Abstract
Resistin-like molecule (RELM) α belongs to a family of secreted mammalian proteins that have putative immunomodulatory functions. Recent studies have identified a pathogenic role for RELMα in chemically-induced colitis through effects on innate cell populations. However, whether RELMα regulates intestinal adaptive immunity to enteric pathogens is unknown. Here, we employed Citrobacter rodentium as a physiologic model of pathogenic Escherichia coli-induced diarrheal disease, colitis and Th17 cell responses. In response to Citrobacter, RELMα expression was induced in intestinal epithelial cells, infiltrating macrophages and eosinophils of the infected colons. Citrobacter-infected RELMα−/− mice exhibited reduced infection-induced intestinal inflammation, characterized by decreased leukocyte recruitment to the colons and reduced immune cell activation compared to wild-type mice. Interestingly, Citrobacter colonization and clearance were unaffected in RELMα−/− mice, suggesting that the immune stimulatory effects of RELMα following Citrobacter infection were pathologic rather than host-protective. Further, infected RELMα−/− mice exhibited decreased CD4+ T cell expression of the pro-inflammatory cytokine IL-17A. To directly test whether RELMα promoted Citrobacter-induced intestinal inflammation via IL-17A, infected WT and IL-17A−/− mice were treated with recombinant RELMα. RELMα treatment of Citrobacter-infected WT mice exacerbated intestinal inflammation and IL-17A expression whereas IL-17A−/− mice were protected from RELMα-induced intestinal inflammation. Finally, infected RELMα−/− mice exhibited reduced levels of serum IL-23p19 compared to WT mice, and RELMα−/− peritoneal macrophages showed deficient IL-23p19 induction. Together, these data identify a pro-inflammatory role for RELMα in bacterial-induced colitis and suggest that the IL-23/Th17 axis is a critical mediator of RELMα-induced inflammation.
INTRODUCTION
The intestine is continuously exposed to a multitude of antigens, including commensal bacteria and potentially dangerous pathogens. In response to intestinal pathogen infection, the initiation of a mucosal immune response, including activation of immune cells such as macrophages and T cells as well as production of effector cytokines, is essential for host protection and survival. However, if this inflammatory response is engaged against innocuous antigens or sustained beyond pathogen clearance, it can lead to inflammatory disorders such as food allergies and inflammatory bowel disease. Delineating the cellular and molecular pathways that regulate intestinal immunity and/or intestinal inflammation could allow for the design of new strategies to achieve the optimal balance between promoting host immunity while limiting excessive inflammation.
Resistin-like molecule (RELM) α is upregulated in several infectious and inflammatory settings including helminth infection, allergic airway inflammation and colitis (1–5). RELMα belongs to the RELM family of secreted mammalian proteins, including two human RELM proteins, both of which are upregulated in several inflammatory disorders (6–9). In an experimental model of chemically-induced colitis with dextran sodium sulfate (DSS), RELMα was pathogenic and promoted the activation of innate immune cells in the intestine, including macrophages and eosinophils (2, 3). In addition to activation of the innate immune response, we have previously demonstrated a potent immunomodulatory role for RELMα in regulating CD4+ T cell responses, suggesting that RELMα regulates adaptive immunity (10). Further, other studies have identified essential functions for the related protein RELMβ in intestinal inflammation models and immunity to intestinal helminth pathogens (11–13). However, whether RELMα modulates adaptive immune responses in intestinal immunity and inflammation is unknown.
Employing both DSS and Citrobacter rodentium as models of injury-induced or infection-induced intestinal inflammation, respectively, we demonstrate a pathogenic role for RELMα in promoting colitis through stimulating adaptive CD4+ T cell responses, and provide data that suggest RELMα is an upstream regulator of the pro-inflammatory cytokine IL-17A. Following exposure to DSS, RELMα−/− mice were protected from excessive intestinal inflammation, and ameliorated disease severity was associated with reduced CD4+ T cell-derived IL-17A.
To test if the immune-stimulatory effects of RELMα in the colon may be beneficial for host adaptive immunity to enteric pathogens, we employed the natural gastrointestinal pathogen of mice Citrobacter rodentium. Citrobacter belongs to the group of attaching and effacing bacteria including enteropathogenic (EPEC) and enterohemorrhagic (EHEC) Escherichia coli, which are major causative agents of diarrheal diseases (14). Diarrheal diseases affect an estimated 1.5 billion individuals each year and the associated dehydration is the second most common cause of infant mortality globally (15, 16). Immunity to Citrobacter is dependent on innate and adaptive immunity and several immune factors including IL-17A (17–21). In addition to defining the critical factors that are important for resistance to enteric bacterial infections, Citrobacter infection has been used as a model for IBD, as it induces colonic inflammation characterized by crypt hyperplasia, thickening of the mucosa and inflammatory cell infiltrate in WT mice (22).
Following Citrobacter infection, RELMα expression was upregulated early and expressed at the site of infection by epithelial cells, infiltrating macrophages and eosinophils. Employing RELMα−/− mice and through administration of recombinant RELMα, we demonstrate that RELMα promoted intestinal antigen presenting cell activation, Citrobacter-specific Th17 cell responses and intestinal inflammation. Although Th17 cells are necessary for optimal immunity to Citrobacter (18), RELMα−/− mice did not exhibit significant differences in Citrobacter clearance compared to wild-type (WT) mice. Critically, Citrobacter-specific Th1 cell responses in RELMα−/− mice were not impaired, and mice could successfully eradicate Citrobacter, suggesting that targeting RELMα to prevent intestinal inflammation may not significantly compromise host intestinal immunity to bacterial pathogens. Further, RELMα-mediated intestinal inflammation was abrogated in IL-17A−/− mice. These data place RELMα upstream of IL-17A and suggest that RELMα-directed inflammation requires IL-17A expression. Finally, infected RELMα−/− mice exhibited reduced serum levels of Th17-associated cytokine IL-23p19 compared to infected WT mice, and peritoneal macrophages isolated from naïve RELMα−/− mice showed impaired LPS-induced IL-23p19 expression, suggesting that RELMα promotes Th17 cell responses through stimulatory effects on macrophages. In conclusion, using two models of intestinal inflammation, we present data that identifies a previously unrecognized pathway where RELMα exacerbates colitis through the IL-23/IL-17A immune axis.
MATERIALS AND METHODS
Mice
WT C57BL/6 mice were purchased from the Jackson laboratory or bred in-house. RELMα−/− mice were generated as previously described (10). IL-17A−/− mice were kindly provided by Y. Iwakura, and bred at the University of Pennsylvania. Mice were maintained in a specific pathogen-free facility. All experiments were carried out under the guidelines of the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Dextran sodium sulfate
Dextran sodium sulfate (MP Biomedicals, Solon, OH) was added to drinking water at 5% weight/volume throughout the course of the experiment. Mice were monitored daily for morbidity (piloerection, lethargy), weight loss and rectal bleeding. Severity of colitis (1–4) was scored as an average of the following parameters: A/ Feces: normal, 0; pasty, semi-formed, 1; sticky, 2; sticky with some blood, 3; completely liquid, bloody or unable to defecate after 10 minutes, 4; B/ Rectal bleeding: no blood, 0; visible blood in rectum, 1–2; visible blood on fur, 3–4; C/ General appearance: normal, 0; piloerect, 1; lethargic and piloerect, 2; lethargic and hunched, 3; motionless, sickly, 4.
