Abstract
The early detection of many human diseases is crucial if they are to be treated successfully. Therefore, the development of imaging techniques that can facilitate early detection of disease is of high importance. Changes in the levels of enzyme expression are known to occur in many diseases, making their accurate detection at low concentrations an area of considerable active research. Activatable fluorescent probes show immense promise in this area. If properly designed they should exhibit no signal until they interact with their target enzyme, reducing the level of background fluorescence and potentially endowing them with greater sensitivity. The mechanisms of fluorescence changes in activatable probes vary. This review aims to survey the field of activatable probes, focusing on their mechanisms of action as well as illustrating some of the in vitro and in vivo settings in which they have been employed.
Keywords: Enzyme, Probe, Activatable, Optical, Fluorescent
INTRODUCTION
Enzymes are known to play a fundamental role in the pathology of several major human diseases, making the development of techniques to accurately detect specific enzymes crucial as they can be used to study their role in disease progression. This information can then be used not only to further our understanding of the disease but also to develop diagnostic tests that allow for more accurate diagnoses, potentially at an earlier stage of the disease. The motivation is especially strong for cancer, where the level of enzyme expression can be indicative of the tumor’s aggressiveness and susceptibility to a certain treatment. Furthermore sensitive enzymatic detection could play a major role in facilitating earlier detection of cancers, in turn positively impacting on their prognosis.
Optical fluorescence imaging has been widely used to study biological processes in vitro and in vivo. It is highly sensitive, does not require the use of radioactive materials and, after the recent development of fluorescence molecular tomography (FMT), it can now be used to gain quantitative information in an in vivo setting. This technique requires a fluorescent probe capable of accurately reporting the location of its target enzyme. The precise fluorescent properties of many fluorophores are dependent on their molecular structure and their microenvironment (e.g. their proximity to other fluorophores). This allows for the design of probes which exhibit significant changes in their spectroscopic properties upon interaction with a certain enzymes. If this change results in an enhancement of emission at a certain wavelength, then the probe can be described as ‘activatable’. This endows these compounds with several advantages, the greatest of which is a significant reduction in background signal which is hugely beneficial in both in vitro and in vivo settings. For example, when using probes that are always ‘on’ in vivo, it is often necessary to wait for several hours for probes to be excreted so that the background fluorescence is at a low enough level to give a high target-to-background signal ratio. This is not the case with activatable probes, typically allowing them to be interrogated more rapidly after systemic administration. In many situations it is also desirable that the probe reports on the functionality of the target enzyme to prevent false positive results due to non-active enzymatic precursors. This can be facilitated by the use of probes whose fluorescent signal is intricately linked to the catalytic function of their target. These advantageous characteristics have led to the development of a wide range of activatable probes and the continued efforts to improve upon their design has made this field an area of considerable active research.
This review will detail the mechanisms by which the fluorescence of these probes is activated and give a broad overview of their biomedical applications. The majority have been developed for use in vitro, however particular attention will be focused on those that are designed to function either in cellulo or in vivo, as it is in these settings that the most biologically relevant information can be attained. It should be noted that there are extra requirements of such probes compared to those that are strictly for in vitro use. Most importantly their emission wavelengths should be in the near-infrared (NIR) region, as this represents a window where autofluorescence from biological molecules is at its lowest [1]. This requirement is especially strict for whole animal (including human) imaging, where the scattering and absorption of photons by tissues and biological molecules (e.g. water and hemoglobin) must also be kept to a minimum [1,2]. The probe should also be able to access enzymes in their native locations. Hence it must be able to cross biological membranes to access enzymes in cellulo and, if used in vivo, must exhibit a pharmacokinetic profile that allows it to reach the native site of enzymatic action in high enough concentrations to provide a detectable signal. Finally, the activated probe should ideally exhibit strong fluorescence under physiological conditions. Although many of the probes described in this review do not meet these criteria, their inclusion ensures that the full range of activation mechanisms is presented.
DUAL LABELED PROBES
One of the most common probe designs used to measure enzyme activity is outlined schematically in Fig (1). In the quenched state a complementary fluorophore and quencher pair is attached to either end of a cleavable enzyme substrate. Upon enzymatic cleavage of the substrate, the fluorophore and quencher are separated and emission from the reporter is restored, producing a signal that is directly correlated to the activity of the enzyme.
Fig. 1.

Mechanism of fluorescence enhancement for dual-labeled activatable probes.
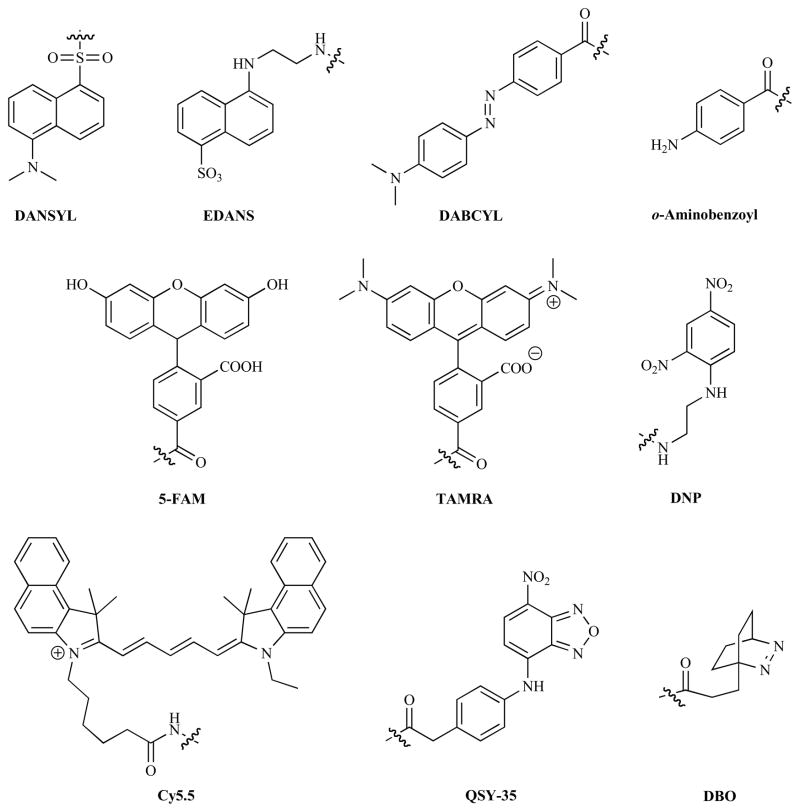
The first examples of such probes were reported in the early 1970s, with many of them taking advantage of the inherent fluorescent properties of the amino acid tryptophan. In one of the earliest examples, Latt et al. attached a 5-(dimethylamino)napthalene-1-sulfonyl (DANSYL) group to the N-terminus of a Gly-Trp dipeptide. The quenched fluorescent signal from the amino acid could be recovered upon hydrolysis with carboxypeptidase A, resulting in a 100-fold increase in fluorescence [3]. Shortly thereafter the first probe synthesized with a non-natural donor and acceptor, napthylene and anthrocene respectively, was reported [4]. Once again cleavage of the dipeptide, in this case by trypsin, resulted in the recovery of the fluorescence emission from the donor. Others used tryptophan or tyrosine as quenchers for introduced organic fluorophores such as 9,10-dioxa-syn-3,4,6,7-tetramethylbimane [5,6]. A major limitation to of these designs is the requirement that a bulky organic fluorophore to be conjugated to the P1 or P1′ residue, limiting the range of enzymes which could be analyzed. However the introduction by Kraft and co-workers of 5-(2′-aminoethyl)aminonapthalene sulfonic acid (EDANS) and 4-(4′-dimethylaminobenzeneazo)benzoyl (DABCYL), a fluorophore/quencher pair where effective quenching was mediated by Förster resonance energy transfer (FRET), alleviated this problem. They constructed a probe to target HIV-1 associated protease where an octapeptide was flanked by these two fluorophores [7]. Upon cleavage by the enzyme an increase in EDANS emission of 40-fold was observed, proving the efficacy of the probe. The choice of this pair of fluorophores was determined after several candidates had been screened, with their quenching efficacy ascribed to the excellent spectral overlap between the EDANS emission and DABCYL absorption along with the long fluorescent lifetime of EDANS (13 ns) and the high extinction coefficient of DABCYL [8].
Other FRET pairs which have also been commonly incorporated into this type of probe include o-aminobenzoyl and N′-2,4-dinitrophenyl ethylenediamine (DNP) [13,25,50,95] or 3-nitrotyrosine [12,29,65,71,96–98]; benzoxazole derivatives and 3-nitrotyrosine [99]; napthylmethylidene and EDANS [73]; indolylethyl and EDANS [72]; various coumarin derivatives in conjunction with p-nitroanilide [100], DNP [19,60,61,101], DABCYL and 5-carboxyfluorescein (5-FAM) [77] or N-({4-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]phenyl}acetyl (QSY-35) [52]; 5-FAM and DABCYL [62,65]; pyrene or perylene and DNP [83,84]; tetramethylrhodamine (TAMRA) and black-hole quencher-2 (BHQ-2) [93]; and Cy3 and Cy5 [46]. These fluorophores have been subsequently used to probe the kinetics, substrate specificity and also to screen potential inhibitors of a wide range of enzymes (Table 1).
Table 1.
Selection of Enzymes for which FRET-based Probes have been Reported
| Enzyme Class | Specific Enzyme |
|---|---|
| Aspartate proteases | Renin [9–12], Pepsin [9,13,14], Gastricsin [13,14], Cathepsin D [9,13,15,16], Cathepsin E [16], Plasmepsins [17,18], β-Secretase [19] |
| Serine proteases | Cathepsin G [20–22], Trypsin [23,24], Kallikreins [24–28], Human Cytomegalovirus viral protease [29,30], Hepatitis C viral protease [31], Subtilisins [32,33], Furin [34–36], Proprotein convertase 4 [37], Subtilisin kexin isozyme 1 [38], Elastases [39–41], Signal peptidases [42,43], Dengue virus type II protease [44], Proteinase 3 [45] |
| Metalloproteases | Matrix metalloproteinases [46–49], Tumor necrosis factor-α convertase [50], Thermolysin [51], Dispase [51], Bacillus anthracis lethal factor protease [52], Lysostaphin [53], N-Arginine dibasic convertase [54], Enkephalinase [55], Angiotensin-converting enzyme I [10,56], Aminopeptidase P [57,58], Staphylolysin [39], Neurolysin [59], Pz peptidase [59–61], Neprilysin [59] |
| Cysteine proteases | Papain [24,62–64], Calpain [65], SARS main protease [66,67], Foot-and-mouth viral protease [68], Cathepsin B [24], Cathepsin L [24], Cruzipain [69] |
| Glucoaminidase [70] | |
| Glycosidase | α-Amylase [71,72], Ceramide glycanase [73], Chitinases [74], Chitobiosidases [74], Cellulases [75] |
| Transferases | Glycosyltransferase [76] |
| Phosphodiesterase [77–79] Phospholipase [80–82] | |
| Lipase/Esterase [83–86] | |
| Nuclease [87–91] | |
| Integrase [92] | |
| DNA Polymerase [93,94] |
Despite their success these probes still suffer from limitations. The fluorophores and quenchers are usually conjugated to a peptidic cleavable linker after its solid-phase synthesis. Synthetically this can be challenging as the reactions must be both precise and high-yielding to prevent a high level of background fluorescence from the probe. In addition the organic fluorophores used may not be stable under the acidic conditions needed to cleave peptides from a solid-support, further complicating the production of high purity probes. Fluorescent amino acid derivatives, which can be used directly during solid-phase synthesis, have been developed to facilitate the high yield synthesis of peptide-based probes as they obviate the need for complex conjugations [62,102,103]. These fluorescent amino acids have also been incorporated into fusion proteins, allowing for the convenient production of probes in E. coli [104]. The large hydrophobic nature of the fluorophores can alter the specificity of substrates to enzymes, for example attachment of a DANSYL group at the P3 position can synergistically enhance the rate at which a probe is cleaved by carboxypeptidase A [105]. Hence the use of natural amino acids, especially tyrosine, remains an attractive option if the substrate specificity of the enzyme in question permits it. For example para-nitrophenyl alanine has been reported to be an effective quencher a non-radiative process mediated through a dipole-dipole interaction for tyrosine, especially when it is placed adjacent to it, with increases in tyrosine emission upon cleavage of up to 1000-fold reported [106,107].
Self-quenched probes that target enzymes which do not catalyze a cleavage reaction are self-evidently rare, however an example, outlined in Fig. (3), designed to detect protein kinase A (PKA) has been reported by Law et al. [108]. An amino acid sequence specific for the kinase’s active site was flanked with 5-FAM and TAMRA, with the former linked to the amino acids via a labile disulfide linkage. When dissolved in an aqueous solution the two fluorophores were quenched via static association, but it exhibits a 49-fold increase in emission from 5-FAM upon treatment with a strong reducing agent (dithiothreatol). When added to PKA this labile disulfide linkage is placed within its active site so that it is vulnerable to nucleophilic attack by a free cysteine residue, liberating the 5-FAM and allowing for the recovery of its fluorescence. SDS-PAGE analysis of PKA that had been treated with the probe showed that it had been labeled by TAMRA, validating this proposed mechanism of action. It should be noted that the activation of this probe is no longer directly linked to the enzyme’s function, hence it may also detect non-active forms of the enzyme.
Fig. 3.
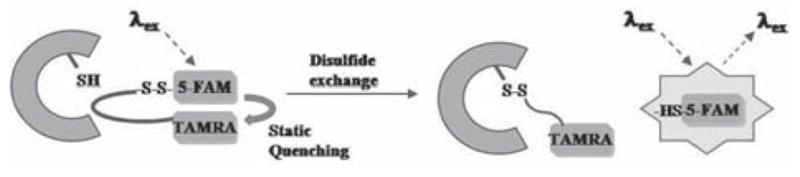
Proposed mechanism of fluorescence enhancement for protein kinase A probe [108].
The mechanism by which quenching is achieved will impact on the performance of a probe, hence a brief description of the main examples is instructive. It should be noted that more than one mechanism can be operated simultaneously. FRET, the mechanism which is most commonly described as being responsible for quenching, that is dependent on the spectral overlap between the donor emission and acceptor absorption, the distance between them and their relative orientation [109]. The efficiency of energy transfer is inversely proportional to (R0)6, where R0 is the distance at which this process is 50% efficient (known as the Förster distance). This strong distance dependence is advantageous for the probes described in this section as it ensures a rapid recovery of fluorescence upon enzymatic cleavage. Static quenching involves the association of fluorophores to form either H- or J-type aggregates (depending on the relative orientation of their dipoles) which can be non-fluorescent. Advantageously, no emission is observed from any of the fluorophores, which obviates the need for narrow bandpath filters to prevent emission from the acceptor being measured if this is the dominant quenching mechanism. In addition static quenching can occur between identical fluorophores, simplifying the synthesis of the probes. Successful protease probes incorporating static quenching from pyrene [110] and xanthene [111] derivatives have been described. However the nature of the been described. However the nature of the interaction requires the substrate sequence of the probe to adopt a conformation that permits the close-association of the dyes, somewhat limiting the scope of this type of probe. Photoinduced electron transfer (PeT) is widely used within the field of small molecule sensors to quench fluorescence, however to date it has not been widely applied to enzymatic sensors. In an isolated example Marmé et al. utilized tryptophan to accept an electron from the excited state of an oxazine fluorophore to construct probes for trypsin [112] and carboxypeptidase A [113]. Hennig and co-workers have used a collision-induced mechanism to quench the emission from a 2,3-diazabicyclo[2.2.2]oct-2-ene (DBO) fluorophore with tyrosine [105,114]. This moiety exhibits an extremely long fluorescence lifetime of around 300 ns when incorporated into a peptidic probe, a characteristic which facilitates collision induced quenching. If time-resolved fluorescence detection is used in conjunction with a long-lived fluorophore like DBO, then up to 5-fold increases in signal to background ratios have been measured (although in theory improvements of several orders of magnitude could be achieved) [105,114]. In addition DBO is small and hydrophilic when compared to many other fluorophores, making it less likely to interfere with the action of its target enzyme. However despite the long lifetime of DBO such a design is clearly not well-suited to a rigid substrate.
The probes described up to this point have been used for the detection of their target enzymes outside of their native environment, in other words neither in cellulo or in vivo. For a probe to be successfully deployed in these scenarios it must be both membrane permeable and exhibit a fluorescent signal that is distinct from the biological background. For example any signal from a probe that relies upon the emission from a tryptophan residue is likely to be masked by the large background signal from native proteins. To illustrate this point George et al. compared a (7-methoxycoumarin-4-yl)acetyl/DNP FRET pair to a longer wavelength Cy3/Cy5 pair in a high throughput assay for biological samples and found that the Cy3/Cy5 system was able to give a 3-fold improvement in fluorescence recovery [46]. This meant that only 0.5% of the library was unanalyzable due to autofluorescence compared to 11.9% for the other pair.
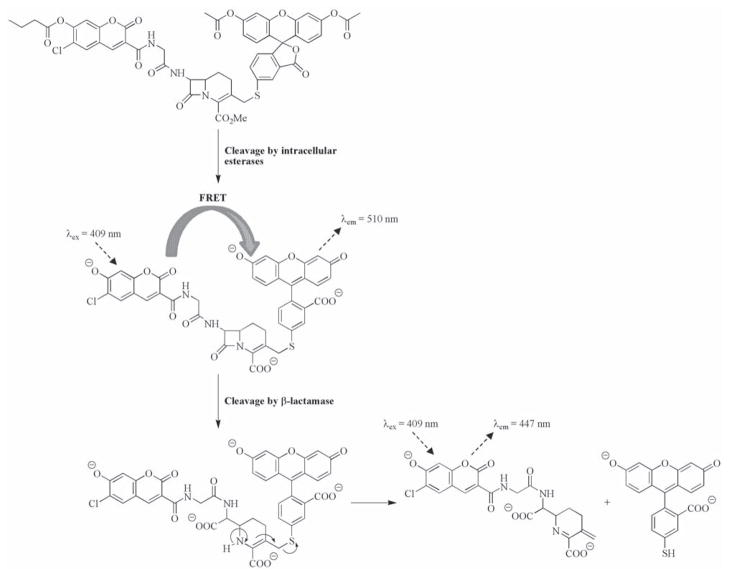
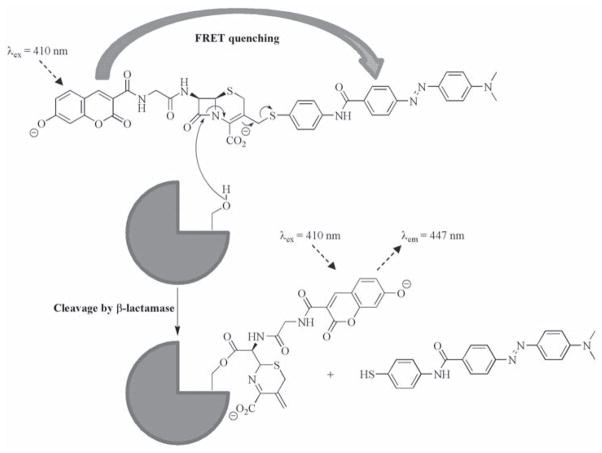
Some of the first dual-labeled probes specifically designed for use in live cells targeted β-lactamase (Bla), an enzyme commonly used as a reporter gene for transfection assays. The first example was reported by Zlokarniak et al., who linked a 7-hydroxycoumarin-based donor to a fluorescein acceptor via a cephalosporin moiety [115]. Cleavage of the β-lactam functionality reveals an amine which subsequently triggers the ejection of the fluorescein group (see Fig. (4)) causing the loss of FRET and hence the ratio of emission from the 7-hydroxycoumarin compared to the fluorescein is increased 70-fold. Importantly the probe could be delivered to cells as a neutral membrane permeable analogue, and then converted into the active probe by intra-cellular esterases. When applied to Jurkat cells which had previously been transfected with differing levels of Bla, the rate at which the cells acquired the blue color characteristic of probe activation was directly related to the expression of the enzyme, proving its efficacy in cellulo. The same basic structural motifs were conserved in a probe developed by Xing et al., however they used it to tether Cy5 to the dark quencher QSY-21 to produce a compound with an increase in fluorescence of 57-fold upon exposure to Bla, allowing detection of the enzyme at concentrations of 190 fM [116]. A fully acetylated D-glucosamine derivative was applied to C6 gliomal cells stably transfected with Bla resulting in a 10-fold increase in fluorescence compared to the wild-type cells. Recently Mizukami et al. reported a ‘suicide’ FRET based Bla probe in which interaction with the enzyme’s active site caused both the release of the DABCYL quencher and the formation of a stable covalent linkage between a serine residue in the active site and the coumarin reporter (see Fig. (5)) [117]. Whilst useful for direct protein labeling, such an approach prohibits any signal amplification due to the cleavage of several probes by the same enzyme, limiting the sensitivity of the probe.
Fig. 4.
β-Lactamase probe designed by Zlokarniak et al. for use in cellulo [115].
Fig. 5.
A ‘suicide’ FRET probe for β-lactamase [117].
