Abstract
Recent findings show that zinc is an important factor necessary for regulating the meiotic cell cycle and ovulation. However, the role of zinc in promoting oocyte quality and developmental potential is not known. Using an in vivo model of acute dietary zinc deficiency, we show that feeding a zinc deficient diet (ZDD) for 3–5 days before ovulation (preconception) dramatically disrupts oocyte chromatin methylation and preimplantation development. There was a dramatic decrease in histone H3K4 trimethylation and global DNA methylation in zinc deficient oocytes. Moreover, there was a 3–20 fold increase in transcript abundance of repetitive elements (Iap, Line1, Sineb1, Sineb2), but a decrease in Gdf9, Zp3 and Figla mRNA. Only 53% and 8% of mature eggs reached the 2-cell stage after IVF in animals receiving a 3 and 5 day ZDD, respectively, while a 5 day ZDD in vivo reduced the proportion of 2-cells to 49%. In vivo fertilized 2-cell embryos cultured in vitro formed fewer (38%) blastocysts compared to control embryos (74%). Likewise, fewer blastocyst and expanded blastocyst were collected from the reproductive tract of zinc deficient animals on day 3.5 of pregnancy. This could be due to a decrease in Igf2 and H19 mRNA in ZDD blastocyst. Supplementation with a methyl donor (SAM) during IVM restored histone H3K4me3 and doubled the IVF success rate from 17% to 43% in oocytes from zinc deficient animals. Thus, the terminal period of oocyte development is extremely sensitive to perturbation in dietary zinc availability.
Keywords: zinc, oocyte, embryo, methylation
Introduction
Development of healthy embryos and progeny is critically dependent on the quality of the gametes and an optimal uterine environment. The quality of the maternal epigenome, in particular, contributes significantly to promoting optimal embryonic development and postnatal health (Corry et al., 2009; Hales et al., 2011). The epigenome of the oocyte is dramatically remodeled during oogenesis. As the oocyte nears ovulation, major changes in chromatin structure and biochemistry take place to prepare for fertilization and embryonic development. These changes include reorganization of the chromatin from a non-surrounded nucleolus (NSN) to a surrounded nucleolus (SN) configuration where the chromatin becomes tightly packed around the nucleolus (Debey et al., 1993; Mattson and Albertini, 1990; Zuccotti et al., 1995). Chromatin modifications are an important component of epigenetic programming in the oocyte. These include methylation of histone proteins and methylcytosine residues on the DNA. Global DNA methylation is low in early oogenesis and peaks as oocytes reach full size (Kageyama et al., 2007; Kim et al., 2003). DNA methylation is necessary to establish imprinted (monoallelic) gene expression (Bartolomei and Tilghman, 1997). Maternal imprints are established during oogenesis at selected imprint control regions (ICR) (Lucifero et al., 2004; Lucifero, 2002). Most imprinted genes are hypermethylated on the maternal allele, with fewer, such as the Igf2/H19 loci, hypermethylated on the paternal allele (Davis et al., 1999; Tremblay et al., 1997). Relative hypomethylation in the growing oocyte helps to maintain a high rate of transcription (Bouniol-Baly et al., 1999; De La Fuente and Eppig, 2001; De La Fuente et al., 2004). High transcription in oocytes is necessary to produce and store the large quantity of maternal RNAs and proteins (Schultz and Wassarman, 1977; Sternlicht and Schultz, 1981; Wassarman et al., 1979) that are needed as maternal factors until embryonic genome activation. However, because of the extensive hypomethylation, many of the transcripts produced in the oocyte are of repetitive sequences, such as the mouse transcript (MT) family of retrotransposons, which accounts for up to 14% of transcripts in fully-grown oocyte (Evsikov et al., 2004; Mehlmann et al., 2004; Peaston et al., 2004; Peaston et al., 2007). Later in oogenesis, transcription of repetitive elements including, intracisternal A particle (Iap), long interspersed nuclear element 1 (Line1) and short interspersed nuclear element (SINEb) are silenced by DNA methylation (Lees-Murdock et al., 2003; Rollins et al., 2006; Walsh et al., 1998). Failure to re-methylate repetitive elements can lead to aberrant expression and/or genome instability including the increase in DNA double-strand breaks (Hedges and Deininger, 2007). DNA methylation also causes global transcriptional silencing of cellular genes in fully-grown oocyte (Bouniol-Baly et al., 1999; De La Fuente and Eppig, 2001; Miyara et al., 2003). Histone methylation and acetylation are another type of epigenetic modification regulated during oocyte growth and maturation. Acetylation of histone 3 (K9 and K18), histone 4 (K5 and K12) increase as the oocyte nears ovulation and the chromatin rearranges into a SN configuration (Kageyama et al., 2007). Likewise di- and tri-methylation of histone H3K4 and H3K9 increases in fully-grown oocytes (Kageyama et al., 2007). In the one-celled zygote, histone methylation progressively increases in the paternal pronucleus as the paternal genome is reprogrammed after fertilization (Arney et al., 2002; Lepikhov and Walter, 2004; Liu et al., 2004). Deletion of genes encoding histone or DNA methyltransferase enzymes severely compromises oocyte development and fertility (Andreu-Vieyra et al., 2010; Howell et al., 2001; Kaneda et al., 2004). Thus, chromatin methylation is an important component of epigenetic programming during oogenesis.
Zinc has recently been recognized as an important factor required for completion of meiosis and egg activation in vitro (Bernhardt et al., 2011; Kim et al., 2010; Kong et al., 2012; Suzuki et al., 2010b; Tian and Diaz, 2012) and for follicle rupture and completion of meiosis in vivo (Tian and Diaz, 2012). Numerous studies have shown that zinc deficiency during pregnancy causes abnormal embryo and fetal development and poor progeny health (Apgar, 1985; Keen et al., 2003; Uriu-Adams and Keen, 2010). In other tissues, zinc deficiency decreases histone and DNA methylation (Breksa Iii and Garrow, 2002; Wallwork and Duerre, 1985). However, the consequence of zinc depletion during the final period of oogenesis on chromatin methylation, fertilization and preimplantation development has not been examined. Thus, given that, 1) zinc deficiency is known to decreased chromatin methylation in other tissues and 2) chromatin methylation is increasing in the oocyte during antral follicular development, we hypothesize that preconception zinc deficiency will decrease chromatin (DNA and histone) methylation and impair fertilization and preimplantation development. The findings provide evidence in support of this hypothesis and establish the period of final oocyte growth as a critical transition in oocyte epigenetic programming that is sensitive to perturbation in whole body zinc homeostasis.
Materials and Methods
Animal model of zinc deficiency
Female CD1 mice (Mus musculus) were obtained from the research colony of the investigators. To study effects of acute in vivo zinc deficiency before ovulation on oocyte epigenetic programming and embryonic development, newly weaned 18 day old mice were housed on wire-bottom racks in polycarbonate cages and given a control diet (29 mg zinc/kg) based on AIN76 (MP Biomedicals, Solon, OH) or a zinc deficient diet (ZDD), which is the same diet as the control with zinc omitted from the mineral mix (<1 mg zinc/kg). Diets were given for 3 or 5 days before ovulation. A 5 day treatment was chosen because in previous (Tian and Diaz, 2012) and preliminary work (data not shown, longer treatments with a zinc deficient diet cause a decrease in ovulation. A 3 day treatment was used in some experiments to show a dosage effect of a shorter dietary treatment. Animals were primed with 5 IU eCG (National Hormone and Peptide Program, NIDDK) 2 days before the end of the dietary treatment period. Fully-grown GV stage oocytes were collected by gentle puncture of follicles with 25 ga needles. To collect mature eggs, animals were primed with eCG for 48 hours and induced to ovulate with 5 IU hCG. Ovulated oocytes were collected from the oviduct 13 hours later. To examine embryonic development, animals were induced to ovulate with eCG/hCG and bred to a fertile male. Only animals with a mating plug on the morning after mating were used for subsequent analyses. Preimplantation embryos were flushed from the reproductive tract on days 1.5 (2 cell) and 3.5 (blastocysts). Importantly, at the time of hCG, animals receiving a zinc-deficient diet were switched back to a control diet for the duration of the experiment. To examine fertilization ability in vitro, female mice were superovulated at the end of a 3 or 5 day dietary treatment as described above. Ovulated oocytes were recovered from the oviduct and fertilized in vitro as described previously (O'Brien et al., 2003). Briefly, expanded cumulus-oocyte complexes were released from the oviduct and directly transferred to fertilization dish containing sperm (~10X106/ml) collected from the tail of the epididymis of a fertile male. The medium used was MEM with 3 mg/ml BSA as described previously (O'Brien et al., 2003). Complexes were incubated for 5 hours, washed of excess sperm and cultured for an additional 20 hours (24 hours total). At the end of culture, the proportion of 2-cell embryos was determined as a measure of fertilization. Pronuclear stage embryos were collected 8 hours after fertilization in vitro. Animals were maintained according to the Guide for the Care and Use of Laboratory Animals (Institute for Learning and Animal Research). All animal use was reviewed and approved by the IACUC committee at The Pennsylvania State University.