Citrobacter rodentium infection model
Mice were infected by oral gavage with 0.2 ml of an overnight culture in Luria broth containing approximately 5 × 108 CFU of wild-type C. rodentium as previously described (19). Where indicated, control PBS or recombinant RELMα (10 µg, Peprotech) was injected intraperitoneal in 100µL volumes. For bacterial counts, fecal pellets were collected, weighed, homogenized in PBS and serial dilutions were plated on MacConkey Agar (Sigma) and incubated overnight at 37°C. Bacterial colonies were counted the following day.
Histological staining
At necropsy, 1 cm section of the distal colon was removed and flushed with PBS followed by fixing in 4% paraformaldehyde and wax embedded, or frozen in OCT for cryosections. 5 µm sections were prepared and stained for H&E or with alcian blue-periodic acid Schiff’s reagent (PAS). Blinded clinical scoring of Citrobacter-infected mice was performed according to the following criteria: crypt hyperplasia (1–5) and mural inflammation/edema (1–5). For immunofluorescence (IF) and immunohistochemistry (IH), sections were dewaxed and stained with rabbit polyclonal anti-RELMα (Peprotech). For IF, dewaxed sections were stained with anti-RELMα and biotinylated anti-Siglec-F (R&D systems), followed by secondary staining with Cy2-conjugated anti-rabbit secondary antibody and Cy3-conjugated streptavidin (Jackson Laboratory), and counterstaining with DAPI (Molecular Probes). For IH, the DAB Peroxidase Substrate kit (Vector Lab) was used according to manufacturer’s instructions.
Isolation of immune cells for analysis
At necropsy, mesenteric lymph nodes and spleens were harvested and single cell suspensions prepared. For lamina propria cell isolation, the colon was harvested, flushed with PBS, and cut into 1 cm pieces. To strip epithelial cells, colonic tissue was incubated with shaking in 5% FBS, 1mM EDTA, 1mM DTT in PBS at 37°C for 20 minutes. Intestinal epithelial leukocytes were further isolated by shaking for 20 minutes in 1mM EDTA/PBS. To obtain lamina propria lymphocytes, the remaining tissue was digested in a shaker with collagenase/dispase (Roche, 0.5mg/mL) and DNAse Type IV (Sigma, 30µg/mL) for 30 minutes followed by isolation of live cells by Percoll gradient. Recovered cells were stained with Aqua live/dead stain (Molecular Probes) followed by standard surface staining for flow cytometric analysis with fluorochrome-conjugated antibodies (eBioscience, BD Bioscience). For RELMα intracellular staining, cells were fixed and permeabilized using the fixation/ permeabilization kit (eBioscience), and biotinylated anti-RELMα (1 µg/mL; Peprotech) followed by fluorochrome-conjugated streptavidin.
For peritoneal macrophage cultures, peritoneal lavage cells from naïve mice were recovered by thorough washing of the peritoneal cavity with 10 mL PBS. Peritoneal cells were plated in 96-well flat-bottomed plates at 1×105 cells/well for 1–2 hours, washed in warm media to enrich for adherent peritoneal macrophages, and stimulated with LPS (25 ng/mL, Sigma) and IFNγ (20 ng/mL). At time-points indicated, supernatants were recovered for analysis by ELISA, and cells resuspended in RLT buffer (Qiagen) for RNA isolation.
Cytokine analysis
To examine CD4+ T cell activation, single cell suspensions from spleens and/or mesenteric lymph nodes were stimulated for 4 hours ex vivo with PMA (50 ng/mL), Ionomycin (500 ng/mL) and Brefeldin A (10 µg/mL) (all from Sigma-Aldrich), or cultured for 48 to 72 hours in medium alone, freeze-thawed Citrobacter antigen (30µg/mL) or αCD3/αCD28 (eBioscience, 1µg/mL) followed by a brief (4-hour) PMA/Ionomycin stimulation in the presence of BFA. Cells were surface and intracellular stained with the combination of fluorochrome antibodies as indicated (obtained from eBioscience and BD Biosciences) using the Cytofix/Cytoperm kit according to manufacturer’s instructions (BD Biosciences). Stained cells were acquired on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.). To confirm analysis of CD4+ T cells, cells were also examined for CD3 and/or TCRβ surface expression. For restimulation cultures, cell-free supernatants were recovered and cytokine production measured by sandwich ELISA. RELMα ELISAs were performed on serum recovered by cardiac puncture at necropsy, or on 1 cm distal colonic tissue mechanically homogenized in PBS. For RELMα ELISA, anti-RELMα capture antibody and biotinylated anti-RELMα detection antibody (both from Peprotech) were used.
Real-time RT PCR
Colonic tissue RNA was isolated by TRIzol (Invitrogen) and peritoneal macrophage RNA by the RNeasy kit (Qiagen) in accordance with the manufacturer’s instructions. cDNA was generated and analyzed by real-time PCR using SYBR Green technology (Applied Biosystems) with customized primers (Qiagen). Reactions were run on the GeneAmp 7500 Sequence Detection System (Applied Biosystems). Results were standardized to the housekeeping gene β-actin.
Statistical analysis
Results represent the mean ± S.E.M. of individual animals or replicate wells. Statistical significance was determined by the two-tailed Student’s t test, one-way ANOVA or two-way ANOVA using Prism GraphPad software (version 4). Results were considered significant when *P<0.05.
RESULTS
RELMα promotes DSS-induced intestinal inflammation and Th17 cell responses
Previous studies reported that RELMα was pro-inflammatory in response to DSS, where it promoted innate immune cell activation and pro-inflammatory chemokine and cytokine expression in DSS-exposed mice (2, 3). Since DSS-induced intestinal inflammation is mediated both by innate and adaptive immune cells (23), and given recent findings that RELMα regulates CD4+ T cell responses (10), we first examined whether, in addition to regulation of innate immune cell activation, RELMα also regulated CD4+ T cell responses in this model.
Following 5% DSS treatment in the drinking water as a model for acute DSS colitis, wild-type (WT) C57BL/6 mice exhibited increased expression of Retnla (the gene encoding RELMα) in the colon (Fig S1A), and recruitment of RELMα+ cells to the lamina propria (Fig. S1B). Consistent with previous studies showing that RELMα expression promoted intestinal inflammation, RELMα−/− mice exhibited less severe DSS-induced weight loss (Fig. S1C) and reduced disease severity at day 7, as measured by fecal consistency, rectal bleeding and general appearance (Fig. S1D). Histological examination of colonic tissue sections from day 7 DSS-treated mice revealed that RELMα−/− animals were protected from DSS-induced colonic lesions and demonstrated normal crypt architecture, lack of ulceration and less severe inflammatory cell infiltration than WT controls (Fig. S1E).
Intestinal inflammation resulting from 5% DSS treatment is associated with CD4+ Th1 and Th17 cell activation (24, 25). To test whether RELMα regulated these helper T cell subsets, mesenteric lymph node cells (mLN) from DSS-treated WT or RELMα−/− mice were polyclonally stimulated and IFN-γ and IL-17A production examined by ELISA. In comparison to DSS-treated WT mice, mLN cells from DSS-treated RELMα−/− mice exhibited equivalent IFN-γ production but significantly reduced IL-17A production (Fig. S1F). Further, intracellular flow cytometric analysis revealed significantly reduced CD4+ T cell-derived IL-17A in the absence of RELMα (Fig. S1G). Associated with reduced Th17 cell responses in RELMα−/− mice, real-time PCR analysis of the colons of DSS-treated WT and RELMα−/− mice revealed reduced expression of factors associated with Th17 cell polarization including Rorc, Il23a and Il17a (Fig. S1H). Collectively, this provides the first demonstration that RELMα contributes to CD4+ Th17-mediated inflammation and implicates RELMα in exacerbating intestinal inflammation following DSS exposure.
