Abstract
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi, and it affects as many as 10 million people in North and South America, where it represents a major public health problem. T. cruzi is a parasite with high genetic diversity, and it has been grouped into 6 discrete typing units (DTUs), designated as T. cruzi I (TcI) to T. cruzi VI (TcVI). Mexican isolates from humans and from vector insects have been primarily found to be TcI, and these isolates are likely to be the strains that cause the clinical manifestations observed in Mexico. However, genetic characterization and drug susceptibility assays are limited in Mexican TcI strains. In this work, 24 Mexican T. cruzi strains, obtained primarily from humans, were studied with 7 locus microsatellites and mini-exon gene by PCR. Also, drug susceptibility was evaluated by growth and mobility assays. All of the human strains belonged to TcI, and they could be further grouped through microsatellite analysis into 2 subgroups (microsatellite genotypes 1 and 2), which were not related to the host clinical status or biological origin of the strain. Two strains, both from wild mammals, belonged to the TcII–TcVI groups; these strains and the CL Brener strain constituted microsatellite genotype 3. The number of alleles in each locus was lower than reported for South American strains, and a departure from the Hardy–Weinberg equilibrium was observed. The susceptibility of these strains to nifurtimox and benznidazole was heterogeneous. T. cruzi strains characterized as microsatellite genotypes 2 and 3 were significantly more susceptible to benznidazole than strains of microsatellite genotype 1. Only 1 Mexican strain resistant to both drugs was found in this study.
Key Words: Trypanosome, Chagas disease, Genetics, Nifurtimox, Benznidazole
Introduction
Trypanosoma cruzi, the causal agent of Chagas disease, is present in several Latin American countries and 10 million infected inhabitants have been reported (World Health Organization 2010). In Mexico, a seroprevalence of 1.6% was reported several years ago (Velasco-Castrejón et al. 1992). However, some communities and blood banks showed seroprevalence rates higher than previously reported (Rangel-Flores et al. 2001, Galavíz-Silva et al. 2009).
The genetics of T. cruzi have been revised, and this species has been regrouped into 6 discrete typing units (DTUs) designated as TcI–TcVI (Zingales et al. 2009). Subgroups of TcI have also been reported, using mini-exon gene sequences and microsatellite analysis (Macedo et al. 2001, Falla et al. 2009, Llewelyn et al. 2009, Ocaña-Mayorga et al. 2010, Barnabé et al. 2011).
However, Mexican T. cruzi strains have not been characterized by these molecular markers. This characterization is important because differences in the biological features between Mexican and South American TcI strains, such as metacyclogenesis and growth in vitro, have been reported (López-Olmos et al. 1998).
The association of genetic markers with drug susceptibility in T. cruzi has been studied in some South American strains (Murta et al. 1998, Gomes et al. 2003). However, only a few studies have focused on the susceptibility of Mexican T. cruzi strains to nifurtimox or benznidazole, and the Mexican T. cruzi strains used in these studies have not been well characterized genetically (León-Pérez et al. 2007).
The purpose of the present work was to characterize Mexican human TcI strains through the analysis of 7 microsatellite loci and the mini-exon gene and to assay their susceptibility to nifurtimox and benznidazole in vitro.
Materials and Methods
Parasite culture
Epimastigotes of 20 Mexican T. cruzi strains from humans with different clinical conditions (acute cases, chronic asymptomatic cases, and chronic chagasic cardiomyopathy) were obtained from different geographical areas of Mexico. Additionally, 2 samples from vectors (Triatoma barberi and Triatoma picturata) and 2 from wild mammals (Philander opossum and Didelphis virginiana) were also studied (Table 1). Epimastigotes were cultured in liver infusion and tryptose medium (LIT medium), supplemented with 10% fetal bovine serum (FBS) and 25 μ/mL of hemin. Cultures of parasites were maintained at 28°C according to López-Olmos et al. (1998). Two South American reference strains were included in this study—Silvio (TcI) and CL Brener (TcVI).
Table 1.