A number of papers have reported the development of FRET based probes that target various members of the phospholipase A2 (PLA2) family of enzymes. An early example in this area utilized DNP to quench a boron-dipyrromethane (BODIPY) probe and proved able to detect cytosolic PLA2 in zebrafish embryos and larvae [118,119]. Feng et al. used a BODIPY/DABCYL FRET pair for their phospholipase probe, which was able to distinguish MDCK-D1 cells where the activity of cytosolic PLA2 had been induced from untreated ones [120]. Schultz and co-workers used 7-nitrobenzo-2-oxa-1,3-diazole amine and the 2-hydroxy derivative of nile red in a series of probes which they tested against several phospholipases [121,122]. They also employed S-acetyl-2-thioethyl groups to mask the charged phosphate functionalities and ensure facile cell entry. Their optimal probe proved active in vitro, where it could detect the inhibition of PLA2 activity in Medaka fish embryos by either arachidonyl fluorophosphate or bromoenollactone [121]. Another reporter enzyme that is widely used is β-galactoside and FRET based probes have also been developed to detect it. Komatsu et al. used the β-galactoside catalyzed cleavage of a β-galactopyranosyl-protected phenol to trigger the loss of fluorescein via a 1,6-quinonemethode shift, revealing the fluorescence from a 7-hydroxycoumarin moiety [123]. The probe functioned well in LacZ-positive cells, however it had to be introduced via microinjection, a significant drawback. In addition the probe forms a stable covalent bond with the active site of the enzyme ruling out any potential for signal amplification.
In order to use these probes in vivo they must incorporate NIR fluorophores, which in turn relies upon finding molecules able to quench them efficiently. Tung and co-workers developed a bisazulenyl derived dark quencher and used it in conjunction with AlexaFluor-680 in a caspase-3 sensitive probe whose increase in fluorescence was around twice that of its DABCYL quenched analogue [124]. The same group later reported a cycloheptapolymethine molecule, termed NIRQ820, as a novel quencher for Cy5.5. This FRET pair was used to flank a MMP-7 sensitive peptide sequence to probe for the overexpression of this enzyme in a human fibrosarcoma tumor model [125]. A 4-fold increase in Cy5.5 emission was measured however a negative control containing a scrambled amino acid sequence gave a 2-fold increase, demonstrating the potential for significant background signals due to non-specific cleavage of this type of probe. A similar polymethine quencher was reported by Peng et al., which proved able to quench over 97.5% of the emission from several NIR fluorophores [126]. Their optimal probe, which was aimed at caspase-3, showed an 8-fold increase in fluorescence in Jurkat cells treated with the pro-apoptotics anisomycin or camptothecin compared to untreated cells.
Other groups have used unnatural substrate groups to improve the specificity of their FRET probes, aiming to prevent the non-specific hydrolysis that had been observed previously by Tung’s group. Bogyo and co-workers based their design on peptide acyloxymethylketones, a motif which had previously been employed for non-quenched probes during proteomic studies of cathepsin B and cathepsin L. Cy5.5 and QSY21 were conjugated either side of the cleavable peptide linkage to give the activatable probe, which was tested in mice bearing MDA-MB-231 tumors [127]. When compared to a non-quenched activity probe it gave a significantly lower background signal and was able to achieve its optimal signal to background ratio more rapidly. The successful covalent labeling of the cathepsins was demonstrated ex vivo by SDS-PAGE, however, as with Komatsu et al.’s β-galactoside ‘suicide’ probe, the sensitivity of this probe is limited as it irreversibly binds to the enzyme’s active site. Watzke et al. chemically modified known inhibitors of specific cathepsins by replacing their electrophilic attachment points with a labile peptide bond to give a quenched probe which does not form a permeable covalent attachment to the enzyme (see Fig. (6)) [128]. Their membrane permeable analogue, functionalized with a BODIPY TMR-X/QSY-7 FRET pair, selectively targeted cathepsins S and L over cathepsins B and K in vitro and was able to stain HaCaT cells in a pattern that was consistent with the lysosomal expression of the target enzymes within these cells.
Fig. 6.
Cathepsin specific FRET probe based on the chemical structure of a known inhibitors of this enzyme [128].
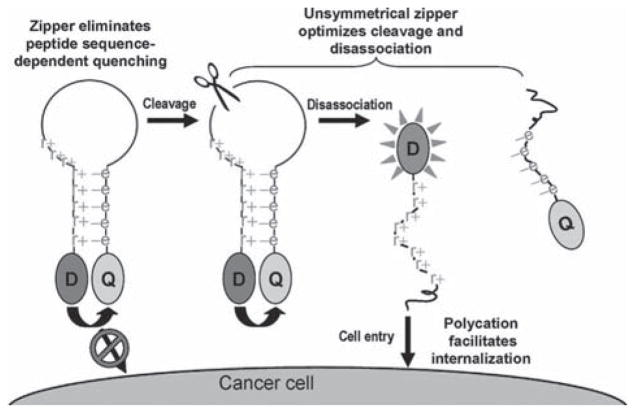
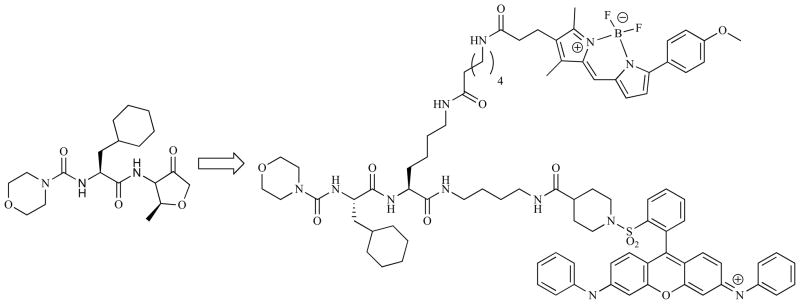
Cell penetrating peptide sequences have been used to improve the cellular uptake of self-quenched probes. For example Bullock et al. tagged a Tat-peptide sequence onto the N-terminus of their peptide based probe which also contained an AlexaFluor-647/QSY-21 FRET pair. Selective activation by either caspase-3 or -7 resulted in fluorescence recovery at a more rapid rate than a non-targeted caspase (170- and 3.5-fold respectively), and, when incubated with KB3-1 tumor cells in which apoptosis had been induced with vinblastine activation of the fluorescence was only observed in the absence of the caspase inhibitor C6-ceramide. Chen et al. recently published a probe in which the cell-penetrating peptide, as well as the NIR fluorophore, was activatable in what was termed a ‘zipper’ probe [129]. Their ingenious design is outlined in Fig. (7). Advantageously, electrostatic interactions between the cationic D-arginine and anionic D-glutamic acid sequences holds the FRET pair in close proximity, allowing for highly efficient quenching. Upon cleavage of the substrate sequence the probe dissociates as the electrostatic interactions are overcome, releasing the now activated fluorophore attached to the cell penetrating peptide sequence which facilitates its cell entry. Such a design relies upon careful optimization of the length of the charged amino acid sequences to ensure that the probe adopts the correct configuration initially and then dissociates promptly when cleaved. An amino acid substrate sequence for MMP-7 was incorporated into this design and was activated and internalized by MT1 breast carcinoma cells.
Fig. 7.
Schematic of ‘zipper’ molecular beacon designed by Chen et al. Reprinted with permission from [129]. Copyright 2009 American Chemical Society.
The vast majority of FRET based probes rely on interrupting this energy transfer upon interaction with an enzyme, however this is not the case with all such probes. Takakusa et al. have devised a probe where the removal of 3′- and 6′-O-phosphate groups from a fluorescein acceptor converts it to the quinoid form, which is then able to fluoresce when the molecule is excited at the λabs maximum for coumarin due to FRET [130]. A membrane permeable derivative of this probe was able to distinguish between human umbilical vein endothelial cells treated with an inhibitor of protein tyrosine phosphatase and those that had not by measuring the ratio of emission from fluorescein and coumarin fluorophores.
Quantum dots have found widespread use within biological imaging due to their favorable optical properties which include tunable emission spectra, a continuous absorption band, high extinction coefficients and quantum yields, long fluorescence lifetimes and excellent photostability [131,132]. Their use in activatable enzymatic probes has been facilitated by the conjugation of various quenchers to their surface via cleavable linkers. Typically organic fluorophores with suitable absorption spectra have been used in FRET-based systems to detect enzymes such as collagenase [133,134], Bla [135], caspase-1 [134], thrombin [134], trypsin [134,136] and nucleases [137]. Other more unusual quenchers including gold nanoparticles [138], mCherry fluorescent protein [139], a quinone moiety which quenches via a PeT mechanism [140] and metal complexes [141] have also been used in similar probe designs. To date the in vitro use of quantum dot-based probes remains scarce, possible due to concerns over their toxicity, although Shi et al. were able to use their collagenase probe to distinguish breast cancer cells from benign ones using their FRET-based quantum dot probe [133]. To the best of our knowledge no activatable probe which incorporates a quantum dot has yet been tested in vivo.
MACROMOLECULAR DUAL- OR MULTI-LABELED PROBES
Whilst many of the low molecular weight probes described in the previous section have proven to be effective in vitro, their progress into live animals is hampered by the rapid rate at which they are excreted by the kidneys, via filtration through the glomerulus, after systemic administration. However macromolecules which have molecular weights of over 30–50 kDa (the precise figure is also dependent on their chemical composition, architecture and flexibility) are too large to pass through these membranes in the kidney and hence they are able to persist in vivo, allowing them to reach their site of action via the circulatory system [142,143].
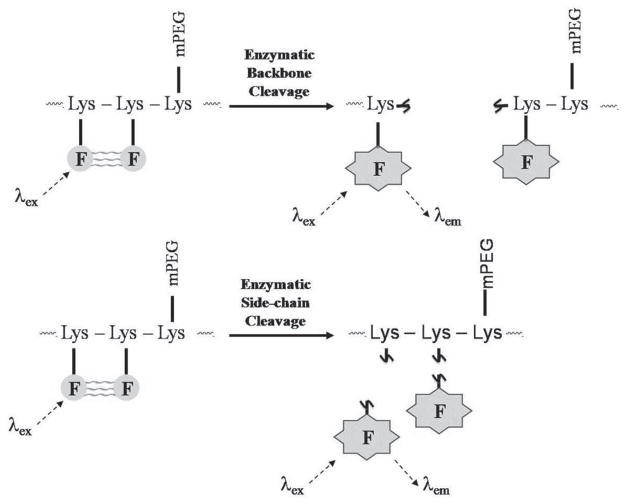
Early examples in this area used proteins such as casein and bovine serum albumin as the macromolecular skeleton, labeling them with several molecules of the same organic fluorophore (BODIPYs and fluoresceins were both used) that self-quenched due to their close proximity [144,145]. When the protein-dye conjugate was digested by a proteolytic enzyme, the fluorescence from the dyes was recovered. Similarly it has been found that a fluorescent derivative of adenosine, 1,N6-ethanoadenosine, self-quenches when polymerized allowing for the synthesis of ribonuclease probes [146]. The protein-based probes were extremely limited in that they were not targeted to any specific protease. This problem was addressed with a series of protease-specific probes developed by Weissleder and co-workers, schematic representations of which are shown in Fig. (8).
Fig. 8.
Mechanism of fluorescence enhancement of poly-L-lysine-based self-quenched macromolecular probes for protease detection in vivo.
Initially they chose a poly-L-lysine (PL) backbone (Mw 35.5 kDa) in which around 30% of the ε-amino groups were functionalized with methoxypoly(ethylene glycol) (mPEG). This crucial adaptation raises the circulation time of the resultant graft co-polymer by preventing opsonization by plasma proteins and subsequent uptake by the reticuloendothelial system, giving it a blood half-life of 20.6 ± 2.3 hours when tested in human patients [147]. Initially around 11 Cy5.5 fluorophores (an optimized number) were conjugated via a stable amide linkage to the remaining free ε-amino groups to form a probe capable of detecting cathepsins B, H and L as well as trypsin via degradation of the PL backbone [148]. The resulting construct exhibited 15-fold less fluorescence than the corresponding concentration of free Cy5.5 and 95% of fluorescence was recovered upon treatment with trypsin. In vitro a noticeable increase in fluorescence was observed after just 15 minutes incubation on LX-1 tumor cells which could be prevented if the cells were treated with inhibitors for cathepsins B, H and L, but not cathepsins D, demonstrating the specificity of the probe. The same tumor cells were then implanted into the mammary fat pads of mice and could be detected by fluorescence reflectance imaging 24 hours after the injection of the probe. Biodistribution studies with an 111In tagged derivative of the probe confirmed that a significant proportion remained in the blood, however the quenched nature of the probe ensured that no signal was detectable. Indeed the authors postulate that should the probe be degraded prematurely then the low molecular weight of the resulting fragments would ensure their removal by renal filtration, clearing any activated signal from the blood. The proteases which could be targeted with this first type of probe were limited to those able to degrade PL, a restriction that was addressed by the same group in a subsequent paper. The same graft polymer was used as the basis of the probe, however the Cy5.5 fluorophores were now linked to it not via a non-degradable covalent linkage but rather by an amino acid sequence that was a substrate for the target enzyme cathepsins D [149]. The recovery of fluorescence was now triggered not by the degradation of the PL backbone but rather by the cleavage of the spacers between it and the fluorophores, as outlined in the lower panel of Fig. (8). The synthetic methodology developed allowed the attachment of around 24 Cy5.5 molecules per graft co-polymer. Over 99% of their fluorescence was quenched in the construct, allowing for a 350-fold increase upon exposure to chymotrypsin, a superior figure compared to the earlier probe which the authors ascribe to the greater number of fluorophores per polymer. When incubated with 3Y1-AD12 embryonic tumor cells, which had been stably transfected to express cathepsins D, a high fluorescent signal was clearly observed in comparison to the negative probe with a scrambled peptide sequence. Mice bearing both cathepsins D positive and negative 3Y1 tumors were injected with the probe and reflectance imaging demonstrated a clear difference between the two tumors, with the cathepsins D positive one giving a NIR signal 6-fold higher than the negative [150]. The signal to noise ratio between the target tumor and the surrounding tissue was even higher, between 16.2- and 22.8-times, clearly demonstrating the efficacy of the probe. The flexibility of this probe design allows for the incorporation of an array of target amino acid sequences which in turn has led to the development of probes for a range of proteolytic enzymes including MMPs [151], thrombin [152], HIV-1 protease [153], urokinase plasminogen activator [154], caspase-1 [155] and cathepsin K [156]. A later example of a cathepsin K specific probe utilized a poly-D-lysine backbone to prevent its degradation by non-target enzymes, indeed it proved to be stable against trypsin induced degradation for at least 22 hours [157]. The efficacy of both probe designs, and their subsequent commercial availability, has led to their use in a range of in vivo settings including the detection of adenomatous polyps in APCMin/+ mice [158], distinguishing highly aggressive breast cancer cells from less aggressive ones [159], the detection of induced hematomas and thrombi [160], monitoring rheumatoid arthritis [161], the detection of peripheral lung tumors via thoracoscopy [162], assessment of myocardinum healing after an induced infarction [163], the detection of atherosclerosis [156], the visualization of eosinophil activity in response to allergic airway inflammation [164], the detection of early-stage pancreatic cancer [165], early detection of bone loss via visualization of osteoclasts [157], imaging of metastatic ovarian cancer [166], the detection of cardiac allograft rejection [167] and to study the role that MMPs play in cerebral ischemia [168].
One drawback of the graft co-polymer motif is that its precise molecular characteristics are difficult to define accurately due to the inherent polydispersity of the PL backbone as well as the poor synthetic control over the site of mPEG and fluorophore attachment. In contrast, dendrimers represent a class of macromolecules with precise molecular weights that are more amenable to controlled derivatization, making them attractive as scaffolds for multi-labeled probes. Tung and co-workers used a multiple antigenic peptide core and functionalized it with four peptide-PEG-CyTE-777 units to give a probe whose fluorescence was quenched via the formation of H-dimers [169]. Cathepsin S was able to mediate a 70-fold increase in fluorescence, in comparison to the non-target enzymes cathepsin L and K which showed only minimal increases (< 2.3-fold), demonstrating that this architecture retained the sensing abilities of their earlier PL-based probe. Matrisian and co-workers conjugated around 6 Cy5.5 molecules to the peripheral amines of a fourth generation poly(amido amine) (PAMAM) dendrimer, which had previously been conjugated to a single PEG chain (Mw 5 kDa), via a peptidic substrate for MMP-7 [170]. AlexaFluor-750 fluorophores were then conjugated to some of the remaining free amino groups via non-cleavable bonds to give a ratiometric probe whose Cy5.5 emission increased by 5-fold in response to MMP-7 treatment. This probe proved able to detect MMP-7 positive tumor cell implants in athymic mice and spontaneous MMP-7 positive tumors in APCmin mice. Ternon et al. used a dendritic architecture to allow a single DABCYL molecule to quench three DANSYL molecules [171]. Although the quenching efficiency was lower than for a simple linear probe (84% compared to 97%) the greater number of fluorophores in the construct resulted in a fluorescence enhancement of 28.4-fold compared to just 6.1 for the linear control. A later paper from the same group used self-quenching fluoresceins, all linked to the dendritic structure via a range of peptide sequences, to screen those sequences for their specificity for trypsin and papain, with increases in fluorescence of up to 300-fold observed [172]. However neither probe was tested in either an in cellulo or an in vivo setting.
Conjugated polymers (CPs) are fluorescent materials that have found widespread use as sensors due to their ability to amplify quenching events. The theory behind this, as well as a thorough summary of sensors based upon it, can be found in a recent review [173]. In brief, an exciton can travel along the CP, visiting a number of individual units (the precise number depends on the mobility of the exciton and its lifetime). Should the quencher be present at any one of those sites then a loss of fluorescence will occur. It is this ability of the exciton to ‘test’ a number of possible sites that results in the amplification effect compared to the analogous small molecule.
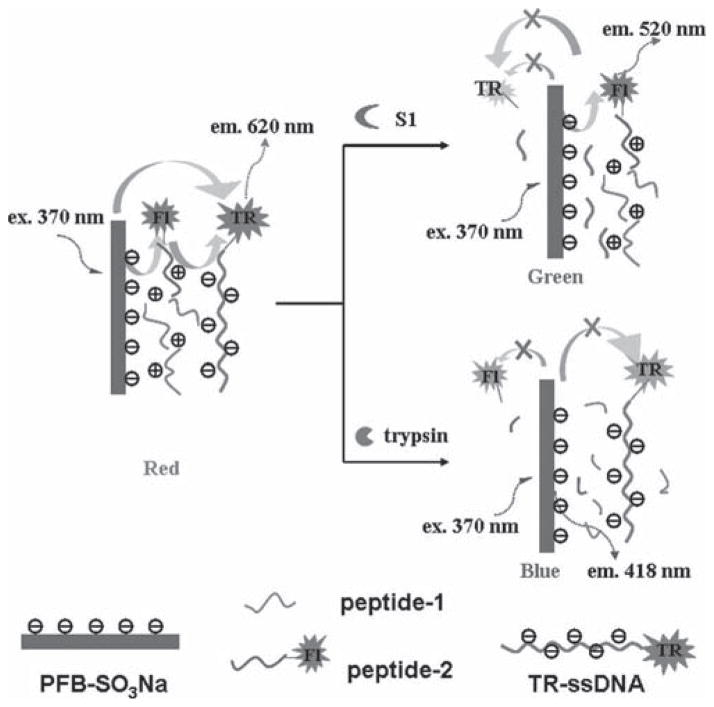
To apply this effect in enzyme detection, cleavable linkers with a quencher are linked to a conjugated polyelectrolyte (CPE – charged groups are required to solubilize the polymer is aqueous solutions), as shown in Fig. (9). Whitten and co-workers developed the first such assay, coating latex microspheres with a cationic poly(phenylene ethylene) (PPE), a biotin binding protein (e.g. streptavidin) and doping them with a small amount of biotin-R-phycoerythrin to give beads that exhibited a sharp emission at 576 nm upon excitation at 440 nm [174]. They then used a peptide sequence flanked with biotin and either QSY-7 or a diazo moiety to quench its fluorescence. Unfortunately when the target enzyme was added to the microspheres there was no increase in fluorescence, which the author’s ascribed to the peptide sequence being sterically hindered. The authors also investigated the use of PEG spacers within the quencher unit to try to expose the peptide sequence to the enzyme, but to no avail. When the peptide-based quenching unit was incubated with the target enzyme prior to the addition of the microspheres the assay was functional, able to detect attomolar concentrations of enterokinase as well as caspase-3 or -7 and β-secretase down to nanomolar concentrations. However such a probe design, with the required pre-incubation step, is not suited for in cellulo or in vivo applications, although it has been used successfully to measure β-secretase activity in cell extracts. Pinto et al. developed a solution based approach, quenching the fluorescence of an anionic sulfated PPE with a cationic peptide that is linked to p-nitroanilide and a substrate sequence for peptidase or thrombin [175]. With a 4-fold increase in CPE emission this probe can detect its target enzyme at concentrations as low as 50 pM. The ionic nature of the interaction between the quencher and the CPE means that the assays were performed in low salt (1 mM) buffers, hence it is not clear if they would still be effective when used in a more complex biological setting. Wosnick et al. developed a protease sensor that covalently attached DNP to the PPE backbone via a PEG-peptide linkage [176]. Addition of trypsin resulted in a 10-fold increase in CPE emission, not observed if a known trypsin inhibitor (benzamidine hydrochloride) was added or if a non-peptide containing analogue of the probe was used. In comparison a small molecule analogue of the CPE exhibited only a 2.9-fold increase in fluorescence, showing the benefit of the amplified quenching effect. However it was also noted that the gain in fluorescence for the CPE was strongly dependent on the surrounding environment, with its gain in emission lessened at higher salt concentrations. Other classes of enzymes apart from proteases have also been detected with CPEs. Feng et al. used a DABCYL group that was linked to a cationic quaternary amine via an ester bond to quench the emission from an anionic sulfated polyfluorene via electrostatic interactions [177]. The addition of acetylcholinesterase to a solution of the probe in PBS caused a 130-fold increase in fluorescence due to cleavage of the ester bond, which could be inhibited using either galanthamine or donepezil. The same group used a similar approach to detect various nucleases. The fluorescence from a cationic CPE was quenched via FRET upon electrostatic complexation with fluorescein-labeled DNA [178]. The action of a nuclease enzyme chopped the DNA into short fragments that were no longer able to form a strong complex with the CPE, hence the FRET signal was lost. Similarly a probe for endonucleases could be constructed if the action of the enzyme cleaved the fluorescein away from the DNA. In all cases though the DNA-fluorescein conjugate was pre-incubated with the enzyme prior to the addition of the CPE probe which, as mentioned already, is not an approach that could be replicated in cellulo. A later paper from the same group detailed the design of a single probe able to detect one of three different nucleases [179]. A Y-shaped DNA molecule, containing three different cleavage sites each specific for a separate nuclease, was labeled with three different fluorophores (fluorescein, Texas red and Cy5) and complexed with a cationic poly(fluorene). The FRET pattern within the probes is dependent on the combination of nucleases in its presence, allowing for the detection of any combination of the three targets simultaneously by monitoring the emission from the CPE and all three fluorophores following excitation of the CPE. Chemburu et al. developed an assay for phospholipase A (PLA2) by coating borosilicate beads with a cationic PPE and then with a 100:1 molar mixture of 1,2-dimyristoyl-sn-glycero-3-[phosphor-rac-(1-glycerol)] and a L-dioleoyl phosphatidylethanolamine-rhodamine (DOPE-rhodamine) conjugate [180]. The CPE emission from this FRET quenched assembly could be almost completely recovered upon exposure to PLA2, which cleaved the DOPE lipid releasing the rhodamine, generating a 12-fold increase in fluorescence. Zhang et al. constructed a three component electrolyte complex, shown schematically in Fig. (10), able to detect the presence of either a nuclease or trypsin [181]. They use a mixture of an anionic poly(fluorene), a fluorescein tagged polyarginine and a Texas red tagged single stranded length of DNA to form a complex in which both the fluorescein and CPE emissions were quenched via FRET. Addition of trypsin caused the complete break-up of the complex, resulting in a strong increase in CPE emission, whereas addition of a nuclease only resulted in loss of the Texas-red-DNA component and hence a rise in the emission from fluorescein was observed. However the dominant effect of either enzyme was a loss of emission from the Texas red, with fluorescence enhancements of 2–3 fold observed, limiting the sensitivity of this approach.