Immunofluorescence
Immunofluorescence staining of control and zinc-deficient oocytes and fertilized eggs was conducted essentially as described previously (Tian and Diaz, 2012; Tian et al., 2010). Briefly, COC were collected from eCG-primed mice (48 hours) and manually denuded by gentle pipetting to remove the cumulus cells. Oocytes and embryos from in vitro fertilization experiments were fixed immediately in 4% PFA for 30 minutes. After fixing, oocytes and embryos were permeabilized with PBS (0.05% Tween and 0.5% Triton-X) for 15 min at room temperature followed by washing (3×) in PBST (1% BSA)and then blocked in goat serum blocking buffer (PBS, 2% goat serum, 1% BSA, 0.1% Triton-X and 0.05% Tween 20) for 1 hour followed immediately by incubation with anti-trimethylated histone H3K4 (1:400, Cell Signaling) or anti-dimethylated histone H3K4 (1:400, Cell Signaling) for 1 hour. For 5-methylcytosine (5-Mec) detection, oocytes and embryos were fixed for 15 min with 4% PFA and treated with 2N HCl for 30 min and then neutralized with Tris-HCl (PH=8) for 15 minutes. After washing in PBST, cells were immunostained with antibody against 5-methylcytosine (1:200, Calbiochem) for 1 hour. After washing in PBST, oocytes and embryos were incubated with goat anti-rabbit IgG Alexa Fluor 594 or goat anti-mouse IgG Alexa Fluor 546 for 1 hour followed by washing with PBST and was mounted in DAPI anti-fade gold (Invitrogen) on glass slides with etched rings to prevent them from being ruptured by the cover slip. Slides were imaged with a fluorescent microscope (AxioScope 2 Plus, Leica, Bannockburn, IL). Fluorescence intensity was determined with Image J software. All values within a replicate were normalized to the average integrated pixel density of the control oocytes.
Isolation of total RNA and qPCR
Total RNA was isolated from 40–50 oocytes and 10 blastocysts using the RNAeasy micro or mini kits (Qiagen, Valencia, CA), respectively. Total RNA was reverse transcribed into cDNA as described previously (Diaz et al., 2006) using the Quantitek cDNA synthesis kit (Qiagen, Valencia, CA). Quantification of Gdf9, Bmp15, Figla, Zp3, Nobox, Igf2, H19, Ddx4, Nr4a1 and Pou5f1 mRNAs was conducted using gene specific primers (Table 1) and Rpl19 mRNA as the normalizer as described previously (Livak and Schmittgen, 2001; Tian and Diaz, 2012). Only one product of the appropriate size was identified for each set of primers and all amplification products were sequenced to confirm specificity. Primers for repetitive elements (Iap, Line1, Sneb1, Sineb2 and Mt) were validated previously (Su et al., 2012). Experiments were repeated 3–5 times and values shown are the mean ±SEM.
Table 1.
Primer sequences used for qPCR.
| Gene symbol | Forward | Reverse |
|---|---|---|
| Igf2 | AGGGGAGCTTGTTGACACG | GGGTATCTGGGGAAGTCGTC |
| H19 | CATGTCTGGGCCTTTGAA | TTGGCTCCAGGATGATGT |
| Ddx4 | CCCATTGTATTAGCAGGACGA | GCGACTGGCAGTTATTCCAT |
| Nr4a1 | CACAGCTTGGGTGTTGATGT | GCTCCTTCAGACAGCTAGCAA |
| Gdf9 | CTACAATACCGTCCGGCTCT | CAAGTGTTCCATGGCAGTCA |
| Bmp15 | ACACAGTAAGGCCTCCCAGA | GATGAAGTTGATGGCGGTAAA |
| Figla | ACAGAGCAGGAAGCCCGTA | GTCAGAGGGTCTGCCACTGT |
| Nobox | AGGGACGTTCCTGGCAGT | GCTGCTTGCTTGGTAGTCCT |
| Iap | ACAAGAAAAGAAGCCCGTGA | GCCAGAACATGTGTCAATGG |
| Line1 | GAGACATAACAACAGATCCTGA | AACTTTGGTACCTGGTATCTG |
| Sineb1 | GTGGCGCACGCCTTTAATC | GACAGGGTTTCTCTGTGTAG |
| Sineb2 | GAGATGGCTCAGTGGTTAAG | CTGTCTTCAGACACTCCAG |
| Mt | TGTTAAGAGCTCTGTCGGATGTTG | ACTGATTCTTCAGTCCCAGCTAAC |
| Rpl19 | TTCAAAAACAAGCGCATCCT | CTTTCGTGCTTCCTTGGTCT |
Statistical analysis
Results from ovulation rate, the number of 2-cell stage embryos, blastocysts and qPCR were analyzed by either student’s t-test or two-way ANOVA followed by Tukey's HSD post hoc test if a positive F test was detected. Proportional data was transformed (arcsine) before analysis. The JMP 7.1 statistical analysis software (SAS, Cary, NC) and Microsoft Excel were used for analyses.
Results
Zinc deficiency causes epigenetic defects in oocytes
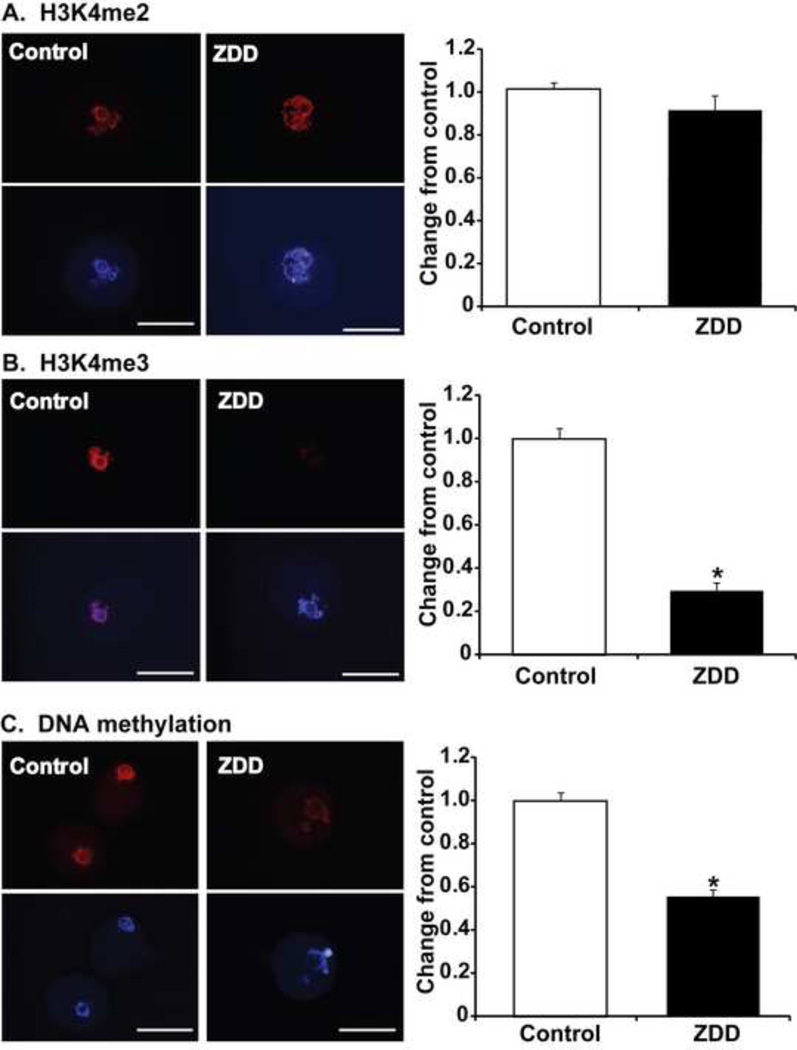
To establish that oocyte epigenetic programming is altered in zinc deficient oocytes, we used immunofluorescence staining and measurements of nuclear fluorescence intensity in GV-stage oocytes from control and zinc deficient animals to measure differences in chromatin methylation. Dimethylation of H3K4 was not altered by a 5 day treatment with a zinc deficient diet (Figure 1A). However, little or no staining was observed for trimethylated histone H3K4 in zinc deficient oocytes (Figure 1B). To determine if DNA methylation might also be affected by zinc deficiency, an antibody against 5-methylcytosine (5-MeC) was used to detect global DNA methylation. Surprisingly, DNA methylation was also dramatically reduced in zinc deficient oocytes compared to control oocytes (Figure 1C).
Figure 1. Effect of zinc deficiency on chromatin methylation in GV-stage oocytes.
Representative images and nuclear fluorescence intensity of histone H3K4 dimethylation (H3K4me2) (A), histone H3K4 trimethylation (H3K4me3) (B) and DNA methylation (C) in GV stage oocytes from animals receiving a control or zinc deficient diet (ZDD) for 5 days. Red=methylated histone or 5-methylcytosine, Blue=DNA (DAPI). *P<0.05, N=3–4 (10–20 oocytes/replicate). Scale bar = 50 µm.