Origin and Genotype of Mexican Trypanosoma cruzi Strains
| Strain nomenclature | Geographical origin | Biological origin | Clinical statusa | T. cruzigroupb | Microsatellite genotype |
|---|---|---|---|---|---|
| TBAR/MX/0000/Querétaro | Querétaro | T. barberi | TcI | 1 | |
| TPIR/MX/0000/Guaymas | Sonora | T. picturata | TcI | 1 | |
| MHOM/MX/1994/INC1 | Oaxaca | Human | CCC | TcI | 1 |
| MHOM/MX/1994/INC5 | Guanajuato | Human | CCC | TcI | 1 |
| MHOM/MX/2000/INC6 | Oaxaca | Human | CCC | TcI | 1 |
| MHOM/MX/2001/INC7 | Veracruz | Human | CCC | TcI | 1 |
| MHOM/MX/2001/INC8 | Veracruz | Human | CCC | TcI | 1 |
| MHOM/MX/2001/INC9 | Guerrero | Human | CCC | TcI | 1 |
| MHOM/MX/0000/H4 | Yucatán | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2000/INC10 | Guanajuato | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/0000/EA | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor1 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor4 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor5 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor6 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor8 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor9 | Morelos | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/1994/Ninoa | Oaxaca | Human | Asymptomatic | TcI | 1 |
| MHOM/MX/2001/Mor3 | Morelos | Human | Asymptomatic | TcI | 2 |
| MHOM/MX/1998/JJO | Jalisco | Human | Acute case | TcI | 2 |
| MHOM/MX/1998/JRA | Jalisco | Human | Acute case | TcI | 1 |
| MHOM/MX/0000/H1 | Yucatán | Human | Acute case | TcI | 1 |
| MPHI/MX/1991/Ver3 | Veracruz | P. opossum | c | 3 | |
| MDID/MX/1991/Ver6 | Veracruz | D. virginiana | c | 3 |
CCC indicates chronic chagasic cardiopathy cases; asymptomatic indicates chronic chagasic asymptomatic or indeterminate cases.
According to nomenclature by Zingales et al. (2009).
Similarity to CL Brener strain by mini-exon gene analysis and microsatellite products.
DNA isolation
A total of 20 mL of parasite culture with 40–60×106 parasites/mL was used to isolate DNA. This isolation was performed following extraction with phenol-chloroform from cell pellets, as previously reported (Macedo et al. 1992). The DNA obtained was maintained at −20°C until use.
Mini-exon gene PCR
The intergenic region of the T. cruzi mini-exon gene was amplified using 3 oligonucleotides, as previously described (Souto et al. 1996). The amplification products were resolved for 30 min in 1.5% agarose gels at 115 volts and stained with ethidium bromide for 15 min. The gels were photographed with a Gel Logic 200 transilluminator (Kodak, USA). The size of amplicons was determined by comparison with a DNA ladder of 100 bp (Invitrogen, USA).
Microsatellite assay
Seven pairs of previously described microsatellite primers were used: SCLE11, MCL05, MCLG10, MCLF10, MCLE01, MCLE08, and SCLE10 (Oliveira et al. 1998). PCR was performed using a reaction mixture in a final volume of 50 μL containing 20 pmol of each primer, 0.2 mM of each deoxyribonucleotide triphosphate (dNTP; Invitrogen, USA), 1× PCR buffer, 4 mM MgCl2 (Invitrogen, USA), 200 ng of DNA template, and 0.5 U of Platinum Taq DNA polymerase (Invitrogen, USA). The cycling conditions were standardized as 94°C for 10 min, 95°C for 1 min, 59°C for 2 min, and 72°C for 2 min. This cycle was followed by 30 cycles of a denaturation step at 95°C for 1 min; an annealing step at 59°C for 1 min (for SCLE11, MCLG10, MCLF10, SCLE10, and MCLE01), 57°C for 1 min (for MCL05), or 61°C for 1 min (for MCLE08) and an extension step at 72°C for 1 min. The amplification products were resolved with 6% non-denaturing acrylamide gels stained with ethidium bromide. Analysis of amplicons was performed as described above.