Citrobacter rodentium infection induces local and systemic RELMα expression
Although Th17 cell activation and IL-17A production are associated with multiple inflammatory diseases including colitis, arthritis and asthma, IL-17A expression is necessary for host immunity to several fungal and bacterial pathogens (18, 26–29). To test the hypothesis that RELMα-mediated IL-17A expression is host-protective in the context of infection-induced inflammation, we employed Citrobacter rodentium, a natural bacterial pathogen of mice that colonizes the colon, induces intestinal inflammation (22) and requires IL-17A for optimal clearance (18, 20).
Quantification of Citrobacter loads in infected WT C57BL/6 mice revealed detectable Citrobacter colonization at day 3 post-infection, maximal bacterial burden at day 9 post-infection and clearance by day 21 post-infection (Fig. 1A). Colons from naïve and Citrobacter-infected mice were recovered for Retnla mRNA and RELMα protein quantification. Compared to naïve mice, RELMα expression in the colon was upregulated at days 3 and 9 post-infection, correlating with maximal fecal Citrobacter burdens (Fig. 1B, C). Examination of RELMα protein expression by immunohistochemistry of colon tissue sections revealed Citrobacter-induced RELMα expression in the intestinal crypts and lamina propria (Fig. 1D). High power magnification of anti-RELMα immunofluorescent-stained sections (Fig. 1E, green) confirmed staining by intestinal epithelial cells in the crypts (C) and by macrophages and Siglec-F+ eosinophils in the lamina propria (LP). In addition to expression of RELMα locally at mucosal sites, previous reports have shown that RELMα can be detected systemically (2). Quantification of RELMα serum levels by ELISA revealed that Citrobacter infection induced increased and maintained systemic RELMα production (Fig. 1F).
Figure 1. Citrobacter rodentium infection induces RELMα expression.
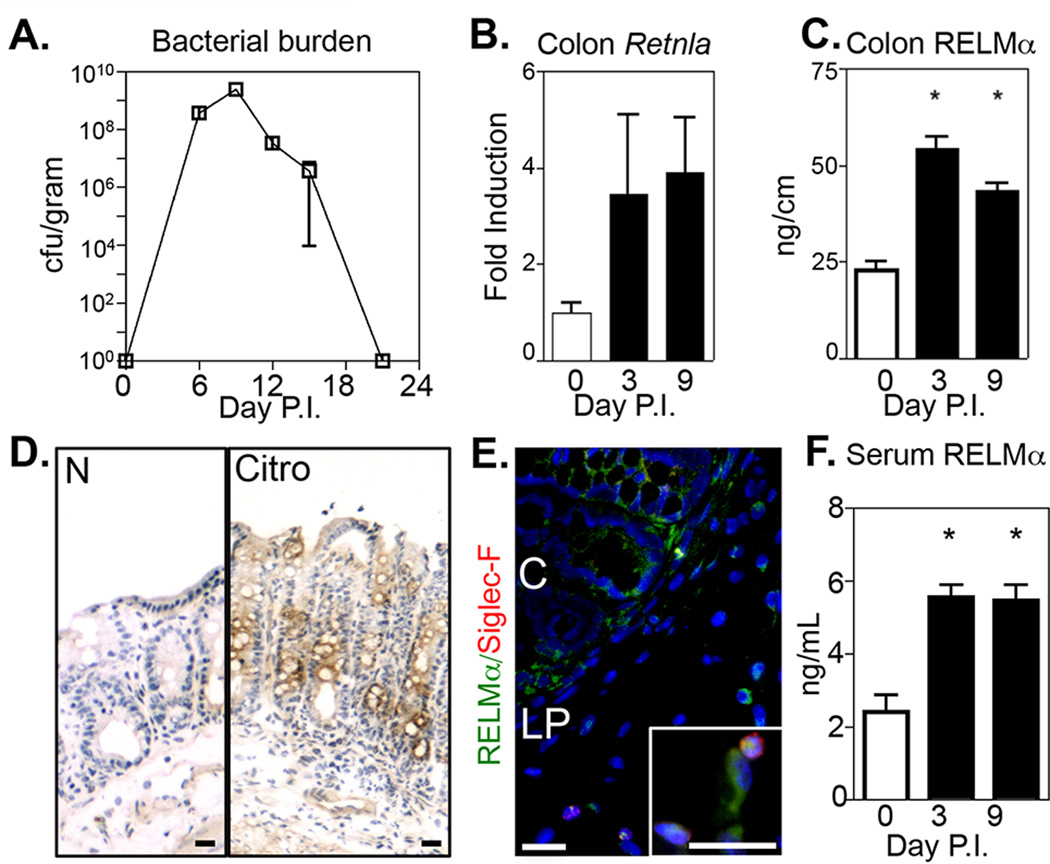
C57BL/6 mice were infected with Citrobacter. A. Bacterial burdens in the feces were measured. B. Retnla mRNA in colon tissue was quantified by real-time PCR as fold induction over naïve. C. RELMα protein was quantified in naïve and Citrobacter-infected colon tissue homogenate by ELISA. D–E. Localization of RELMα expression in the colon was examined by immunohistochemistry (D, RELMα, brown) and by immunofluorescent staining (E, RELMα, green; Siglec-F, red; DAPI, blue) at day 6 post-infection. C, crypt; LP, lamina propria. Bar, 20 µm. F. RELMα serum levels. * P<0.05. Data are representative of 3 experiments with 2–4 mice per group.
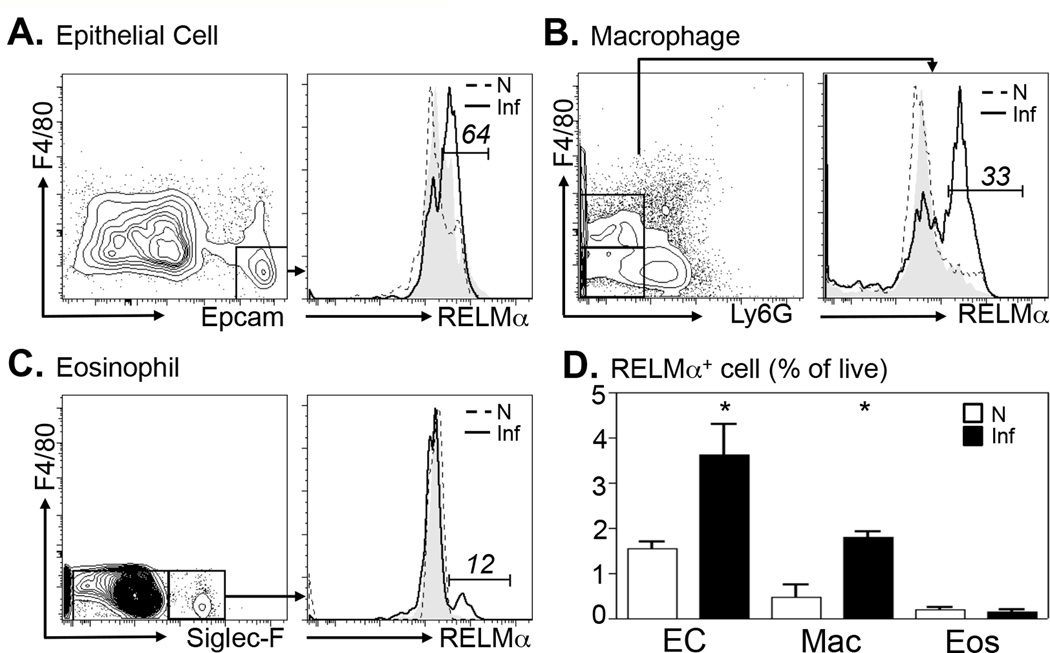
To quantify the cell populations in the colon that express RELMα following Citrobacter infection, cells isolated from the colonic tissue of naïve or day 10 Citrobacter-infected mice were analyzed by intracellular RELMα staining. To control for background staining and inherent autofluorescence of colonic cell preparations, colon cells from infected RELMα−/− mice were also examined. Viable cells were gated, and intestinal epithelial cells were determined according to Epcam surface expression (Fig. 2A). Colonic epithelial cells (EC) from naïve mice (Fig. 2A, dashed histogram) did not exhibit significant positive RELMα staining in comparison to control RELMα−/− EC (grey histogram). Following Citrobacter infection, there was a noticeable shift in RELMα+ cells with 64% of EC staining positive for RELMα (black histogram). In addition, colonic F4/80+Ly6G− macrophages (Fig. 2B) and F4/80−SiglecF+ eosinophils (Fig. 2C) exhibited infection-induced RELMα expression. Given previous studies showing dendritic cell expression of RELMα (5, 30), CD11c+F4/80− colon cells were examined but no RELMα positive staining above background was observed (data not shown). To determine the relative contribution of RELMα by EC, macrophages (Macs) and eosinophils (Eos), the frequency of RELMα+ cells as a percentage of live cells was quantified (Fig. 2D). Although significant infection-induced increases in RELMα expression by EC and Macs were observed, there was no obvious contribution of RELMα by eosinophils due to the low frequency of this population in the infected colons. Together, these data employ two separate methods to identify epithelial cells and macrophages as dominant cellular sources of RELMα in the colon following exposure to Citrobacter.
