Summary
Proteins with annealing activity are newly identified ATP-dependent motors that can rewind RPA-coated complementary single-stranded DNA bubbles. AH2 (annealing helicase 2, also named as ZRANB3) is the second protein with annealing activity, the function of which is still unknown. Here, we report that AH2 is recruited to stalled replication forks and that cells depleted of AH2 are hypersensitive to replication stresses. Furthermore, AH2 binds to PCNA, which is crucial for its function at stalled replication forks. Interestingly, we identified a HARP-like (HPL) domain in AH2 that is indispensible for its annealing activity in vitro and its function in vivo. Moreover, searching of HPL domain in SNF2 family of proteins led to the identification of SMARCA1 and RAD54L, both of which possess annealing activity. Thus, this study not only demonstrate the in vivo functions of AH2, but also reveal a common feature of this new subfamily of proteins with annealing activity.
Keywords: AH2, ZRANB3, HARP, SMARCAL1, PCNA, replication fork, annealing activity
Introduction
Cells have evolved a coordinated network of responses involving cell cycle checkpoint control, DNA replication, and DNA repair for the maintenance of genomic integrity. S phase cells are particularly prone to damage because many types of DNA lesions, such as ultraviolet light-induced pyrimidine dimers, DNA cross-links, and alkylated DNA bases, delay the progression of replication forks (Niedernhofer et al., 2005; Tercero and Diffley, 2001). In these situations, cells activate a replication stress-response pathway to protect the stability of stalled forks in order to assist fork restart, as continuous fork stalling may lead to the dissociation of DNA polymerases and other replisome components, and eventually to fork collapse (Branzei and Foiani, 2009). Disruption of such proper S phase control could lead to the generation of single stranded DNA (ssDNA), inappropriate processing and/or cleavage of stalled replication forks by nucleases, and aberrant recombinations and chromosomal translocations (Kolodner et al., 2002). Accordingly, many human genetic syndromes caused by mutations in genes whose products are central to protect genome integrity during S phase often lead to cancer predisposition (Hickson, 2003; Kastan and Bartek, 2004).
Proteins with annealing activity are a newly identified class of proteins, that rewind RPA (replication protein A) bound complementary ssDNA in an ATP-dependent manner (Yusufzai and Kadonaga, 2008). HARP (HepA-related protein, also known as SMARCAL1) is the first identified protein with annealing activity (Yusufzai and Kadonaga, 2008). HARP is a distant member of the SNF2 family of proteins (Coleman et al., 2000; Eisen et al., 1995; Flaus et al., 2006). Mutations in HARP are responsible for an autosomal recessive disorder known as Schimke immunoosseous dysplasia (SIOD) (Boerkoel et al., 2000; Schimke et al., 1971; Spranger et al., 1991). Recently, several groups including ours reported that HARP is recruited to stalled replication forks via its direct interaction with RPA, helps to stabilize stalled replication forks and facilitates DNA repair during replication. These studies also demonstrated that the annealing activity of HARP is required for its in vivo function at replication forks and proposed that SIOD syndrome may be caused by the destabilization of replication forks during cell proliferation (Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009). While it still remains unclear how HARP catalyzes annealing activity, we reported that the conserved tandem HARP domain in HARP protein dictates its annealing activity (Ghosal et al., 2011). Recently, Yusufzai et al. reported a second protein with annealing activity, which they named AH2 for annealing helicase 2 (Yusufzai and Kadonaga, 2010). Like HARP, AH2 is a member of the SNF2 family. It catalyzes the ATP-dependent rewinding of RPA-bound complementary ssDNA, but does not exhibit any detectable helicase activity (Yusufzai and Kadonaga, 2010). However, the biological function of this new protein with annealing activity remains unknown.
In this study, we reported that AH2 is recruited to sites of DNA damage and depletion of AH2 sensitizes cells to replication stress. We identified an HARP-like domain in AH2, which endows AH2 with the annealing activity. In addition, we found that AH2 coordinates with HARP to stabilize stalled replication forks. Finally, we have broadened this family of proteins with annealing activity by identifying two additional HARP-like domain-containing SNF2 proteins, namely, RAD54L and SMARCA1, which possess ATP-driven annealing activity.
Results
AH2 is recruited to sites of DNA damage
Since HARP is recruited to stalled replication forks in response to replication stress (Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009), we first tested whether AH2 would also participate in cellular response to replication stress and/or other types of DNA damage. Thus, we generated polyclonal anti-AH2 antibody. As shown in Figure 1A, endogenous AH2 was recruited to DNA damage sites following laser-induced micro-irradiation and colocalized with single strand DNA binding protein RPA. Similarly, discrete foci of Flag-tagged AH2, which colocalized with RPA, were readily detected in cells following hydroxyurea (HU) treatment (Figure 1B), indicating that AH2 is involved in DNA damage response.
Figure 1. AH2 is involved in cellular response to DNA damage.
(A) HeLa cells were laser-microirradiated and analyzed by immunostaining with AH2 and RPA2 antibodies. (B) HeLa cells were transfected with the plasmid encoding SFB-tagged AH2. Immunostaining experiments were performed 6 hr after HU treatment using indicated antibodies. Foci-positive transfected cells were quantified by counting a total of 100 transfected cells with positive staining. Data are presented as mean ± s.d. from 3 different experiments. (C) Knock-down efficiency of AH2 using specific shRNAs was confirmed by immunoblotting of lysates prepared from HeLa cells expressing the indicated shRNA. (D–G) Survival curves in response to increasing doses of HU (D), CPT (E), MMC (F) and IR (G) for indicated cell lines are presented. Cell survival assays were performed as described in the Experimental Procedures. Data are presented as mean ± s.d. from three different experiments. See also Figure S1.