Fig. 9.
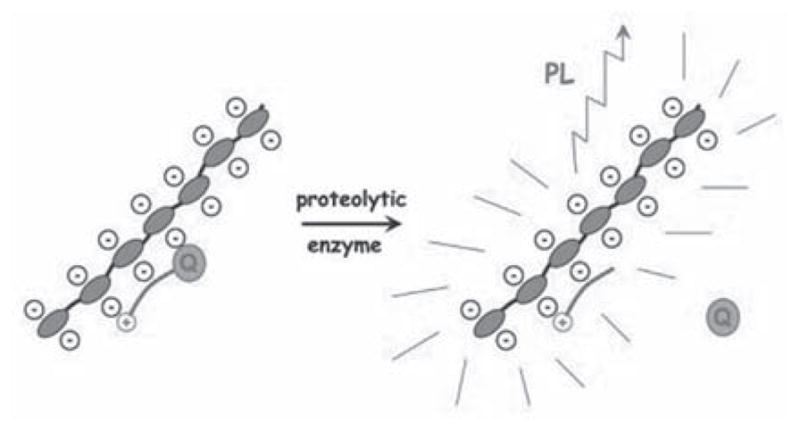
Conjugated polyelectrolyte-based protease sensor. Reprinted with permission from [175]. Copyright 2004 National Academy of Sciences.
Fig. 10.
Zhang et al.’s conjugated polymer-based probe designed to detect both proteases and nucleases. Reprinted with permission from [181]. Copyright 2009 American Chemical Society. Key: PFB-SO3Na = poly(9,9-bis(43-sulfonatobutyl)fluorene-alt-1,4-phenylene) sodium salt, S1 = nuclease, TR = texas red, Fl = fluorescein.
An et al. incorporated their FRET acceptors into the backbone of their CPEs, synthesizing both cat- and an-ionic poly(fluorene)s containing 2,1,3-benzothiadiazole units. Upon aggregation of these polymers using the oppositely charged biological molecule (either polyR6 or ATP) only emission from the azole units was measured [182]. Increases in CPE emission of 120% and 22-fold were ob- served for alkaline phosphatase and trypsin respectively. However assays had to be performed in the presence of at least 20% DMF to prevent the hydrophobic CPEs from aggregating via hydrophobic interactions, indicating that this design may not function well in cellulo. Two papers have reported that the Cu2+ induced quenching of anionic polythiophenes can be reversed via the complexation of the metal with either amino acids or ATP, and have used this phenomenon to design assays for the presence of trypsin and adenylate kinase, however it is unlikely that the initial complex would be stable in a complex biological environment which would leave the probe vulnerable to producing false positive results [183,184]. The fluorescent properties of CPEs are highly dependent of their exact conformation. This relationship has been used to develop a range of probes that exhibit distinct spectral changes upon interaction with certain enzymes, which can be used to both sense the protein in question and to probe its conformation [185,186]. However such probes are not ‘activity-based’ and hence will not be discussed in detail, although it is interesting to note that they have been used successfully in vivo. To date though no CPE-based activatable probe has been used in cellulo or in vivo. The emission from a CPE is dependent on its surroundings, particularly its ionic strength, and they are also prone to aggregation in aqueous solutions which can also affect their optical properties. As such the translation of their undoubted ability to sense enzymes with high sensitivity to a bio- logically relevant setting is likely to be non-trivial.
FLUOROGENIC COMPOUNDS
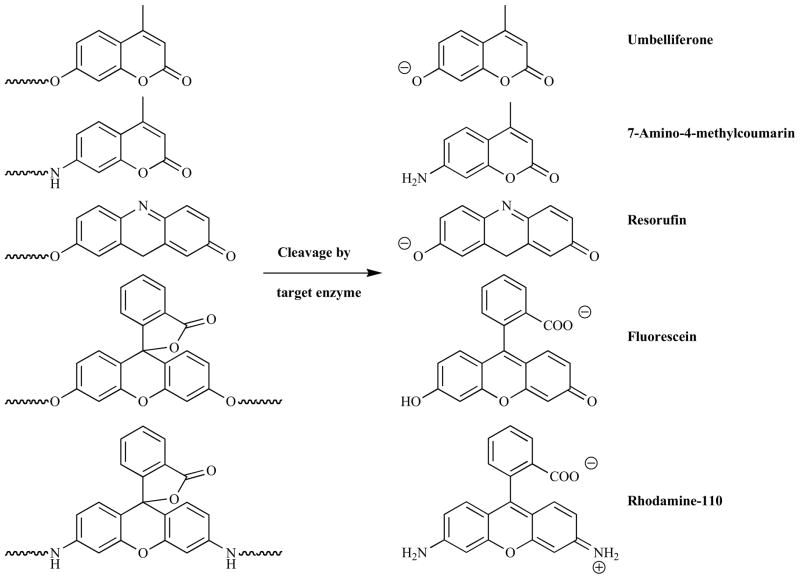
The probes described thus far all rely upon the interaction between two fluorophores to achieve their quenched ‘off’ state, however another broad range of compounds employ just a single fluorophore. Conceptually these are even simpler than the dual-fluorophore systems, with the action of the target enzymes converting the non-fluorescent probe into a fluorescent reporter. Many utilize a cleaving reaction to achieve the desired conversion, a selection of which are shown in Fig. (11).
Fig. 11.
Inactive and fluorescent forms of common fluorogenic probes.
Three groups of fluorophores have dominated research in this area: coumarins, phenoxazinones and xanthene-based compounds. The fluorescent properties of the 4-alkyl-7-heteroatom-coumarin derivatives widely used in fluorogenic probes are known to depend strongly upon the degree of intramolecular charge transfer (ICT) [187]. As such the conversion of the 7-ester/amide compounds to the analogous phenolate/amine species is accompanied by a significant increase in the ICT which red-shifts the fluorescence. Therefore, as long as the excitation and emission wavelengths are chosen judiciously, significant fluorescent enhancements can be observed. The emission from phenoxazines (e.g. resorufin) shows a similar dependence on the degree of ICT. Fluorescein and rhodamine, the most common probes based on a xanthene motif, do not absorb in the visible region of the spectra in their lactone forms as the conjugation of the molecule is interrupted [188,189]. This form can be stabilized via the conversion of either the phenolic alcohols (fluorecein) or amines (rhodamine) into the analogous carboxyl species. Once these carboxy functionalities have been cleaved to reveal the free phenolic alcohol/amine then the lactone is rapidly converted to the free carboxyl, restoring the full conjugation and resulting in strong fluorescence. In aqueous solutions both fluorescein and rhodamine-110 (the most commonly used derivative in this area) exhibit absorption maxima around 490 nm and strong emission around 520 nm [188,189].
In early reports, dating from the early 1960s, coumarin-based probes were simple 4-methylumbelliferyl and resorufin derivatives used for the detection of esterases, lipases, chymotrypsin, acylases, phosphatases and glucosidases [190–193]. Around a decade later 7-amino-4-methylcoumarin (AMC) fluorophores were introduced, which demonstrated several advantages over their 7-hydroxy analogues [194,195]. Both the free amino compounds and their amidic derivatives exhibited strong fluorescence, however the excitation and emission maxima were red-shifted from 325 and 395 nm to 345 and 445 nm upon cleavage allowing for increases in emission of 500-fold to be measured. Umbelliferyl requires a high pH (~10) to achieve its maximal fluorescence due to the relatively high pKa of the phenolic alcohol, but in comparison AMC derivatives can be monitored successfully at around pH 8, far closer to physiological conditions. In addition, the cleavable linkage is an amide instead of an ester, providing greater stability against premature hydrolysis and hence a lower background signal. The use of an amide as the cleavable linker also provides a more biomimetic handle for the attachment of amino acids, allowing for the development of probes with greater specificity to many proteases. Taken together the use of umbelliferyl, resorufin and AMC fluorophores encompasses a great number of enzymatic probes (see Table 2).
Numerous synthetic modifications have been made to the coumarin and phenoxazine moieties to improve their performance (Fig. (12)). Electron withdrawing groups, for example 4-trifluoroacetyl (1) [249,250], 4-cyano [251], 3-carboxylate [252], 6,8-difluoro (2) [225,253–257], and 6-chloro functionalities [252,256] have been used to lower the pKa of the umbelliferyl phenolic alcohol and hence produce probes that fluoresce strongly at physiological pH. When tested against analogous umbelliferyl compounds these probes show up to 10-fold improvements in emission at around pH 7 [249,255]. Another issue that impedes the use of these fluorophores in live cells or animals is the short wavelength of their emission, hence several analogues with red-shifted emissions have been reported. Tung et al. constructed a β-galactoside probe with a 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one) fluorophore whose emission was red-shifted by 50 nm upon release [258]. Although the intact probe still showed strong fluorescence this change in optical properties was sufficient to use effectively in cellulo and also to detect LacZ positive tumor implants in a mouse model, although the overlap between the absorption and emission spectra did limit its sensitivity. Subsequently the same group tested several novel fluorophores based on a benzo[a]phenoxazine motif (4), with their optimal design incorporating hydrophilic sulfonate groups, to discourage H-dimer formation, and exhibiting a high fluorescent intensity at long wavelengths (650–730 nm), a high molar absorption coefficient and pH independent emission in the physiologically relevant range [259]. Short amino-protected (necessary to prevent premature hydrolysis) peptide sequences could be conjugated to the dye to produce effective probes for the peptidases trypsin, leucine aminopeptidase and dipeptidyl peptidase IV which exhibited increases in fluorescence of 6–7 fold in the presence of their target enzymes [259,260]. The membrane permeable nature of the coumarin fluorophores allows them to diffuse out of cells, potentially leading to misleading results as the reporter is found outside the cell where it was released. 7-Aminocoumaryl-4-methanesulfonic acid has been shown to be retained intracellularly whilst showing similar optical properties to AMC, however the probes that incorporate it cannot pass freely into cells, limiting its applicability as it must be microinjected into its site of action [261–263]. 7-Amino-4-chloromethylcoumarins have been shown to enter cells where they react with intracellular thiols via nucleophilic displacement of the chloro-functionality to form a membrane impermanent species that cannot diffuse back out of the cell [264]. A peptide-functionalized derivative was able to successfully detect calpain in rat hepatocytes, and the fluorescence was shown to be localized in the cell’s cytoplasm and to be strongly dependant on the level of intracellular glutathione, both observations that are consistent with its proposed mechanism of action. Mitra and Barrios synthesized a phosphocoumarin derived amino acid (5) that could be used during solid-phase synthesis, enabling the pro-fluorophore to be placed precisely within a certain amino acid sequence [265,266]. This adaptation allows for the production of probes specific to individual protein phosphatases, the activities of which are dependent on the local environment of their target amino acids.
Fig. 12.
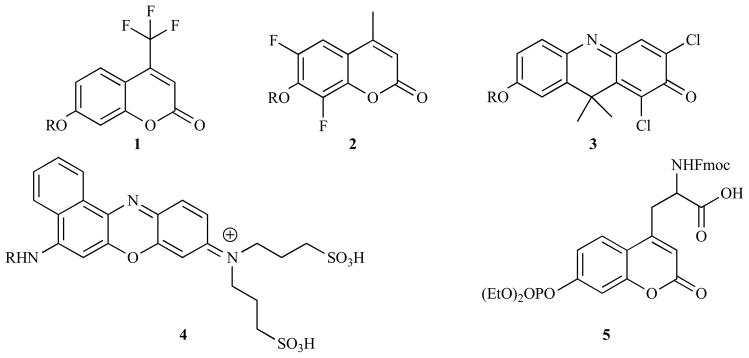
Coumarin and phenoxazine derivatives with improved spectroscopic or chemical properties as fluorogenic probes.
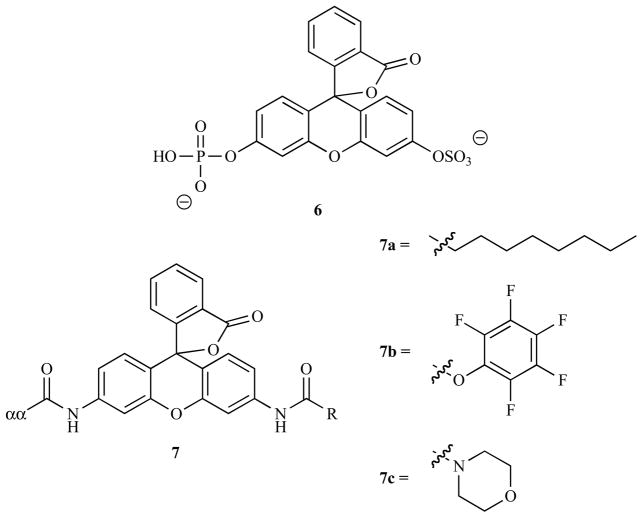
Fluorescein-based compounds, specifically the di-acylated derivatives, were amongst some of the earliest fluorogenic enzyme probes reported, able to detect lipase, acylases and chymotrypsin [267]. Rhodamine, the amino analogue of fluorecein, was introduced as a fluorogenic motif in 1983 [268] and, as with the introduction of AMC, it permitted the direct use of amino acid sequences to trigger the activation of the probe, increasing the range of enzymes that could be analyzed. When compared to the analogous AMC probes in this original paper from 1983 the authors reported that it was 50–280 fold more effective as a result of a combination of its higher molar fluorescence coefficient, the faster rate of its enzymatic hydrolysis and its greater affinity for the target enzymes [268]. Additionally both of these xanthene derivatives display longer wavelength absorption and emission spectra, higher extinction coefficients [269] and are generally more cell permeable than coumarin-based compounds [270], making them more suited for in cellulo applications. Whilst several other papers have confirmed the superiority of xanthene-based probes over coumaryls, it should be noted that this is not always the case. For example Soukharev et al. reported that their 6,8-difluoroumbelliferyl probe for certain organophosphatases exhibited faster hydrolysis kinetics than the analogous fluorescein compound, making it around 100-times more sensitive for their assays [225]. Examples of enzymes targeted by xanthene-based probes are shown in Table 3.
A major issue with these fluorophores is that they are bi-functional, with each cleavage resulting in an increase in fluorescence. This phenomenon complicates the accurate quantification of enzymatic activity, hence several groups have blocked one site with a non-cleavable group. Advantageously, this presents an opportunity to endow the probe with additional desirable properties. For example Wang et al. screened several mono-phosphate derivatives of fluorescein for their activity against protein tyrosine phosphates and were able to identify the sulfonated compound 6 which exhibited a higher affinity for this enzyme, raising the efficacy of the probe [283]. Similarly Zaikova et al. reported optimized probes for phosphatidylinositol-specific phospholipase C [284]. Cai and coworkers chose to functionalize their rhodamine-based probe for caspases with an N-octyloxycarbonyl group (7a) to increase its cell permeability [285]. In a later paper the same group used a 2,3,4,5-tetrafluorobenzoyl moiety to produce a probe (7b) that was both cell permeable and reactive towards nucleophiles [286]. It was postulated that this activated ester would react with intracellular proteins, preventing the efflux of the released fluorophore from the cell. Indeed when compared to rhodamine this moiety was shown to retain the fluorescence intracellularly, meaning that despite its smaller fluorescence increase on cleavage it was a more effective probe, able to detect caspase-3 in apoptotic Jurkat T cells. Lorey et al. screened a range of haloalkane and maleimide groups as electrophilic handles for proteins, with their optimal design permitting the assessment of dipeptidyl peptidase activity in single cells [287]. A limitation of these probes is their reduced sensitivity due to the incomplete recovery of fluorescence from the cleavage of just one of the N-acyl groups. Wang et al. tackled this issue by using a morpholine blocking group (7c), that favored the free lactone form of the dye upon removal of the caspase-sensitive peptide sequence [288]. This probe retained the excellent cell permeability of previous mono-functionalized rhodamine derivatives, however it also showed 90% of the fluorescence of R-110 upon cleavage. When tested side-by-side against the analogous bis(peptidyl) rhodamine in apoptotic Jurkat cells it was found to be three times more sensitive, potentially due to the high increase in fluorescence caused by the cleavage of just a single bond.
Not all fluorescein-based probes are quenched by the formation of the spirolactone moiety. Nagano and co-workers have developed several probes which employ PeT as the quenching mechanism. The free carboxylic acid form of fluorescein can be treated as two separate conjugated systems as the orientation of the xanthene group is orthogonal to that of the benzene group (Fig. (14a)). If the HOMO of the benzene moiety is sufficiently high in energy, then it can donate an electron to the excited state of the xanthene, effectively acting as an reducing agent and hence quenching the xanthene’s radiative emission [290]. Building on this work they realized that the primary role of the carboxylic acid group in fluorescein was to keep the xanthene and benzene groups orthogonal, in other words its effect was based on sterics rather than electronics. This led them to replace the carboxylic acid with other functional groups and to place other functionalities around the benzene ring. They established that electron donating groups encouraged PeT and hence were better quenchers [289]. They also found that neutral xanthene groups were easier to quench than the analogous anionic forms, hence by carefully choosing the oxidation potential of the benzene they were able to design a probe for β-galactoside that was non-fluorescent until it was cleaved enzymatically [289]. A later paper introduced pendant esters (8) that were cleaved intracellularly to ensure that the fluorophore was retained in cellulo [291]. This probe was tested in an in vivo model and proved able to detect peritoneal SHIN3 tumors as small as 200-μm in diameter following intra-peritoneal injection, with fluorescence enhancements of 33-fold compared to the surrounding tissue. It is also possible to design quenching mechanisms based around the PeT from the excited xanthene to the benzene group [290], and this was exploited by the same group to construct a probe for glutathione transferase (9). Nucleophilic displacement of an aromatic nitro group by glutathione, catalyzed by the transferase enzyme, raised the energy of the LUMO on benzene preventing it from reduction by xanthene and resulting in a significant fluorescent enhancement [292]. This probe was used successfully in HL60 cells to show the inhibition of glutathione by N-ethylmaleimide using flow cytometry.
Fig. 14.
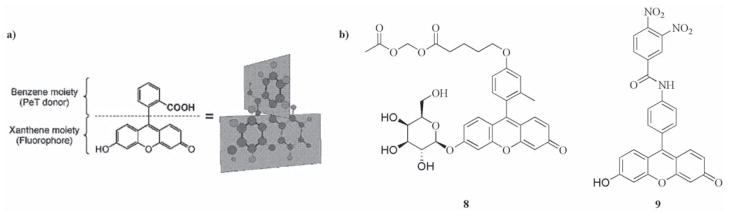
a) Schematic representation of orthogonal conjugated moieties in fluorescein. Reproduced with permission from [289]. Copyright 2005 American Chemical Society. b) Examples of fluorogenic probes based on PeT quenching mechanism in fluorescein derivatives.