Preconception zinc deficiency causes aberrant gene expression in oocytes
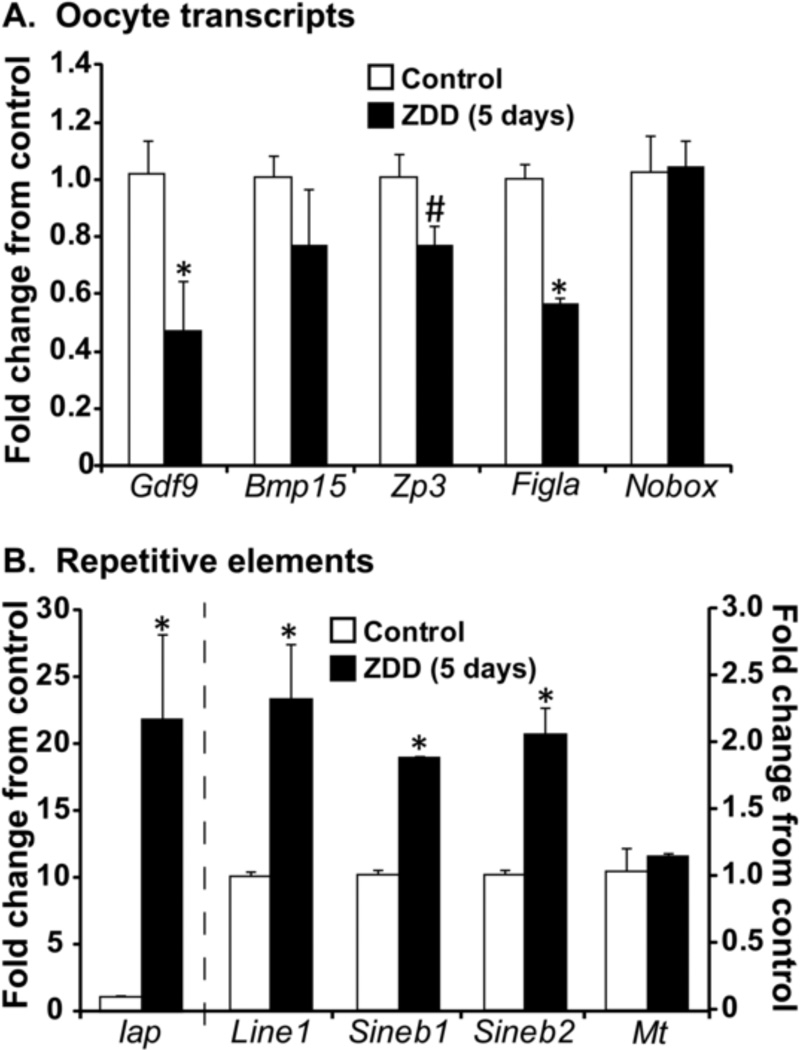
Epigenetic defects, particularly reduced DNA methylation could interfere with global transcriptional silencing and/or silencing of repetitive elements in oocytes. The expression of several highly abundant and important oocyte transcripts was measured by qPCR as an indirect measure of transcriptional silencing. Concentration of Gdf9 and Figla mRNA were decreased by ~50% and while Zp3 mRNA was only moderately decreased ((P=0.08), Figure 2A) in zinc deficient oocytes. The concentration of Bmp15 and Nobox mRNA were not altered by zinc deficiency in oocytes. In contrast, there were significant increases in concentrations of transcripts for various repetitive elements. Iap transcripts increased more than 20 fold while Line1, Sineb1 and Sineb2 transcripts increased 2–3 fold in zinc deficient oocytes. In contrast, Mt transcripts did not differ in zinc deficient oocytes compared to controls (Figure 2B).
Figure 2. Effect of zinc deficiency on transcript expression in GV-oocytes.
A. Relative (fold change) concentration of oocyte-specific transcripts (Gdf9, Bmp15, Zp3, Figla, and Nobox) in control and ZDD oocytes. (B) Relative (fold change) concentration of repetitive elements (Iap, Line1, Sineb1, Sineb2 and MT) (B) in control and ZDD oocytes. *P<0.05, #P=0.08, N=4 (10 oocytes/replicate).
Fertilization potential in vitro is decreased by preconception zinc deficiency
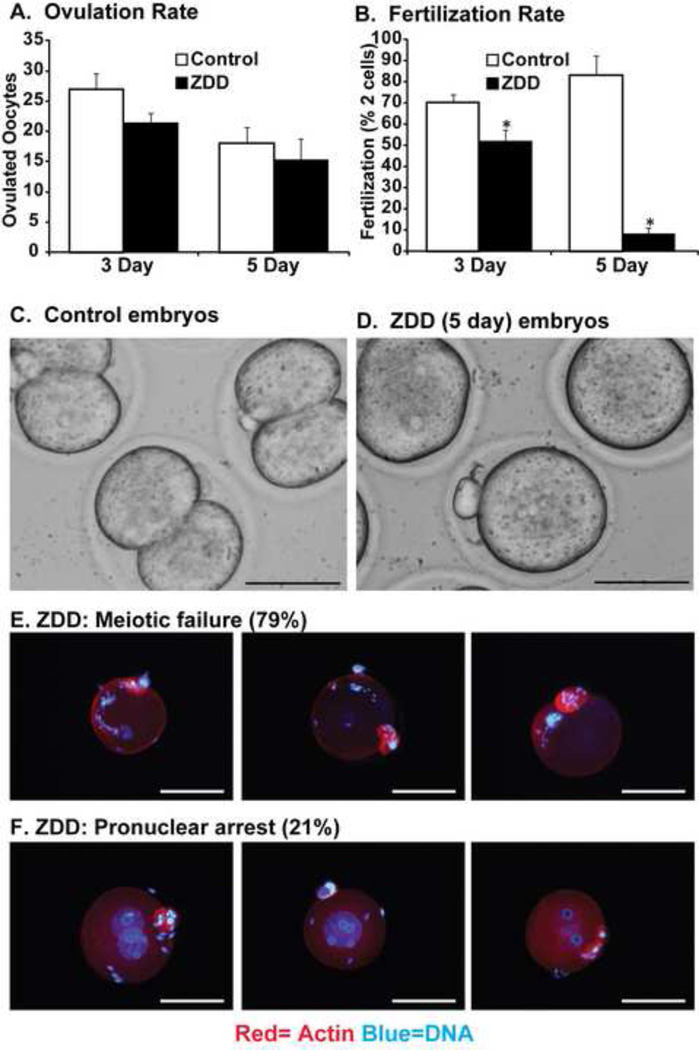
To test for detrimental effects of zinc deficiency (3 or 5 day) on fertilization that are independent of effects on the oviduct and uterus, the fertilization potential of control and zinc- deficient oocytes was determined in vitro. Ovulation rate did not differ in either control of zinc deficient groups treated for 3 or 5 days (Figure 3A). However, fertilization rate (proportion of mature eggs that progress to the 2-cell stage) was dramatically decreased in the ZDD group from 70% to 52% in the 3 day treatment group and from 83% to 8% in the 5 day treatment group (Figure 3B-D). The dramatic collapse in fertilization rate after a 5 day zinc deficient treatment in fertilized eggs was caused by a failure to reach metaphase II (no polar body, 79%) or by a failure of pronucleus fusion (pronuclei present, 21%) (Figure 3E-F).
Figure 3. Effect of zinc deficiency on in vitro fertilization.
The number of ovulated oocytes (A), proportion of 2-cell embryos (B) and representative images (C-D) 24 hours after in vitro fertilization from animals receiving a control or zinc deficient diet (ZDD) for 3 or 5 days. Defective meiosis (E) and pronuclear arrest (F) in ZDD fertilized eggs. Blue = DNA, Red = Actin. *Significant difference by student’s t-test, P<0.05, (N=4; 50–60 eggs/replicate). Proportion of 2-cell embryos were transformed (arcsine) before analysis. Scale bar = 50 µm (panel 3C-D) and 100 µm (panels 3E-F).
To determine if chromatin methylation is altered by zinc deficiency in fertilized eggs, oocytes from control or zinc-deficient animals (5 days) were fixed 8 hours after fertilization in vitro. Embryos were immunostained with an antibody recognizing H3K4me3. Embryos from control animals showed the typical dimorphic pattern of intense immunostaining for H3K4me3 in the maternal pronucleus with little staining evident in the paternal pronucleus (Figure 4). The fertilized zinc deficient eggs showed disorganized chromatin characteristic of meiotic failure. However, the sperm nucleus did manage to enter the oocyte indicating that some early steps in fertilization still occur in zinc deficient oocytes.
Figure 4. Effect of zinc deficiency on histone H3K4 trimethylation in fertilized eggs.
Representative images of fluorescent immunostaining for histone H3K4 trimethylation 8 hours after in vitro fertilization of ovulated eggs from animals receiving a control or zinc deficient diet (ZDD) for 5 days. (N=3, 10–20 oocytes/replicate). Red=trimethylated histone, Blue=DNA (DAPI), Green=Actin. Scale bar = 50 µm.