Depletion of AH2 leads to increased sensitivity to DNA damage
Localization of AH2 to sites of DNA damage suggested a possible role of AH2 in DNA damage response. Thus, we examined the effect of AH2 depletion on cell survival following DNA damage. Two lentiviral shRNAs specifically targeting AH2 worked well in HeLa cells (Figure 1C). HU treatment reduces the production of deoxyribonucleotides via inhibition of ribonucleotide reductase, therefore causing replication stress (Platt, 2008). Cells with lentiviral shRNA-mediated AH2 depletion showed a marked hypersensitivity to HU treatment (Figure 1D). We also observed increased sensitivity of AH2 depleted cells to the topoisomerase I inhibitor camptothecin (CPT) and the DNA cross-linking reagent mitomycin C (MMC) (Figure 1E, F), whereas we only detected mild sensitivity to ionizing radiation (Figure 1G). Re-expression of wild-type AH2 in AH2-depleted cells rescued cellular sensitivity to CPT and MMC (Figure S1A–B). Together, these data suggested that AH2 is mainly involved in maintaining cell viability when cells are challenged with agents that induce replication stress.
AH2 is a PCNA-binding protein
We do not know exactly how AH2 acts in replication stress pathway. HARP is the first known protein with annealing activity and it is recruited to stalled replication forks by its direct interaction with RPA via a conserved N-terminal motif (Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009). However, AH2 does not bind RPA (Yusufzai and Kadonaga, 2010). Thus, it would be interesting to determine whether there is a specific partner of AH2 at stalled replication forks and/or sites of DNA damage. We took an unbiased approach and performed tandem affinity purification (TAP) using cell lysates prepared from 293T cells stably expressing triple-epitope (S-protein, Flag and streptavidin-binding peptide) tagged AH2 (SFB-AH2). Mass spectrometry analysis revealed that the major AH2-associated protein is proliferating cell nuclear antigen (PCNA) (Figure 2A). PCNA is a small protein of 261 residues. Our mass spectrometry analysis identified many PCNA-derived peptides, suggesting a strong interaction between AH2 and PCNA. We first performed co-immunoprecipitation experiment and verified the in vivo interaction between endogenous AH2 and PCNA (Figure 2B). Second, we included several other SNF2 family proteins such as SMARCD1, SMARCA1, HELLS, RAD54L and HARP as controls and showed that only SFB-AH2 strongly co-precipitated with endogenous PCNA (Figure 2C), indicating that the interaction between AH2 and PCNA was specific.
Figure 2. AH2 is a PCNA-binding protein.

(A) Tandem affinity purification was performed using 293T cells stably expressing tagged AH2. The results from mass spectrometry analysis are shown in the table. (B) Association of endogenous AH2 with PCNA in 293T cells was analyzed by immunoprecipitation using anti-AH2 antibody and immunoblotting using antibodies as indicated. (C) AH2 specifically interacts with PCNA. 293T cells were transfected with plasmids encoding SFB-tagged AH2 or other SNF2 family proteins: SMARCD1, SMARCA1, HELLS, RAD54L or HARP. Co-precipitation was carried out using S-protein beads and immunoblotting was performed using antibodies as indicated.
The interaction between AH2 and PCNA via a conserved PIP box is important for AH2 function in the cell
A majority of PCNA-binding proteins interact with PCNA via a characteristic PCNA-interacting motif (PIP box), which was first identified in the cyclin-dependent kinase inhibitor 1A, also called p21 or Cip1 (Gulbis et al., 1996). After scanning the amino acid sequence of the whole AH2 protein, we identified a potential PIP box in the middle region of AH2 protein (Figure 3A), which clearly fits the consensus sequence of PIP box: Q-x-x-(L/V/I/M)-x-x-(F/Y)-(F/Y). We next generated a deletion mutant lacking the entire PIP box (dPIPB) and two point mutants with mutations within the PIP box (Q519A and F525A). As shown in Figure 3B, only wild-type AH2 and not any of the three PIP box mutants could bind to endogenous PCNA, suggesting that AH2 interacts with PCNA via this conserved PIP box motif. However, the interaction between AH2 and PCNA did not change following HU or CPT treatment (Figure S2A).
Figure 3. AH2 interacts with PCNA via a conserved PIP box.
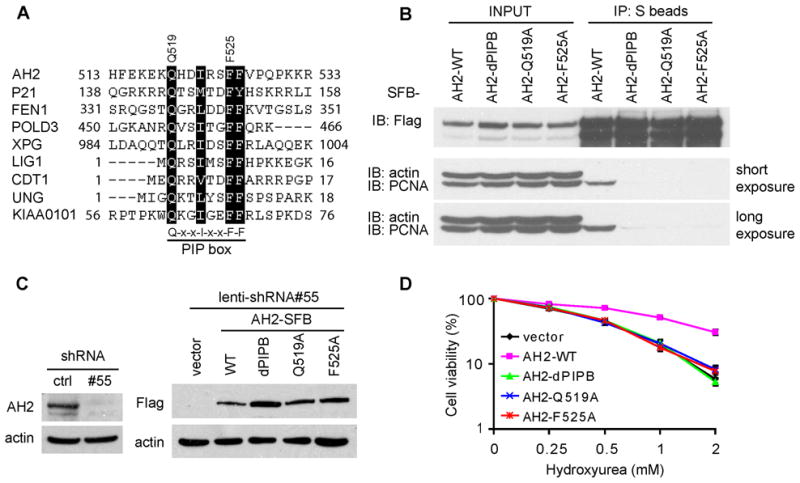
(A) AH2 contains a PIP box that is highly conserved among some other PCNA interacting proteins. The consensus sequence of PIP box is indicated. (B) The PIP box of AH2 is required for AH2 to bind to PCNA. 293T cells were transfected with plasmids encoding SFB-tagged wild-type AH2 or three PIP box mutants (dPIPB, Q519A and F525A) of AH2. Co-precipitation was carried out using S-protein beads and immunoblotting was performed using antibodies as indicated. (C) AH2-depleted (AH2 shRNA#55) HeLa derivative cell lines stably expressing shRNA-resistant wild-type AH2 or three PIP box mutants (dPIPB, Q519A and F525A) of AH2 were generated. The empty vector was included as control. The endogenous and exogenous AH2 expression was confirmed by immunoblotting using indicated antibodies. (D) PIP box mutants of AH2 could not rescue HU hypersensitivity of AH2-depleted cells. Survival curves are shown for indicated cell lines in response to increasing doses of HU. Data are presented as mean ± s.d. from three different experiments. See also Figure S2.