Figure 2. Citrobacter rodentium infection induces RELMα expression in epithelial cells, macrophages and eosinophils.
Colons of naïve (N, dashed) or day 10-infected (Inf, solid line) WT mice were recovered for flow cytometric analysis of intracellular RELMα. Plots shown were previously gated on live cells. A. Epithelial cells (EC) were gated as Epcam+F4/80− cells. B. Macrophages (Mac) were gated as F4/80+Ly6G− cells. C. Eosinophils (Eos) were gated as Ly6GF4/80−SiglecF+ cells. Numbers indicated represent frequency of positive cells in infected WT mice. For control RELMα staining (filled grey graph), gated epithelial cells (A), macrophages (B) or eosinophils (C) from day 10-infected RELMα−/− mice were stained with anti-RELMα. D. RELMα+ cell populations in the colon as a proportion of live cells was measured. *P<0.05. Data from 3 naïve or infected mice per group are representative of 3 separate experiments.
RELMα−/− mice are resistant to Citrobacter-induced colitis
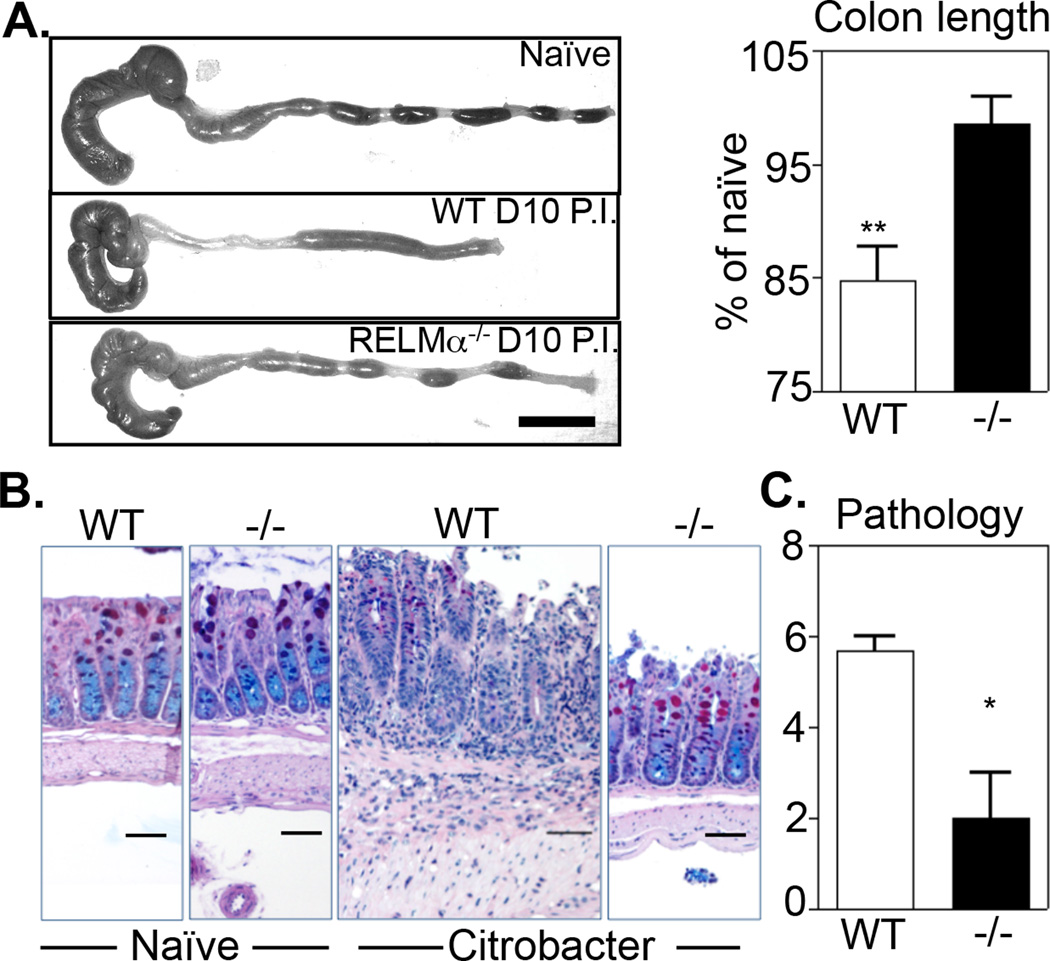
To examine whether the up-regulation of RELMα played a functional role in enteric bacterial infection, WT or RELMα−/− mice were infected with Citrobacter rodentium and sacrificed at day 10 post-infection, a time point that correlates with maximal bacterial burden, intestinal inflammation and RELMα expression. Macroscopic examination of the colons of naïve and infected mice revealed that infected WT mice exhibited characteristic signs of Citrobacter-induced colonic inflammation, including loose stools and significant colon shortening (Fig. 3A). In contrast, infected RELMα−/− mice exhibited minimal signs of infection-induced inflammation with fecal pellet consistency and colon lengths that were comparable to naïve mice. Examination of PAS/Alcian blue-stained colonic tissue sections did not reveal histological differences between naive WT and RELMα−/− colons at steady state (Fig. 3B, left panels). Citrobacter infection induced severe colitis in WT mice characterized by leukocyte infiltration in the lamina propria and submucosa, reduced mucin production, crypt hyperplasia, and thickening of the muscularis externa. In contrast, colons from RELMα−/− mice were minimally affected and exhibited little to no intestinal inflammation (Fig. 3B, right panels). Blind clinical scoring of colon sections from infected WT and RELMα−/− mice confirmed that there was significantly reduced Citrobacter-induced intestinal inflammation in the absence of RELMα (Fig. 3C). Taken together, these data reveal a pro-inflammatory function for RELMα in enteric bacterial infection.
Figure 3. RELMα−/− mice are resistant to Citrobacter-induced colitis.