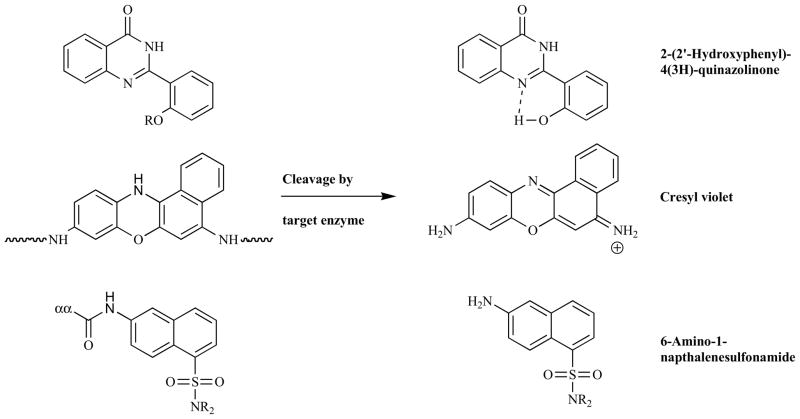
Numerous other fluorophores have also found use as enzymatic probes (see Fig. (15) for representative examples). Haugland and co-workers have reported a series of derivatives of 2-(2′-hydroxyphenyl)-4(3H)-quinazolinone that yield fluorescent precipitate due to intramolecular hydrogen bonding when their phenolic alcohol is revealed via the action of a target enzyme. The dye is photostable, exhibits a large Stoke’s shift and has found use in histochemical stains for phosphatases [293] and β-glucuronidase [294,295]. Advantageously, the fluorescent precipitate is not able to diffuse away from its site of release, producing sharper staining compared to soluble fluorophores. Cresyl violet, a di-amino derivative of resorufin, exhibits red-shifted absorption and emission maxima (591 and 628 nm respectively) relative to its di-peptidyl derivatives (488 and 570 nm), which has led to its use in cellulo as a probe for dipeptidyl peptidase IV [296] and cathepsin B [297] via the attachment of peptide sequences specific for these enzymes. When compared directly to the analogous rhodamine compound it was found to be less stable, however it also showed a far higher specificity for dipeptidyl peptidase IV, demonstrating the effect that the fluorophore can have on this property [298]. Mann and coworkers introduced 6-amino-1-napthalenesulfonamide as a fluorogenic reporter with emission at 470 nm that is 99.9% quenched upon conjugation to an amino acid sequence specific to the target enzyme [299]. Besides varying the amino acid sequence, it was also shown that the substituents on both sulfonamide and napthalene moieties have a substantial influence on the probe. Hence large libraries with variations in both of these areas allowed the authors to identify compounds with high specificity towards various different proteolytic enzymes [299–302]. Cen et al. functionalized a nucleic acid dye, NucView488, with the anionic caspase-3 specific peptide sequence DEVD to give a compound that could no longer intercalate into DNA due to electrostatic repulsion [303]. Removal of the peptidic portion of the molecule by caspase-3 regenerated the free dye, which was then able to fluoresce upon interaction with DNA. The probe specifically detected apoptotic HeLa and Jurkat cells via flow cytometry and microscopy, where localization of the signal in the cell’s nuclei was observed. Other groups which have been employed as fluorogenic reporters include 3-amino-9-ethyl-carbazole [304,305], 2,3-dicyanohydroquinone [306], 2-napthol and 7-methoxynapthylamine [307] and substituted furan-2-ones [308].
Fig. 15.
Other selected fluorogenic reporting groups.
In order to maintain a low background signal a fluorogenic probe must be hydrolytically stable at a physiologically relevant pH. However the low pKa of many of the phenolic fluorophores makes them excellent leaving groups, which renders their ester linkages susceptible to premature hydrolysis. Hence self-immolative linkers, which facilitate the linkage of the enzymatically cleavable bond to the fluorophore via a bond that is more hydrolytically stable, have been developed (Fig (16)). These linkers also place the large fluorophore moieties at a distance from the cleavable linker, potentially preventing any adverse steric effects on the rate of the enzymatic reaction. Several strategies that use added reagents, for example NaIO4 or BSA, to cause the break-down of the linker have been reported. However this section will focus on those species which can release their fluorophore spontaneously. One of the most common motifs employed is the ‘trimethyl lock’ (10), an example of which is shown in Fig. (16). Removal of the acetyl groups by an esterase reveals a phenolic alcohol which rapidly participates in an intra-molecular cyclization to form a lactone, driven by the unfavorable steric interactions between the three methyl groups [309]. The electron-rich phenol derivative is a relatively poor leaving group, stabilizing this ester bond, and the linkage to the fluorophore itself is now an amide hence this probe is stable for months in phosphate buffered saline (PBS). When tested in Dulbecco’s modified Eagle’s medium, it showed no increase in fluorescence over three hours, as opposed to fluorescein diacetate which was hydrolyzed within minutes. Similar results were observed when the two probes were tested on HeLa cells, with the trimethyl lock probe showing only intra-cellular fluorescence with no signal from the media. The same immolative linker was later used with coumaryl and cresyl violet fluorophores [310]. As with previous xanthene derivatives it was also desirable to produce a simple mono-reactive compound, hence the mono-morpholine analogue was synthesized and proved able to retain 35% of the fluorescence of rhodamine upon cleavage [311]. The same paper also detailed the synthesis of a maleimide functionalized compound which the authors were able to conjugate to RNase. The resultant bioconjugate was found to be less stable than the parent compound, which was ascribed to its proximity to nucleophilic residues on the protein, however it could be stored at pH 5 and was used successfully to track the enzyme in HeLa cells. Yatzeck et al. also used a trimethyl lock linker to adapt a mono-morpholine rhodamine based probe for the sensing of a cytochrome P450 isozyme, producing a probe that was tested successfully in lung carcinoma A549 cells after the induction of enzyme expression with 2,3,7,8-tetrachlorodibenzo-p-dioxin [312]. The trimethyl lock can also be adapted to produce probes for enzymes that cannot usually be targeted with xanthene- and coumarin-based compounds. Huang and co-workers used the reduction of an electrophilic quinone by DT diaphorase (DTP) to form the phenolic alcohol required for the self-immolation and were able to develop two probes that were stable for several months in PBS but showed a rapid increase in fluorescence in the presence of DTP [313,314]. Ho et al. used a p-benzyloxycarbonyl linker (12) to allow the cleavage of an O-glycoside linker by β-galactoside to release their previously developed NIR fluorophore [315]. An additional glycine unit was required as the direct attachment of the benzylic alcohol via a carbamate bond did not quench the fluorophore effectively. Another common immolative linker is the p-aminobenzyl alcohol, which was first used in this context by Jones et al. as a spacer to produce a probe for prostate specific antigen (13) [316]. Later Renard and coworkers used the same motif in order to use phenolic fluorophores in conjunction with penicillin G acylase (PGA) sensitive- and peptidic-triggers [317,318]. Interestingly they noted that one of their dyes of choice, 7-hydroxy-9H-(9,9-dimethylacridin-2-one), was not stable in the presence of glutathione, undergoing conversion to a non-fluorescent product [317]. Hence in their later paper they introduced a 7-hydroxycoumarin-hemocyanine hybrid dye that exhibited high chemical stability, along with a high extinction coefficient, reasonable quantum yield and long wavelength absorption and emission (573 and 636 nm) to give a probe that showed high stability in PBS and significant fluorescent enhancement in the presence of PGA [318]. The same group also investigated the use of an N-acylhemithioaminal linker and synthesized two PGA sensitive probes, one containing carbonate linkages (11) and the other with carbamates [241]. They found that the carbonates were more susceptible to non-specific cleavage in PBS, favoring the use of carbamates despite the slower kinetics it exhibited with PGA. The self-immolating concept has recently been extended to macromolecules with the synthesis of both dendrimers [319] and polymers [320] that disintegrate in response to a single cleavage event. If several fluorogenic compounds are released as they break-up then a significant amplification of the signal can be achieved. This concept has been utilized in two types of fluorogenic probe to date. In the first, exposure of a free amine functionality by PGA triggered a series of 1,6-azaquinone methide and 1,4-quinone methide eliminations, along with a cyclization, to release the two reporter molecules one of which was fluorescent (6-aminoquinoline) [321]. The second example uses a self-immolative polymer that releases multiple reactive aza-quinone methide units upon enzymatic cleavage. These reactive units can then conjugate to nearby nucleophiles from the enzymes, resulting in effective labeling [322]. Importantly the emission is significantly red-shifted during this process, potentially allowing for this system to be adapted as an in vivo enzymatic probe. Although the use of self-immolating macromolecules is still in its infancy the potential for large amplifications of a single cleavage event marks them as promising systems for increasing the sensitivity of molecular probes.
Fig. 16.
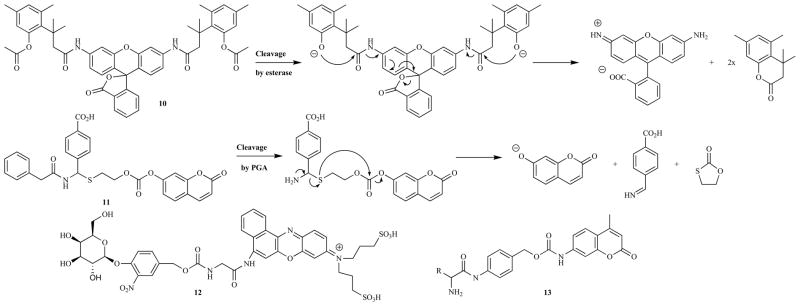
Examples of fluorogenic probes which incorporate self-immolative linkers.
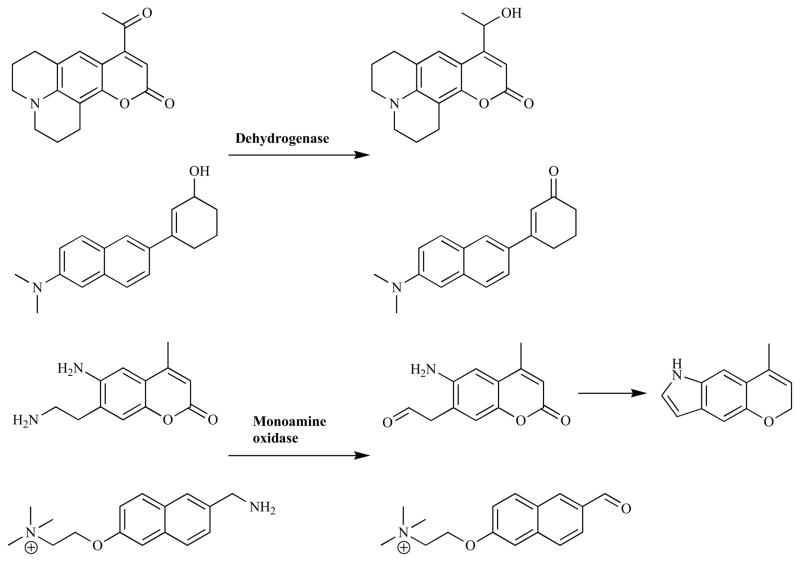
Although enzymatic triggers that rely on cleavage reactions dominate the field of fluorogenic probes, there have been examples of other classes of reactions (Fig. (17)). The conversion of an electron-rich aromatic aldehydes or ketones to the analogous alcohol profoundly affects the electronics of the aromatic group and can lead to significant changes in the fluorescent properties of the molecule [323]. This forms the basis of a novel probe design for various dehydrogenase enzymes. The precise structure of the aromatic system determines the specificity of the compound for a particular ticular isozyme, allowing for the development of highly specific probes [323–325]. Yee et al. screened a range of probes to identify a highly selective compound for AKR1C, and they were able to demonstrate the efficacy of this membrane permeable compound in COS-1 cells [326]. Froemming et al. used the opposite reaction, the conversion of an alcohol to a ketone, to activate their fluorescent probe [327]. The placement of an amine functionality in the 6-napthyl position set-up a ‘push-pull’ system upon the formation of a ketone allowing the molecule to fluoresce via ICT. This process was inefficient in aqueous solutions, however in cellulo a bright yellow-green emission was observed due to the activated probe migrating to a hydrophobic cellular membrane that favored ICT. Monoamine oxidases catalyze the conversion of an amine to an aldehyde via an imine intermediate. Chen et al. used this aldehyde functionality to form an indole from a 3-amino-coumaryl species, negating the twisted-ICT based quenching to give a probe whose fluorescence at 524 nm increase by 200 times in the presence of monoamine oxidases [328]. Ling et al. employed computational analysis to aid their design of a bovine plasma amine oxidase probe which was based on the formation of a ‘push-pull’ fluorophore upon reaction with the target enzyme. After several iterations they identified a successful compound, containing a pendant cationic amine that formed strong electrostatic interactions with anionic residues in the enzyme’s active site and hence raised the specificity of the probe [329].
Fig. 17.
Examples of non-cleavage-based fluorogenic enzyme probes.
CONCLUSIONS
Activatable fluorescent probes have been successfully designed to target a wide range of enzymes through several ingenious mechanisms, allowing for their routine detection in vitro. Many of the probes have also proved to be effective in cellulo, however to date relatively few have progressed to use in animal or human imaging. The in vivo environment is far more complex, requiring stricter control over the probe’s specificity, its spectroscopic properties and crucially its pharmacokinetics. As demonstrated by Weissleder and co-workers, it is in these in vivo models that the role of enzymes in disease pathology will likely be discovered. The continued development of novel probes that are able to function in these most demanding of environments represents a significant challenge, however it is one that must be met in order to facilitate a greater understanding of enzymes and their role in diseases.
Fig. 2.
A selection of organic fluorophores and quenchers commonly used in dual- and multi-labeled probes.
Fig. 13.
Xanthene derivatives with improved spectroscopic and chemical properties as fluorogenic probes.
Table 2.
Selection of Enzymes for which Coumarin- and Phenoxazines-based Probes have been Reported
| Enzyme Class | Specific Enzyme |
|---|---|
| Serine proteases | Human cytomegalovirus protease [196], Subtilisin [197], Trypsin [198,199], Furin [200], Prolyl oligopeptidase [201] |
| Metalloproteases | Aminopeptidases [202,203] |
| Cysteine proteases | Gingipain [204], Cathepsin B [205], separase [206], papain [198] |
| Glycosidases | Sialidases [207,208], endo-α-1,2-Mannosidase [209], Chitinases [210,211], Glucosidases [212,213], Galactosidase [214], endo-β-Xylosidase [215], Xylanases [216,217] |
| Phosphodiesterase [218]/Phosphatases [219–226] | |
| Other hydrolases | Penicillin G acylase [227], Glycoasparaginase [228,229], Glycolipid hydrolase [230] |
| Transferases | N-acetyltransferase [231], γ-glutamyltranspeptidase [232], Tissue transglutaminase [233] |
| Lyases | Tryptophanase [234] |
| Oxoreductases | Baeyer-Villiger monooxygenase [235], Cytochrome P450s [236–248] |
Table 3.
Selection of Enzymes for which Xanthene-Based Probes have been Reported
| Enzyme Class | Specific Enzyme |
|---|---|
| Serine proteases [271] | Plasmin [272], Thrombin [272], Subtilisin [37], Tripeptidyl peptidase I [273], Hepatitis C viral protease [274], chymotrypsin [274] |
| Metalloproteases | Aminopeptidases [275,276] |
| Cysteine proteases | Caspases [269,270], Cathepsin B [274], Cathepsin C [277], Cathepsin K [278], SARS main protease [279], papain [274] |
| Glycosidases | β-glucosidase [213] |
| Phosphodiesterase/Phosphatases [225,280] | |
| Lipase/esterase [86] | |
| Other hydrolases | β-N-Acetylglucoaminidase [281], |
| Transferases | Glutathione transferase [282] |
| Oxoreductases | cytochrome P450s [246] |
Acknowledgments
This work was supported in part by the National Institutes of Health RO1 CA 135626-01 and Friends for an Earlier Breast Cancer Test.
ABBREVIATIONS
- 5-FAM
5-Carboxyfluorescein
- AMC
7-Amino-4-methylcoumarin
- ATP
Adenosine triphosphate
- BHQ-2
Black-hole quencher-2
- Bla
β-Lactamase
- BODIPY
Boron-dipyrromethene
- BSA
Bovine serum albumin
- CP
Conjugated polymer
- CPE
Conjugated polyelectrolyte
- DABCYL
4-(4′-Dimethyl-aminobenzeneazo)benzoyl
- DANSYL
5-(Dimethylamino) napthalene-1-sulfonyl
- DBO
2,3-Diazabicyclo[2.2.2]oct-2-ene
- DNA
Deoxyribonucleic acid
- DNP
Dinitrophenol
- DTP
DT diaphorase
- EDANS
5-(2′-Aminoethyl)aminonapthalene sulfonic acid
- FMT
Fluorescence molecular tomography
- FRET
Förster resonance energy transfer
- HIV
Human immunodeficiency virus
- HOMO
Highest occupied molecular orbital
- ICT
Intramolecular charge transfer
- LUMO
Lowest unoccupied molecular orbital
- MMP
Matrix metalloproteinase
- mPEG
Methoxypoly(ethylene glycol)
- NIR
Near infra-red
- PAMAM
Poly(amido amine)
- PBS
Phosphate buffered saline
- PEG
Poly(ethylene glycol)
- PeT
Photoinduced electron transfer
- PGA
Penicillin G acylase
- PKA
Protein kinase A
- PL
Poly-L-lysine
- PLA2
Phospholipase A2
- PPE
Poly(phenylene ethylene)
- R0
Förster distance
- SARS
Severe acute respiratory syndrome
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TAMRA
Tetramethylrhodamine
References
- 1.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9(1):123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 2.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardinal oxygen sufficiency and circulatory parameters. Science. 1977;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 3.Latt SA, Auld DS, Vallee BL. Fluorescence determination of carboxypeptidase A activity based on electronic energy transfer. Anal Biochem. 1972;50:56–62. doi: 10.1016/0003-2697(72)90485-x. [DOI] [PubMed] [Google Scholar]
- 4.Carmel A, Zur M, Yaron A, Katchalski E. Use of substrates with fluorescent donor and acceptor chromophores for the kinetic assay of hydrolases. FEBS Lett. 1973;30(1):11–14. doi: 10.1016/0014-5793(73)80607-6. [DOI] [PubMed] [Google Scholar]
- 5.Sato E, Sakashita M, Kanaoka Y, Kosower EM. XIV. Novel fluorogenic substrates for microdetermination of chemotrypsin and aminopeptidase: bimane fluorescence appears after hydrolysis. Bioorg Chem. 1988;16:298–306. [Google Scholar]
- 6.Sato E, Hattori H, Kanaoka Y. Fluorogenic bimane substrates with dabsyl group for endopeptidases; chemotrypsin, collagenase and thermolysin. J Pharmacobio-Dyn. 1991;14:599–604. doi: 10.1248/bpb1978.14.599. [DOI] [PubMed] [Google Scholar]
- 7.Mayatoshi ED, Wang GT, Krafft GA, Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990;247(4945):954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 8.Wang GT, Mayatoshi ED, Huffaker J, Krafft GA. Design and synthesis of new flurogenic HIV protease substrates based on resonance energy transfer. Tetrahedron Lett. 1990;31(45):6493–6496. [Google Scholar]
- 9.Juliano MA, Filira F, Gobbo M, Rocchi R, Del Nery E, Juliano L. Chromogenic and fluorogenic glycosylated and acetylglycosylated peptides as substrates for serine, thiol and aspartyl peptidases. J Peptide Res. 1999;53:109–119. doi: 10.1034/j.1399-3011.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Jullien ND, Cuniasse P, Georgiadis D, Yiotakis A, Dive V. Combined use of selective inhibitors and fluorogenic substrates to study the specificity of somatic wild-type angiotensin converting enzyme. FEBS J. 2006;273:1772–1781. doi: 10.1111/j.1742-4658.2006.05196.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang GT, Chung CC, Holzman TF, Krafft GA. A continuous fluorescence assay of renin activity. Anal Biochem. 1993;210:351–359. doi: 10.1006/abio.1993.1207. [DOI] [PubMed] [Google Scholar]
- 12.Yan ZH, Ren KJ, Wang Y, Chen S, Brock TA, Rege AA. Development of intramolecularly quenched fluorescent peptides as substrates of angiotensin-converting enzyme 2. Anal Biochem. 2003;312:141–147. doi: 10.1016/s0003-2697(02)00461-x. [DOI] [PubMed] [Google Scholar]
- 13.Filippova IY, Lysogorskaya EN, Anisimova VV, Suvorov LI, Oksenoit ES, Stepanov VM. Fluorogenic peptide substrates for assay of aspartyl proteinases. Anal Biochem. 1996;234:113–118. doi: 10.1006/abio.1996.0062. [DOI] [PubMed] [Google Scholar]
- 14.Gomes R, Batista R, Almeida A, Fonseca D, Juliano L, Hial V. A fluorimetric method for the determination of pepsin activity. Anal Biochem. 2003;316:11–14. doi: 10.1016/s0003-2697(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Gulnik SV, Suvorov LI, Majer P, Collins J, Kane BP, Johnson DG, Erikson JW. Design of sensitive fluorogenic substrates for human cathepsin D. FEBS Lett. 1997;413:379–384. doi: 10.1016/s0014-5793(97)00886-7. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda Y, Kageyama T, Akamine A, Shibata M, Kominami E, Uchiyama Y, Yamamoto K. Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J Biochem. 1999;125:1137–1143. doi: 10.1093/oxfordjournals.jbchem.a022396. [DOI] [PubMed] [Google Scholar]