Since PCNA has been implicated as a replication stress sensor and participates in DNA repair, we further tested whether the binding of AH2 to PCNA is required for its function in vivo. We generated constructs encoding shRNA-resistant SFB-tagged wild-type or three PIP box mutants of AH2 so that we were able to express exogenous AH2 when the endogenous AH2 was depleted by shRNA (Figure 3C). As shown in Figure 3D, the expression of wild-type AH2 rescued HU hypersensitivity in AH2-depleted cells. However, the three PIP box mutants (dPIPB, Q519A and F525A) failed to do so (Figure 3D), although all of them were still localized to nucleus (Figure S2B). These data indicate that the PCNA-binding activity is required for AH2 to function in vivo.
A C-terminal conserved region in AH2 is required for its tethering to DNA damage sites
Since binding to PCNA is important for AH2 function, we next determined whether this binding was required for the localization of AH2 to DNA damage sites. Surprisingly, the dPIPB mutant, which lacks the entire PIP box, was still able to form discrete foci following HU treatment (Figure 4A). Consistently, AH2 also formed foci in PCNA-depleted cells (Figure S2C–E), suggesting that PCNA binding is not essential for AH2 focus formation.
Figure 4. A C-terminal conserved region in AH2 is required for its tethering to DNA damage sites.
(A) HeLa cells were transfected with the plasmid encoding SFB-tagged AH2 and dPIPB mutant. Immunostaining experiments were performed 6 hr after HU treatment using indicated antibodies. (B) Schematic representation of wild type and mutant AH2 used in this study. Four distinct conserved regions are presented: SNF2 helicase domain (residues 45–498); PIP box (residues 510–528); HARP-like (HPL) domain (residues 712–820); and HNH motif (residues 1011–1051). (C and D) Experiments were performed as in (A). (E) Foci-positive transfected cells were quantified by counting a total of 100 transfected cells with positive staining. Data are presented as mean ± s.d. from 3 different experiments. (F) Deletion of C-terminal conserved region (dC80) failed to rescue HU hypersensitivity in cells with AH2 depletion. AH2 depleted (AH2 shRNA#55) HeLa derivative cell lines stably expressing shRNA-resistant wild-type AH2 or dC80 mutant were generated. The empty vector was included as control. Survival curves are shown for indicated cell lines in response to increasing doses of HU. Data are presented as mean ± s.d. from three different experiments. (G) The endogenous and exogenous AH2 expression was confirmed by immunoblotting using indicated antibodies. See also Figure S2 and S3.
Alignment of human AH2 protein sequence with its orthologs from other species revealed four conserved regions (Figure 4B; also see Figure S3): (1) a SNF2 helicase domain (residues 45–498); (2) a PIP box (residues 510–528); (3) an unknown conserved region (residues 735–859, which we later identified as a HARP-like domain); and (4) a C-terminal region (residues 899–1079) containing a putative HNH motif (residues 1011-1051), which is commonly found in bacteria and fungi and is often associated with nuclease activity. By domain mapping, we found that the very C-terminal region (residues 89–1079) is required for AH2 foci formation (Figure 4C and 4D). Interestingly, deletion of the C-terminal region markedly reduced AH2 foci formation, from ~75% (WT) to ~3% (dC80) (Figure 4E). When the PIP box was deleted from the C-terminal deletion mutant (dC80), AH2 foci formation was completely disrupted (Figure 4E), suggesting that while the C-terminus of AH2 plays a major role in determining AH2 focus localization, the association with PCNA also has an accessory function in this process. Moreover, the dC80 mutant could not rescue HU hypersensitivity in AH2-depleted cells (Figure 4F and 4G), indicating that the focus formation activity of AH2 is also important for its function in response to replication stress.
HARP-like domain dictates the annealing activity of AH2 and is required for its function in vivo
Our previous study on HARP revealed that a conserved tandem HARP domain dictates the annealing activity of HARP (Ghosal et al., 2011). We were excited to report that the alignment of HARP domains with AH2 revealed a putative ‘HARP-like’ domain (HPL) in AH2 protein (residues 712–820), which overlaps with the unknown conserved region described above (Figure 4B). This led us to test whether this putative ‘HARP-like’ domain is critical for AH2 functions.
We generated an internal deletion mutant of AH2 (dHPL; deleted of residues 712–820), which lacked the HPL domain, and a truncated mutant of AH2 (AH2N; residues 1–540), which contained the N-terminal half of the protein including the entire SNF2 helicase domain (Figure 4B). We purified wild-type and the AH2 mutant proteins (see Figure S4A) and examined their annealing activity using an assay described previously (Yusufzai and Kadonaga, 2008). As shown in Figure 5A, RPA-coated DNA was incubated with the indicated proteins. Following incubation, reaction products were deproteinized, resolved in agarose gel, and stained with ethidium bromide. As shown in Figure 5B, HARP and wild-type AH2, but not the AH2N or dHPL mutant of AH2, catalyzed the annealing of RPA-coated complementary DNA in the presence of ATP. To determine the minimal region within the dHPL domain that is required for AH2 annealing activity, we generated four internal deletion mutants within the HPL domain (dHPLF1, dHPLF2, dHPLF3 and dHPLF4; see Figure S4A–B) and found that all four failed to display any annealing activity (see Figure S4C), indicating that the integrity of the 108-residue HPL domain is required for AH2 annealing activity. Moreover, fork DNA stimulated the ATPase activity of wild-type AH2 as well as AH2 mutants (Figure 5C), suggesting that while the HPL domain is required for the annealing activity of AH2, it does not affect AH2 ATPase activity. Because the SNF2 domain is known to bind and hydrolyse ATP (Fan et al., 2005), we generated controls with mutations in the Mg2+-binding pocket and the ATP-binding site in the SNF2 domain of AH2 (AH2-MBM and AH2-ABM respectively; see Figure S4A) and found that these mutants did not catalyze the ATP-dependent annealing reaction (Figure 5D).
Figure 5. HARP-like domain dictates the annealing activity and the in vivo function of AH2.