Genetic and phylogenetic analysis
The presence, absence, number, and size of microsatellite PCR products in different strains were observed for the construction of a microsatellite binary data matrix. The phylogenetic reconstruction was performed with the MrBayes 3.1.2. program, using the Bayes theorem and simulation model of Markov Chain Monte Carlo (MCMC) to calculate the posterior probabilities of the trees. Analyses were performed for a data set of 1 million generations, with sampling trees every 1000 generations. Trees with a probability with a lower score than those at stationary phase (burn in) were discarded from the analysis. Generated trees reaching the stationary phase were collected and used to build a majority rule consensus tree as reported previously (Huelsenbeck et al. 2001). The Arlequin program (version 2000) was used for calculating the Hardy–Weinberg data, as described previously (Schneider et al. 2000).
Drug susceptibility assays
Epimastigotes were obtained after 5 days of cultivation (log phase of growth), and 1×106 parasites/mL were seeded in 24-well culture plates (Costar, USA) in LIT medium supplemented as mentioned above. Benznidazole (N-benzyl-2-nitro-1-imidazolacetamide; Roche, Switzerland) and nifurtimox (tetrahydro-3-methyl-4-[(5-nitro-furfurylidene)amine]-2-methyl-tetrahydro-1,4-thiazine-4,4-dioxide; Bayer, Germany) were dissolved in dimethylsulfoxide (DMSO; Sigma, USA), and serial dilutions were made ranging from 0.038 to 384.6 μM in the cultures. Controls with DMSO alone (1% v/v) were included in all of the experiments. The plated parasites were incubated at 28°C for 24 and 48 h. Three independent experiments were performed for each evaluation.
IC50 determination
The number and motility of parasites were determined by counting in a Neubauer chamber, and the data were used to construct dose–response curves. The drug concentration inhibiting 50% of parasite growth (IC50G) or motility (IC50M) was determined using a formula previously reported by Villarreal et al. (2004).
Statistical analysis
The statistical significance between IC50 values was determined with a paired 2-tailed t-test (p<0.05), as reported previously (Mezencev et al. 2009).
Results
Mini-exon PCR
The characteristic 350-bp product was obtained in 22 (91.7%) of the 24 strains, indicating that they belonged to DTU TcI as Silvio strain (Table 1). Only 2 strains (Ver3 and Ver6) presented a 300-bp product, indicating that they do not belong to TcI. By mini-exon gene PCR, it is not possible to discriminate among DTUs TcII through TcVI.
Microsatellite analysis
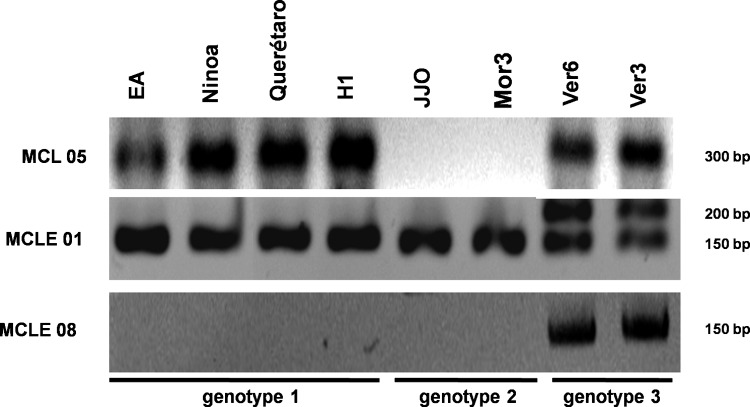
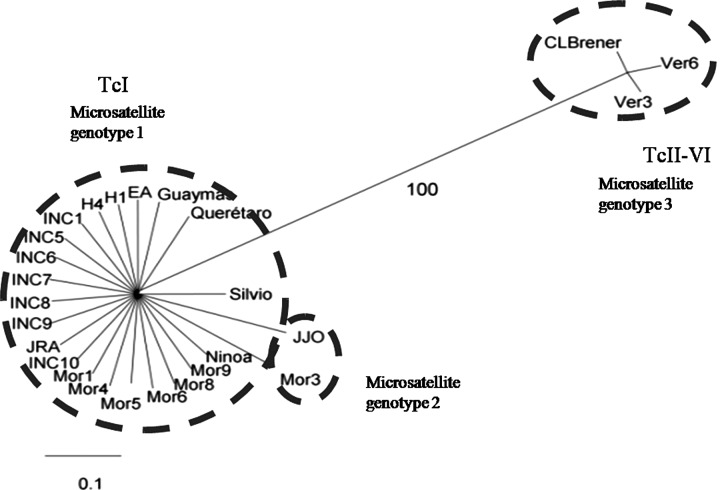
