt. = weight, Lt. = length, HC = head circumference
Similar to findings from previous studies [14,15,30,31], a positive correlation was found between the levels of testosterone and cortisol in neonates and in mothers. Circulating testosterone and cortisol are both synthesized in adrenal tissue in neonates and women, and thus, this finding appeared to be reasonable. In comparison with mothers, neonates had a higher testosterone level and a higher cortisol level. This finding corroborates those of previous studies [14,15] in amniotic fluid, fetal blood, and cord blood that the neonatal period is one of testosterone surges, which are also observed in the second trimester and puberty [18].
This study has strengths. First, we determined the levels of salivary testosterone and cortisol concurrently in neonates and mothers given that measurement of testosterone in children and women is reportedly technically challenging because of the low concentrations [25]. Second, we collected saliva samples from each participant without using any cotton, commercial collection devices, or oral stimulants because they may introduce assay artifacts [32]. This study also has limitations. First, it is a preliminary study in nature. As such, the sample size has not been powered with a preset effect size. Second, we used only postnatal maternal and neonatal saliva to reduce major prenatal confounders such as antenatal steroid use; however, had we used both cord blood and postnatal saliva that it may have been more informative.
In conclusion, the level of postnatal salivary testosterone is a more reliable marker in assessing neonatal health and growth outcomes compared to salivary cortisol. Further research on both testosterone and cortisol measurements at various stages during the neonatal period may elucidate further these associations.
Acknowledgments
This study was supported by a grant from the NICHD, NIH (R21HD066186) and a Dean’s Scholar Award from the School of Nursing at the University of Alabama at Birmingham to the first author.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000 Nov;83(3):F182–5. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geschwind N, Behan P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5097–100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimshaw GM, Bryden MP, Finegan J-AK. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsychology. 1995;9(1):68–79. [Google Scholar]
- 4.Witelson SF, Nowakowski RS. Left out axons make men right: a hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia. 1991;29(4):327–33. doi: 10.1016/0028-3932(91)90046-b. [DOI] [PubMed] [Google Scholar]
- 5.Smith LL, Hines M. Language lateralization and handedness in women prenatally exposed to diethylstilbestrol (DES) Psychoneuroendocrinology. 2000 Jul;25(5):497–512. doi: 10.1016/s0306-4530(00)00005-6. [DOI] [PubMed] [Google Scholar]
- 6.Geschwind NGAM. Cerebral lateralization: biological mechanisms, associations, and pathology. Cambridge, Mass: MIT Press; 1987. [Google Scholar]
- 7.Bartels DB, Kreienbrock L, Dammann O, Wenzlaff P, Poets CF. Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed. 2005 Jan;90(1):F53–9. doi: 10.1136/adc.2004.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AA. Male sex and intraventricular hemorrhage. Pediatr Crit Care Med. 2006 Jan;7(1):40–4. doi: 10.1097/01.pcc.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]
- 9.Nunez JL, McCarthy MM. Sex differences and hormonal effects in a model of preterm infant brain injury. Ann N Y Acad Sci. 2003 Dec;1008:281–4. doi: 10.1196/annals.1301.032. [DOI] [PubMed] [Google Scholar]
- 10.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005 Jul;146(7):3185–93. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 11.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004 Feb;145(2):790–8. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 12.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009 Jan;85(1):65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar P, Bergman K, Fisk NM, O’Connor TG, Glover V. Amniotic fluid testosterone: relationship with cortisol and gestational age. Clin Endocrinol (Oxf) 2007 Nov;67(5):743–7. doi: 10.1111/j.1365-2265.2007.02955.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. J Neuroendocrinol. 2008 Apr;20(4):489–96. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 15.Gitau R, Adams D, Fisk NM, Glover V. Fetal plasma testosterone correlates positively with cortisol. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;90(2):F166–9. doi: 10.1136/adc.2004.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003 Spring;15(2):431–49. [PubMed] [Google Scholar]
- 17.Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, et al. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009:144–8. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005 Feb;46(2):198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- 19.Booth A, Johnson DR, Granger DA, Crouter AC, McHale S. Testosterone and child and adolescent adjustment: the moderating role of parent–child relationships. Dev Psychol. 2003;39(1):85–98. doi: 10.1037//0012-1649.39.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and vocabulary size in 18- and 24-month-old infants. Infant Behav Dev. 2001;24(4):418–24. [Google Scholar]
- 21.Brazy JE, Goldstein RF, Oehler JM, Gustafson KE, Thompson RJ., Jr Nursery neurobiologic risk score: levels of risk and relationships with nonmedical factors. J Dev Behav Pediatr. 1993 Dec;14(6):375–80. [PubMed] [Google Scholar]
- 22.Holditch-Davis D, Scher M, Schwartz T. Respiratory development in preterm infants. J Perinatol. 2004 Oct;24(10):631–9. doi: 10.1038/sj.jp.7211150. [DOI] [PubMed] [Google Scholar]
- 23.Achermann JC. development of the reproductive systems. In: Brook CGD, Clayton PE, Brown RS, Savage MO, editors. Clinical pediatric endocrinology. 5. Chap 8. Malden, Massachsetts: Blackwell Publishing, Inc.; 2005. pp. 153–70. Available from: http://www.loc.gov/catdir/enhancements/fy0802/2006273369-t.html. Materials specified: Table of contents only http://www.loc.gov/catdir/enhancements/fy0802/2006273369-t.html. [Google Scholar]
- 24.Chada M, Prusa R, Bronsky J, Kotaska K, Sidlova K, Pechova M, et al. Inhibin B, follicle stimulating hormone, luteinizing hormone and testosterone during childhood and puberty in males: changes in serum concentrations in relation to age and stage of puberty. Physiol Res. 2003;52(1):45–51. [PubMed] [Google Scholar]
- 25.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007 Feb;92(2):405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 26.Davison S. Salivary testing opens a Pandora’s box of issues surrounding accurate measurement of testosterone in women. Menopause. 2009 Jul-Aug;16(4):630–1. doi: 10.1097/gme.0b013e3181a8f914. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilova N, Lindau ST. Salivary sex hormone measurement in a national, population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2009 Nov;64(Suppl. 1):i94–i105. doi: 10.1093/geronb/gbn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergada I, Milani C, Bedecarras P, Andreone L, Ropelato MG, Gottlieb S, et al. Time course of the serum gonadotropin surge, inhibins, and anti-Mullerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006 Oct;91(10):4092–8. doi: 10.1210/jc.2006-1079. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007 May;261(5):453–60. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 30.Bateup HS, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women’s competition. Evol Hum Behav. 2002;23(3):181–92. [Google Scholar]
- 31.Vicennati V, Ceroni L, Genghini S, Patton L, Pagotto U, Pasquali R. Sex difference in the relationship between the hypothalamic–pituitary–adrenal axis and sex hormones in obesity. Obesity (Silver Spring) 2006 Feb;14(2):235–43. doi: 10.1038/oby.2006.30. [DOI] [PubMed] [Google Scholar]
- 32.Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Horm Behav. 1999 Feb;35(1):18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]