(A) Schematic diagram of the annealing activity assay. Plasmid DNA is incubated with purified RPA and topoisomease I to generate partially unwound DNA molecules with stably-bound RPA. Addition of SDS and proteinase K to the partially unwound DNA to inactivate and deproteinize topoisomerase I will result in the generation of negatively supercoiled DNA due to unwinding of the DNA by RPA. However, if any protein with annealing activity rewinds the partially unwound DNA in the presence of topoisomerase I, the resulting DNA would be relaxed prior to SDS treatment and deproteinization. (B) HARP-like (HPL) domain dictates the annealing activity of AH2. The annealing helicase assay was carried out as described in the Experimental Procedures, with 60 nM HARP, AH2, AH2N or dHPL in the presence (top panel) or absence (bottom panel) of ATP. Uridine triphosphate (UTP) was used as control in the absence of ATP. All reactions contained DNA, RPA, and topoisomerase I. (C) ATPase activities of both wild-type and mutant AH2 were stimulated by fork DNA. Graph shows percentage of ATP hydrolyzed against concentration of fork DNA, with mean ± s.d. calculated from three independent experiments. (D) Mutations in the Mg2+ binding pocket (AH2-MBM) or ATP binding site (AH2-ABM) of AH2 abolish AH2 annealing activity. The annealing helicase assay was carried out with 60 nM AH2, AH2-MBM and AH2-ABM in the presence of ATP. All reactions contained DNA, RPA, and topoisomerase I. (E) PIP box and HNH motif of AH2 does not contribute to the annealing activity of AH2. The annealing helicase assay were carried similar to those described in (B), with indicated proteins. (F) Deletion of HPL domain failed to rescue HU hypersensitivity in cells with AH2 depletion. AH2-depleted (AH2 shRNA#55) HeLa derivative cell lines stably expressing shRNA-resistant wild-type AH2 or HPL domain deleted mutant (dHPL) of AH2 were generated. The empty vector was included as control. Survival curves are shown for indicated cell lines in response to increasing doses of HU. Data are presented as mean ± s.d. from three different experiments. (G) The endogenous and exogenous AH2 expression was confirmed by immunoblotting using indicated antibodies. See also Figure S4.
Since AH2 interacts with PCNA, we also asked whether the PCNA-binding domain is important for the biochemical activity of AH2. We assayed the annealing activity and ATPase activity of the mutant AH2 protein lacking the PIP box (dPIPB) as well as the mutant lacking the C-terminal conserved region (dC80). We found that dPIPB and dC80 mutants of AH2 displayed normal annealing activity (Figure 5E; also see Figure S4A) and ATPase activity (Figure 5B), suggesting that the PCNA-binding domain and the C-terminal conserved region of AH2 do not influence the in vitro biochemical activities of AH2.
Interestingly, only expression of the shRNA-resistant wild-type AH2, and not expression of the dHPL mutant, which lacks the HPL domain, rescued HU hypersensitivity in AH2-depleted cells (Figure 5F and 5G). These results, together with our previous study (Ghosal et al., 2011), suggest that HARP or HARP-like domains dictate the annealing activity and functions of a subset of SNF2 domain-containing proteins.
AH2 and HARP coordinate to protect stalled replication forks
Cells with HARP depletion display increased spontaneous DNA damage and hypersensitivity to replication stress (Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009). To better decipher the relationship between AH2 and HARP, we tested whether AH2 depletion could also trigger DNA damage by destabilizing stalled replication forks. The knockdown of AH2, HARP or both was confirmed by immunoblotting (Figure 6A). The appearance of RPA foci could be used as marker for the emergence of ssDNA regions in the cell. Indeed, increased RPA foci were observed in AH2 knockdown cells, although this increase was not as robust as that observed in HARP-depleted cells (Figure 6B). Similar results were obtained when we used γH2AX foci formation as the readout for DNA damage (Figure 6B). Interestingly, RPA and γH2AX foci were considerably increased in AH2 and HARP double knockdown cells (Figure 6B). Consistently, cells with depletion of both AH2 and HARP showed higher sensitivity to replication stress (Figure 6C). These results suggest that AH2 and HARP coordinate activity in response to replication stress.
Figure 6. AH2 and HARP coordinate to protect stalled replication forks.
(A) Knock-down efficiency of AH2 shRNA and HARP siRNA was confirmed by immunoblotting using lysates prepared from HeLa cells in which the indicated shRNA or siRNA had been introduced. (B) Double knockdown of HARP and AH2 considerably induces RPA and γH2AX foci. HeLa cells were introduced with indicated shRNAs or siRNAs. Seventy-two hours later, cells were subjected to immunostaining using indicated antibodies. The quantification of foci-positive cells was performed by counting a total of 200 cells per sample. Data are presented as mean ± s.d. from 3 different experiments. (C) Double knockdown of HARP and AH2 enhances HU sensitivity in HeLa cells. Cell survival assays were performed as described in the Experimental Procedures. Data are presented as mean ± s.d. from three different experiments. (D) Schematic representation of the labeling protocol for DNA fiber analysis of replication forks. Hela cells were pulse labeled with IdU, treated with 2 mM HU for 6 h, and released into medium containing IdU. DNA fibers were prepared on slides and immunolabeled with antibodies to detect the modified thymidine analogs incorporated into the DNA. Representative images of replication forks are shown. (E) Quantification of the percentage of newly initiated replication at the indicated time point following the removal of HU. The percentage of newly initiated replication sites was determined by dividing the number of CIdU-containing tracts by the total of all tracts (IdU-containing tracts + CIdU-containing tracts + IdU and CIdU containing tracts). (F) Quantification of the percentage of stalled replication forks was determined by counting IdU containing tracts. (G) Quantification of the percentage of newly fired origins after the removal of HU was determined by counting CIdU-containing tracts. For all the above quantifications, 100 DNA fibers were counted in each slide and the experiment was repeated three times. The means and s.d. (bars) of three independent experiments are shown with P< 0.001. See also Figure S4 and S5.