In vivo fertilization and blastocyst development is impaired by preconception zinc deficiency
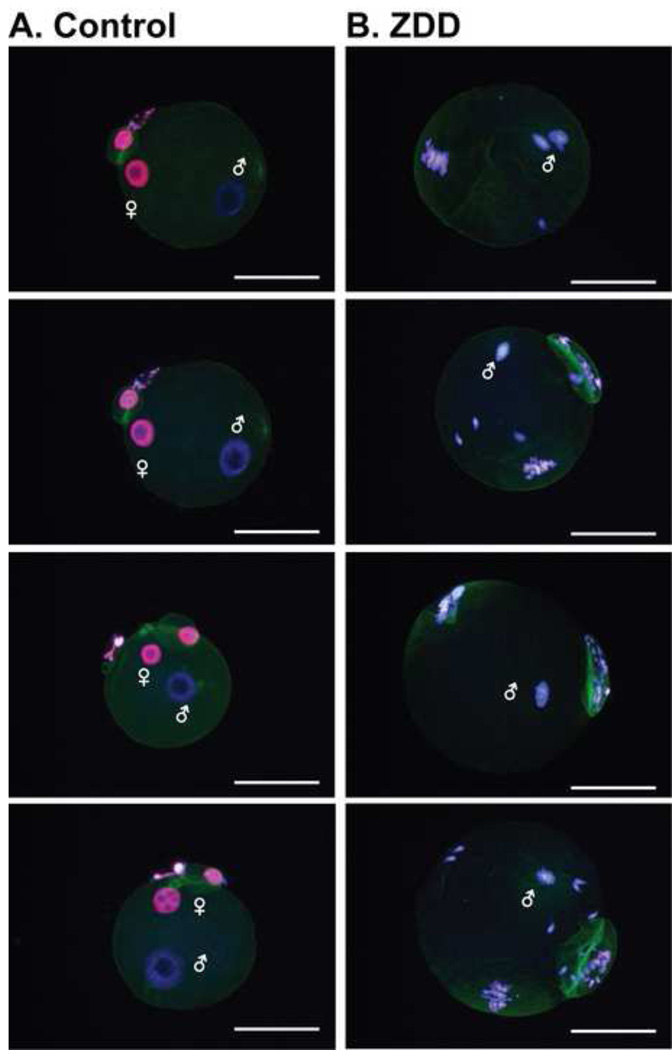
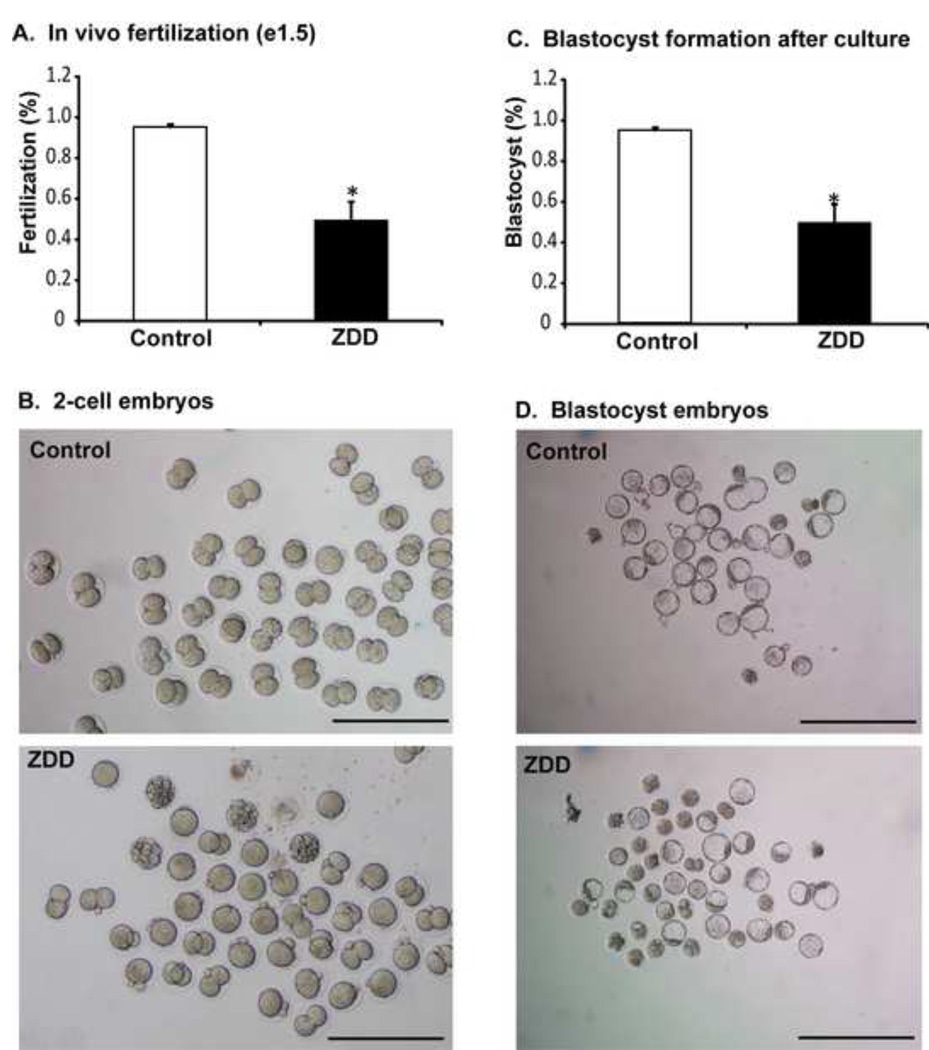
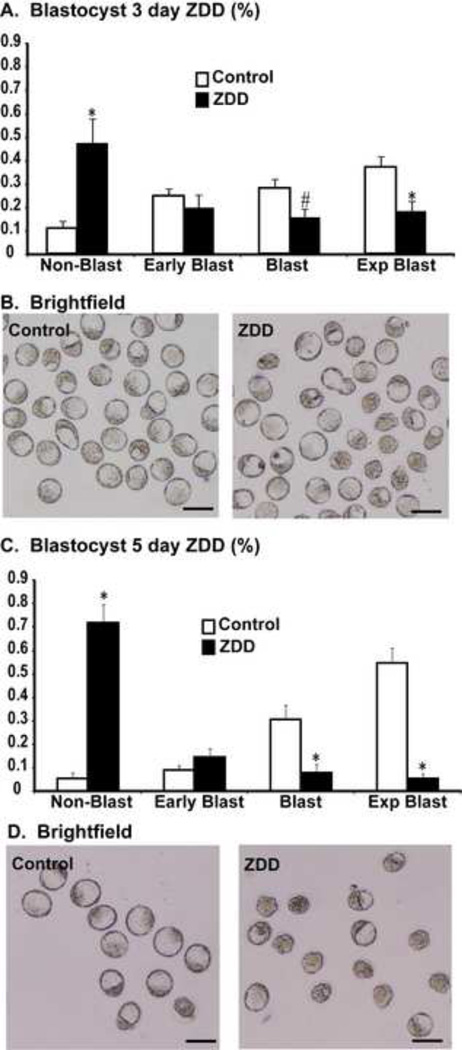
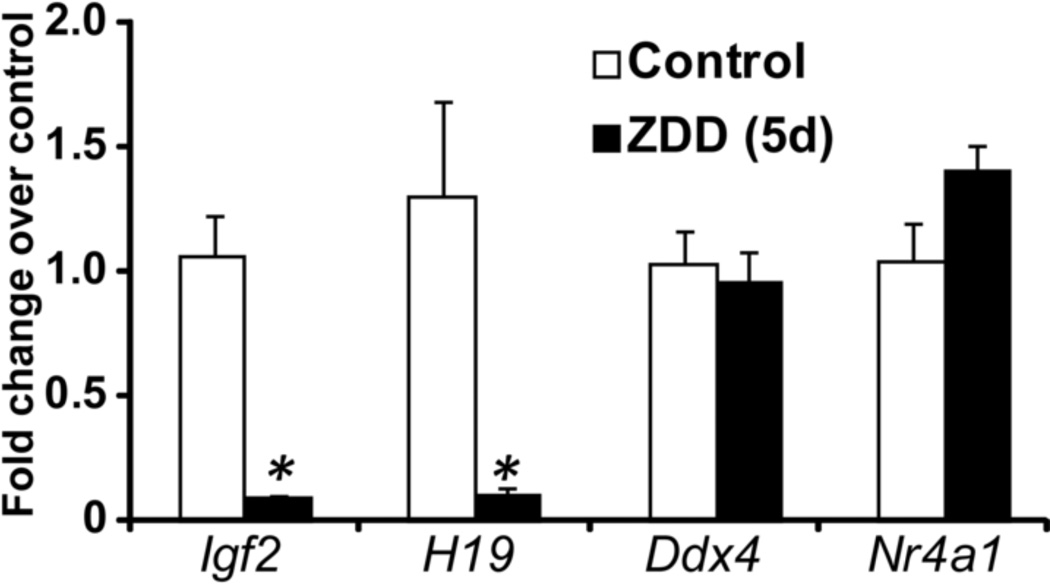
In contrast to the dramatic decrease in fertilization rate in vitro, fertilization in vivo was not as dramatically affected by zinc deficiency. In vivo fertilization rate was 95% in control animals, but was reduced to 49% in ZDD animals (Figure 5A-B). However, the 2-cell embryos from zinc deficient animals were less competent than control embryos. Embryos were cultured in KSOM medium to determine the rate of blastocyst formation. Only 38% of 2-cell embryos from zinc deficient animals reached the blastocyst stage after in vitro culture compared to 74% of control embryos (Figure 5C-D). To test if blastocyst development in vivo is also affected, animals were fed a control diet or zinc deficient diet for 3 and 5 days before ovulation and mated to a fertile male as above. On day 3.5 after mating, embryos were flushed from the reproductive tract. There was no difference in the total number of embryos recovered from the reproductive tract in either experiment (3 day, control 38±5, ZDD 46±8; 5 day, control 19±3, ZDD 19±2; N=4–5). However, the proportion of blastocyst and expanded blastocysts were reduced, while the proportion or non-blastocyst (one cell to morula) and early blastocysts were increased in zinc deficient animals (Figure 6). The decrease in proportion of blastocysts and expanded blastocysts could be related to a much lower level of Igf2 mRNA in zinc deficient blastocyst embryos (Figure 7). Expression of the linked transcript, H19, was also decreased in zinc deficient embryos, but the non-imprinted transcripts, Ddx4 (vasa) and Nr4a1 were not affected by zinc deficiency in blastocyst embryos (Figure 7). Importantly, transcripts analyzed in figure 7 were measured in fully-formed blastocyst embryos from control and ZDD animals. Thus, the decrease in Igf2/H19 mRNA is not due to an obvious difference in developmental stage.