- 17.Capobianco JA, Lerner CG, Goldman RC. Application of a fluorogenic substrate in the assay of proteolytic activity and in the discovery of a potent inhibitor of Candida albicans aspartic proteinase. Anal Biochem. 1992;204:96–102. doi: 10.1016/0003-2697(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 18.Gopi HN, Ravindra G, Pal PP, Pattanaik P, Balaram H, Balaram P. Proteolytic stability of β-peptide bonds probed using quenched fluorescent substrates incorporating a hemoglobin cleavage site. FEBS Lett. 2003;535:175–178. doi: 10.1016/s0014-5793(02)03885-1. [DOI] [PubMed] [Google Scholar]
- 19.Ermolieff J, Loy JA, Koelsch G, Tang J. Proteolytic activation of recombinant pro-memapsin 2 (pro-β-secretase) studies with new fluorogenic substrates. Biochemistry-US. 2000;39:12450–12456. doi: 10.1021/bi001494f. [DOI] [PubMed] [Google Scholar]
- 20.Réhault S, Brillard-Bourdet M, Juliano MA, Juliano L, Gauthier F, Moreau T. New, sensitive fluorogenic substrates for human cathepsin G based on the sequence of serpin-reactive site loops. J Biol Chem. 1999;274(20):13810–13817. doi: 10.1074/jbc.274.20.13810. [DOI] [PubMed] [Google Scholar]
- 21.Attucci S, Korkmaz B, Juliano L, Hazouard E, Girardin C, Brillard-Bourdet M, Réhault S, Anthonioz P, Gauthier F. Measurement of free and membrane bound cathepsin G in human neutrophils using new sensitive fluorogenic substrates. Biochem J. 2002;366(3):965–970. doi: 10.1042/BJ20020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesner A, Wysocka M, Guzow K, Wiczk W, Legowska A, Rolka K. Development of sensitive cathepsin G substrate using combinatorial chemistry methods. Anal Biochem. 2008;375:306–312. doi: 10.1016/j.ab.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Grahn S, Ullmann D, Jakubke HD. Design and synthesis of fluorogenic trypsin peptide substrates based on resonance energy transfer. Anal Biochem. 1998;265:225–231. doi: 10.1006/abio.1998.2902. [DOI] [PubMed] [Google Scholar]
- 24.Melo RL, Alves LC, Del Nery E, Juliano L, Juliano MA. Synthesis and hydrolysis by cysteine and serine proteases of short internally quenched fluorogenic peptides. Anal Biochem. 2001;293(71):77. doi: 10.1006/abio.2001.5115. [DOI] [PubMed] [Google Scholar]
- 25.Chagas JR, Juliano L, Prado ES. Intramolecularly quenched fluorogenic tetrapeptide substrates for tissue and plasma kallikreins. Anal Biochem. 1991;192:419–425. doi: 10.1016/0003-2697(91)90558-b. [DOI] [PubMed] [Google Scholar]
- 26.Almeida PC, Chagas JR, Cezari MHS, Juliano MA, Juliano L. Hydrolysis by plasma kallikrein of fluorogenic peptides derived from prorenin processing site. Biochim Biophys Acta. 2000;1479:83–90. doi: 10.1016/s0167-4838(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois L, Brillard-Bourdet M, Deperthes D, Juliano MA, Juliano L, Tremblay RR, Dubé JY, Gauthier F. Serpin-derived peptide substrates for investigating the substrate specificity of human tissue kallikreins hK1 and hK2. J Biol Chem. 1997;272(47):29590–29595. doi: 10.1074/jbc.272.47.29590. [DOI] [PubMed] [Google Scholar]
- 28.Oliva MLV, Santomauro-Vaz EM, Andrade SA, Juliano MA, Pott VJ, Sampaio MU, Sampaio CAM. Synthetic peptides and fluorogenic substrates related to the reactive site sequence of Kunitz-type inhibitors isolated from Bauhinia: interaction with human plasma kallikrein. Biol Chem. 2001;382:109–113. doi: 10.1515/BC.2001.016. [DOI] [PubMed] [Google Scholar]
- 29.Bonneau PR, Plouffe C, Pelletier A, Wernic D, Poupart MA. Design of fluorogenic peptide substrates for human cytomegalovirus protease based on structure-activity relationship studies. Anal Biochem. 1998;255:59–65. doi: 10.1006/abio.1997.2445. [DOI] [PubMed] [Google Scholar]
- 30.Holskin BP, Bukhtiyarova M, Dunn BM, Baur P, de Chastonay J, Pennington MW. A continuous fluorescence-based assay of human cytomegalovirus protease using a peptide substrate. Anal Biochem. 1995;226:148–155. doi: 10.1006/abio.1995.1264. [DOI] [PubMed] [Google Scholar]
- 31.Taliani M, Bianchi E, Narjes F, Fossatelli M, Urbani A, Steinkuehler C, De Francesco R, Pessi A. A continuous assay of hepatitis C virus protease based on resonance energy transfer depsipeptide substrates. Anal Biochem. 1996;240:60–67. doi: 10.1006/abio.1996.0331. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen-Brahms I, Jansson AM, Meldal M, Breddam K, Bock K. Silyl protection in the solid-phase synthesis of N-linked glycopeptides. Preparation of glycosylated fluorogenic substrates for subtilisins. Bioorg Med Chem. 1994;2(11):1153–1167. doi: 10.1016/s0968-0896(00)82067-2. [DOI] [PubMed] [Google Scholar]
- 33.Meldal M, Svendsen I. Direct visualization of enzyme inhibitors using a portion mixing inhibitor library containing a quenched fluorogenic peptide substrate. Part 1. Inhibitors for subtilisin carlsberg. J Chem Soc Perk T. 1995;1:1591–1596. [Google Scholar]
- 34.Angliker H, Neumann U, Molloy SS, Thomas G. Internally quenched fluorogenic substrate for furin. Anal Biochem. 1995;224:409–412. doi: 10.1006/abio.1995.1058. [DOI] [PubMed] [Google Scholar]
- 35.Jean F, Basak A, DiMaio J, Seidah MG, Lazure C. An internally quenched fluorogenic substrate of prohormone convertase 1 and furin leads to a potent prohormone convertase inhibitor. Biochem J. 1995;307:689–695. doi: 10.1042/bj3070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazure C, Gauthier D, Jean F, Boudreault A, Seidah MG, Bennett HPJ, Hendy GN. In vitro cleavage of internally quenched fluorogenic human proparathyroid hormone and proparathyroid-related peptide substrates by furin. J Biol Chem. 1998;273(15):8572–8580. doi: 10.1074/jbc.273.15.8572. [DOI] [PubMed] [Google Scholar]
- 37.Basak S, Chrétien M, Mbikay M, Basak A. In vitro elucidation of substrate specificity and bioassay of proprotein convertase 4 using intramolecularly quenched fluorogenic peptides. Biochem J. 2004;380(2):505–514. doi: 10.1042/BJ20031405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basak A, Chrétien M, Seidah MG. A rapid fluorometric assay for the proteolytic activity of SKI1/S1P based on the surface glycoprotein of the Hemorrhagic fever lassa virus. FEBS Lett. 2002;514:333–339. doi: 10.1016/s0014-5793(02)02394-3. [DOI] [PubMed] [Google Scholar]
- 39.Elston C, Wallach J, Saulnier J. New continuous and specific fluorometric assays for Pseudomonas aeruginosa elastase and LasA protease. Anal Biochem. 2007;368:87–94. doi: 10.1016/j.ab.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 40.Korkmaz B, Attucci S, Moreau T, Godat E, Juliano L, Gauthier F. Design and use of highly specific substrates of neutrophil elastase and proteinase 3. Am J Resp Cell Mol. 2004;30(6):801–807. doi: 10.1165/rcmb.2003-0139OC. [DOI] [PubMed] [Google Scholar]
- 41.Korkmaz B, Attucci S, Hazouard E, Ferrandière M, Jourdan ML, Brillard-Bourdet M, Juliano L, Gauthier F. Discriminating between the activities of human neutrophil elastase and proteinase 3 using serpin-derived fluorogenic substrates. J Biol Chem. 2002;277(42):39074–39081. doi: 10.1074/jbc.M202918200. [DOI] [PubMed] [Google Scholar]
- 42.Rao S, Bockstael K, Nath S, Engelborghs Y, Anné J, Geukins N. Enzymatic investigation of the Staphylococcus aureus type I signal peptidase SpsB - implications for the search for novel antibiotics. FEBS J. 2009;276:3222–3234. doi: 10.1111/j.1742-4658.2009.07037.x. [DOI] [PubMed] [Google Scholar]
- 43.Peng SB, Zheng F, Angleton EL, Smiley D, Carpenter J, Scott JE. Development of an internally quenched fluorescent substrate and a continuous fluorometric assay for Streptococcus pneumoniae signal peptidase I. Anal Biochem. 2001;293(88):95. doi: 10.1006/abio.2001.5102. [DOI] [PubMed] [Google Scholar]
- 44.Niyomrattanakit P, Yahorava S, Mutule I, Mutulis F, Petrovska R, Prusis P, Katzenmeier G, Wikberg JES. Probing the substrate specificity of the Dengue virus type 2 NS3 serine protease by using internally quenched fluorescent peptides. Biochem J. 2006;397(1):203–211. doi: 10.1042/BJ20051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wysocka M, Lesner A, Guzow K, Mackiewicz L, Legowska A, Wiczk W, Rolka K. Design of selective substrates of proteinase 3 using combinatorial chemistry methods. Anal Biochem. 2008;378:208–215. doi: 10.1016/j.ab.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 46.George J, Teear ML, Norey CG, Burns DD. Evaluation of an imaging platform during the development of a FRET protease assay. J Biomol Screen. 2003;8(1):72–80. doi: 10.1177/1087057102239778. [DOI] [PubMed] [Google Scholar]
- 47.Lauer-Fields JL, Broder T, Sritharan T, Chung L, Nagase H, Fields GB. Kinetic analysis of matrix metalloproteinase activity using fluorogenic triple-helical substrates. Biochem. 2001;40(19):5795–5803. doi: 10.1021/bi0101190. [DOI] [PubMed] [Google Scholar]
- 48.Lauer-Fields JL, Kele P, Sui G, Nagase H, Leblanc RM, Fields CG. Analysis of matrix metalloproteinase triple-helical peptidase activity with substrates incorporating fluorogenic L- or D-amino acids. Anal Biochem. 2003;321:105–115. doi: 10.1016/s0003-2697(03)00460-3. [DOI] [PubMed] [Google Scholar]
- 49.Welch AR, Holman CM, Browner MF, Gehring MR, Kan CC, Van Wart HE. Purification of human matrilysin produced in Escherichia coli and characterization using a new optimized fluorogenic peptide substrate. Arch Biochem Biophys. 1995;324(1):59–64. doi: 10.1006/abbi.1995.9929. [DOI] [PubMed] [Google Scholar]
- 50.Jin G, Huang X, Black R, Wolfson M, Rauch C, McGregor H, Ellestad G, Cowling R. A continuous fluorometric assay for tumor necrosis factor-α converting enzyme. Anal Biochem. 2002;302:269–275. doi: 10.1006/abio.2001.5549. [DOI] [PubMed] [Google Scholar]
- 51.Weimer S, Oertel K, Fuchsbauer HL. A quenched fluorescent dipeptide for assaying dispase- and thermolysin-like proteases. Anal Biochem. 2006;352:110–119. doi: 10.1016/j.ab.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 52.Cummings RT, Salowe SP, Cunningham BR, Wiltsie J, Park YW, Sonatore LM, Wisniewski D, Douglas CM, Hermes JD, Scolnick EM. A peptide based fluorescence resonance energy transfer assay for Bacillus anthrasis lethal factor protease. Proc Natl Acad Sci USA. 2002;99(10):6603–6606. doi: 10.1073/pnas.062171599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warfield R, Bardelang P, Saunders H, Chan WC, Penfold C, James R, Thomas NR. Internally quenched peptides for the study of lysostaphin: an antimicrobial protease that kills Staphylococcus aureus. Org Biomol Chem. 2006;4:3626–3638. doi: 10.1039/b607999g. [DOI] [PubMed] [Google Scholar]
- 54.Csuhai E, Juliano MA, St Pyrek J, Harms AC, Juliano L, Hersh LB. New fluorogenic substrates for N-arginine dibasic convertase. Anal Biochem. 1999;269:149–154. doi: 10.1006/abio.1999.4033. [DOI] [PubMed] [Google Scholar]
- 55.Carvalho KM, Boileau G, Camargo ACM, Juliano L. A highly selective assay for neutral endopeptidase based on the cleavage of a fluorogenic substrate related to Leuenkephalin. Anal Biochem. 1996;237:167–173. doi: 10.1006/abio.1996.0224. [DOI] [PubMed] [Google Scholar]
- 56.Araujo MC, Melo RI, Del Nery E, Alves MFM, Juliano MA, Casarini DE, Juliano L, Carmona AK. Internally quenched fluorogenic substrates for angiotensin I-converting enzyme. J Hypertens. 1999;17(5):665–672. doi: 10.1097/00004872-199917050-00010. [DOI] [PubMed] [Google Scholar]
- 57.Hawthorne SJ, Harriott P, Lim J, Turner AJ, Walker B, Williams CH. Evaluation of some fluorogenic substrates for continuous assay of aminopeptidase P. Anal Biochem. 1997;253:13–17. doi: 10.1006/abio.1997.2320. [DOI] [PubMed] [Google Scholar]
- 58.Stöckel-Maschek A, Stiebitz B, Koelsch R, Neubert K. A continuous fluorometric assay for aminopeptidase P detailed analysis of product inhibition. Anal Biochem. 2003;322:60–67. doi: 10.1016/S0003-2697(03)00464-0. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira V, Campos M, Hemerly JP, Ferro ES, Camargo ACM, Juliano MA, Juliano L. Selective neurotensin-derived internally quenched fluorogenic substrates for neurolysin (EC 3.4.24.16): comparison with thimet oligopeptidase (EC 3.4.24.15) and neprilysin (EC 3.4.24.11) Anal Biochem. 2001;292:257–265. doi: 10.1006/abio.2001.5083. [DOI] [PubMed] [Google Scholar]
- 60.Knight CG. A quenched fluorescent substrate for thimet peptidase containing a new fluorescent amino acid, DL-2-amino-3-(7-methoxy-4-coumaryl)propionic acid. Biochem J. 1991;274:45–48. doi: 10.1042/bj2740045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tisljar U, Knight CG, Barrett AJ. An alternative quenched fluorescent substrate for Pz-peptidase. Anal Biochem. 1990;186:112–115. doi: 10.1016/0003-2697(90)90582-t. [DOI] [PubMed] [Google Scholar]
- 62.Burchak ON, Mugherli L, Chatelain F, Balakirev MY. Fluorescein-based amino acids for solid phase synthesis of fluorogenic protease substrates. Bioorg Med Chem. 2006;14:2559–2568. doi: 10.1016/j.bmc.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Echeverria C, Rich DH. New intramolecularly quenched fluorogenic peptide substrates for the study of the kinetic specificity of papain. FEBS Lett. 1992;297(1–2):100–102. doi: 10.1016/0014-5793(92)80336-f. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Echeverria C, Rich DH. Kinetic studies of papain: effect of P3′ substituents and donor/acceptor pairs of intramolecularly quenched fluorogenic substrates. Lett Pept Sci. 1995;2:77–82. [Google Scholar]
- 65.Mittoo S, Sundstrom LE, Bradley M. Synthesis and evaluation of fluorescent probes for the detection of calpain activity. Anal Biochem. 2003;319:234–238. doi: 10.1016/s0003-2697(03)00324-5. [DOI] [PubMed] [Google Scholar]
- 66.Kuo CJ, Chi YH, Hsu JTA, Laing PH. Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate. Biochem Bioph Res Co. 2004;318:862–867. doi: 10.1016/j.bbrc.2004.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kao RK, To APC, Ng LWY, Tsui WHW, Lee TSW, Tsoi HW, Yuen KY. Characterization of SARS-CoV main protease and identification of biologically active small molecule inhibitors using a continuous fluorescence-based assay. FEBS Lett. 2004;576:325–330. doi: 10.1016/j.febslet.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaulent AM, Fahy AS, Know SR, Birtley JR, Roque-Rosell N, Curry S, Leatherbarrow RJ. A continuous assay for foot-and-mouth disease virus 3C protease activity. Anal Biochem. 2007;368:130–137. doi: 10.1016/j.ab.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 69.Serveau C, Lalmanach G, Hirata I, Scharfstein J, Juliano MA, Gauthier D. Discrimination of cruzipain, the major cysteine protease of Trypanosoma cruzi, and mammalian cathepsins B and L, by a pH-inducible fluorogenic substrate of trypanosomal cysteine proteinases. Eur J Biochem. 1999;259:275–280. doi: 10.1046/j.1432-1327.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee KB, Lee YC. Preparation of bifluorescent-labeled glycopeptides for glycoamidase assay. Method Enzymol. 1997;278:512–519. doi: 10.1016/s0076-6879(97)78027-3. [DOI] [PubMed] [Google Scholar]
- 71.Ferro V, Meldal M, Bock K. Synthesis of 2- and 2′-O-acetylated maltotriosides as potential fluorescence-quenched substrates for α-amylase. J Chem Soc Perk T. 1994;1(15):2169–2176. [Google Scholar]
- 72.Payre N, Cottaz S, Driguez H. Chemoenzymatic synthesis of a modified pentasaccharide as a specific substrate for a sensitive assay of α-amylase by fluorescence quenching. Angew Chem Int Ed. 1995;34(11):1239–1241. [Google Scholar]
- 73.Matsuoka K, Nishimura SI, Lee YC. A bi-fluorescence-labeled substrate for ceramide glycanase based on fluorescence energy transfer. Carbohyd Res. 1995;276:31–42. doi: 10.1016/0008-6215(95)00238-o. [DOI] [PubMed] [Google Scholar]
- 74.Cottaz S, Brasme B, Driguez H. A fluorescence quenched chitopentaose for the study of endo-chitinases and chitobiosidases. Eur J Biochem. 2000;267:5593–5600. doi: 10.1046/j.1432-1327.2000.01624.x. [DOI] [PubMed] [Google Scholar]
- 75.Armand S, Drouillard S, Schülein M, Henrissat B, Driguez H. A bi-functionalized fluorogenic tetrasaccharide as a substrate to study cellulases. J Biol Chem. 1997;272(5):2709–2713. doi: 10.1074/jbc.272.5.2709. [DOI] [PubMed] [Google Scholar]
- 76.Washiya K, Furuike T, Nakajima F, Lee YC, Nishimura SI. Design of fluorogenic substrates for continuous assay of sialyltransferase by resonance energy transfer. Anal Biochem. 2000;283:39–48. doi: 10.1006/abio.2000.4632. [DOI] [PubMed] [Google Scholar]
- 77.Takakusa H, Kikuchi K, Urano Y, Sakamoto S, Yamaguchi K, Nagano T. Design and synthesis of an enzyme-cleavable sensor molecule for phosphodiesterase activity based on fluorescence resonance energy transfer. J Am Chem Soc. 2002;124(8):1653–1657. doi: 10.1021/ja011251q. [DOI] [PubMed] [Google Scholar]
- 78.Berkessel A, Riedl R. Fluorescence reporters for phosphodiesterase activity. Angew Chem Int Ed. 1997;36(13–14):1481–1483. [Google Scholar]
- 79.Sato E, Yoshikawa M, Kanaoka Y. New fluorogenic substrates for phosphodiesterase I. Chem Pharm Bull. 1990;38(8):2287–2289. doi: 10.1248/cpb.38.2287. [DOI] [PubMed] [Google Scholar]
- 80.Rose TM, Prestwich GD. Fluorogenic phospholipids as head-group selective reporters of phospholipase A activity. ACS Chem Biol. 2006;1(2):83–92. doi: 10.1021/cb5000014. [DOI] [PubMed] [Google Scholar]
- 81.Thuren T, Virtanen JA, Somerharju PJ, Kinnunen PKJ. Phospholipase A2 assay using an intramolecularly quenched pyrene-labeled phospholipid analogue as a substrate. Anal Biochem. 1988;170:248–255. doi: 10.1016/0003-2697(88)90115-7. [DOI] [PubMed] [Google Scholar]
- 82.Rose TM, Prestwich GD. Synthesis and evaluation of fluorogenic substrates for phospholipase D and phospholipase C. Org Lett. 2006;8(12):2575–2578. doi: 10.1021/ol060773d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y, Babiak P, Reymond JL. Low background FRET substrates for lipases and esterases suitable for high throughput screening under basic (pH 11) conditions. Org Biomol Chem. 2006;4:1746–1754. doi: 10.1039/b601151a. [DOI] [PubMed] [Google Scholar]
- 84.Zandonella G, Haalck L, Spener F, Faber K, Paltauf F, Hermetter A. Enantiomeric perylene-glycerolipids as fluorogenic substrates for a dual wavelength assay of lipase activity and stereoselectivity. Chirality. 1996;8:481–489. doi: 10.1002/(SICI)1520-636X(1996)8:7<481::AID-CHIR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 85.Duque M, Graupner M, Stütz H, Wicher I, Zechner R, Paltauf F, Hermetter A. New fluorogenic triacylglycerol analogs as substrates for the determination and chiral discrimination of lipase activities. J Lipid Res. 1996;37:868–876. [PubMed] [Google Scholar]
- 86.Caturla F, Enjo J, Bernabeu MC, Le Serre S. New fluorescent probes for testing combinatorial catalysts with phosphodiesterase and esterase activities. Tetrahedron. 2004;60:1903–1911. [Google Scholar]
- 87.Li JJ, Geyer R, Tan W. Using molecular beacons as a sensitive fluorescence assay for enzymatic cleavage of single-stranded DNA. Nucleic Acids Res. 2000;28(11):e52. doi: 10.1093/nar/28.11.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray PC, Fortner A, Darbha GP. Gold nanoparticle based FRET assay for the detection of DNA cleavage. J Phys Chem B. 2006;110:20745–20748. doi: 10.1021/jp065121l. [DOI] [PubMed] [Google Scholar]
- 89.Jordan JA, Faas FJ, Braun ER, Trucco M. Exonuclease-released fluorescence detection of human parovirus B19 DNA. Mol Diagn. 1996;1(4):321–328. doi: 10.1054/MODI00100321. [DOI] [PubMed] [Google Scholar]