WT or RELMα−/− mice were infected with Citrobacter for 10 days. A. Infection-induced colon shortening was determined and quantified as % of uninfected controls. Bar, 1 cm. B–C. Histologic examination of infection-induced inflammation in PAS/Alcian blue stained sections of distal colonic tissue was performed and scored. Bar, 50 µm. Data are representative of 4 experiments with 3–4 infected mice per group.**P<0.01. *P<0.05.
Ameliorated Citrobacter-induced intestinal inflammation in RELMα−/− mice is associated with reduced immune cell activation and lower Th17 cell responses
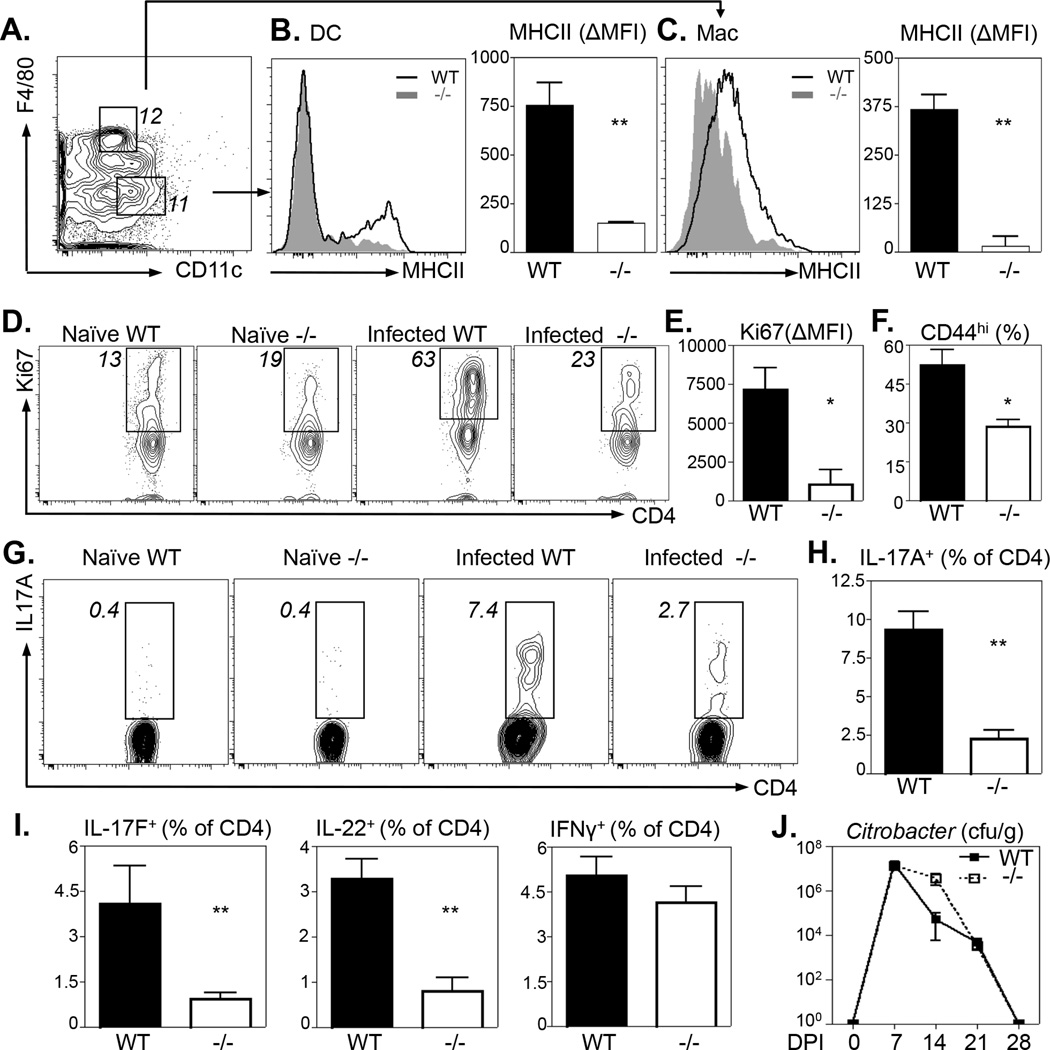
Previous studies demonstrated an immunomodulatory role for RELMα on antigen presenting cells and T cells (2, 10). We therefore examined if the ameliorated intestinal inflammation observed in RELMα−/− mice correlated with reduced intestinal dendritic cell (DC), macrophage and CD4+ T cell activation. First, no significant differences in the frequency of DCs, monocytes or macrophage populations were detected between Citrobacter-infected WT and RELMα−/− mice (Fig. S2A–C). Additionally, there were no obvious differences in surface MHCII expression in macrophages or DCs isolated from the colons of naïve WT or RELMα−/− mice (Fig. S2D–E). However, following Citrobacter infection, colonic DCs from WT mice exhibited increased surface MHCII expression (Fig. 4A–B, black histogram) compared to DCs isolated from RELMα−/− mice (Fig. 4B, grey histogram). Quantification of infection-induced MHCII expression by ΔMFI revealed that DCs from the colon of RELMα−/− mice exhibited significantly reduced activation in response to Citrobacter infection (Fig. 4B, right). In addition to defective DC activation, RELMα−/− colonic macrophages also exhibited significantly reduced MHCII up-regulation compared to WT mice (Fig. 4C).
Figure 4. Ameliorated Citrobacter-induced intestinal inflammation in RELMα−/− mice is associated with reduced immune cell activation and Th17 cell responses.
WT and RELMα−/− mice were infected with Citrobacter for 10 days followed by leukocyte preparations of the colonic tissue. A. Gating strategy of live, CD45+Ly6G−Ly6C− for CD11c+ Dendritic Cells (DC) and F4/80+ macrophages (Mac). Number denotes frequency of gated cells. B–C. DCs (B) and Macs (C) were analyzed for MHC class II expression. Delta Mean Fluorescence Intensity (ΔMFI) was calculated as the difference between naïve and infected MHCII MFI. D–F. CD4+CD3+ T cells from the mLN were analyzed ex vivo for expression of Ki67 (D–E) and CD44 (F). G–I. mLN from Citrobacter-infected WT and RELMα−/− mice were stimulated with Citrobacter antigen for 48 hours followed by intracellular cytokine staining for CD4+ T cell-derived IL-17A (G–H), IL-17F, IL-22 and IFNγ (I). J. Citrobacter burdens from fecal pellets harvested from WT and RELMα−/− mice throughout the course of infection. **P<0.01, *P<0.05. Data from 3–4 naïve or infected mice per group are representative of 3 experiments.