Figure 5. Effect of zinc deficiency on in vivo fertilization and blastocyst formation.
Proportion of 2-cell embryos (A), representative images (B) of embryos recovered on day 1.5 of pregnancy from animals receiving a control or zinc deficient diet for 5 days. Proportion of blastocyst embryos (C) and representative images (D) of 2-cell embryos in panel A, cultured for 3 days in KSOM medium. *Significant by student’s t-test, P<0.05, N=4–5 animals. Scale bar = 250 µm (panel B), 500 µm (panel D).
Figure 6. Effect of zinc deficiency on preimplantation development in vivo.
A. Proportion of embryos at different developmental stages including, non-blastocysts (single cell to morula), early blastocyst, blastocyst and expanded blastocyst collected from control and 3 day ZDD groups on 3.5 days after mating. B. Representative images of embryos from the 3 day treatment group. C. Proportion of embryos collected at different developmental stages collected from animals in the 5 day treatment group. C. Representative images of embryos from the 5 day treatment group (Control and ZDD). *Significant by student’s t-test, P<0.05, N=4–5 animals, group. Scale bar = 100 µm
Figure 7. Effect of zinc deficiency on blastocyst gene expression.
Relative (fold change) concentration of Igf2, H19, Ddx4 and Nr4a1 mRNA in blastocyst embryos recovered from animals receiving a control and ZDD for 5 days. Relative levels of mRNA measured by qPCR using the 2-ddct method. *Significant difference by student’s t-test, P<0.05, N=4 (10 blastocyst/replicate).
Supplementation with SAM during IVM restores histone H3K4me3 and improves fertilization potential of zinc deficient oocytes
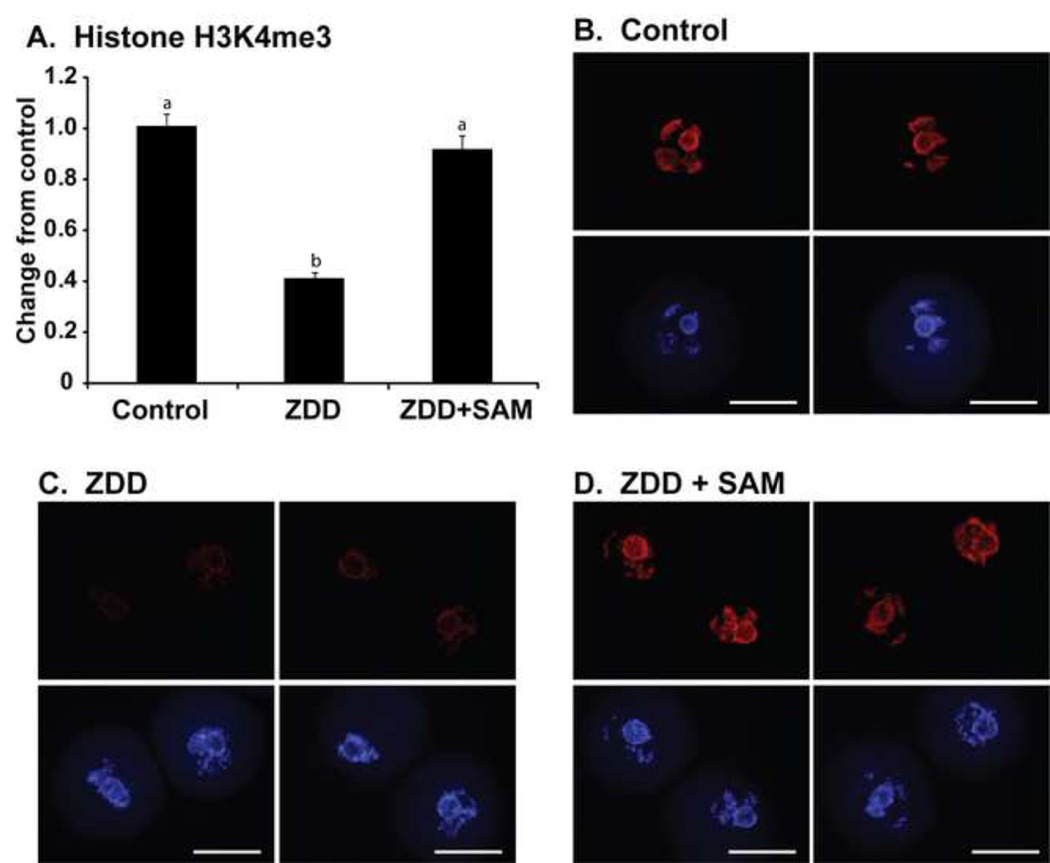
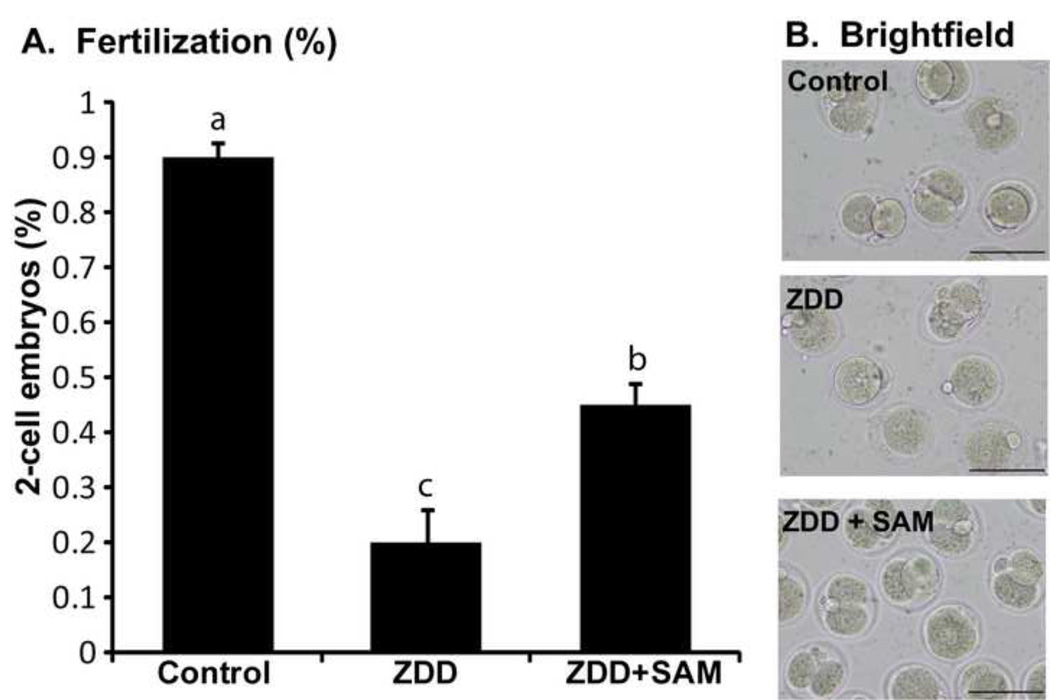
The dramatic decrease in chromatin methylation in oocytes from animals receiving a zinc deficient diet is alleviated by supplementation with the methyl donor s-adenosylmethionie (SAM). Mice were given a zinc deficient diet for 5 before ovulation. The COC were collected from animals primed with eCG and were cultured in MEM-alpha medium alone (control), medium only (ZDD) or medium supplemented with 100 µM SAM (ZDD+SAM) for 24 hours. At the end of culture, oocytes were fixed and immunostained for histone H3K4me3. As expected, control oocytes had robust H3K4me3, but zinc deficient oocytes did not (Figure 8). However, zinc deficient oocytes cultured with SAM had levels of H3K4me3 similar to control oocytes (Figure 8). To test if SAM can increase the proportion of zinc-deficient oocytes progressing to the 2-cell stage, COC were collected from eCG-primed animals fed a control or zinc deficient diet for 5 days and matured in vitro. Control embryos have a high fertilization rate of 95% which was reduced to 19% in zinc deficient oocytes (Figure 9). However, zinc deficient oocytes matured in the presence of SAM had a 45% fertilization rate (Figure 9).