Eighteen strains from humans and 2 strains isolated from vectors were named microsatellite genotype 1 (Table 1). Two strains isolated from humans (JJO and Mor3) were different from microsatellite genotype 1 in the MCL05 locus, and these strains were named microsatellite genotype 2. Furthermore, the Ver3 and Ver6 strains were labeled microsatellite genotype 3 by their different amplified profile (Fig. 1). No more than 3 alleles were found in each locus, ranging between 90 and 450 bp (Table 2). Microsatellite data from the population genetic analysis showed departures from the Hardy–Weinberg equilibrium (p<0.001). Differences were found between the observed and expected heterozygosity in 5 out of 7 microsatellite loci (Table 2). The statistical tests for microsatellite loci showed that these loci were strongly associated with homozygosity within the strains (p<0.001). A phylogenetic tree was built using the microsatellite data, which demonstrated a clear separation of 2 groups with high posterior probability values (Fig. 2).
FIG. 1.
PCR products obtained with the primers indicated (left) of the different Mexican strains of T. cruzi (indicated above). The presence or absence and the product sizes were used to determine genotypes. A representative experiment is shown.
Table 2.
Microsatellites of Mexican DTU TcI Strains
| Marker | Repeat sequencea | Size allele (bp)b | Number of alleles found | Heterozygocity observed/expected |
|---|---|---|---|---|
| MCLG 10 | (CA)8 | 90* 300* 200** |
3 | 0.88/0.61 |
| SCLE 11 | (AC)9 | 140* 160** |
2 | 0.00/0.24 |
| SCLE 10 | (GT)2(TG)10 | 250* 450** |
2 | 0.00/0.24 |
| MCLF 10 | (CA)2A(CA)14 | 150* 200** |
2 | 0.00/0.24 |
| MCLE 08 | (CA)2AA(CA)12 | 150** | 1 | ML |
| MCLE 01 | (CA)9 | 150* 200** |
2 | 0.11/0.14 |
| MCL 05 | (TC)9(GT)4 | 300*** | 1 | ML |
As reported by Oliveira et al. (1998)
Products observed in microsatellite genotype 1 and 2 (*), genotype 3 (**), and genotype 1 and 3 (***).
DTU, Discrete typing units; ML, monomorphic locus.
FIG. 2.
Phylogenetic tree of Mexican T. cruzi strains. The microsatellites data matrix was analyzed with the MrBayes program. Values of the nodes indicate the posterior probability by Markov Chain Monte Carlo (MCMC) analysis.
Effect of benznidazole and nifurtimox on parasite growth and mobility
Nine Mexican strains of T. cruzi were selected to evaluate their susceptibility to nifurtimox and benznidazole, based on the clinical status of infected patients and the geographic origin of the host. Six strains from microsatellite genotype 1, 2 from microsatellite genotype 2, and 1 from microsatellite genotype 3 were chosen.
The Mexican strains of T. cruzi had different susceptibilities to these drugs. The greatest effect of the drugs on motility and growth was reached after treatment for 48 h. Benznidazole treatment showed that Mexican strains belonging to microsatellite genotypes 2 and 3 (JJO, Mor 3, and Ver6) had IC50G values that were significantly lower than those of other strains (Table 3). Silvio and H1 were the only strains with an IC50G value significantly higher than the other strains. When mobility was analyzed, some strains required concentrations higher than 384.6 μM to reach their IC50M.