To investigate the role of AH2 at stalled replication forks more directly, we analyzed replication fork progression using DNA fibers. Cells were first pulse-labeled with modified thymidine analog iododeoxyuridine (IdU), mock treated or treated with HU to induce replication stalling, and then labeled with chlorodeoxyuridine (CIdU) to visualize fork restart. Replication forks that restarted following HU treatment were visualized as a stretch of IdU incorporation (Figure 6D, labeled in red) followed by a stretch of CIdU incorporation (Figure 6D, labeled in green). Tracts showing only IdU incorporation (Figure 6D, red only) indicate stalled or collapsed forks that did not restart replication following the removal of HU. Replication origins that fired after removal of HU showed only CIdU incorporation (Figure 6D, green only). If this new DNA synthesis occurred at sites of previously stalled DNA replication forks, CIdU-containing tracts would colocalize with IdU-containing tracts (yellow, Figure 6D). We counted CIdU- and IdU-containing tracts as well as the total number of IdU-containing tracts and determined the recovery of DNA synthesis from stalled replication sites. In untreated wild-type cells, 88%±8% of IdU-containing tracts colocalized with CIdU. In the absence of replication stress, depletion of AH2, HARP, or both showed a modest effect on fork progression (Figure 6E and 6F). However, restart of replication forks following HU treatment was notably reduced in cells depleted of AH2 or HARP when compared with that in control cells. Furthermore, cells with AH2 and HARP double knockdown showed a more severe defect in replication restart (Figure 6E and 6F). New origin firing after HU treatment, however, was not significantly affected upon AH2 and/or HARP depletion (Figure 6G). Together, these data indicate that AH2 is involved in stabilizing stalled replication fork following replication stress, either by preventing the irreversible collapse of stalled forks or directly mediating the reinitiating of DNA replication.
Since cells depleted of AH2, HARP or both were sensitive to replication stress, we tested whether AH2 or HARP is involved in replication checkpoint control. As shown in Figure S5, while we observed increased phospho-CHK1 in AH2, HARP, and double knockdown cells without treatment, there was no obvious change in phospho-CHK1 level in these cells following HU or CPT treatment. Our data suggest that while AH2 or HARP depletion results in destabilization of stalled replication forks, they do not participate in replication checkpoint control.
Identify additional proteins with annealing activity containing HARP-like domain
The identification of a second protein with annealing activity in DNA damage response pathway highlights the importance of this newly discovered enzyme activity in genome maintenance, and suggests the possibility that there are still more such proteins. Both HARP and AH2 are distant members of the SNF2 family of ATP-dependent molecular motor proteins (Coleman et al., 2000; Flaus et al., 2006; Yusufzai and Kadonaga, 2008, 2010). However, because annealing activity is not a general property of SNF2 family proteins, we decided to identify additional proteins with annealing activity within the SNF2 family by examining them for the characteristic HARP or HARP-like domain. Excitingly, alignment of HARP domains with other SNF2 family proteins revealed at least two proteins, RAD54L and SMARCA1, which contain putative ‘HARP-like’ domains ranging from residues 558–692 and 153–282, respectively (Figure 7A, and Figure S6A–C). By comparing amino acid sequences of RAD54L or SMARCA1 from different species, we found that these putative ‘HARP-like’ domains are highly conserved (Figure S6A–B). We therefore examined if RAD54L and SMARCA1 would display any annealing activity in vitro. We found that just like AH2, RAD54L and SMARCA1 rewound RPA bound complementary ssDNA in the presence of ATP (Figure 7B; also see Figure S4A), and hydrolyzed ATP with increasing concentrations of fork DNA (Figure 7C). As controls, two mutants of the Mg2+ binding pocket and ATP binding site within the SNF2 domain of RAD54L, RAD54L-MBM and RAD54L-ABM, abrogated the ATP dependent annealing activity of RAD54L (Figure 7D). BRG1, another member of the SNF2 family that lacks the HARP or HARP-like domain, did not display any annealing activity (Figure 7B), but was able to hydrolyze ATP in the presence of DNA (Figure 7C). These data suggest that HARP and HARP-like domains determine the unique annealing activity of these SNF2 proteins, although the underlying mechanism remains unknown.
Figure 7. Identification and validation of additional proteins with annealing activity that contain HARP-like domain.
(A) Schematic representation of HARP-like domain-containing proteins RAD54L and SMARCA1. (B) The annealing helicase assay was carried out using 60 nM AH2, dHPL, RAD54L, SMARCA1 or BRG1 in the presence (top panel) or absence (bottom panel) of ATP. (C) ATPase activity of AH2, RAD54L, SMARCA1, and BRG1 was assayed as described above. Graph shows percentage of ATP hydrolyzed against concentration of fork DNA, with mean ± s.d. calculated from three independent experiments. (D) Mutations in the Mg2+ binding pocket (RAD54L-MBM) or the ATP binding site (RAD54L-ABM) of RAD54L eliminate its annealing activity. The annealing helicase assay was carried out with 60 nM RAD54L, RAD54L-MBM or RAD54L-ABM in the presence of ATP. All reactions contained DNA, RPA, and topoisomerase I. See also Figure S6.
Discussion
In conclusion, this study demonstrated that 1) AH2 is a PCNA-interacting annealing helicase that participates in cellular response to replication stress; 2) AH2 is recruited to DNA damage sites upon replication stress and coordinates with HARP to stabilize stalled replication forks; 3) HARP or HARP-like domains (HPL) are common features of proteins with annealing activity. We also identified a subset of the proteins in the SNF2 family that contain HARP-like domains and possess annealing activity. We expect that our study will promote new discoveries on proteins with annealing activity and help uncover the physiological functions of this new class of enzymes.
In response to spontaneous or replication stress, replication forks stall and allow the formation of ssDNA regions coated with RPA. We and others have reported that HARP, the first identified protein with annealing activity, is recruited to these stalled replication forks through its interaction with RPA, and exerts its annealing activity to prevent further separation of double-stranded DNA and thus stabilize stalled replication forks (Yuan et al., 2009). In the absence of HARP and its annealing activity, extensive ssDNA regions are generated at stalled replication forks, where they are easily attacked by nucleases. Thus the forks collapse and DNA breaks emerge. In this study, we showed that AH2, another protein with annealing activity, can be recruited to stalled replication forks via a different mechanism and coordinate with HARP to play partially redundant roles in stabilizing stalled replication forks and preventing fork collapse.
When we looked at the schematic diagrams of human HARP and AH2, we can see the unique features of HARP include the N-terminal RPA-binding motif, two tandem HARP domains, and a SNF2 helicase domain at the C-terminal half; AH2 has SNF2 helicase domain at its N-terminal half, a PIP box in the middle, a HARP-like domain, and the C-terminal conserved domain containing an HNH motif. The data in this study together with our previous reports demonstrate that the HARP or HARP-like domain, when combined with SNF2 helicase domain, endows a protein with annealing activity (Ghosal et al., 2011). Unlike HARP, AH2 does not contain a potential RPA-binding motif, and can not associate with RPA (Yusufzai and Kadonaga, 2010). Our tandem affinity purification of AH2 revealed that the major AH2-associated protein is PCNA (Figure 2A).