Figure 8. Effect of SAM supplementation on histone methylation.
A. Immunostaining for histone H3K4me3 in GV oocytes from animals receiving a control or zinc deficient diet (ZDD) and cultured in medium alone (Control and ZDD) or medium containing 100 µM SAM (ZDD+SAM) for 24 hours. *Significant difference by ANOVA followed by Tukey’s HSD test, P<0.05, N=3 replicates with 10–15 oocytes/group. Scale bar = 50 µm.
Figure 9. SAM rescue during IVF.
Proportion of 2-cell embryos (A) and representative images (B) of in vitro matured and fertilized oocyte from control, ZDD and ZDD animals supplemented with SAM (100 µM) during IVM (16 hours). *Significant difference by ANOVA followed by Tukey’s HSD test, P<0.05, N=3 replicates with 20–30 COC/group. Scale bar = 50 µm.
Discussion
Nutritional deficiencies during pregnancy can impair development and postnatal health by altering specific epigenetic marks (Burdge et al., 2007; Cooney et al., 2002; Kim et al., 2009; Waterland and Jirtle, 2003; Wu et al., 2004). Previously, we reported that dietary zinc deficiency caused multiple problems in ovarian function during the periovulatory period, including failure to complete meiosis and a lack of cumulus expansion and ovulation (Tian and Diaz, 2012). The experimental design and findings of the current study are summarized in figure 10 and show that maternal zinc deficiency during a very narrow window just before ovulation disrupts oocyte epigenetic programming including a decrease in DNA and histone methylation and associated increase in expression of repetitive elements. These epigenetic defects along with previously shown meiotic defects (Tian and Diaz, 2012) severely compromise fertilization and preimplantation embryonic development. However, whether zinc deficiency acts directly on the oocyte or indirectly through other mechanisms to decrease chromatin methylation in the oocyte is not known. In vitro supplementation with s-adenosylmethionine, a methyl donor, restored histone methylation and improved fertilization rate of zinc deficient eggs. Our challenge now is to fully uncover the precise pathways and proteins affected by zinc deficiency that lead to decreased oocyte developmental potential. Understanding these zinc-mediated mechanisms will provide new insights into the regulation of oocyte quality and fertility.
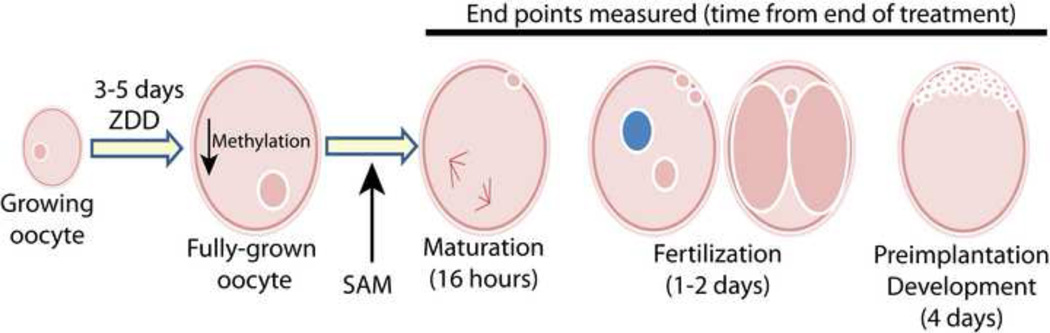
Figure 10. Schematic showing timing of treatment in relation to endpoints examined.
Dietary treatments were applied to growing oocytes just entering antral stage of development around 18 days of age. Treatments were stopped 48 hours after the end of eCG, which encompasses the period of antral follicular growth. Effects on maturation, fertilization and preimplantation development were determined. Previous research showed that TPEN treatment disrupts completion of meiosis I, but effects of in vivo zinc deficiency on fertilization and preimplantation development have not been reported.
Experiments using dietary zinc deficiency during early pregnancy have produced mixed results on fertility. In rats fed a zinc deficient diet on days 1–4 of gestation, fewer blastocyst embryos formed (Hurley and Shrader, 1975) suggesting delayed growth. However, an earlier study from the same group, using a similar treatment period (day 1–5 of gestation), found that a zinc deficient diet caused only minor effects on fertility and embryonic development at birth (Hurley et al., 1971). In mice, a zinc deficient diet given one day before mating to one day after mating (3 days) did not disrupt blastocyst development (Peters et al., 1991). These observations in rodents suggest that any defect induced by short-term (3–4 days) dietary zinc deficiency in early pregnancy can be rescued by subsequent restoration of dietary zinc. In contrast to these earlier studies, treatment with a zinc deficient diet during the 3–5 days before conception caused a dramatic decrease in maturation, fertilization and preimplantation development. Previous work clearly shows that zinc is essential for completion of meiosis I (Bernhardt et al., 2011; Kim et al., 2010; Tian and Diaz, 2012). In the present study, we show a decrease in the fertilization rate of 25% and 90% after a 3 and 5 day treatment with a zinc deficient diet. Much of this decrease is due to a failure to complete meiosis, but the observed incidence of pronuclear formation (21%) and the appearance of partially decondensed sperm heads in the cytoplasm of fertilized zinc deficient eggs suggests that early steps in fertilization can still occur. Similarly, oocytes matured in the presence of a zinc chelator, TPEN, and fertilized in vitro progressed to the pronuclear stage and exhibited calcium oscillations, but did not divide to form 2-cell embryos (Kim et al., 2010). Clearly zinc deficient eggs, whether in vivo or in vitro, can undergo early stages of fertilization but become arrested before the 2-cell stage. The pronuclear stage arrest in zinc deficient oocytes could also be the result of premature egg activation. Normally, zinc increases during maturation and is required for establishment of metaphase II arrest. At fertilization, zinc is actively exported from the egg which allows resumption of the cell cycle and egg activation (Bernhardt et al., 2012a; Kim et al., 2011). Interestingly, our results show that zinc deficient eggs are more susceptible to fertilization failure in vitro than in vivo. What causes this difference in fertilization potential is unknown, but does suggest that women with marginal or acute zinc deficiency may have lower success rates during ART procedure and may benefit from additional zinc supplementation. In addition to meiotic and fertilization defects, zinc deficient oocytes have reduced developmental competence to the blastocyst stage after fertilization. On day 1.5 after mating, only 43% of 2-cell embryos form zinc deficient eggs reached the blastocyst stage when cultured in vitro compared to 95% of controls embryos. On day 3.5 after mating there were fewer blastocyst and expanded blastocysts in zinc deficient animals compared to controls suggesting a slower rate of development. The decreased expression of the growth promoting gene, Igf2 could account for some of the defects in preimplantation development, but how dietary zinc deficiency results in decreased Igf2/H19 expression in the embryo remains to be determined. Collectively, these results firmly establish that preconception zinc status is an important determinant of maturation, fertilization and preimplantation development.
The findings support the hypothesis that preconception dietary zinc deficiency decreases oocyte developmental potential, but the mechanism responsible for this effect is unknown. The decrease in oocyte H3K4me3 could account for many of the defects observed in zinc deficient oocytes. Recently, the lysine methyltransferase protein, mixed lineage leukemia 2 (MLL2), was shown to catalyze the trimethylation of H3K4 in oocytes (Andreu-Vieyra et al., 2010). Deletion of MLL2 in oocytes abolished both tri- and dimethylation, but not monomethylation of H3K4. These epigenetic changes were associated with ovulation failure, lack of transcriptional silencing, increased expression of repetitive elements, and infertility. This phenotype is remarkably similar to our dietary model of zinc deficiency where there is ovulation failure (Tian and Diaz, 2012), a decrease in H3K4 trimethylation and an increase in expression of repetitive elements (present study). MLL2 is a zinc binding protein (Bach et al., 2008) and could be directly affected by a lack of zinc in the oocyte. However, this hypothesis remains to be tested directly.