Given the defective antigen presenting cell activation in RELMα−/− mice, we examined if local CD4+ T cell proliferation and activation was altered. Ki67 staining of mLN CD4+ T cells revealed infection-induced increases in the frequency of Ki67+ CD4+ T cells from WT mice that were reduced in RELMα−/− mice (Fig. 4D). Additionally, the delta mean fluorescent intensity of Ki67 expression was significantly reduced in the infected RELMα−/− mice compared to infected WT mice, suggesting that there were proliferative defects on a per cell basis (Fig. 4E). Associated with reduced CD4+ T cell proliferation, the frequency of activated CD44hiCD4+ T cells isolated from the colons was significantly reduced in infected RELMα−/− mice compared to infected WT mice (Fig. 4F). Given that RELMα−/− mice exhibited a specific defect in Th17 cell activation following DSS-induced colitis (Fig. S1), we next examined Citrobacter-specific Th17 cell responses in infected WT or RELMα−/− mice. Cells were isolated from the mLN and re-stimulated with Citrobacter antigen for 48 hours and assessed for IL-17A production by intracellular cytokine staining. WT mice exhibited a robust population increase of infection-induced CD4+ IL-17A+ T cells (Fig. 4G, H). In contrast, infected RELMα−/− mice exhibited decreased frequencies of IL-17A producing CD4+ T cells compared to infected WT mice (Fig. 4G, H). In addition, CD4+ T cell-derived IL-17F and IL-22 but not IFN-γ were also reduced in Citrobacter antigen-stimulated mLN cells from infected RELMα−/− mice (Fig. 4I). Given the reduced proliferative capacity of the RELMα CD4+ T cells, these data suggest that following Citrobacter antigen stimulation, the proliferating CD4+ T cells in RELMα−/− mice preferentially express IFNγ. Collectively, these data identify an immunostimulatory role for RELMα in promoting bacterial infection-induced intestinal macrophage and Th17 cell activation. Since immunity to Citrobacter is dependent on macrophage activation and on Th17 cells (20, 31), we hypothesized that the reduced Citrobacter-specific immune cell response in RELMα−/− mice may result in increased Citrobacter burden. Although there was a modest delay in Citrobacter elimination in RELMα−/− mice at day 14 post-infection (Fig. 4J), we observed no significant differences in the kinetics of Citrobacter colonization and clearance between WT or RELMα−/− mice, suggesting that in the context of enteric bacterial infection, the immunostimulatory effects of RELMα contribute to inflammatory pathology rather than a critical host-protective function.
Recombinant RELMα treatment induces Citrobacter-induced colitis
To determine whether the dampened intestinal inflammation seen in RELMα−/− mice could be restored by exogenous administration of recombinant RELMα, Citrobacter-infected RELMα−/− mice were injected intraperitoneally with PBS or recombinant RELMα throughout infection. Despite high concentrations of administered recombinant RELMα, rRELMα-treated mice had much lower RELMα levels than WT mice (Fig. 5A, compare to Fig. 1F). However, histologic examination of H&E-stained colon tissue sections from PBS and RELMα-treated mice revealed exacerbated Citrobacter-induced intestinal lesions following RELMα treatment characterized by increased crypt hyperplasia, submucosal edema (Fig. 5B, arrow), and leukocyte infiltration (Fig. 5B, box) relative to PBS treated mice. Finally, treatment of Citrobacter-infected RELMα−/− mice with recombinant RELMα was sufficient to induce significantly increased intestinal inflammation compared to PBS treated mice (Fig. 5C). Collectively, this data suggest that RELMα directly contributes to intestinal inflammation during Citrobacter infection.
Figure 5. Recombinant RELMα induces Citrobacter-induced colitis.
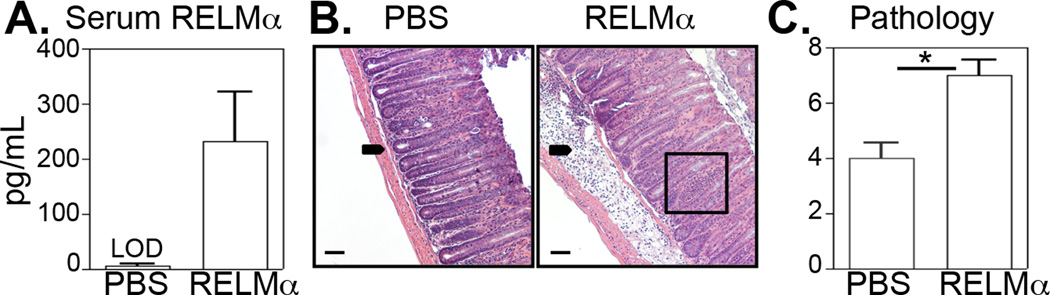
Citrobacter-infected RELMα−/− mice were treated with PBS, rRELMα (10µg) at days 0, 3, 6 and 9 post-infection and sacrificed at day 10. A. RELMα serum levels. B. H&E-stained sections of colonic tissue from PBS or rRELMα-treated Citrobacter-infected mice. C. Pathology score based on histological sections shown in (B). Bar, 50 µm. Arrow, submucosal inflammation. Box, crypt inflammation. LOD, limit of detection. *P<0.05. Data are representative of 2 experiments of three to five mice per group.
RELMα-induced intestinal inflammation following Citrobacter infection is dependent on IL-17A
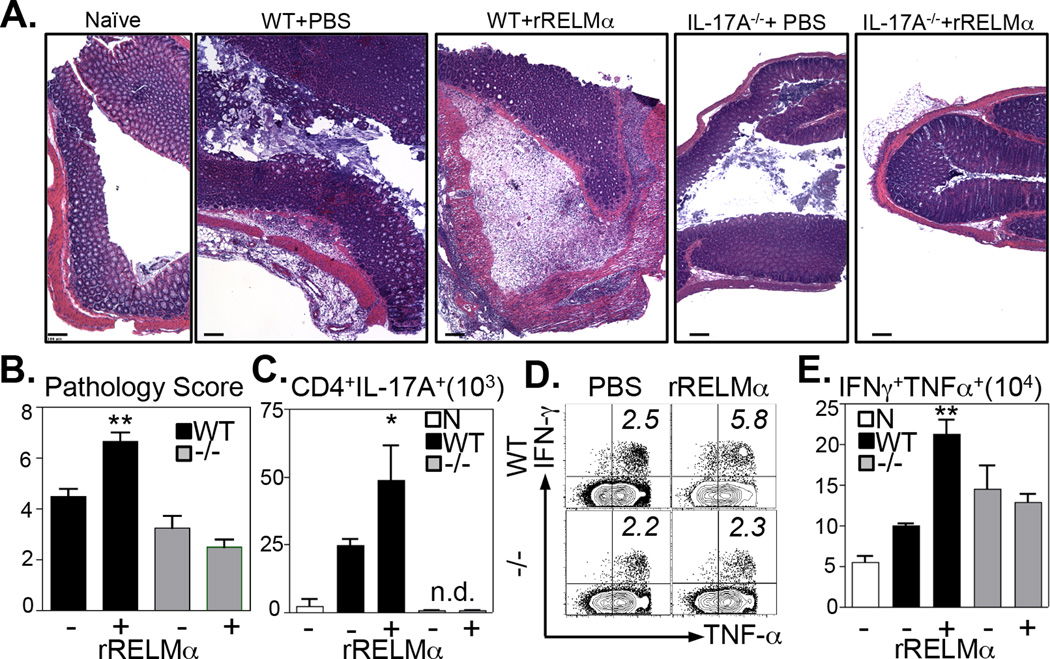
Employing RELMα−/− mice in two models of intestinal inflammation, these data have revealed a previously unrecognized function for RELMα in influencing Th17 cell responses. However, Citrobacter-infected RELMα−/− mice also exhibited reduced macrophage activation and CD4+ T cell proliferation, suggesting that RELMα may promote intestinal inflammation via mechanisms other than IL-17A production. To test this hypothesis, Citrobacter-infected WT and IL-17A−/− mice were treated with recombinant RELMα and examined at day 10 post-infection for intestinal inflammation and T cell activation. In WT mice, Citrobacter infection induced characteristic colonic lesions consisting of leukocyte infiltration, submucosal edema, and crypt hyperplasia and treatment of WT mice with RELMα exacerbated Citrobacter-induced inflammation (Fig. 6A, left panels). In contrast, infected IL-17A−/− mice exhibited less severe intestinal inflammation, edema, and crypt hyperplasia, consistent with the known pro-inflammatory function of IL-17A (Fig. 6A, right panels). Strikingly, unlike WT mice, RELMα treatment of IL-17A−/− mice did not exacerbate Citrobacter-associated intestinal inflammation, suggesting that IL-17A is a necessary mediator of RELMα directed inflammation. Blind pathology scoring confirmed that RELMα treatment significantly increased the severity of Citrobacter-induced inflammation in WT mice but not IL-17A−/− mice (Fig. 6B). To examine the effect of RELMα treatment on CD4+ T cell activation, CD4+ T cells were stimulated ex vivo with PMA/Ionomycin and stained for intracellular cytokines. Compared to naïve control WT mice, there was an increase in the frequency of CD4+ T cell-derived IL-17A following Citrobacter infection, which was enhanced with RELMα treatment (Fig. 6C). To examine CD4+ T cell activation in infected IL-17A−/− mice, CD4+ T cell-derived IFNγ and TNFα were quantified (Fig. 6D, E). Whereas RELMα treatment of infected WT mice resulted in the increased frequency (Fig. 6D, top panels) and total number (Fig. 6E) of IFNγ+TNFα+ co-producers, RELMα treatment had no effect on CD4+ T cells from infected IL-17A−/−mice (Fig. 6D bottom panels, E). Together, these data suggest that RELMα-induced intestinal inflammation following Citrobacter infection is dependent on IL-17A.