Table 3.
Drug Inhibitory Concentration at 48 Hours
| |
|
Benznidazole (μM) |
Nifurtimox (μM) |
||
|---|---|---|---|---|---|
| Strain | MGa | IC50G | IC50M | IC50G | IC50M |
| Silvio | 1 | 63.3±22.8b | NF | 73.4±23b | NF |
| MHOM/MX/0000/EA | 1 | 0.7±0.02b,c | 5.7±1.1 | 7±0.8 | 6.5±0.15 |
| MHOM/MX/0000/H1 | 1 | 61.99±14.17b | NF | 59.2±11b | NF |
| MHOM/MX/2001/INC9 | 1 | 7.1±3.9 | NF | 4.5±1.19 | 10.2±1.7c |
| MHOM/MX/2000/INC10 | 1 | 12.27±4.2 | NF | 5±0.7 | 9±2.7c |
| MHOM/MX/1994/Ninoa | 1 | 8.21±1.8 | NF | 0.21±0.18b,c | NF |
| TBAR/MX/0000/Querétaro | 1 | 4.29±2.9 | 17.7±11.6 | 0.38±0.3b | 0.9±0.3b |
| MHOM/MX/1998/JJO | 2 | 0.38±0.3b,c | 0.85±0.3c | 7.7±3.5 | NF |
| MHOM/MX/2001/Mor3 | 2 | 0.8±0.5b,c | 3.7±2.8c | 7.24±1.8 | NF |
| MDID/MX/1991/Ver6 | 3 | 0.04±0.01b,c | 0.07±0.09b,c | 0.68±0.5b | 6±0.8 |
| CL Brener | 3 | 5.54±0.44 | 69.92±3.4b,c | 0.51±0.2b,c | 6.38±0.01 |
Microsatellite genotype according to Table 1.
Statistical differences (p<0.05) respect other strains with the same drug.
Statistical differences (p<0.05) comparing the same strain and parameter with different drugs.
IC, Inhibitory concentration; NF, IC50M higher than 384.6 μM.
In the presence of nifurtimox, low IC50G values were obtained for the Ninoa, Querétaro, Ver6, and CL Brener strains (p<0.05). When the IC50G values of benznidazole versus nifurtimox were compared, JJO, Mor 3, Ver6, and EA were more susceptible to benznidazole. In contrast, the Ninoa strain was more susceptible to nifurtimox. The comparison of IC50M values showed that parasite motility was more affected in JJO, Mor 3, and Ver6 by benznidazole, whereas INC9 and INC10 were more susceptible to nifurtimox. Finally, the Silvio and H1 strains were considered resistant to both drugs in vitro because more than 50 μM was needed to affect their growth and motility.
Discussion
Six DTUs of T. cruzi have been identified and designated as TcI–TcVI (Zingales et al. 2009). In Mexico, most T. cruzi strains have been genetically determined to belong to TcI (Bosseno et al. 2002, León-Pérez et al. 2007, Gómez-Hernández et al. 2011) and TcII–TcVI strains have been reported only in a recent serological study, in wild mammals and the feces of vectors (López-Olmos et al. 1998, Bosseno et al. 2009, Risso et al. 2011). In this work, we confirmed that the Mexican T. cruzi strains isolated from humans belonged to TcI, as reported in Guatemala, Colombia, and Venezuela (Añez et al. 2004, Ruíz-Sánchez et al. 2005, Falla et al. 2009). In the present work, 2 other strains from wild mammals belonging to TcII–TcVI groups were also identified, but their association with any specific DTU remains to be determined.
Several strains of TcI from Latin American countries have been analyzed with microsatellite analysis, and at least 2 subgroups have been established (Macedo et al. 2001, Llewellyn et al. 2009). In the present work, the analyzed TcI Mexican strains were grouped into 2 microsatellite genotypes. Most strains were grouped as microsatellite genotype 1, and only 2 strains were subgrouped as microsatellite genotype 2. These strains were not geographically related. Only 2 strains (Ver3 and Ver6) were microsatellite genotype 3. However, a more careful characterization is needed to determinate to which genetic group (TcII–TcVI) they belong.