- 90.Jiang X, Belasco JG. Catalytic activation of multimeric RNase E and RNase G by 5′-monophosphorylated RNA. Proc Natl Acad Sci USA. 2004;101(25):9211–9216. doi: 10.1073/pnas.0401382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zelenko O, Neumann U, Brill W, Pieles U, Moser HE, Hofsteenge J. A novel fluorogenic substrate for ribonucleases. Synthesis and enzymatic characterization. Nucleic Acids Res. 1994;22(14):2731–2739. doi: 10.1093/nar/22.14.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He HQ, Ma XH, Liu B, Zhang XY, Chen WZ, Wang CW, Cheng SH. High-throughput real-time assay based on molecular beacons for HIV-1 integrase 3′-processing reaction. Acta Pharm Sinic. 2007;28(6):811–817. doi: 10.1111/j.1745-7254.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 93.Dorjsuren D, Wilson DM, III, Beard WA, McDonald JP, Austin CP, Woodgate R, Wilson SH, Simeonov A. A real time fluorescence method for enzymatic characterization of specialized human DNA polymerases. Nucleic Acids Res. 2009;37(19):e128. doi: 10.1093/nar/gkp641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Q, Li Q. Characterization of the 5′ to 3′ nuclease activity of Thermus aquticus DNA polymerase on fluorogenic double-stranded probes. Mol Cell Probe. 2009;23:188–194. doi: 10.1016/j.mcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Rehault S, Brillard-Bourdet M, Juliano MA, Juliano L, Gauthier F, Moreau T. New, Sensitive Fluorogenic Substrates for Human Cathepsin G Based on the Sequence of Serpin-Reactive Siter Loops. J Biol Chem. 1999;274(20):13810–13817. doi: 10.1074/jbc.274.20.13810. [DOI] [PubMed] [Google Scholar]
- 96.St Hilaire PM, Willert M, Juliano MA, Juliano L, Meldal M. Fluorescence-quenched solid phase combinatorial libraries in the characterization of cysteine protease substrate specificity. J Comb Chem. 1999;1:509–523. doi: 10.1021/cc990031u. [DOI] [PubMed] [Google Scholar]
- 97.Meldal M, Svendsen I, Juliano L, Juliano MA, Del Nery E, Scharfstein J. Inhibition of cruzipain visualized in a fluorescence quenched solid- phase inhibitor library assay. D-amino acid inhibitors for cruzipain, cathepsin B and cathepsin L. J Peptide Sci. 1998;4:83–91. doi: 10.1002/(sici)1099-1387(199804)4:2<83::aid-psc124>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 98.Meldal M, Svendsen I, Breddam K, Auzanneau FI. Portion-mixing peptide libraries of quenched fluorogenic substrates for complete subsite mapping of endoprotease specificity. Proc Natl Acad Sci USA. 1994;91:3314–3318. doi: 10.1073/pnas.91.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szabelski M, Rogiewicz M, Wiczk W. Fluorogenic peptide substrates containing benzoxazol-5-yl-alanine derivatives for kinetic assay of cysteine proteases. Anal Biochem. 2005;342:20–27. doi: 10.1016/j.ab.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 100.Charitos C, Tzougraki C, Kokotos G. Synthesis and fluorescence properties of intramolecularly quenched fluorogenic p-nitroanilides containing coumarin or quinolinone derivatives as fluorophores. J Peptide Res. 2000;56:373–381. doi: 10.1034/j.1399-3011.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- 101.Kokotos G, Tzougraki C. Synthesis and study of intramolecularly quenched fluorogenic substrates containing aminocoumarin or aminoquinolinone-type fluorophores. J Chem Soc Perk T. 1991;2(4):495–499. [Google Scholar]
- 102.Berthelot T, Talbot JC, Lain J, Déleris G, Latxague L. Synthesis of Nε-(7-diethylaminocoumarin-3-carboxyl)- and Nε-(7-methoxycoumarin-3-carboxyl)-L-Fmoc lysine as tools for protease cleavage detection by fluorescence. J Peptide Sci. 2005;11:153–160. doi: 10.1002/psc.608. [DOI] [PubMed] [Google Scholar]
- 103.Lohse J, Nielsen PE, Harrit N, Dahl O. Fluorescein conjugated lysine monomers for solid phase synthesis of fluorescent peptides and PNA oligomers. Bioconj Chem. 1997;8:503–509. doi: 10.1021/bc9700704. [DOI] [PubMed] [Google Scholar]
- 104.Anderson RA, Zhou J, Hecht SM. Fluorescence resonance energy transfer between unnatural amino acids in a structurally modified dihydrofolate reductase. J Am Chem Soc. 2002;124:9674–9675. doi: 10.1021/ja0205939. [DOI] [PubMed] [Google Scholar]
- 105.Hennig A, Roth D, Enderle T, Nau WM. Nanosecond time-resolved fluorescence protease assays. Chem Bio Chem. 2006;7:733–737. doi: 10.1002/cbic.200500561. [DOI] [PubMed] [Google Scholar]
- 106.Peranteau AG, Kuzmic P, Angell Y, Garcia-Echeverria C, Rich DH. Increase in the fluorescence upon the hydrolysis of tyrosine peptides: application to proteinase assays. Anal Biochem. 1995;227:242–245. doi: 10.1006/abio.1995.1276. [DOI] [PubMed] [Google Scholar]
- 107.Yonezawa H, Uchikoba T, Arima K, Kaneda M. Fluorogenic substrates for cathepsin D. Biosci Biotechnol Biochem. 1999;63(8):1471–1474. doi: 10.1271/bbb.63.1471. [DOI] [PubMed] [Google Scholar]
- 108.Law B, Weissleder R, Tung CH. Mechanism-Based Fluorescent Reporter for Protein Kinase A Detection. Chem Bio Chem. 2005;6:1361–1367. doi: 10.1002/cbic.200500027. [DOI] [PubMed] [Google Scholar]
- 109.Jares-Erijman E, Jovin TM. FRET imaging. Nat Biotech. 2003;21(11):1387–1395. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- 110.Ahn T, Kim JS, Choi HI, Yun CH. Development of peptide substrates for trypsin based on monomer/excimer fluorescence of pyrene. Anal Biochem. 2002;306:247–251. doi: 10.1006/abio.2002.5717. [DOI] [PubMed] [Google Scholar]
- 111.Packard BZ, Toptygin DD, Komoriya A, Brand L. Profluorescent protease substrates: intramolecular dimers described by the exciton model. Proc Natl Acad Sci USA. 1996;93:11640–11645. doi: 10.1073/pnas.93.21.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marmé N, Knemeyer JP, Wolfrum J, Sauer M. Highly sensitive protease assay using fluorescence quenching of peptide probes based on photoinduced electron transfer. Angew Chem Int Ed. 2004;43:3798–3801. doi: 10.1002/anie.200453835. [DOI] [PubMed] [Google Scholar]
- 113.Marmé N, Weston KD, Staudt J, Spatz J, Knemeyer JP. A fluorescence-based assay for exopeptidases using self-quenched peptide probes with single-molecule sensitivity. Int J Environ An Ch. 2005;85(9–11):741–751. [Google Scholar]
- 114.Hennig A, Florea M, Roth D, Enderle T, Nau WM. Design of peptide substrates for nanosecond time-resolved fluorescence assays of proteases: 2,3-diazabicyclo [2.2.2]oct-2-ene as a non-invasive fluorophore. Anal Biochem. 2007;360:255–265. doi: 10.1016/j.ab.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 115.Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, Whitney M, Roemer K, Tsien RY. Quantification of transcription and clonal selection of single living cells with β-lactamase as reporter. Science. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- 116.Xing B, Khanamiryan A, Rao J. Cell permeable near-infrared fluorogenic substrates for imaging β-lactamase activity. J Am Chem Soc. 2005;127:4158–4159. doi: 10.1021/ja042829+. [DOI] [PubMed] [Google Scholar]
- 117.Mizukami S, Watanabe S, Hori Y, Kikuchi K. Covalent protein labeling Based on a noncatalytic β-lactamase and a designed FRET substrate. J Am Chem Soc. 2009;131(14):5016–5017. doi: 10.1021/ja8082285. [DOI] [PubMed] [Google Scholar]
- 118.Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 119.Hendrickson HS, Hendrickson EK, Johnson ID, Farber SA. Intramolecularly quenched BODIPY-labeled phospholipid analogs in phospholipase A2 and platelet activating factor acetylhydrolase assays and in vivo fluorescence imaging. Anal Biochem. 1999;276:27–35. doi: 10.1006/abio.1999.4280. [DOI] [PubMed] [Google Scholar]
- 120.Feng L, Manabe K, Shope JC, Widmer S, DeWald DB, Prestwich GD. A real-time fluorogenic phospholipase A2 assay for biochemical and cellular activity measurements. Chem Biol. 2002;9:795–803. doi: 10.1016/s1074-5521(02)00168-0. [DOI] [PubMed] [Google Scholar]
- 121.Wichmann O, Wittbrodt J, Schultz C. A small molecule FRET probe to monitor phospholipase A2 activity in cells and organisms. Angew Chem Int Ed. 2006;45(3):508–512. doi: 10.1002/anie.200500751. [DOI] [PubMed] [Google Scholar]
- 122.Wichmann O, Gelb MH, Schultz C. Probing phospholipase A2 with fluorescent phospholipid substrates. Chem Bio Chem. 2007;8(13):1555–1569. doi: 10.1002/cbic.200600462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Komatsu T, Kikuchi K, Takakusa H, Hanaoka K, Ueno T, Kamiya M, Urano Y, Nagano T. Design and synthesis of an enzyme activity-based labeling molecule with fluorescence spectral change. J Am Chem Soc. 2006;128:15946–15947. doi: 10.1021/ja0657307. [DOI] [PubMed] [Google Scholar]
- 124.Pham W, Weissleder R, Tung CH. An azulene dimer as a near-infrared quencher. Angew Chem Int Ed. 2002;41(19):3659–3662. doi: 10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 125.Pham W, Choi Y, Weissleder R, Tung CH. Developing a peptide based near-infrared molecular probe for protease sensing. Bioconj Chem. 2004;15:1403–1407. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 126.Peng X, Chen H, Draney DR, Volcheck W, Schutz-Geschwender A, Olive DM. A non-fluorescent broad-range quencher dye for Förster resonance energy transfer assays. Anal Biochem. 2009;388:220–228. doi: 10.1016/j.ab.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 127.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Non-invasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3(10):668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 128.Watzke A, Kosec G, Kindermann M, Jeske V, Nestler HP, Turk V, Turk B, Wendt KU. Selective activity-based probes for cysteine cathepsins. Angew Chem Int Ed. 2008;47:406–409. doi: 10.1002/anie.200702811. [DOI] [PubMed] [Google Scholar]
- 129.Chen J, Liu TWB, Lo PC, Wilson BC, Zheng G. “Zipper” molecular beacons: a generalized strategy to optimize the performance of activatable protease probes. Bioconj Chem. 2009;20(10):1836–1842. doi: 10.1021/bc900207k. [DOI] [PubMed] [Google Scholar]
- 130.Takakusa H, Kikuchi K, Urano Y, Kojima H, Nagano T. A novel design method of ratiometric fluorescent probes based on fluorescence resonance energy transfer switching by spectral overlap integral. Chem Eur J. 2003;9(7):1479–1485. doi: 10.1002/chem.200390167. [DOI] [PubMed] [Google Scholar]
- 131.Bentolila LA, Ebenstein Y, Weiss S. Quantum Dots for In Vivo Small Animal Imaging. J Nucl Med. 2009;50(4):493–496. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Resch-Genger U, Grabolle M, Caviliere-Jaricot S, Nitschke R, Nann T. Quantum Dots versus Organic Dyes as Fluorecent Labels. Nat Methods. 2008;5(9):763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 133.Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z. Synthesis and Applcation of Quantum Dots FRET Based Protease Sensors. J Am Chem Soc. 2006;128:10378–10379. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 134.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE, Mattoussi H. Proteolytic Activity Monitored by Fluorescence Resonance Energy Transfer Through Quantum-Dot-Peptide Conjugates. Nat Mat. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 135.Xu C, Xing B, Rao J. A Self-Assembled Quantum Dot Probe for Detecting β-Lactamase Activity. Biochem Bioph Res Co. 2006;344:931–935. doi: 10.1016/j.bbrc.2006.03.225. [DOI] [PubMed] [Google Scholar]
- 136.Shi L, Rosenzweig N, Rosenzweig Z. Luminescent Quantum Dots Fluorescence Resonance Energy Transfer-Based Probes for Enzymatic Activity and Enzymatic Inhibitors. Anal Chem. 2007;79:208–214. doi: 10.1021/ac0614644. [DOI] [PubMed] [Google Scholar]
- 137.Huang S, Xiao Q, He ZK, Liu Y, Tinnefeld P, Su XR, Peng XN. A High Sensitive and Specific QDs FRET Bioprobe for MNase. Chem Comm. 2008;(45):5990–5992. doi: 10.1039/b815061c. [DOI] [PubMed] [Google Scholar]
- 138.Chang E, Miller JS, Sun J, Yu WW, Colvin VL, Drezek R, West JL. Protease Activated Quantum Dot Probes. Biochem Bioph Res Co. 2005;334:1317–1321. doi: 10.1016/j.bbrc.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 139.Boeneman K, Mei BC, Dennis AM, Bao G, Deschamps JR, Mattoussi H, Medintz IL. Sensing Caspase 3 Activity with Quantum Dot-Fluorescent Protein Assemblies. J Am Chem Soc. 2009;131:3828–3829. doi: 10.1021/ja809721j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gill R, Freeman R, Xu JP, Willner I, Winograd S, Shweky I, Banin U. Probing Biocatalytic Transformations with CdSe-ZnS QDs. J Am Chem Soc. 2006;128:15376–15377. doi: 10.1021/ja066636t. [DOI] [PubMed] [Google Scholar]
- 141.Medintz IL, Pons T, Tramell SA, Grimes AF, English DS, Blanco-Canosa JB, Dawson PE, Mattoussi H. Interactions between Redox Complexes and Semi-Conductor Quantum Dots Coupled via a Peptide Bridge. J Am Chem Soc. 2008;130:16745–16756. doi: 10.1021/ja805456x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fox ME, Szoka FC, Fréchet JMJ. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Accounts Chem Res. 2009;42(8):1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duncan R. The Dawning Era of Polymer Therapeutics. Nat Rev Drug Disc. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 144.Jones LJ, Upson RH, Haugland RP, Panchuk-Voloshina N, Zhou M, Haugland RP. Quenched BODIPY dye-Labeled casein substrates for the assay of protease activity by direct fluorescence measurement. Anal Biochem. 1997;251:144–152. doi: 10.1006/abio.1997.2259. [DOI] [PubMed] [Google Scholar]
- 145.Homer KA, Beighton D. Fluorometric determination of bacterial protease activity using fluorescein isothiocyanate-labeled proteins as substrates. Anal Biochem. 1990;191(133):137. doi: 10.1016/0003-2697(90)90399-t. [DOI] [PubMed] [Google Scholar]
- 146.Fitzgerald PC, Hartley RW. Polyethenoadenosine phosphate as a fluorogenic substrate for barnase. Anal Biochem. 1993;214:544–547. doi: 10.1006/abio.1993.1536. [DOI] [PubMed] [Google Scholar]
- 147.Callahan RJ, Bogdanov A, Jr, Fischman AJ, Brady TJ, Weissleder R. Pre-clinical evaluation and phase I clinical trial of a 99mTc-labeled synthetic polymer used in blood pool imaging. Am J Roentgenol. 1998;171(1):137–143. doi: 10.2214/ajr.171.1.9648777. [DOI] [PubMed] [Google Scholar]
- 148.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease activated near-infrared fluorescent probes. Nat Biotech. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 149.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconj Chem. 1999;10(5):892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 150.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- 151.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7(6):743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 152.Tung CH, Gerszten RE, Jaffer FA, Weissleder R. A novel near-infrared fluorescence sensor for detection of thrombin activation in blood. Chem Bio Chem. 2002;3(2–3):207–211. doi: 10.1002/1439-7633(20020301)3:2/3<207::AID-CBIC207>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 153.Shah K, Tung CH, Chang CH, Slootweg E, O’Loughlin T, Breakefield XO, Weissleder R. In vivo imaging of HIV protease activity in amplicon vector-transduced gliomas. Cancer Res. 2004;64(1):273–278. doi: 10.1158/0008-5472.can-03-1123. [DOI] [PubMed] [Google Scholar]
- 154.Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Design, synthesis, and characterization of urokinase plasminogen-activator-sensitive near-infrared reporter. Chem Biol. 2004;11(1):99–106. doi: 10.1016/j.chembiol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 155.Messerli SM, Prabhakar S, Tang Y, Shah K, Cortes ML, Murthy V, Weissleder R, Breakefield XO, Tung CH. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia. 2004;6(2):95–105. doi: 10.1593/neo.03214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 157.Kozloff KM, Quinti L, Patntirapong S, Hauschka PV, Tung CH, Weissleder R, Mahmood U. Non-invasive optical detection of cathepsin-K mediated fluorescence reveals osteoclast activity in vitro and in vivo. Bone. 2009;44:190–198. doi: 10.1016/j.bone.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marten K, Bremer C, Khazaie K, Sameni M, Sloane B, Tung CH, Weissleder R. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002;122:406–414. doi: 10.1053/gast.2002.30990. [DOI] [PubMed] [Google Scholar]
- 159.Bremer C, Tung CH, Bogdanov A, Jr, Weissleder R. Imaging of differing protease expression in breast cancers for detection of aggressive tumor phenotypes. Radiology. 2002;222(3):814–818. doi: 10.1148/radiol.2223010812. [DOI] [PubMed] [Google Scholar]
- 160.Jaffer FA, Tung CH, Gerszten RE, Weissleder R. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. Arterioscl Throm Vas. 2002;22(11):1929–1935. doi: 10.1161/01.atv.0000033089.56970.2d. [DOI] [PubMed] [Google Scholar]
- 161.Wunder A, Tung C-H, Müller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis. Arthritis Rheum. 2004;50(8):2459–2465. doi: 10.1002/art.20379. [DOI] [PubMed] [Google Scholar]
- 162.Figueiredo JL, Alencar H, Weissleder R, Mahmood U. Near infrared thoracoscopy of tumoral protease activity for improved detection of peripheral lung cancer. Int J Cancer. 2006;118:2672–2677. doi: 10.1002/ijc.21713. [DOI] [PubMed] [Google Scholar]
- 163.Nahrendorf M, Sosnovik DE, Waterman P, Swirski SK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leokocyte recruitment and protease activity in the healing myocardinal infarct. Circ Res. 2007;100(8):1218–1225. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 164.Cortez-Retamozo V, Swirski SK, Waterman P, Yuan H, Figueiredo JL, Newton AP, Upadhyay R, Vinegoni C, Kohler RH, Blois J, Smith A, Nahrendorf M, Josephson L, Weissleder R, Pittet MJ. Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation. J Clin Invest. 2008;118(12):4058–4067. doi: 10.1172/JCI36335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.von Burstin J, Eser S, Seidler B, Meining A, Bajbouj M, Mages J, Lang R, Kind AJ, Schnieke AE, Schmid RM, Schneider G, Saur D. Highly sensitive detection of early-stage pancreatic cancer by multimodal near-infrared molecular imaging in living mice. Int J Cancer. 2008;123:2138–2147. doi: 10.1002/ijc.23780. [DOI] [PubMed] [Google Scholar]
- 166.Sheth RA, Upadhyay R, Stangenberg L, Sheth R, Weissleder R, Mahmood U. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol Oncol. 2009;112:616–622. doi: 10.1016/j.ygyno.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Christen T, Nahrendorf M, Wildgruber M, Swirski SK, Aikawa E, Waterman P, Shimizu K, Weissleder R, Libby P. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Klohs J, Baeva N, Steinbrink J, Bourayou R, Boettcher C, Royl G, Megow D, Dirnag U, Priller J, Wunder A. In vivo near-infrared fluorescence imaging of matrix metalloprotein activity after cerebral ischemia. J Cerebr Blood F Met. 2009;29:1284–1292. doi: 10.1038/jcbfm.2009.51. [DOI] [PubMed] [Google Scholar]
- 169.Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J Med Chem. 2006;49(15):4715–4720. doi: 10.1021/jm051001a. [DOI] [PubMed] [Google Scholar]
- 170.Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008;7(3):118–131. [PMC free article] [PubMed] [Google Scholar]
- 171.Ternon M, Bradley M. Assay amplification-multiple valent fluorophores. Chem Comm. 2003;(19):2402–2403. doi: 10.1039/b308421c. [DOI] [PubMed] [Google Scholar]
- 172.Ternon M, Díaz-Mochón JJ, Belsom A, Bradley M. Dendrimers and combinatorial chemistry - tools for fluorescent enhancement in protease assays. Tetrahedron. 2004;60:8721–8728. [Google Scholar]