Methylation of DNA on cytosine residues is another epigenetic modification that is important for oocyte programming and was decreased by dietary zinc deficiency. DNA methylation is low in growing oocytes, but increases steadily as the oocyte nears ovulation (Kageyama et al., 2007; Kim et al., 2003). High levels of DNA methylation are established at imprinting control regions of imprinted loci (Bartolomei and Tilghman, 1997). DNA methylation is also essential for suppression of repetitive elements in the oocyte (Lees-Murdock et al., 2003; Rollins et al., 2006). Failure to silence repetitive sequences can cause aberrant expression of nearby genes (Dolinoy et al., 2007) and/or genome instability (Hedges and Deininger, 2007). For example, methylation of the intracisternal A-particle (Iap) prevents inappropriate expression of the nearby Agouti gene. However, IAP becomes active when hypomethylated and drives ectopic expression of the Agouti gene and associated increase in obesity and other metabolic defects in mice (Blewitt et al., 2006; Morgan et al., 1999). In the oocyte, repetitive elements often serve as alternative promoters for other genes (Evsikov et al., 2004; Mehlmann et al., 2004; Peaston et al., 2004; Peaston et al., 2007). Global DNA methylation was decreased significantly in zinc deficient oocytes and this likely contributed to the failure to repress transcription of repetitive elements. On the other hand, the lack of dramatic effects on cellular transcripts (Figure 4A) suggests that a global transcriptional de-repression did not occur in zinc deficient oocytes. Further studies using transcriptional run-on assay, a direct measure of transcriptional activity, are needed to determine the effects of zinc deficiency on global transcription in the oocyte. Nevertheless, aberrant gene expression caused by increased activity of repetitive elements together with decreased DNA and histone methylation could be a major cause of developmental failure of zinc deficient oocytes.
An open question is how does dietary zinc deficiency cause methylation defects in the oocyte. Methylation of DNA and histone proteins requires the biosynthesis of the universal methyl donor, s-adenosylmethionine or (SAM) (Loenen, 2006). During methylation reactions, SAM is converted to S-adenosyl homocysteine (SAH), which is reversibly converted to homocysteine and then to methionine. Methionine then serves as the substrate to regenerate SAM. The recycling of homocysteine to SAM is under control of two zinc-dependent enzymes, betaine-homocysteine methyltransferase (BHMT) and 5-methyl tetrahydrofolate homocysteine methyltransferase (MTR) (Koutmos et al., 2008; Loenen, 2006). BHMT and MTR catalyzed the transfer of methyl groups to homocysteine to re-generate methionine. This is the key step in the re-cycling of SAM from methionine. Both SAH and homocysteine are potent inhibitors of methylation reactions (De Cabo et al., 1995; de la Haba and Cantoni, 1959; Hoffman et al., 1980). Therefore, the efficient conversion of homocysteine to methionine is essential to prevent build-up of these inhibitory metabolites. Inhibition of BHMT decreases SAM and increases homocysteine concentration in the liver (Strakova et al., 2011), thus not only decreasing methyltransferase substrate (SAM), but also increasing a potent inhibitor of methyltransferase activity (SAH). Similarly, zinc deficiency decreases DNA and histone methylation in the liver (Breksa Iii and Garrow, 2002; Wallwork and Duerre, 1985), possibly by limiting SAM availability for methylation reactions and/or increasing inhibition by SAH/homocysteine. The full complement of enzymes involved in methionine and SAM biosynthesis are expressed in oocytes (Benkhalifa et al., 2008; Ikeda et al., 2010). We propose a working hypothesis that zinc deficiency inhibits production of SAM in the oocyte resulting in reduced methylation capacity and hence reduced DNA and histone methylation. Another possibility is that SAM produced in the liver, which is a major site for SAM biosynthesis, could supply SAM to the oocyte through the circulation. Thus, it is equally possible that zinc deficiency could inhibit hepatic SAM biosynthesis and thus indirectly affect SAM concentration in the oocyte. Further work is needed to distinguish between these two possibilities. Regardless of whether SAM in the oocyte is produced locally or originates from the liver, supplementation of oocytes from zinc deficient animals with SAM during maturation restored histone methylation and greatly improved fertilization success. Thus, SAM depletion appears to be one way that dietary zinc deficiency degrades oocyte quality. There is evidence in the literature that regulation of SAM/SAH metabolism has a significant effect on fertility. High homocysteine concentrations in follicular fluid are associated with decreased oocyte developmental potential in women undergoing assisted reproduction (Ebisch et al., 2006; Steegers-Theunissen et al., 1993) and in women with PCOS (Berker et al., 2009). Whether high follicular fluid SAH concentrations are associated with epigenetic alterations is not known, but certainly warrants further research.
Zinc deficiency is common in many parts of the world (Wuehler et al., 2005) and in minority and poor populations in the US (Schneider et al., 2007). Impaired liver function or liver damage caused by alcoholism also results in systemic zinc deficiency (Flynn et al., 1981; Sullivan and Lankford, 1965). Our findings show that acute dietary zinc deficiency before ovulation dramatically decreases oocyte quality and developmental potential and is associated with defects in epigenetic programming possibly caused by local or systemic SAM depletion. These observations and the previously shown effects of zinc deficiency on the meiotic cell cycle (Bernhardt et al., 2012b; Kim et al., 2011; Kim et al., 2010; Kong et al., 2012; Tian and Diaz, 2012) could lead to improved methods of assisted reproductive procedures by modulating zinc and/or SAM availability during oocyte maturation and fertilization. The present findings add further evidence that even transient dietary deficiencies can affect fertility. Future research will examine how zinc deficiency leads to reduces SAM biosynthesis and whether other metabolic pathways are affected. Finally, it will be very interesting to determine the impact of preconception zinc deficiency on postimplantation development and postnatal health.
Highlights.
Acute zinc deficiency before ovulation causes decreased chromatin methylation
Fertilization and preimplantation development is impaired in zinc deficient oocytes
Zinc deficiency increases expression of repetitive elements in oocytes
Methyl donor supplementation partially restores fertilization potential of zinc deficient oocytes
Acknowledgments
Funding: Research was supported by start-up funds from the College of Agricultural Sciences at The Pennsylvania State University and NIH HD057283 to FJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreu-Vieyra CV, et al. MLL2 Is Required in Oocytes for Bulk Histone 3 Lysine 4 Trimethylation and Transcriptional Silencing. PLoS Biol. 2010;8:e1000453. doi: 10.1371/journal.pbio.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apgar J. Zinc and Reproduction. Annual Review of Nutrition. 1985;5:43–68. doi: 10.1146/annurev.nu.05.070185.000355. [DOI] [PubMed] [Google Scholar]
- Arney KL, et al. Histone methylation defines epigenetic asymmetry in the mouse zygote. International Journal of Developmental Biology. 2002;46:317–320. [PubMed] [Google Scholar]
- Bach C, et al. Alterations of the CxxC domain preclude oncogenic activation of mixed-lineage leukemia 2. Oncogene. 2008;28:815–823. doi: 10.1038/onc.2008.443. [DOI] [PubMed] [Google Scholar]
- Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet. 1997;31:493–525. doi: 10.1146/annurev.genet.31.1.493. [DOI] [PubMed] [Google Scholar]
- Benkhalifa M, et al. Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertility and Sterility. 2008;93:1585–1590. doi: 10.1016/j.fertnstert.2009.02.081. [DOI] [PubMed] [Google Scholar]
- Berker Bl, et al. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Human Reproduction. 2009;24:2293–2302. doi: 10.1093/humrep/dep069. [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, et al. Zinc Requirement During Meiosis I–Meiosis II Transition in Mouse Oocytes Is Independent of the MOS-MAPK Pathway. Biology of Reproduction. 2011;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, et al. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod. 2012a;86:114. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML. A Zinc-Dependent Mechanism Regulates Meiotic Progression in Mammalian Oocytes. Biology of Reproduction. 2012b doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamberg DL, et al. Effect of zinc deficiency in hens on hatchability and embryonic development. Proc Soc Exp Biol Med. 1960;104:217–220. doi: 10.3181/00379727-104-25784. [DOI] [PubMed] [Google Scholar]
- Blewitt ME, et al. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006;2:e49. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouniol-Baly C, et al. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–587. doi: 10.1095/biolreprod60.3.580. [DOI] [PubMed] [Google Scholar]
- Breksa Iii AP, Garrow TA. Random Mutagenesis of the Zinc-Binding Motif of Betaine-Homocysteine Methyltransferase Reveals That Gly 214 Is Essential. Archives of Biochemistry and Biophysics. 2002;399:73–80. doi: 10.1006/abbi.2001.2751. [DOI] [PubMed] [Google Scholar]
- Burdge GC, et al. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–1046. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cooney CA, et al. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Corry GN, et al. Epigenetic regulatory mechanisms during preimplantation development. Birth Defects Research Part C: Embryo Today: Reviews. 2009;87:297–313. doi: 10.1002/bdrc.20165. [DOI] [PubMed] [Google Scholar]
- Davis TL, et al. Acquisition of the H19 methylation imprint occurs differentially on the parental alleles during spermatogenesis. Genomics. 1999;58:18–28. doi: 10.1006/geno.1999.5813. [DOI] [PubMed] [Google Scholar]
- De Cabo SF, et al. Molecular and cytological evidence of S-adenosyl-L-homocysteine as an innocuous undermethylating agent in vivo. Cytogenet Cell Genet. 1995;71:187–192. doi: 10.1159/000134104. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, et al. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol. 2004;275:447–458. doi: 10.1016/j.ydbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- de la Haba G, Cantoni GL. The Enzymatic Synthesis of S-Adenosyl-l-homocysteine from Adenosine and Homocysteine. Journal of Biological Chemistry. 1959;234:603–608. [PubMed] [Google Scholar]