The effect of nifurtimox and benznidazole on parasite motility and growth was evaluated. Only the Mexican strain H1 was resistant to both drugs, according to the criteria established in previous studies, because it had IC50G and IC50M values higher than 50 μM (Nirdé et al. 1995, Villarreal et al. 2005). The IC50M and IC50G values for the other Mexican strains were heterogeneous, and they were comparable with values reported for South American strains, such as Tulahuen 2 (TcIV), Dm28c (TcI), Silvio (TcI), Esmeraldo (TcII), and Y (TcII) (Cinque et al. 1998, Vieites et al. 2008, Wilkinson et al. 2008, Luna et al. 2009).
We found a possible correlation between genetic characteristics (microsatellite genotype 2) and susceptibility to the drug benznidazole, described previously only in South American strains (Murta et al. 1998, Gomes et al. 2003, Toledo et al. 2003).
Previous studies reported the effect of both drugs either on parasite mobility or growth. It has been shown that loss of mobility in vitro can be correlated with the death of the parasite (Jacobs et al. 2003). However, this parameter can only give an incomplete picture of the trypanocidal effect, because it has also been reported that some compounds affect only the mobility and the growth and viability of the parasite are not affected (Arantes et al. 2011). It has also been demonstrated that some compounds with anti-T. cruzi activity can affect growth but not mobility, allowing the parasite to survive (Zaverucha et al. 2003).
In this work, the effect of nifurtimox and benznidazole on both parameters was evaluated to determine if differences existed between them. We show the importance of evaluating both mobility and growth to determine the real trypanocidal effect of the drugs because growth can be affected more than motility in some strains. It would be important to assess the effect of any drug on both parameters, particularly those recently proposed for the control of the parasite.
In the future, it will be important to study the role of genes such as type I nitroreductase (NTR) and OYE in benznidazole susceptibility in Mexican strains and to study possible markers of this biological response (Murta et al. 2006, Mejia-Jaramillo et al. 2011). In addition, it will be interesting to analyze whether microsatellite genotypes 1 and 2 described in the present study can be confirmed by a different set of genetic markers.
Acknowledgments
We wish to thank Octavio Fernandes for the mini-exon gene primers and Dr. Ricardo Alejandre for providing the Guaymas T. cruzi strain. This work was supported by DGAPA-UNAM grant number IN206512 and Instituto de Ciencia y Tecnología del Distrito Federal, grant number PICSA 10–130.
Author Disclosure Statement
No competing financial interests exist.
References
- Añez N. Crisante G. da Silva FM. Rojas A, et al. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas´ disease. Trop Med Int Healt. 2004;9:1319–1326. doi: 10.1111/j.1365-3156.2004.01333.x. [DOI] [PubMed] [Google Scholar]
- Arantes JM. Francisco AF. de Abreu VPM. Silva M, et al. Trypanosoma cruzi: Desferrioxamine decreases mortality and parasitemia in infected mice through a trypanostatic effect. Exp Parasitol. 2011;128:401–408. doi: 10.1016/j.exppara.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Barnabé C. De Meus T. Noireau F. Bosseno MF, et al. Trypanosoma cruzi discrete typing units (DTUs): Microsatellite loci and population genetics of DTUs TcV and TcI in Bolivia and Peru. Inf Gen Evol. 2011;11:1752–1760. doi: 10.1016/j.meegid.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Bosseno MF. Barnabé C. Magallon E. Lozano F, et al. Predominance of Trypanosoma cruzi lineage I in Mexico. J Clin Microbiol. 2002;40:627–632. doi: 10.1128/JCM.40.2.627-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseno MF. Barnabé C. Ramírez-Sierra MJ. Kengne P, et al. Wild ecotopes and food habits of Triatoma longipennis infected by Trypanosoma cruzi lineages I and II in Mexico. Am J Trop Med Hyg. 2009;80:988–991. [PubMed] [Google Scholar]