Figure 6. RELMα-induced intestinal inflammation is dependent on IL-17A.
Citrobacter-infected WT or IL-17A−/− mice were treated intraperitoneally with PBS or 10µg recombinant RELMα at days 0, 3, 6 and 9 post-infection and sacrificed at day 10. A. H&E-stained colonic tissue sections of naïve or infected mice were examined. Bar, 100µm. B. Infected mice were scored for intestinal inflammation. C–E. Splenocytes were stimulated ex vivo with PMA/Ionomycin. C. Number of CD44hiCD4+ T cells expressing IL-17A. D. Expression of IFN-γ and TNF-α by CD44hiCD4+ T cells. E. Number of CD4+CD44hi splenocytes that are IFN-γ+TNF-α+ co-producers. n.d., not detected; N, WT naïve. *P<0.05, **P<0.01. Data are representative of 2 experiments (Naïve, n=4; Inf, n=7).
Macrophages from RELMα−/− mice exhibit impaired production of IL-23p19
Given the selective impairment in Citrobacter-induced Th17 cell responses in the absence of RELMα, we hypothesized that RELMα−/− mice may exhibit impaired expression of IL-23, a critical cytokine for the development and maintenance of CD4+ Th17 cells. Consistent with this, IL-23p19 levels in the serum of Citrobacter-infected RELMα−/− mice were significantly reduced compared to infected WT mice (Fig. 7A). Together, these data suggest that the immunostimulatory effects of RELMα act through promoting the IL-23/Th17 immune axis; however, whether RELMα was required for CD4+ Th17 cell differentiation or for activation of antigen presenting cells such as macrophages was unknown. In vitro Th17 cell polarized splenocyte cultures from WT or RELMα−/− mice revealed no defect in IL-17A production by RELMα−/− CD4+ T cells (data not shown).
Figure 7. Macrophages from RELMα−/− mice exhibit impaired production of IL-23p19.
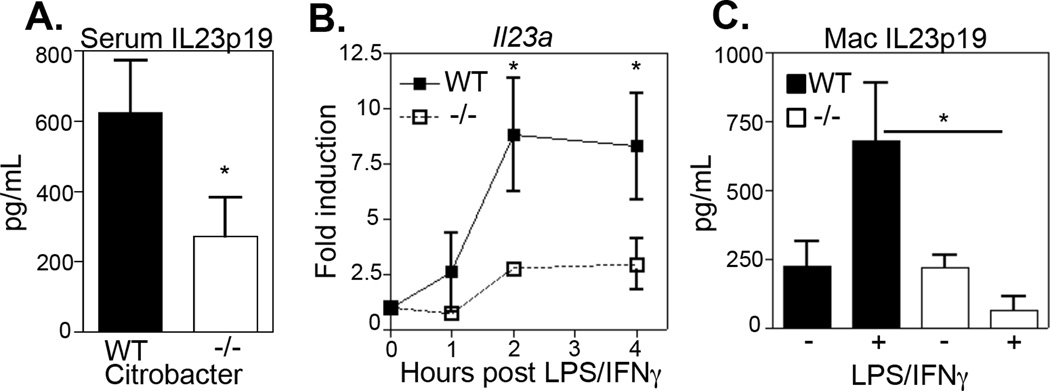
A. IL-23p19 serum levels in Citrobacter-infected WT and RELMα−/− was measured. B–C. Adherent peritoneal macrophages from naïve WT or RELMα−/− mice were treated overnight with media or activated with LPS/IFN-γ. B. At timepoints indicated cells were recovered for IL23a real-time RT-PCR analysis. C. Overnight supernatants were recovered for measurement of IL-23p19 by ELISA. *P<0.05. Data are representative of two separate experiments with three to four replicate wells.
Since cytokine-mediated Th17 polarization was not affected in RELMα−/− CD4+ T cells in vitro, and we had observed impaired macrophage activation in vivo following Citrobacter infection, we tested the hypothesis that intrinsic defects in RELMα−/− macrophages could explain the impaired infection-induced Th17 cell response. Peritoneal macrophages from naïve WT or RELMα−/−mice were treated ex vivo with the bacterial ligand LPS and immunostimulatory IFN-γ and assayed for expression of inflammatory cytokines IL-12p40, TNFα and IL-23p19. Although there was no significant difference in LPS/IFN-γ induced TNFα and IL-12p40 by RELMα−/− peritoneal macrophages (data not shown), there was a selective impairment in the ability of RELMα−/− macrophages to up-regulate expression of IL-23p19 RNA transcripts (Fig. 7B) and protein (Fig. 7C). Collectively, the data presented here demonstrate that macrophages in RELMα−/− mice exhibit intrinsic defects in IL-23p19 expression, which may have important consequences for CD4+ Th17 development and intestinal inflammation.
DISCUSSION
RELMα is a constitutively expressed protein that has been associated with multiple infectious and inflammatory responses. Previous work has demonstrated dysregulated RELMα expression in insulin resistance, helminth infection, type 2-associated lung inflammation and chemically-induced colitis (2, 3, 10, 30, 32). These studies have identified that in these various settings, RELMα plays a critical role in glucose homeostasis, can interact directly with CD4+ Th2 cells to limit inflammation and can activate myeloid innate immune responses following intestinal injury. However, the role of RELMα in coordinating a Th17 immune response and its potential function in response to bacterial infection were unknown. Here, we report that following infection with Citrobacter, a murine model for EPEC/EHEC intestinal diseases in humans, RELMα exacerbates intestinal inflammation. Collectively, this study demonstrates several key findings that contribute to our understanding of this immunomodulatory molecule (Fig. S3). First, we demonstrate that intestinal epithelial cells and macrophages are potent sources of RELMα in the colon of Citrobacter-infected mice. Second, using both RELMα−/− mice and treatment with exogenous RELMα, we show that RELMα promotes antigen presenting cell and CD4+ T cell activation at the site of infection, and that genetic deletion of RELMα limits infection-induced colitis. Third, we identify the IL-23/Th17 immune axis as a downstream effector pathway that mediates RELMα-induced intestinal inflammation. Specifically, this study identifies a new secreted factor that influences intestinal disease following enteric bacterial infection. Additionally, our findings suggest that targeting this protein, the cell-types that express it or the downstream effector pathways may offer new therapies to alleviate the symptoms of EPEC/EHEC intestinal diseases.