- 173.Thomas SW, Joly GD, Swager TM. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem Rev. 2007;107(4):1339–1386. doi: 10.1021/cr0501339. [DOI] [PubMed] [Google Scholar]
- 174.Kumaraswamy S, Bergstedt T, Shi X, Rininsland F, Kushon S, Xia W, Ley K, Achyuthan K, McBranch D, Whitten D. Fluorescent-Conjugated Polymer Superquenching Facilitates Highly Sensitive Detection of Proteases. Proc Natl Acad Sci USA. 2004;101(20):7511–7515. doi: 10.1073/pnas.0402367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pinto MR, Schanze KS. Amplified Fluorescence Sensing of Protease Activity with Conjugated Polyelectrolytes. Proc Natl Acad Sci USA. 2004;101(20):7505–7510. doi: 10.1073/pnas.0402280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wosnick JH, Mello CM, Swager TM. Synthesis and Application of Poly(phenylene Ethylene)s for Bioconjugation: A Conjugated Polymer-Based Fluorogenic Probe for Proteases. J Am Chem Soc. 2005;127:3400–3405. doi: 10.1021/ja043134b. [DOI] [PubMed] [Google Scholar]
- 177.Feng F, Tang Y, Wang S, Li Y, Zhu D. Continuous Fluorometric Assays for Acetylcholinesterase Activity and Inhibition with Conjugated Polyelectrolytes. Angew Chem Int Ed. 2007;46:7882–7886. doi: 10.1002/anie.200701724. [DOI] [PubMed] [Google Scholar]
- 178.Feng F, Tang Y, He F, Yu M, Duan X, Wang S, Li Y, Zhu D. Cationic Conjugated Polymer/DNA Complexes for Amplified Fluorescence Assays of Nucleases and Methyltransferases. Adv Mater. 2007;19:3490–3495. [Google Scholar]
- 179.Feng X, Duan X, Liu L, Feng F, Wang S, Li Y, Zhu D. Fluorescence Logic-Signal-Based Multiplex Detection of Nucleases with the Assembly of a Cationic Conjugated Polymer and Branched DNA. Angew Chem Int Ed. 2009;48:5316–5321. doi: 10.1002/anie.200901555. [DOI] [PubMed] [Google Scholar]
- 180.Chemburu S, Ji E, Casana Y, Wu Y, Burande T, Schanze KS, Lopez GP, Whitten D. Conjugated Polyelectrolyte Supported Bead Based Assays for Phospholipase A2 Activity. J Phys Chem B. 2008;112:14492–14499. doi: 10.1021/jp803358j. [DOI] [PubMed] [Google Scholar]
- 181.Zhang Y, Wang Y, Liu B. Peptide-mediated energy transfer between an anionic water-soluble conjugated polymer and texas red labeled DNA for protease and nuclease activity study. Anal Chem. 2009;81:3731–3737. doi: 10.1021/ac802488m. [DOI] [PubMed] [Google Scholar]
- 182.An L, Tang Y, Feng F, He F, Wang S. Water-Soluble Conjugated Polymers for Continuous and Sensitive Fluorescence Assays for Phosphatase and Peptidase. J Mater Chem. 2007;17:4147–4152. [Google Scholar]
- 183.Liu Y, Schanze KS. Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal Chem. 2008;80(22):8605–8612. doi: 10.1021/ac801508y. [DOI] [PubMed] [Google Scholar]
- 184.An L, Liu L, Wang S. Label-free, homogeneous, and fluorescence “turn-on” detection of protease using conjugated polyelectrolytes. Biomacromol. 2009;10(2):454–457. doi: 10.1021/bm801036h. [DOI] [PubMed] [Google Scholar]
- 185.Nilsson KPR, Hammarström P. Luminescent Conjugated Polymers: Illuminating the Dark Matters of Biology and Pathology. Adv Mater. 2008;20:2639–2645. [Google Scholar]
- 186.Ambade AV, Sandanaraj BS, Klaikherd A, Thayumanavan S. Fluorescent Polyelectrolytes as Protein Sensors. Polym Int. 2007;56:474–481. [Google Scholar]
- 187.de Silva AP, Gunarate N, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Signaling recognition events with fluorescent sensors and switches. Chem Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 188.Martin MM, Lindqvist L. The pH dependence of fluorescein fluorescence. J Lumin. 1975;10:381–390. [Google Scholar]
- 189.Beija M, Afonso CAM, Martinho JMG. Synthesis and application of rhodamine derivatives as fluorescent probes. Chem Soc Rev. 2009;38:2410–2433. doi: 10.1039/b901612k. [DOI] [PubMed] [Google Scholar]
- 190.Guilbault GG, Kramer DN. Resorufin butyrate and indoxyl acetate as fluorogenic substrates for cholinesterase. Anal Chem. 1965;37(1):120–123. doi: 10.1021/ac60220a031. [DOI] [PubMed] [Google Scholar]
- 191.Kramer DN, Guilbault GG. Resorufin acetate as substrate for determination of hydrolytic enzymes at low enzyme and substrate concentrations. Anal Chem. 1964;36(8):1662–1663. [Google Scholar]
- 192.Fernley HN, Walker PG. Kinetic behaviour of calf-intestinal alkaline phosphatase with 4-methylumbelliferyl phosphate. Biochem J. 1965;97(1):95–103. doi: 10.1042/bj0970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sherman WR, Robins E. Some 7-(β-D-glucopyranosyloxy)coumarins for use as fluorogenic substrates. Carbohyd Res. 1968;7:184–192. [Google Scholar]
- 194.Zimmerman M, Yurewicz EC, Patel G. A new fluorogenic substrate for chemotrypsin. Anal Biochem. 1976;70:258–262. doi: 10.1016/s0003-2697(76)80066-8. [DOI] [PubMed] [Google Scholar]
- 195.Zimmerman M, Ashe B, Yurewicz EC, Patel G. Sensitive assays for trypsin, elastase, and chemotrypsin using new fluorogenic substrates. Anal Biochem. 1977;78:47–51. doi: 10.1016/0003-2697(77)90006-9. [DOI] [PubMed] [Google Scholar]
- 196.Bonneau PR, Hasani F, Plouffe C, Malenfant E, LaPlante SR, Guse I, Ogilvie WW, Plante R, Davidson WC, Hopkins JL, Morelock MM, Cordingley MG, Déziel R. Inhibition of human cytomegalovirus protease by monocyclic β-lactam derivatives: kinetic chracterization using a fluorescent probe. J Am Chem Soc. 1999;121(13):2965–2973. [Google Scholar]
- 197.Kanaoka Y, Takahashi T, Nakayama H, Tanizawa K. New fluorogenic substrates for subtilisin. Chem Pharm Bull. 1985;33(4):1721–1724. doi: 10.1248/cpb.33.1721. [DOI] [PubMed] [Google Scholar]
- 198.Kanaoka Y, Takahashi T, Nakayama H, Takada K, Kimura T, Sakakibara S. Synthesis of a key fluorogenic amide, L-arginine-4-methylcoumaryl-7-amide (L-Arg-MCA) and its derivatives. Fluorescence assays for trypsin and papain. Chem Pharm Bull. 1977;25(11):3126–3128. [Google Scholar]
- 199.Kitson TM, Freeman GH. 3,4-Dihydro-3,4,6-trimethyl-2H,8H-pyrano-[3,2g]-1,3-benzoxazin-2,8-dione, a potential fluorogenic reporter group reagent for esterases - synthesis and interaction with chymotrypsin. Bioorg Chem. 2000;28:273–282. doi: 10.1006/bioo.2000.1180. [DOI] [PubMed] [Google Scholar]
- 200.Jean F, Boudreault A, Basak A, Seidah NG, Lazure C. Fluorescent peptidyl substrates as an aid in studying the substrate specificity of human prohormone convertase PC1 and human furin and designing a potent irreversible inhibitor. J Biol Chem. 1995;270(33):19225–19231. doi: 10.1074/jbc.270.33.19225. [DOI] [PubMed] [Google Scholar]
- 201.Noula C, Kokotos G, Barth T, Tzougraki C. New fluorogenic substrates for the study of secondary specificity of prolyl oligopeptidase. J Peptide Res. 1997;49:46–51. doi: 10.1111/j.1399-3011.1997.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 202.Kanaoka Y, Takahashi T, Nakayama H. A new fluorogenic substrate for aminopeptidase. Chem Pharm Bull. 1977;25:362–363. doi: 10.1248/cpb.25.362. [DOI] [PubMed] [Google Scholar]
- 203.Kanaoka Y, Takahashi T, Nakayama H, Ueno T, Sekine T. Synthesis of a new fluorogenic substrate for cystine aminopeptidase. Chem Pharm Bull. 1982;30(4):1485–1487. doi: 10.1248/cpb.30.1485. [DOI] [PubMed] [Google Scholar]
- 204.Abe N, Baba A, Kadowaki T, Okamoto K, Okazaki S, Asao T, Yamamoto K. Design and synthesis of sensitive fluorogenic substrates specific for Lys-gingipain. J Biochem. 2000;128:877–881. doi: 10.1093/oxfordjournals.jbchem.a022826. [DOI] [PubMed] [Google Scholar]
- 205.Melo RL, Barbosa Pozzo RC, Alves LC, Perissutti E, Caliendo G, Santagada V, Juliano L, Juliano MA. Synthesis and hydrolysis by cathepsin B of fluorogenic substrates with the general structure benzoyl-X-ARG-MCA containing non-natural basic amino acids at position X. Biochim Biophys Acta. 2001;1547:82–94. doi: 10.1016/s0167-4838(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 206.Basu D, Zhang N, Panigrahi AK, Horton TM, Pati D. Development and validation of a fluorogenic assay to measure separase enzyme activity. Anal Biochem. 2009;392(133):138. doi: 10.1016/j.ab.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 208.Warner TG, O’Brien JS. Synthesis of 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochem. 1979;18(13):2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 209.Vogel C, Pohlentz G. Synthesis of α-D-glucopyranosyl-(1-3)-α-D-mannopyranosyl-(1-7)-4-methylumbelliferone, a fluorogenic substrate for endo-α-1,2-mannosidase. J Carbohyd Chem. 2000;19(9):1247–1258. [Google Scholar]
- 210.Honda Y, Tanimori S, Kirihata M, Kaneko S, Tokuyase K, Hashimoto M, Watanabe T, Fukamizo T. Kinetic analysis of the reaction catalyzed by chitinase A1 from Bacillus circulans WL-12 toward the novel substrates, partially N-deacetylated 4-methylumbelliferyl chitobiosides. FEBS Lett. 2000;476:194–197. doi: 10.1016/s0014-5793(00)01729-4. [DOI] [PubMed] [Google Scholar]
- 211.Hood MA. Comparison of four methods for measuring chitinase activity and the application of the 4-MUF assay in aquatic environments. J Microbiol Meth. 1991;13:151–160. [Google Scholar]
- 212.Motabar O, Shi ZD, Goldin E, Liu K, Southall N, Sidransky E, Austin CP, Griffiths GL, Zheng W. A new resorufin-based α-glucosidase assay for high-throughput screening. Anal Biochem. 2009;390:79–84. doi: 10.1016/j.ab.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kohen E, Kohen C, Hirschberg JG, Santus R, Grabowski G, Mangel W, Gatt S, Prince J. An in situ study of beta-glucosidase activity in normal and Gaucher fibroblasts with fluorogenic probes. Cell Biochem Funct. 1993;11(3):167–177. doi: 10.1002/cbf.290110304. [DOI] [PubMed] [Google Scholar]
- 214.Shi ZD, Motabar O, Goldin E, Liu K, Southall N, Sidransky E, Austin CP, Griffiths GL, Zheng W. Synthesis and characterization of a new fluorogenic substrate for alpha-galactosidase. Anal Bioanal Chem. 2009;394:1903–1909. doi: 10.1007/s00216-009-2879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Takagaki K, Kon A, Kawasaki H, Nakamura T, Endo M. Preparation and application of a fluorogenic substrate for endo-β-xylosidase. J Biochem Biophys Meth. 1989;19:207–214. doi: 10.1016/0165-022x(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 216.Vršanská M, Nerinckx W, Claeyssens M, Biely P. An alternative approach for the synthesis of fluorogenic substrates of endo-β-(1-4)-xylanases and some applications. Carbohyd Res. 2008;343:541–548. doi: 10.1016/j.carres.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 217.Eneyskaya EV, Ivanen DR, Shabalin KA, Kulminskaya KA, Backinowsky LV, Brummer H, III, Neustroev KN. Chemo-enzymatic synthesis of 4-methylumbelliferyl β-(1-4)-D-xylooligosides: new substrates for β-D-xylanase assays. Org Biomol Chem. 2005;3:146–151. doi: 10.1039/b409583a. [DOI] [PubMed] [Google Scholar]
- 218.van Diggelen OP, Voznyi YV, Keulemans JLM, Schoonderwoerd K, Ledvinova J, Mengel E, Zschiesche M, Santer R, Harzer K. A new fluorometric enzyme assay for the diagnosis of Niemann-Pick A/B, with specificity of natural sphingomyelinase substrate. J Inherit Metab Dis. 2005;28:733–741. doi: 10.1007/s10545-005-0105-y. [DOI] [PubMed] [Google Scholar]
- 219.Aharoni A, Gaidukov L, Yagur S, Toker L, Silman I, Tawfik DS. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc Natl Acad Sci USA. 2004;101(2):482–487. doi: 10.1073/pnas.2536901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Amitai G, Adani R, Yacov G, Yishay S, Teitlboim S, Tveria L, Limanovich O, Kushnir M, Meshulam H. Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity. Toxicology. 2007;233:187–198. doi: 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 221.Amitai G, Adani R, Limanovich O, Teitlboim S, Yishay S, Tveria L, Yacov G, Meshulam H, Raveh L. Characterization of asymmetric fluorogenic phosphonates as probes for developing organophosphorus hydrolases with broader stereoselectivity. Chem Biol Interact. 2008;175:249–254. doi: 10.1016/j.cbi.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 222.Blum MM, Timperley CM, Williams GR, Thiermann H, Worek F. Inhibitory potency against human acetylcholinesterase and enzymatic hydrolysis of fluorogenic nerve agent mimics by human paraoxonase 1 and squid diisopropyl fluorophosphatase. Biochem. 2008;47:5216–5224. doi: 10.1021/bi702222x. [DOI] [PubMed] [Google Scholar]
- 223.Briseño-Roa L, Hill J, Notman S, Sellers D, Smith AP, Timperley CM, Wetherell J, Williams NH, Williams GR, Fersht AR, Griffiths AD. Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents. J Med Chem. 2006;49(1):246–255. doi: 10.1021/jm050518j. [DOI] [PubMed] [Google Scholar]
- 224.Halim M, Yee DJ, Sames D. Imaging induction of cytoprotective enzymes in intact human cells: coumberone, a metabolic reporter for human AKR1C enzymes reveals activation by panaxytriol, an active component of red ginseng. J Am Chem Soc. 2008;130(43):14123–14128. doi: 10.1021/ja801245y. [DOI] [PubMed] [Google Scholar]
- 225.Soukharev S, Hammond DJ. A fluorogenic substrate for detection of organophosphatase activity. Anal Biochem. 2004;327:140–148. doi: 10.1016/j.ab.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 226.Timperley CM, Casey KE, Notman S, Sellers DJ, Williams NE, Williams NH, Williams GR. Synthesis and anticholinesterase activity of some new fluorogenic analogues of organophosphorus nerve agents. J Fluorine Chem. 2006;127:1554–1563. [Google Scholar]
- 227.Ninkovic M, Riester D, Wirsching F, Dietrich R, Schweinhorst A. Fluorogenic assay for penicillin G acylase activity. Anal Biochem. 2001;292:228–233. doi: 10.1006/abio.2001.5078. [DOI] [PubMed] [Google Scholar]
- 228.Voznyi YV, Keulemans JLM, Kleijer WJ, Aula P, Gray GR, van Diggelen OP. Applications of a new fluorimetric enzyme assay for the diagnosis of aspartylglucosaminuria. J Inherit Metab Dis. 1993;16:929–934. doi: 10.1007/BF00711507. [DOI] [PubMed] [Google Scholar]
- 229.Mononen IT, Kaartinen VM, Williams JC. A fluorometric assay for glycosylasparaginase activity and detection of aspartylglycosaminuria. Anal Biochem. 1993;208:372–374. doi: 10.1006/abio.1993.1063. [DOI] [PubMed] [Google Scholar]
- 230.Wiederschain GY, Kozlova IK, Ilyina GS, Mikhaylova MA, Beyer EM. The use of glycosides of 6- and 8-acylamino-4-methylumbelliferone in studies of the specificity and properties of human lysosomal glycolipid hydrolases. Carbohyd Res. 1992;224:255–272. doi: 10.1016/0008-6215(92)84111-5. [DOI] [PubMed] [Google Scholar]
- 231.He W, Voznyi YV, Huijmans JGM, Geilen GC, Karpova EA, Dudukina TV, Zaremba J, van Diggelen OP, Kleijer WJ. Prenatal diagnosis of Sanfilippo disease type C using a simple fluorometric enzyme assay. Prenatal Diag. 1994;14:17–22. doi: 10.1002/pd.1970140104. [DOI] [PubMed] [Google Scholar]
- 232.Sekine T, Itakura H, Namihisa T, Takahashi T, Nakayama H, Kanaoka Y. A novel fluorometric ultramicro determination of serum γ-glutamyltranspeptidase activity. Chem Pharm Bull. 1981;29(11):3286–3289. doi: 10.1248/cpb.29.3286. [DOI] [PubMed] [Google Scholar]
- 233.Gillet SMFG, Pelletier JN, Keillor JW. A direct fluorometric assay for tissue transglutaminase. Anal Biochem. 2005;347:221–226. doi: 10.1016/j.ab.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 234.Linn CP, Mize PD, Hoke RA, Quante JM, Pitner JB. Synthesis of serine-AMC-carbamate: a fluorogenic tryptophanase substrate. Anal Biochem. 1992;200:400–404. doi: 10.1016/0003-2697(92)90486-q. [DOI] [PubMed] [Google Scholar]
- 235.Sicard R, Chen LS, Marsaioli AJ, Reymond JL. A fluorescence based assay for Baeyer-Villiger monooxygenases, hydroxylases and lactonases. Adv Synth Catal. 2005;347:1041–1050. [Google Scholar]
- 236.Renwick AB, Surry D, Price RJ, Lake BG, Evans DC. Metabolism of 7-benzyloxy-4-trifluoromethylcoumarin by human hepatic cytochrome P450 isoforms. Xenobiotica. 2000;30(10):955–968. doi: 10.1080/00498250050200113. [DOI] [PubMed] [Google Scholar]
- 237.Renwick AB, Lewis DFV, Fulford S, Surry D, Williams B, Worboys PD, Cai X, Wang RW, Price RJ, Lake BG, Evans DC. Metabolism of 2,5-bis(trifluoromethyl)-7-benzyloxy-4-trifluoromethylcoumarin by human hepatic CYP isoforms: evidence for selectivity towards CYP3A4. Xenobiotica. 2001;31(4):187–204. doi: 10.1080/00498250110043526. [DOI] [PubMed] [Google Scholar]
- 238.Yamazaki H, Inoue K, Mimura M, Oda Y, Guengerich FP, Shimada T. 7-Ethoxycoumarin O-deethylation catalyzed by cytochromes P450 1A2 and 2E1 in human liver microsomes. Biochem Pharmacol. 1996;51:313–319. doi: 10.1016/0006-2952(95)02178-7. [DOI] [PubMed] [Google Scholar]
- 239.Ekins S, Vandenbranden M, Ring BJ, Gillespie JS, Yang TJ, Gelboin HV, Wrighton SA. Further characterization of the expression in liver and catalytic activity of CPY2B6. J Pharmacol Exp Ther. 1998;286(3):1253–1259. [PubMed] [Google Scholar]
- 240.Marks BD, Goossens TA, Braun HA, Ozers MS, Smith RW, Lebakken C, Trubetskoy OV. High throughput screening assays for CYP2B6 metabolism and inhibition using fluorogenic vivid substrates. AAPS Pharm Sci. 2003;5(2) doi: 10.1208/ps050218. No page numbers given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mayer RT, Netter KJ, Heubel F, Hahnemann B, Buchheister A, Mayer GK, Burke MD. 7-Alkoxyquinolines: new fluorescent substrates for cytochrome P450 monooxygenases. Biochem Pharmacol. 1990;40(7):1645–1655. doi: 10.1016/0006-2952(90)90467-y. [DOI] [PubMed] [Google Scholar]
- 242.Nakamura K, Hanna IH, Cai H, Nishimura Y, Williams KM, Guengerich FP. Coumarin substrates for cytochrome P450 2D6 fluorescence assays. Anal Biochem. 2001;292:280–286. doi: 10.1006/abio.2001.5098. [DOI] [PubMed] [Google Scholar]
- 243.Onderwater RCA, Venhorst J, Commandeur JNM, Vermeulen NPE. Design, synthesis, and characterization of 7-methoxy-4-(aminomethyl)coumarin as a novel and selective cytochrome P450 2D6 substrate suitable for high throughput screening. Chem Res Toxicol. 1999;12:555–559. doi: 10.1021/tx980248q. [DOI] [PubMed] [Google Scholar]
- 244.Ono S, Hatanaka T, Hotta H, Satoh T, Gonzalez FJ, Tsutsui M. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of in vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 1996;26(7):681–693. doi: 10.3109/00498259609046742. [DOI] [PubMed] [Google Scholar]
- 245.Simpson DJ, Unkefer CJ, Whaley TW, Marrone BL. A mechanism-based fluorogenic probe for the cytochrome P-450 cholesterol side chain clevage enzyme. J Org Chem. 1991;56(18):5391–5396. [Google Scholar]
- 246.Stresser DM, Turner SD, Blanchard AP, Miller VP, Crespi CL. Cytochrome P450 fluorimetric substrates: identification of isoform-selective probes for rat CYP2D2 and human CYP3A4. Drug Metab Dispos. 2002;30(7):845–852. doi: 10.1124/dmd.30.7.845. [DOI] [PubMed] [Google Scholar]
- 247.Waxman DJ, Lapenson DP, Aoyama T, Gelboin HV, Gonzalez FJ, Korzekwa K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991;290(1):160–166. doi: 10.1016/0003-9861(91)90602-f. [DOI] [PubMed] [Google Scholar]
- 248.Donato MT, Gómez-Léchon MJ, Castell JV. A microassay for measuring cytochrome P450IA1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Anal Biochem. 1993;213:29–33. doi: 10.1006/abio.1993.1381. [DOI] [PubMed] [Google Scholar]
- 249.Engstler M, Talhouk JW, Smith RE, Schauer R. Chemical synthesis of 4-trifluoromethylumbelliferyl-α-D-N-acetylneuraminic acid glycosidase and its use for the fluorometric detection of poorly expressed natural and recombinant sialidases. Anal Biochem. 1997;250:176–180. doi: 10.1006/abio.1997.2205. [DOI] [PubMed] [Google Scholar]