- Cinque GM. Szajnman SH. Zhong L. Docampo R, et al. Structure-activity relationship of new growth inhibitors of Trypanosoma cruzi. J Med Chem. 1998;41:1540–1554. doi: 10.1021/jm970860z. [DOI] [PubMed] [Google Scholar]
- Falla A. Herrera C. Fajardo A. Montilla M, et al. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Galavíz-Silva L. Molina-Garza DP. González-Santos MA. Mercado-Hernández R, et al. Update on seroprevalence of anti-Trypanosoma cruzi antibodies among blood donors in northeast Mexico. Am J Trop Med Hyg. 2009;81:404–406. [PubMed] [Google Scholar]
- Gomes ML. Ornelas M. Vataru C. Rodríguez N, et al. Trypanosoma cruzi: Genetic group with peculiar biochemical and biological behavior. Mem Inst Oswaldo Cruz. 2003;98:649–654. doi: 10.1590/s0074-02762003000500011. [DOI] [PubMed] [Google Scholar]
- Gómez-Hernández C. Resende-Oliveira K. Nogueira G. Rocha L, et al. Molecular characterization of Trypanosoma cruzi Mexican strains and their behavior in the mouse experimental model. Rev Soc Bras Med Trop. 2011;44:684–690. doi: 10.1590/s0037-86822011005000058. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP. Ronquist F. Nielsen R. Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2231. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- Jacobs T. Bruhn H. Gaworski I. Fleischer B, et al. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrob Agents Chemother. 2003;47:607–613. doi: 10.1128/AAC.47.2.607-613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Pérez F. Gómez-García L. Alejandre-Aguilar R. López R, et al. Mexican Trypanosoma cruzi isolates: In vitro susceptibility of epimastigotes to anti-Trypanosoma cruzi drugs and metacyclic forms to complement-mediated lysis. Vector Borne Zoonot Dis. 2007;7:330–336. doi: 10.1089/vbz.2006.0604. [DOI] [PubMed] [Google Scholar]
- Llewellyn MS. Miles MA. Carrasco HJ. Lewis MD, et al. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLOS Path. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Olmos V. Pérez-Nasser V. Piñero D. Ortega E, et al. Biological characterization and genetic diversity of Mexican isolates of Trypanosoma cruzi. Acta Trop. 1998;69:239–254. doi: 10.1016/s0001-706x(97)00131-9. [DOI] [PubMed] [Google Scholar]
- Luna KP. Hernández IP. Rueda CM. Zorro MM, et al. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole. Biomédica. 2009;29:448–455. [PubMed] [Google Scholar]
- Macedo AM. Martins MS. Chiari E. Pena SDJ. DNA fingerprint of Trypanosoma cruzi: A new tool for characterization of strains and clones. Mol Biochem Parasitol. 1992;55:147–154. doi: 10.1016/0166-6851(92)90135-7. [DOI] [PubMed] [Google Scholar]
- Macedo AM. Pimenta J. Aguiar R. Melo A, et al. Usefulness of microsatellite typing in population genetic studies of Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2001;96:407–413. doi: 10.1590/s0074-02762001000300023. [DOI] [PubMed] [Google Scholar]
- Mejía-Jaramillo AM. Fernández GJ. Palacio L. Triana-Chávez O. Gene expression study using real-time PCR identifies an NTR gene as a major marker of resistance to benznidazole in Trypanosoma cruzi. Parasite Vector. 2011;4:169–181. doi: 10.1186/1756-3305-4-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezencev R. Galizzi M. Kutschy P. Docampo R. Trypanosoma cruzi: Antiproliferative effect of indole phytoalexins on intracellular amastigotes in vitro. Exp Parasitol. 2009;122:66–69. doi: 10.1016/j.exppara.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murta SMF. Gazzinelli RT. Brener Z. Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- Murta SMF. Krieger MA. Montenegro LR. Campos , et al. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;146:151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Nirdé P. Larroque C. Barnabé C. Drug-resistant epimastigotes of Trypanosoma cruzi and persistence of this phenotype after differentiation into amastigotes. C R Acad Sci III. 1995;318:1239–1244. [PubMed] [Google Scholar]