PCNA was originally identified as a DNA sliding clamp that assists replicative DNA polymerases during DNA replication. Upon DNA damage, PCNA is ubiquitinated and provides a platform for recruiting other repair factors as well as specialized translesion polymerases, which operate to bypass bulky DNA adducts during replication (Ciccia and Elledge, 2010; Maga and Hubscher, 2003). PCNA interacts with a large, and growing, number of proteins involved in DNA replication, repair, chromatin assembly and cell cycle control (Maga and Hubscher, 2003; Moldovan et al., 2007). A majority of PCNA-binding proteins interact with PCNA via a characteristic PCNA-interacting motif (PIP box). A classical PIP box was identified in the middle of AH2 protein (Figure 2A). While we found that this PIP box is functionally important for AH2 at the stalled replication forks, it is surprisingly not essential for the recruitment of AH2 to DNA damage sites. The determinant region for AH2 foci formation locates at the conserved C terminus, although it remains unknown how the C-terminus determines the focus localization of AH2.
In addition to the in vitro and in vivo functions of AH2, this study also revealed a common feature of proteins with annealing activity, i.e. a HARP or HARP-like domain (HPL), and identified two additional proteins that possess annealing activity, SMARCA1 and RAD54L. All four proteins belong to the SNF2 family, whose members participate in a variety of processes including chromatin remodeling, transcription, DNA repair, and recombination (Eisen et al., 1995; Flaus et al., 2006). The SNF2 family of proteins possesses ATPase activity with no known helicase activity. It is thus intriguing to see a subset of SNF2 family proteins, namely HARP, AH2, RAD54L and SMARCA1, function as ATP-driven DNA-rewinding motors. HARP and AH2 are both involved in the DNA damage response. RAD54L shares similarity with Saccharomyces cerevisiae Rad54 and has been shown to play a role in homologous recombination-related repair of DNA double-strand breaks (Bugreev et al., 2006; Solinger et al., 2002). SMARCA1 was reported to be a global activator of transcription by altering chromatin structure (Okabe et al., 1992). It would be interesting to understand how these two newly identified proteins with annealing activity function in the cell. Furthermore, there may be other proteins that do not belong to the SNF2 family but still possess annealing activity. Understanding how this newly identified family of proteins with annealing activity functions in normal cellular processes will reveal the distinct and common features of these ATP-driven motors.
Experimental Procedures
Antibodies
Anti-AH2 antibodies were raised by immunizing rabbits with GST-AH2 fusion proteins containing residues 1–300 and residues 541–1079 of human AH2 proteins. Antisera were affinity-purified using AminoLink plus Immobilization and purification kit (Pierce). Anti-β-actin and anti-Flag were obtained from Sigma. Anti-RPA2 was from Abcam. Anti-PCNA (PC10) was obtained from Santa Cruz Biotechnology.
Constructs
All cDNAs were subcloned into pDONR201 (Invitrogen) as entry clones and were subsequently transferred to gateway-compatible destination vectors for the expression of N or C-terminal-tagged fusion protein. All point or deletion mutants were generated using the QuickChange site-directed mutagenesis kit (Stratagene) and verified by sequencing. The AH2 ATP-binding site mutant (AH2-ABM) was generated by changing amino acids 62-GLGKT-66 to 62-AAAAA-66. The AH2 Mg2+-binding site mutant (AH2-MBM) was generated by changing amino acids 157-DESH-160 to 157-AAAA-160. The RAD54L ATP-binding site mutant (RAD54L-ABM) was generated by changing amino acids 186-GLGKT-190 to 186-AAAAA-190. The RAD54L Mg2+-binding site mutant (RAD54L-MBM) was generated by changing amino acids 296-DEGH-299 to 296-AAAA-299.
Cell Culture, transfection, siRNAs and shRNAs
HeLa and 293T cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. Plasmid transfection was performed using polyethylenimine (PEI) reagent. The siRNA transfection was performed using Oligofectamine (Invitrogen) following manufacturer’s instruction. The sequence of HARP siRNA #6 is GCUUUGACCUUCUUAGCAAUU. GIPZ lentiviral non-silencing control shRNA and shRNA target sets were purchased from Open Biosystems. The AH2 targeting sequences are: #55: 5′-CAAGAGATATCATCGATTA-3′, #56: 5′-CGGATTCACATCTATACTA-3′. The PCNA targeting sequences are: #10: 5′-GCTAGTATTTGAAGCACCA-3′, #36: 5′-TGATGGATTTAGATGTTGA-3′. The shRNA-resistant wild-type and mutant AH2 constructs were generated by changing seven nucleotides in the shRNA #55 targeting region (A1683G, A1684C, A1686C, T1689C, C1692T, C1695T, and T1698C substitutions). The shRNAs were packaged into lentiviruses by co-transfecting with packaging plasmids pMD2G and pSPAX2 (kindly provided by Professor Songyang Zhou, Baylor College of Medicine) into 293T cells. 48 hr after transfection, the supernatant was collected for infection of HeLa cells. Infection was repeated twice with an interval of 24 hr to achieve maximal infection efficiency. Infected cells were selected with media containing puromycin (2 μg/ml).
Tandem affinity purification (TAP) of AH2-associated protein complexes
Tandem affinity purification was performed as previously described (Yuan et al., 2009). Briefly, 293T cells were transfected with plasmids encoding SFB-tagged AH2. Cell lines stably expressing tagged proteins were selected and the expression of exogenous proteins was confirmed by immunoblotting and immunostaining. For affinity purification, a total of twenty 10-cm dishes of 293T cells stably expressing SFB-AH2 were collected and lysed with NETN buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, containing 1 μg/ml of each pepstatin A and aprotinin) for 25 min. Crude lysates were cleared by centrifugation, and the supernatants were incubated with 300 μl streptavidin sepharose beads (Amersham Biosciences) for 2 hr at 4°C. The beads were washed three times with NETN and then eluted with 3 mg/ml biotin (Sigma) for 2 hr at 4°C. The eluates were incubated with 100 μl S-protein agarose beads (Novagen) for 2 hr at 4°C and then washed three times with NETN. The proteins bound to beads were eluted by boiling with SDS sample buffer, resolved by SDS-PAGE, visualized by Coommasie blue staining and subjected to mass spectrometry analysis for protein identification (performed by Taplin biological mass spectrometry facility, Harvard university).