- 250.Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- 251.Wolfbeis OS, Koller E, Hochmuth P. The unusually strong effect of a 4-cyano group upon electronic spectra and dissociation constants of 3-substituted 7-hydroxycoumarins. B Chem Soc Jpn. 1985;58:731–734. [Google Scholar]
- 252.Chilvers KF, Perry JD, James AL, Reed RH. Synthesis and evaluation of novel fluorogenic substrates for the detection of bacterial β-galactosidase. J Appl Microbiol. 2001;91:1118–1130. doi: 10.1046/j.1365-2672.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 253.Sun WC, Gee KR, Haugland RP. Synthesis of novel fluorinated coumarins: excellent UV-light excitable fluorescent dyes. Bioorg Med Chem Lett. 1998;8:3107–3110. doi: 10.1016/s0960-894x(98)00578-2. [DOI] [PubMed] [Google Scholar]
- 254.Ge Y, Antoulinakis EG, Gee KR, Johnson I. An ultrasensitive continuous assay for xylanase using the fluorogenic substrate 6,8-difluoro-4-methylumbelliferyl β-D-xylobioside. Anal Biochem. 2007;362:63–68. doi: 10.1016/j.ab.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 255.Gee KR, Sun WC, Bhalgat M, Upson RH, Klaubert DH, Latham KA, Haugland RP. Fluorogenic substrates based on fluorinated umbelliferones for continuous assays of phosphatases and β-galactosidases. Anal Biochem. 1999;273:41–48. doi: 10.1006/abio.1999.4202. [DOI] [PubMed] [Google Scholar]
- 256.Perry JD, James AL, Morris KA, Oliver M, Chilvers KF, Reed RH, Gould FK. Evaluation of novel fluorogenic substrates for the detection of glycosidases in Escherichia coli and enterococci. J Appl Microbiol. 2006;101(5):977–985. doi: 10.1111/j.1365-2672.2006.03018.x. [DOI] [PubMed] [Google Scholar]
- 257.Welte S, Baringhaus KH, Schmider W, Müller G, Petry S, Tennagels N. 6,8-Difluoro-4-methylumbiliferyl phosphate: a fluorogenic substrate for protein tyrosine phosphatases. Anal Biochem. 2005;338:32–38. doi: 10.1016/j.ab.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 258.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of β-galactosidase activity using far red fluorescent switch. Cancer Res. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- 259.Ho NH, Weissleder R, Tung CH. Development of water soluble far-red fluorogenic dyes for enzyme sensing. Tetrahedron. 2006;62:578–585. [Google Scholar]
- 260.Ho NH, Weissleder R, Tung CH. Development of a dual fluorogenic and chromogenic dipeptidyl peptidase IV substrate. Bioorg Med Chem Lett. 2006;16:2599–2602. doi: 10.1016/j.bmcl.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 261.Chiba K, Sato E, Hoshi M. Detection of in vivo proteosome activity in a starfish oocyte using membrane-impermeant substrate. J Biochem. 1997;122(2):286–293. doi: 10.1093/oxfordjournals.jbchem.a021751. [DOI] [PubMed] [Google Scholar]
- 262.Sakaue M, Motoyama Y, Yamamoto K, Shiba T, Teshima T, Chiba K. Quantitative measurement of caspase-3 activity in a living starfish egg. Biochem Bioph Res Co. 2006;350:878–883. doi: 10.1016/j.bbrc.2006.09.119. [DOI] [PubMed] [Google Scholar]
- 263.Sato E, Matsuhisa A, Sakashita M, Kanaoka Y. New water soluble fluorogenic amine. 7-Aminocoumarin-4-methanesulfonic ccid (ACMS) and related substrates for proteinases. Chem Pharm Bull. 1988;36(9):3496–3502. doi: 10.1248/cpb.36.3496. [DOI] [PubMed] [Google Scholar]
- 264.Rosser BG, Powers SP, Gores GJ. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J Biol Chem. 1993;268(31):23593–23600. [PubMed] [Google Scholar]
- 265.Mitra A, Barrios AM. Highly sensitive peptide-based probes for protein tyrosine phosphatase activity utilizing a fluorogenic mimic of phosphotyrosine. Bioorg Med Chem Lett. 2005;15:5142–5145. doi: 10.1016/j.bmcl.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 266.Mitra S, Barrios AM. A series of peptide-based, fluorogenic probes for protein tyrosine phosphatase activity. Anal Biochem. 2007;370:249–251. doi: 10.1016/j.ab.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 267.Guilbault GG, Kramer DN. Fluorometric determination of lipase, acylase, alpha- and gamma-chymotrypsin and inhibitors of these enzymes. Anal Chem. 1964;36(2):409–412. [Google Scholar]
- 268.Leytus SP, Melhado L, Mangel WF. Rhodamine-based compounds as fluorogenic substrates for serine proteases. Biochem J. 1983;209:299–307. doi: 10.1042/bj2090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Liu J, Bhalgat M, Zhang C, Diwu Z, Hoyland B, Klaubert DH. Fluorescent molecular probes V: a sensitive caspase-3 substrate for fluorimetric assays. Bioorg Med Chem Lett. 1999;9:3231–3236. doi: 10.1016/s0960-894x(99)00566-1. [DOI] [PubMed] [Google Scholar]
- 270.Hug H, Los M, Hirt W, Debatin KM. Rhodamine-110 linked amino acids and peptides as substrates to measure caspase activity upon apoptosis induction in intact cells. Biochem. 1999;38:13906–13911. doi: 10.1021/bi9913395. [DOI] [PubMed] [Google Scholar]
- 271.Melhado L, Peltz SW, Leytus SP, Mangel W. p-Guanidinobenzoic acid esters of fluorescein as active site titrants of serine proteases. J Am Chem Soc. 1982;104:7299–7306. [Google Scholar]
- 272.Leytus SP, Patterson WL, Mangel W. New class of sensitive and selective fluorogenic substrates for serine proteases. Biochem J. 1983;215:253–260. doi: 10.1042/bj2150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Steinfeld R, Fuhrmann JC, Gärtner J. Detection of tripeptidyl peptidase I activity in living cells by fluorogenic substrates. J Histochem Cytochem. 2006;54(9):991–996. doi: 10.1369/jhc.5A6900.2006. [DOI] [PubMed] [Google Scholar]
- 274.Mugherli L, Burchak ON, Chatelain F, Balakirev MY. Fluorogenic ester substrates to assess proteolytic activity. Bioorg Med Chem Lett. 2006;16:4488–4491. doi: 10.1016/j.bmcl.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 275.Ganesh S, Klingel S, Kahle H, Valet G. Flow cytometric determination of aminopeptidase activities in viable cells using fluorogenic rhodamine 110 substrates. Cytometry. 1995;20:334–340. doi: 10.1002/cyto.990200409. [DOI] [PubMed] [Google Scholar]
- 276.Grant SK, Sklar JG, Cummings RT. Development of novel assays for proteolytic enzymes using rhodamine-based fluorogenic substrates. J Biomol Screen. 2002;7(6):531–540. doi: 10.1177/1087057102238627. [DOI] [PubMed] [Google Scholar]
- 277.Li J, Petrassi M, Tumunut C, Masick BT, Trussell C, Harris JL. Substrate optimization for monitoring cathepsin C activity in live cells. Bioorg Med Chem. 2009;17:1064–1070. doi: 10.1016/j.bmc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 278.Claveau D, Riendeau D, Mancini JA. Expression, maturation, and rhodamine-based fluorescence assay of human cathepsin K expressed in CHO cells. Biochem Pharmacol. 2000;60:759–769. doi: 10.1016/s0006-2952(00)00381-6. [DOI] [PubMed] [Google Scholar]
- 279.Graziano V, McGrath WJ, DeGruccio AM, Dunn JJ, Mangel W. Enzymatic activity of the SARS coronavirus main proteinase dimer. FEBS Lett. 2006;580:2577–2583. doi: 10.1016/j.febslet.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gee KR. Novel fluorogenic substrates for acid phosphatase. Bioorg Med Chem Lett. 1999;9:1395–1396. doi: 10.1016/s0960-894x(99)00199-7. [DOI] [PubMed] [Google Scholar]
- 281.Kim EJ, Kang DO, Love DC, Hanover JA. Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohyd Res. 2006;341:971–982. doi: 10.1016/j.carres.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ålander J, Johansson K, Dahlström Heuser V, Farebo H, Järvliden J, Abe H, Shibata A, Ito M, Ito Y, Morgenstern R. Characterization of a new fluorogenic substrate for microsomal glutathione transferase 1. Anal Biochem. 2009;390:52–56. doi: 10.1016/j.ab.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 283.Wang Q, Scheigetz J, Gilbert M, Snider J, Ramachandran C. Fluorescein monophosphates as fluorogenic substrates for protein tyrosine phosphatases. Biochim Biophys Acta. 1999;1431:14–23. doi: 10.1016/s0167-4838(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 284.Zaikova TA, Rukavishnikov AV, Birrell GB, Griffith OH, Keana JFW. Synthesis of fluorogenic substrates for continuous assay of phosphatidylinositol-specific phospholipase C. Bioconjug Chem. 2001;12(2):307–313. doi: 10.1021/bc0001138. [DOI] [PubMed] [Google Scholar]
- 285.Cai SX, Zhang HZ, Guastella J, Drewe J, Yang W, Weber E. Design and synthesis of rhodamine-110 derivative and caspase-3 substrate for enzyme and cell-based fluorescence assay. Bioorg Med Chem Lett. 2001;11:39–42. doi: 10.1016/s0960-894x(00)00590-4. [DOI] [PubMed] [Google Scholar]
- 286.Zhang HZ, Kasibhatla S, Guastella J, Tseng B, Drewe J, Cai SX. N-Ac-DEVD-N′-(polyfluorobenzoyl)-R110: novel cell permeable fluorogenic aaspase substrates for the detection of caspase activity and apoptosis. Bioconj Chem. 2003;14(2):458–463. doi: 10.1021/bc0256188. [DOI] [PubMed] [Google Scholar]
- 287.Lorey S, Faust J, Mrestani-Klaus C, Kähne T, Ansorge S, Neubert K, Bühling F. Transcellular proteolysis demonstrated by novel cell surface-associated substrates of dipeptidyl peptidase IV (CD26) J Biol Chem. 2002;277(36):33170–33177. doi: 10.1074/jbc.M200798200. [DOI] [PubMed] [Google Scholar]
- 288.Wang ZQ, Liao J, Diwu Z. N-DEVD-N′-Morpholinecarbonyl-rhodamine 110: novel caspase-3 fluorogenic substrates for cell-based apoptosis assay. Bioorg Med Chem Lett. 2005;15:2335–2338. doi: 10.1016/j.bmcl.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 289.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc. 2005;127(13):4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 290.Ueno T, Urano Y, Setsukinai K, Takakusa H, Kojima H, Kikuchi K, Ohkubo K, Fukuzami S, Nagano T. Rational principles for modulating fluorescence properties of fluorescein. J Am Chem Soc. 2004;126(43):14079–14085. doi: 10.1021/ja048241k. [DOI] [PubMed] [Google Scholar]
- 291.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. An enzymatically activated fluorescence probe for targeted tumor imaging. J Am Chem Soc. 2007;129(13):3918–3929. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fujikawa Y, Urano Y, Komatsu T, Hanaoka K, Kojima H, Terai T, Inoue H, Nagano T. Design and synthesis of highly fluorogenic substrates for glutathione S-transferase and application for activity imaging in living cells. J Am Chem Soc. 2008;130(44):14533–14543. doi: 10.1021/ja802423n. [DOI] [PubMed] [Google Scholar]
- 293.Huang Z, Terpetschnig E, You W, Haugland RP. 2-(2′-Phosphoryloxyphenyl)-4(3H)-quinazolinone derivatives as fluorogenic precipitating substrates of phosphatases. Anal Biochem. 1992;207:32–39. doi: 10.1016/0003-2697(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 294.Zhou M, Upson RH, Diwu Z, Haugland RP. A fluorogenic substrate for β-glucuronidase: applications in fluorometric, polyacrylamide gel and histochemical analysis. J Biochem Biophys Meth. 1996;33:197–205. doi: 10.1016/s0165-022x(96)00026-7. [DOI] [PubMed] [Google Scholar]
- 295.Diwu Z, Lu Y, Upson RH, Zhou M, Klaubert DH, Haugland RP. Fluorescent molecular probes I. The synthesis and biological properties of an ELF β-glucuronidase substrate that yields fluorescent precipitates at the enzymatic active site. Tetrahedron. 1997;53(21):7159–7164. [Google Scholar]
- 296.Van Noorden CJF, Boonacker E, Bissell ER, Meijer AJ, van Marle J, Smith RE. Ala-Pro-cresyl violet, a synthetic fluorogenic substrate for the analysis of kinetic parameters of dipeptidyl peptidase IV (CD26) in individual living rat hepatocytes. Anal Biochem. 1997;252:71–77. doi: 10.1006/abio.1997.2312. [DOI] [PubMed] [Google Scholar]
- 297.Van Noorden CJF, Jonges TGN, van Marle J, Bissell ER, Griffini P, Jans M, Snel J, Smith RE. Heterogeneous suppression of experimentally induced colon cancer metastasis in rat liver lobes by inhibition of extracellular cathepsin B. Clin Exp Metastas. 1998;16:159–167. doi: 10.1023/a:1006524321335. [DOI] [PubMed] [Google Scholar]
- 298.Boonacker E, Elferink S, Bardai A, Fleischer B, Van Noorden CJF. Fluorogenic substrate [Ala-Pro]2-cresyl violet but not Ala-Pro-rhodamine 110 is cleaved specifically by PPIV activity: a study in living Jurkat cells and CD26/PPIV-transfected Jurkat cells. J Histochem Cytochem. 2003;51(7):959–968. doi: 10.1177/002215540305100711. [DOI] [PubMed] [Google Scholar]
- 299.Butenas S, Orfeo T, Lawson JH, Mann KG. Aminonapthalenesulfonamides, a new class of modifiable detecting groups and their use in substrates for serine protease enzymes. Biochem. 1992;31(23):5399–5411. doi: 10.1021/bi00138a023. [DOI] [PubMed] [Google Scholar]
- 300.Butenas S, Ribarik N, Mann KG. Synthetic substrates for human factor VIIa and factor VIIa-tissue factor. Biochem. 1993;32(26):6531–6538. doi: 10.1021/bi00077a006. [DOI] [PubMed] [Google Scholar]
- 301.Butenas S, Drungilaite V, Mann KG. Fluorogenic substrates for activated protein C: substrate structure-efficiency correlation. Anal Biochem. 1995;225:231–241. doi: 10.1006/abio.1995.1148. [DOI] [PubMed] [Google Scholar]
- 302.Butenas S, Kalafatis M, Mann KG. Analysis of tissue plasminogen activator specificity using peptidyl fluorogenic substrates. Biochem. 1997;36(8):2123–2131. doi: 10.1021/bi9617670. [DOI] [PubMed] [Google Scholar]
- 303.Cen H, Mao F, Aronchik I, Fuentes RJ, Firestone GL. DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB J. 2008;22(7):2243–2252. doi: 10.1096/fj.07-099234. [DOI] [PubMed] [Google Scholar]
- 304.Harnois-Pontoni M, Monsigny M, Mayer R. Hydrosoluble fluorogenic substrates for plasmin. Anal Biochem. 1991;193:248–255. doi: 10.1016/0003-2697(91)90017-n. [DOI] [PubMed] [Google Scholar]
- 305.Monsigny M, Kieda C, Maillet T. Assay for proteolytic activity using a new fluorogenic substrate (peptidyl-3-amino-9-ethyl-carbazole); quantitative determination of lipopolysaccharide at the level of one picogram. EMBO J. 1982;1(3):303–306. doi: 10.1002/j.1460-2075.1982.tb01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Miller SPF, French SA, Kaneski CR. Synthesis and characterization of a novel lysosomotropic enzyme substrate that fluoresces at intracellular pH. J Org Chem. 1991;56(1):30–34. [Google Scholar]
- 307.Harris AL, Gregory JS, Maycock AL, Graybill TL, Osifo K, Schmidt SJ, Dolle RE. Characterization of a continuous fluorogenic assay for calpain I. Kinetic evaluation of peptide aldehydes, halomethyl ketones and (acyloxy)methyl ketones as inhibitors of the enzyme. Bioorg Med Chem Lett. 1995;5(4):393–398. [Google Scholar]
- 308.Nicoll-Griffith DA, Chauret N, Houle R, Day SH, D’Antoni M, Silva JM. Use of a benzyloxy-substituted lactone cyclooxygenase-2 inhibitor as a selective fluorescent probe for CYP3A activity in primary cultured rat and human hepatocytes. Drug Metab Dispos. 2004;32:1509–1515. doi: 10.1124/dmd.32.12.. [DOI] [PubMed] [Google Scholar]
- 309.Chandran SS, Dickson KA, Raines RT. Latent fluorophore based on the trimethyl lock. J Am Chem Soc. 2005;127:1652–1653. doi: 10.1021/ja043736v. [DOI] [PubMed] [Google Scholar]
- 310.Lavis LD, Chao YZ, Raines RT. Latent blue and red fluorophores based on the trimethyl lock. Chem Bio Chem. 2006;7(8):1151–1154. doi: 10.1002/cbic.200500559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lavis LD, Chao TY, Raines RT. Fluorogenic label for biomolecular imaging. ACS Chem Biol. 2006;1(4):252–260. doi: 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yatzeck MM, Lavis LD, Chao TY, Chandran SS, Raines RT. A highly sensitive fluorogenic probe for cytochrome P450 activity in live cells. Bioorg Med Chem Lett. 2008;18(5864):5866. doi: 10.1016/j.bmcl.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Huang ST, Lin YL. New latent fluorophore for DT diaphorase. Org Lett. 2006;8(2):265–268. doi: 10.1021/ol052635e. [DOI] [PubMed] [Google Scholar]
- 314.Huang ST, Peng YX, Wang KL. Synthesis of a new long wavelength latent fluorimetric indicator for analytes determination in the DT-diaphorase coupling dehydrogenase assay system. Biosens Bioelectron. 2008;23:1793–1798. doi: 10.1016/j.bios.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 315.Ho NH, Weissleder R, Tung CH. A self-immolative reporter for β-galactosidase sensing. Chem Bio Chem. 2007;8(5):560–566. doi: 10.1002/cbic.200600386. [DOI] [PubMed] [Google Scholar]
- 316.Jones GB, Crasto CF, Mathews JE, Xie L, Mitchell MO, El-Shafey A, D’Amico A, Bubley GJ. An image contrast agent selectively activated by prostate specific antigen. Bioorg Med Chem. 2006;14:418–425. doi: 10.1016/j.bmc.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 317.Richard JA, Meyer Y, Jolivel V, Massonneau M, Dumeunier R, Vaudry D, Vaudry H, Renard PY, Romieu A. Latent fluorophores based on a self-immolative linker strategy and suitable for protease sensing. Bioconjug Chem. 2008;19(8):1707–1718. doi: 10.1021/bc8001997. [DOI] [PubMed] [Google Scholar]
- 318.Richard JA, Massonneau M, Renard PY, Romieu A. 7-Hydroxycoumarin-hemicyanine hybrids: a new class of far-red emitting fluorogenic dyes. Org Lett. 2008;10(19):4175–4178. doi: 10.1021/ol801582w. [DOI] [PubMed] [Google Scholar]
- 319.Amir RJ, Shabat D. Domino dendrimers. Adv Polym Sci. 2006;192:59–93. [Google Scholar]
- 320.Sagi A, Weinstain R, Karton N, Shabat D. Self-immolative polymers. J Am Chem Soc. 2008;130(16):5434–5435. doi: 10.1021/ja801065d. [DOI] [PubMed] [Google Scholar]
- 321.Danieli E, Shabat D. Molecular probe for enzymatic activity with dual output. Bioorg Med Chem. 2007;15:7318–7324. doi: 10.1016/j.bmc.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 322.Weinstain R, Baran PS, Shabat D. Activity-linked labeling of enzymes by self-immolative polymers. Bioconjug Chem. 2009;20(9):1783–1791. doi: 10.1021/bc9002037. [DOI] [PubMed] [Google Scholar]
- 323.Yee DJ, Balsanek V, Sames D. New tools for molecular imaging of redox metabolism: development of a fluorogenic probe for 3α-hydroxysteroid dehydrogenases. J Am Chem Soc. 2004;126(8):2282–2283. doi: 10.1021/ja039799f. [DOI] [PubMed] [Google Scholar]
- 324.Wierzchowski J, Ogiela M, Iwanska B, Shugar D. Selective fluorescent and fluorogenic substrates for purine nucleoside phosphorylases from various sources, and direct fluorimetric determination of enzyme levels in human and animal blood. Anal Chim Acta. 2002;472:63–74. [Google Scholar]
- 325.Wierzchowski J, Holmquist B, Vallee BL. Determination of human serum alcohol dehydrogenase using isozyme-specific fluorescent substrates. Anal Chem. 1992;64:181–186. doi: 10.1021/ac00026a017. [DOI] [PubMed] [Google Scholar]
- 326.Yee DJ, Balsanek V, Bauman DR, Penning TM, Sames D. Fluorogenic metabolic probes for direct activity readout of redox enzymes: selective measurement of human AKR1C2 in living cells. Proc Natl Acad Sci USA. 2006;103(36):13304–13309. doi: 10.1073/pnas.0604672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Froemming MK, Sames D. Harnessing functional plasticity of enzymes: a fluorogenic probe for imaging 17β-HSD10-dehydrogenase, an enzyme involved in Alzheimer’s and Parkinson’s Diseases. J Am Chem Soc. 2007;129(46):14518–14522. doi: 10.1021/ja072601x. [DOI] [PubMed] [Google Scholar]
- 328.Chen G, Yee DJ, Gubernator NG, Sames D. Design of optical switches as metabolic indicators: new fluorogenic probes for monoamine oxidases (MAO A and B) J Am Chem Soc. 2005;127(13):4544–4545. doi: 10.1021/ja0428457. [DOI] [PubMed] [Google Scholar]
- 329.Ling KQ, Sayre LM. Discovery of a sensitive, selective, and tightly binding fluorogenic substrate of bovine plasma amine oxidase. J Org Chem. 2009;74(1):339–350. doi: 10.1021/jo8018945. [DOI] [PMC free article] [PubMed] [Google Scholar]