- Ocaña-Mayorga S. Llewellyn MS. Costales JA. Miles MA, et al. Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLOS Neg Trop Dis. 2010;4:e915. doi: 10.1371/journal.pntd.0000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RP. Broude N. Macedo AM. Cantor CR, et al. Probing the genetic population structure of Trypanosoma cruzi with polymorphic microsatellites. Proc Natl Acad Sci USA. 1998;95:3776–3780. doi: 10.1073/pnas.95.7.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Flores H. Sánchez B. Mendoza-Duarte J. Barnabé C, et al. Serologic and parasitologic demonstration of Trypanosoma cruzi infections in an urban area of central México: Correlation with electrocardiographic alterations. Am J Trop Med Hyg. 2001;65:887–895. doi: 10.4269/ajtmh.2001.65.887. [DOI] [PubMed] [Google Scholar]
- Risso M. Sartor P. Burgos J. Briceño L, et al. Immunological identification of Trypanosoma cruzi lineages in human infection along the endemic area. Am J Trop Med Hyg. 2011;84:78–84. doi: 10.4269/ajtmh.2011.10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruíz-Sánchez R. de León MP. Matta V. Reyes PA, et al. Trypanosoma cruzi isolates from Mexican and Guatemalan acute and chronic chagasic cardiopathy patients belong to Trypanosoma cruzi I. Mem Inst Oswaldo Cruz. 2005;100:281–283. doi: 10.1590/s0074-02762005000300012. [DOI] [PubMed] [Google Scholar]
- Schneider S. Roessli D. Excoffier L. Arlequin ver. 2.000. University of Geneva; Switzerland: 2000. A software for population genetics data analysis. Genetics and Biometry Laboratory. [Google Scholar]
- Souto RP. Fernandes O. Macedo AM. Campbell DA, et al. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Toledo MJO. Bahia MT. Carneiro CM. Martins-Filho OA, et al. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrobial Agents Chemother. 2003;47:223–230. doi: 10.1128/AAC.47.1.223-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Castrejón O. Valdespino JL. Tapia-Conyer R. Salvatierra B, et al. Seropepidemiología de la enfermedad de Chagas en México. Salud Pública Mex. 1992;34:186–196. [PubMed] [Google Scholar]
- Vieites M. Otero L. Santos D. Toloza J, et al. Platinum (II) metal complexes as potential anti-Trypanosoma cruzi agents. J Inorg Biochem. 2008;102:1033–1043. doi: 10.1016/j.jinorgbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Villarreal D. Barnabé C. Sereno D. Tibayrenc M. Lack of correlation between in vitro susceptibility to benznidazole and phylogenetic diversity of Trypanosoma cruzi, the agent of Chagas´ disease. Exp Parasitol. 2004;108:24–31. doi: 10.1016/j.exppara.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Villarreal D. Nirdé P. Hide M. Barnabé C, et al. Differential gene expression in benznidazole-resistant Trypanosoma cruzi parasites. Antimicrobial Agents Chemother. 2005;49:2701–2709. doi: 10.1128/AAC.49.7.2701-2709.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SR. Taylor MC. Horn D. Kelly JM, et al. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci USA. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2010. Geneva, Switzerland: First WHO report on neglected tropical diseases 2010: Working to overcome the global impact of neglected tropical diseases. ISBN 978 92 4 1564090. [Google Scholar]
- Zaverucha T. Calabrese KS. Côrte-Real S. Baetas WC, et al. Trypanosoma cruzi: In vitro morphological alterations induced by actinomycin D. Pharmacology. 2003;67:55–58. doi: 10.1159/000067741. [DOI] [PubMed] [Google Scholar]
- Zingales B. Andrade SG. Briones MRS. Campbell DA, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]