Immunoblotting
Cells were lysed with NETN buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5 % Nonidet P-40) on ice for 30 min. Cleared cell lysates were then collected and boiled in 2× Laemmli buffer and separated by SDS-PAGE. Membranes were blocked in 5% milk in TBST buffer and then probed with antibodies as indicated.
Immunostaining
Cells cultured on coverslips were washed with PBS, pre-extracted with 0.5% Triton solution for 3 min and fixed with 3% paraformaldehyde for 12 min. Coverslips were washed with PBS and then immunostained with primary antibodies in 5% goat serum for 60 min. Coverslips were washed and incubated with secondary antibodies conjugated with Rhodamine or FITC for 60 min. Cells were then stained with DAPI to visualize nuclear DNA. The coverslips were mounted onto glass slides with anti-fade solution and visualized using a Nikon ECLIPSE E800 fluorescence microscope with a Nikon Plan Fluor 60× oil objective lens (NA 1.30) at room temperature. Cells were photographed using a SPOT camera (Diagnostic Instruments, Inc) and analyzed using Photoshop software (Adobe). For micro-irradiation experiments, cells were seeded on 35-mm glass bottom dishes (MatTek Corporation), incubated overnight, and then visualized with a Nikon Elipse TE2000-U inverted microscope. Cells were micro-irradiated with a Micropoint Ablation System (Photonics Instruments, St. Charles, IL, USA) with the laser output set to 35%. An average of 20 cells was micro-irradiated and further cultured for 6 hr prior to immunostaining.
Cell survival assays
A total of 1×103 cells were seeded onto 60-mm dishes in triplicates. Twenty-four hours after seeding, cells were treated with HU, CPT or MMC at indicated concentrations. Medium was replaced 24 hr later and cells were then incubated for 14 days. Resulting colonies were fixed and stained with Coomassie blue. Numbers of colonies were counted using a Gel Doc with Quantity One software (BIORAD).
Replication labeling and DNA fiber spreads
Exponentially growing cells were labeled with 20 μM IdU for 15 min and then incubated with 50 μM thymidine for 15 min to chase out the IdU. After pre-labeling, the cells were treated with 2 mM HU for 6 hr. Cells were then washed with PBS and incubated with 100 μM CIdU for 15 min. DNA spreads were prepared as previously described (Jackson and Pombo, 1998) with some modifications. Briefly, cells were trypsinized and resuspended in PBS at 2.5×105 cells per ml. The labeled cells were diluted 1:8 with unlabeled cells, and 2.5 μl of cells were mixed with 7.5 μl of lysis buffer [200 mM Tris-HCl (pH 7.5), 50 mM EDTA, 0.5% (w/v) SDS] on a glass slide. After 6 min, the slides were tilted at 15° to allow the fibers to spread along the slide. The resulting DNA spreads were air-dried, fixed in 3:1 methanol/acetic acid for 3 min and refrigerated overnight before immunolabeling. DNA on the slides was denatured with 2.5 M HCl for 1 h and then neutralized in 0.1 M Na2B4O7 (pH 8.5). Slides were washed three times in PBS for 5 min, and were blocked in 5% (w/v) BSA and 0.1% (v/v) Tween 20 at 37° C for 1 h. The slides were then incubated at 37 °C with the following antibodies, rinsed three times in PBS with 0.1% (v/v) Tween 20, and then washed three times for 15 min in blocking buffer between each incubation: first, for 1 h in 1:400 rat anti-bromodeoxyuridine (detects CIdU; Abcam BU1/75); second, for 45 min in 1:400 Alexafluor 488-conjugated anti-rat (Molecular Probes A11006); third, for 1 h in 1:500 mouse anti-bromodeoxyuridine (detects IdU; SIGMA B2531). To increase the specificity, slides were washed with high-salt buffer (28 mM Tris-HCl, 500 mM NaCl, 0.5% (v/v) Tween-20). After the high-salt wash, slides were washed with PBS containing 0.1% (v/v) Tween-20 and then incubated for 45 min with Rhodamine conjugated anti-mouse IgG (Jackson Immuno research, 1:500 dilution). Microscopy was carried out using a Nikon 90i microscope. The significance of the difference between the means was determined by Student’s t-test.
Protein Purification
293T cells were transfected with plasmids encoding SFB-tagged full length AH2, dHPL, AH2N, dPIPB, dC80, dHPLF1, dHPF2, dHPF3, dHPF4, AH2MBM, AH2ABM, RAD54L, RAD54LMBM RAD54LABM, SMARCA1, HARP and BRG1 proteins. 48 hr later, cells expressing tagged proteins were lysed with NETN buffer for 20 min at 4°C. Crude lysates were clarified by centrifugation at 14,000 rpm at 4°C for 10 min, and the supernatant was then incubated with streptavidin-conjugated beads (Amersham) for 2 hr at 4°C. The beads were washed (4X) with NETN buffer at 4°C and the bound proteins were eluted with NETN buffer containing 2 mg/ml biotin (Sigma). Eluted protein fractions were analyzed on 7.5% SDS-PAGE and the purified protein was dialyzed against buffer containing 10% [v/v] glycerol, 20 mM HEPES [pH 7.6], 50 mM KCl, 0.01% NP-40, and 1 mM DTT. Recombinant RPA was expressed and purified as described earlier (Yuan et al., 2009). HARP and BRG1 were expressed and purified from Sf9 insect cells as described before (Ghosal et al., 2011).