- Debey P, et al. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36:59–74. doi: 10.1002/mrd.1080360110. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, et al. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, et al. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res. 2007;61:30R–37R. doi: 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]
- Ebisch IMW, et al. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Human Reproduction. 2006;21:1725–1733. doi: 10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- Evsikov AV, et al. Systems biology of the 2-cell mouse embryo. Cytogenet Genome Res. 2004;105:240–250. doi: 10.1159/000078195. [DOI] [PubMed] [Google Scholar]
- Falchuk KH, Montorzi M. Zinc physiology and biochemistry in oocytes and embryos. Biometals. 2001;14:385–395. doi: 10.1023/a:1012994427351. [DOI] [PubMed] [Google Scholar]
- Flynn A, et al. ZINC STATUS OF PREGNANT ALCOHOLIC WOMEN: A DETERMINANT OF FETAL OUTCOME. The Lancet. 1981;317:572–575. doi: 10.1016/s0140-6736(81)92029-8. [DOI] [PubMed] [Google Scholar]
- Hales BF, et al. Epigenetic programming: From gametes to blastocyst. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011;91:652–665. doi: 10.1002/bdra.20781. [DOI] [PubMed] [Google Scholar]
- Hedges DJ, Deininger PL. Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2007;616:46–59. doi: 10.1016/j.mrfmmm.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, et al. S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of L-methionine, L-homocystein, and adenosine. Journal of Biological Chemistry. 1980;255:10822–10827. [PubMed] [Google Scholar]
- Howell CY, et al. Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell. 2001;104:829–838. doi: 10.1016/s0092-8674(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Hurley LS, et al. Teratogenic effects of short-term and transitory zinc deficiency in rats. Teratology. 1971;4:199–204. [Google Scholar]
- Hurley LS, Shrader RE. Abnormal development of preimplantation rat eggs after three days of maternal dietary zinc deficiency. Nature. 1975;254:427–429. doi: 10.1038/254427a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S, et al. Expression of methylation pathway enzymes in bovine oocytes and preimplantation embryos. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 2010;313A:129–136. doi: 10.1002/jez.581. [DOI] [PubMed] [Google Scholar]
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann N Y Acad Sci. 1993;678:178–192. doi: 10.1111/j.1749-6632.1993.tb26121.x. [DOI] [PubMed] [Google Scholar]
- Kageyama S, et al. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85–94. doi: 10.1530/REP-06-0025. [DOI] [PubMed] [Google Scholar]
- Kaneda M, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- Keen CL, et al. The Plausibility of Micronutrient Deficiencies Being a Significant Contributing Factor to the Occurrence of Pregnancy Complications. J. Nutr. 2003;133:1597S–1605S. doi: 10.1093/jn/133.5.1597S. [DOI] [PubMed] [Google Scholar]
- Kim AM, et al. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chemical Biology. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, et al. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, et al. Changes in histone acetylation during mouse oocyte meiosis. Journal of Cell Biology. 2003;162:37–46. doi: 10.1083/jcb.200303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, et al. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem. 2009;20:917–926. doi: 10.1016/j.jnutbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong BY, et al. Zinc Maintains Prophase I Arrest in Mouse Oocytes Through Regulation of the MOS-MAPK Pathway. Biology of Reproduction. 2012 doi: 10.1095/biolreprod.112.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutmos M, et al. Metal active site elasticity linked to activation of homocysteine in methionine synthases. Proceedings of the National Academy of Sciences. 2008;105:3286–3291. doi: 10.1073/pnas.0709960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Murdock DJ, et al. Methylation dynamics of repetitive DNA elements in the mouse germ cell lineage. Genomics. 2003;82:230–237. doi: 10.1016/s0888-7543(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Lepikhov K, Walter J. Differential dynamics of histone H3 methylation at positions K4 and K9 in the mouse zygote. BMC Developmental Biology. 2004;4:12. doi: 10.1186/1471-213X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Regulation of histone H3 lysine 9 methylation in oocytes and early pre-implantation embryos. Development. 2004;131:2269–2280. doi: 10.1242/dev.01116. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loenen WA. S-adenosylmethionine: jack of all trades and master of everything? Biochem Soc Trans. 2006;34:330–333. doi: 10.1042/BST20060330. [DOI] [PubMed] [Google Scholar]
- Lucifero D, et al. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet. 2004;13:839–849. doi: 10.1093/hmg/ddh104. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- Madgwick S, et al. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. The Journal of Cell Biology. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25:374–383. doi: 10.1002/mrd.1080250411. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Miyara F, et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Molecular Reproduction & Development. 2003;64:458–470. doi: 10.1002/mrd.10233. [DOI] [PubMed] [Google Scholar]
- Morgan HD, et al. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, et al. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Peaston AE, et al. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev Cell. 2004;7:597–606. doi: 10.1016/j.devcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Peaston AE, et al. Genome plasticity in the mouse oocyte and early embryo. Biochem Soc Trans. 2007;35:618–622. doi: 10.1042/BST0350618. [DOI] [PubMed] [Google Scholar]
- Peters JM, et al. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc Soc Exp Biol Med. 1991;198:561–568. doi: 10.3181/00379727-198-43289. [DOI] [PubMed] [Google Scholar]
- Rollins RA, et al. Large-scale structure of genomic methylation patterns. Genome Res. 2006;16:157–163. doi: 10.1101/gr.4362006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, et al. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes & Development. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JM, et al. The Prevalence of Low Serum Zinc and Copper Levels and Dietary Habits Associated with Serum Zinc and Copper in 12- to 36-Month-Old Children from Low-Income Families at Risk for Iron Deficiency. Journal of the American Dietetic Association. 2007;107:1924–1929. doi: 10.1016/j.jada.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Wassarman PM. Specific changes in the pattern of protein synthesis during meiotic maturation of mammalian oocytes in vitro. Proc Nat Acad Sci USA. 1977;74:538–541. doi: 10.1073/pnas.74.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Sachdev HP. Effect of gestational zinc deficiency on pregnancy outcomes: summary of observation studies and zinc supplementation trials. Br J Nutr. 2001;2(85 Suppl):S101–S108. doi: 10.1079/bjn2000301. [DOI] [PubMed] [Google Scholar]
- Shoji S, et al. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. Embo J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, et al. Study on the presence of homocysteine in ovarian follicular fluid. Fertil Steril. 1993;60:1006–1010. doi: 10.1016/s0015-0282(16)56401-2. [DOI] [PubMed] [Google Scholar]
- Sternlicht AL, Schultz RM. Biochemical studies of mammalian oogenesis: kinetics of accumulation of total and poly(A)-containing RNA during growth of the mouse oocyte. Journal of Experimental Zoology. 1981;215:191–200. doi: 10.1002/jez.1402150209. [DOI] [PubMed] [Google Scholar]
- Strakova J, et al. Inhibition of betaine-homocysteine S-ethyltransferase in rats causes hyperhomocysteinemia and reduces liver cystathionine Î2-synthase activity and methylation capacity. Nutrition Research. 2011;31:563–571. doi: 10.1016/j.nutres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-Q, et al. MARF1 Regulates Essential Oogenic Processes in Mice. Science. 2012;335:1496–1499. doi: 10.1126/science.1214680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JF, Lankford HG. Zinc Metabolism and Chronic Alcoholism. The American Journal of Clinical Nutrition. 1965;17:57–63. doi: 10.1093/ajcn/17.2.57. [DOI] [PubMed] [Google Scholar]
- Suzuki T, et al. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010a;137:3281–3291. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, et al. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development. 2010b;137:2659–2669. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc Depletion Causes Multiple Defects in Ovarian Function during the Periovulatory Period in Mice. Endocrinology. 2012;153:873–886. doi: 10.1210/en.2011-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, et al. Localization of phosphorylated SMAD proteins in granulosa cells, oocytes and oviduct of female mice. Gene Expression Patterns. 2010;10:105–112. doi: 10.1016/j.gep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, et al. A 5' 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol Cell Biol. 1997;17:4322–4329. doi: 10.1128/mcb.17.8.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu-Adams JY, Keen CL. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2010;89:313–325. doi: 10.1002/bdrb.20264. [DOI] [PubMed] [Google Scholar]
- Wallwork JC, Duerre JA. Effect of Zinc Deficiency on Methionine Metabolism, Methylation Reactions and Protein Synthesis in Isolated Perfused Rat Liver. The Journal of Nutrition. 1985;115:252–262. doi: 10.1093/jn/115.2.252. [DOI] [PubMed] [Google Scholar]
- Walsh CP, et al. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat Genet. 1998;20:116–117. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, et al. Protein synthesis during meiotic maturation of mouse oocytes in vitro. Synthesis and phosphorylation of a protein localized in the germinal vesicle. Dev Biol. 1979;69:94–107. doi: 10.1016/0012-1606(79)90277-x. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, et al. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Wuehler S, et al. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutrition. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, et al. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–485. doi: 10.1002/mrd.1080410410. [DOI] [PubMed] [Google Scholar]