Although RELMα is a signature gene of alternatively activated macrophages and has important roles in helminth infection and allergy, its function in other inflammatory environments is less well characterized. For the first time, we have examined whether RELMα is involved in the immune response to a pathogenic bacterial infection and demonstrate a critical role for RELMα expression in promoting infection-induced inflammation. These findings are consistent with a previous report demonstrating that RELMα−/− mice were protected from DSS-induced colitis and extend our knowledge of how RELMα contributes to intestinal immunity and tissue inflammation. Importantly, our studies demonstrate that although RELMα−/− mice exhibited diminished Citrobacter-specific Th17 cell responses, they did not suffer from impaired immunity to Citrobacter. Thus, in this study we have effectively demonstrated that host-protective adaptive immunity can be uncoupled from tissue-damaging inflammation mediated by RELMα and Th17 cell responses in a model of infection-induced colitis.
Given the importance of IL-17A in clearance of Citrobacter infection (18, 20), we were surprised that RELMα−/− mice successfully cleared their bacteria. However, although the frequency is decreased compared to WT mice, infected RELMα−/− animals do generate a pool of Citrobacter-responsive CD4+ Th17 cells, as well as equivalent Citrobacter-specific Th1 cell responses (Fig. 4). Indeed, the protective role of antigen-specific CD4+ Th1 cells has been demonstrated and mice lacking IFNγ-producing CD4+ T cells demonstrated greater weight loss and fecal bacterial burden following Citrobacter infection (33). The combination of these responses may be sufficient for successful Citrobacter clearance in infected RELMα−/− mice. In addition to selective defects in IL-17A cytokine expression, CD4+ T cells from the colon and draining mLN of RELMα−/− mice exhibited striking defects in their activation and proliferation, as examined by CD44 and Ki67 staining. RELMα is highly mitogenic in certain lung inflammation models (34), and we have previously shown that RELMα can bind CD4+ T cells (10). We tested the hypothesis that intrinsic RELMα expression was necessary for Th17 differentiation and/or proliferation through in vitro polarization assays, and although we did not observe defects in RELMα−/− CD4+ T cells in this setting, it is possible that in in vivo inflammatory conditions RELMα may affect local T cell activation and proliferation.
Since direct effects of RELMα deletion in CD4+ T cells were not the apparent cause of the diminished Citrobacter-specific Th17 response in RELMα−/− mice, we tested the influence of RELMα expression on innate immune cell populations that could ultimately influence the quality of the adaptive immune response. We demonstrate here that Citrobacter infection induced up-regulation of RELMα in colonic macrophages and eosinophils as well as non-hematopoietic intestinal epithelial cells in WT animals. Quantification of the contribution of RELMα expressing innate immune cell populations demonstrated that following Citrobacter infection, macrophages were the primary source of hematopoietic-derived RELMα. Previous studies have shown increased RELMα expression in the lung in response to bacterial LPS (35), and we have previously proposed that RELMα may be induced directly in response to injury (36). The Citrobacter-induced expression of RELMα in the colon that we report here may therefore be triggered by Citrobacter LPS and/or as a consequence of the injury induced by pathogenic bacterial infection. Consistent with this hypothesis and previous reports, we show here that RELMα expression is also induced in the intestine in response to chemically induced injury with DSS.
To determine whether the infection-induced up-regulation of RELMα in colonic macrophages had a functional role, we examined whether RELMα−/− macrophage activation or function were impaired in response to bacterial stimulation. Indeed, following Citrobacter infection, colonic RELMα−/− macrophages failed to up-regulate MHCII to the same extent as WT mice. In addition, RELMα−/− macrophages displayed selective defects in their ability to express the Th17-associated cytokine IL-23 following bacterial ligand stimulation. Previous studies have shown that RELMα treatment of macrophages in vitro induces JNK signaling and pro-inflammatory cytokine expression (3). Thus, this data suggests that RELMα promotes CD4+ T cell IL-17A expression via macrophage activation and polarization. Taken together with our previous studies demonstrating that RELMα plays a critical role in limiting type 2 inflammation, our current data provokes the hypothesis that RELMα may act as an immunological rheostat and play a role in tuning the type of immune response generated following infection. Importantly, our results suggest that targeting RELMα may be beneficial for ameliorating intestinal inflammation without compromising intestinal immunity to enteric bacteria.
Critically, RELMα-induced intestinal inflammation was abrogated in the absence of IL- 17A, demonstrating that IL-17A is downstream of the pro-inflammatory function of RELMα. In contrast to most pathogens, where infection-induced T cell activation occurs 1–2 weeks post-infection, recent studies reported that Citrobacter induces a significant population of CD4+ TCRβ+ IL-17A producing T cells at the infection site as early as day 4 post-infection (20). The early induction of RELMα at the site of infection is consistent with the possibility that RELMα directly influences this early Th17 cell response to Citrobacter infection.
Collectively, the results presented here reveal a previously unrecognized role for RELMα in enteric bacterial infection, and uncovers a new pathway by which RELMα promotes intestinal inflammation via an IL-23/IL-17A-dependent inflammatory pathway. These findings suggest that immunotherapies targeting RELMα may provide a way to limit intestinal inflammation without significantly impairing mucosal Th17 immune responses.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank David Artis for advice and support and members of the Artis laboratory for helpful discussion and critical reading of the manuscript.
Financial Support
This work was supported by the National Institutes of Health (NIH) AI091759 (MGN), NIH T32RR007063 & K08DK093784 (TA), NIH DP5OD012116 (GFS), the Crohn’s and Colitis Foundation of America’s William and Shelby Modell Family Foundation Research Award (MGN), Irvington Institute Postdoctoral Fellowship of the Cancer Research Insitute (LCO), National Health and Medical Research Council Overseas Biomedical Fellowship 613718 (PRG), American Australian Association Education Fund (PRG), Crohn's and Colitis Foundation of Canada (BAV) and CIHR operating grant (MOP-115180 to BAV). We thank the Vet School Pathology Service, the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory and the Molecular Pathology, and the Imaging Core of the Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) for technical support. The ACC Flow Cytometry and Cell Sorting Shared Resource is partially supported by NCI Comprehensive Cancer Center Support Grant (#2-P30 CA016520).
Abbreviations used
- DC
denditic cell
- DSS
Dextran sodium sulfate
- mLN
mesenteric lymph node
- RELM
Resistin-like molecule
REFERENCES
- 1.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale F, Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. Embo J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 7.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 8.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-beta induction and role in pulmonary fibrosis. Journal of immunology. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Jr, Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, Cohn SM, Hecht G, Lazar MA, Haczku A, Wu GD. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2293. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rothenberg ME. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nature reviews. Microbiology. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 15.World Gastroenterology Organisation. Acute Diarrhea. 2008 [Google Scholar]
- 16.World Health Organization. Diarrhoea: Why children are still dying and what can be done. 2009 [Google Scholar]
- 17.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Vallance BA, Deng W, Knodler LA, Finlay BB. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect Immun. 2002;70:2070–2081. doi: 10.1128/IAI.70.4.2070-2081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nature medicine. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, Kim JY, Oh KI. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World journal of gastroenterology : WJG. 2006;12:302–305. doi: 10.3748/wjg.v12.i2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. The Journal of experimental medicine. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, Saris CJ, Artis D. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181:4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes and infection / Institut Pasteur. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. The Journal of infectious diseases. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 30.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS pathogens. 2009;5 doi: 10.1371/journal.ppat.1000393. e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infection and immunity. 2010;78:2653–2666. doi: 10.1128/IAI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1077. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Zheng L, Kang Q, Dodd OJ, Langer J, Li B, Wang D, Li D. Upregulation of hypoxia-induced mitogenic factor in bacterial lipopolysaccharide-induced acute lung injury. FEBS Lett. 2006;580:2207–2215. doi: 10.1016/j.febslet.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.