Annealing activity assay
The annealing activity assay was carried out as described previously (Yusufzai and Kadonaga, 2008), with the following modifications: 0.2 μg supercoiled pUC19 DNA was incubated with 2.5 μg of purified RPA in the presence of 40 mM Tris-HCl (pH 8.0), 20 mM NaCl, 1 mM EDTA, 5 mM MgCl2, and 5 mM DTT at 37°C for 45 min. The reaction mixture was then treated with 2 units E. coli topoisomerase I (New England BioLabs) and incubated at 37°C for 30 min. The indicated proteins (at 60 nM) were added, and the mixture was incubated further at 37°C for 30 min. The reaction was terminated with a solution of SDS-EDTA and deproteinized with proteinase K (1 μg/μL). The products were extracted with an equal volume of chloroform and resolved on 1.2% agarose gel at 40 V/cm for 5 hr. The gel was then stained with ethidium bromide.
ATPase assay
Reaction mixtures (20 μL) containing 20 mM Tris-HCl (pH 8.0), 50 mM NaCl, 5 mM MgCl2, 0.2 mg/mL BSA, 0.1 mM DTT, 50 nM [γ-32P] ATP, 5 nM protein, and the indicated concentration of fork DNA, which was generated as previously described (Ghosal et al., 2011), were incubated at 30°C for 60 min. The reactions were terminated by adding EDTA to a final concentration of 15 mM. The products were extracted once with an equal volume of chloroform. A 2.5 μL portion of the extracted sample from each reaction mixture was spotted onto a polyethyleneimine cellulose thin-layer chromatography sheet and developed in 0.5 M LiCl, 1 M HCOOH, and 1 mM EDTA. The sheets were dried and visualized by autoradiography. The band intensities corresponding to [γ-32P] ATP and inorganic 32Pi were quantified using UVI-BandMap software.
Supplementary Material
Highlights.
AH2 is a protein with annealing activity and functions at stalled replication forks.
AH2 binds to PCNA, which is important for its function in vivo.
HARP or HARP-like domain is a common feature of proteins with annealing activity.
Acknowledgments
We thank all members of the Chen’s laboratory for their advice and technical assistance, especially Wenqi Wang. We thank Henry Adams and the Genetics department microscopy CORE facility at MD Anderson Cancer Center. This work was supported in part by grants from the National Institutes of Health to J.C. (CA089239, CA092312, and CA100109). J.C. is also a recipient of an Era of Hope Scholar award from the Department of Defense (W81XWH-05-1-0470) and is a member of MD Anderson Cancer Center (CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 2009;23:2405–2414. doi: 10.1101/gad.1839909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerkoel CF, O’Neill S, Andre JL, Benke PJ, Bogdanovic R, Bulla M, Burguet A, Cockfield S, Cordeiro I, Ehrich JH, et al. Manifestations and treatment of Schimke immuno-osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr. 2000;159:1–7. doi: 10.1007/s004310050001. [DOI] [PubMed] [Google Scholar]
- Branzei D, Foiani M. The checkpoint response to replication stress. DNA Repair (Amst) 2009;8:1038–1046. doi: 10.1016/j.dnarep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Mazina OM, Mazin AV. Rad54 protein promotes branch migration of Holliday junctions. Nature. 2006;442:590–593. doi: 10.1038/nature04889. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Bredemeyer AL, Sowa ME, Terret ME, Jallepalli PV, Harper JW, Elledge SJ. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 2009;23:2415–2425. doi: 10.1101/gad.1832309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MA, Eisen JA, Mohrenweiser HW. Cloning and characterization of HARP/SMARCAL1: a prokaryotic HepA-related SNF2 helicase protein from human and mouse. Genomics. 2000;65:274–282. doi: 10.1006/geno.2000.6174. [DOI] [PubMed] [Google Scholar]
- Eisen JA, Sweder KS, Hanawalt PC. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res. 1995;23:2715–2723. doi: 10.1093/nar/23.14.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Trotter KW, Archer TK, Kingston RE. Swapping function of two chromatin remodeling complexes. Mol Cell. 2005;17:805–815. doi: 10.1016/j.molcel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal G, Yuan J, Chen J. The HARP domain dictates the annealing helicase activity of HARP/SMARCAL1. EMBO Rep. 2011;12:574–580. doi: 10.1038/embor.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbis JM, Kelman Z, Hurwitz J, O’Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Pombo A. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol. 1998;140:1285–1295. doi: 10.1083/jcb.140.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kolodner RD, Putnam CD, Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002;297:552–557. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Okabe I, Bailey LC, Attree O, Srinivasan S, Perkel JM, Laurent BC, Carlson M, Nelson DL, Nussbaum RL. Cloning of human and bovine homologs of SNF2/SWI2: a global activator of transcription in yeast S. cerevisiae. Nucleic Acids Res. 1992;20:4649–4655. doi: 10.1093/nar/20.17.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358:1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- Postow L, Woo EM, Chait BT, Funabiki H. Identification of SMARCAL1 as a component of the DNA damage response. J Biol Chem. 2009;284:35951–35961. doi: 10.1074/jbc.M109.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RN, Horton WA, King CR. Chondroitin-6-sulphaturia, defective cellular immunity, and nephrotic syndrome. Lancet. 1971;2:1088–1089. doi: 10.1016/s0140-6736(71)90400-4. [DOI] [PubMed] [Google Scholar]
- Solinger JA, Kiianitsa K, Heyer WD. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol Cell. 2002;10:1175–1188. doi: 10.1016/s1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- Spranger J, Hinkel GK, Stoss H, Thoenes W, Wargowski D, Zepp F. Schimke immuno-osseous dysplasia: a newly recognized multisystem disease. J Pediatr. 1991;119:64–72. doi: 10.1016/s0022-3476(05)81040-6. [DOI] [PubMed] [Google Scholar]
- Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- Yuan J, Ghosal G, Chen J. The annealing helicase HARP protects stalled replication forks. Genes Dev. 2009;23:2394–2399. doi: 10.1101/gad.1836409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kadonaga JT. HARP is an ATP-driven annealing helicase. Science. 2008;322:748–750. doi: 10.1126/science.1161233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kadonaga JT. Annealing helicase 2 (AH2), a DNA-rewinding motor with an HNH motif. Proc Natl Acad Sci U S A. 2010;107:20970–20973. doi: 10.1073/pnas.1011196107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kong X, Yokomori K, Kadonaga JT. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev. 2009;23:2400–2404. doi: 10.1101/gad.1831509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







