Abstract
The fruit fly Drosophila melanogaster is increasingly utilized as an alternative to costly rodent models to study human diseases. Fly models exist for a wide variety of human conditions, such as Alzheimer's and Parkinson’s Disease, or cardiac function. Advantages of the fly system are its rapid generation time and its low cost. However, the greatest strength of the fly system are the powerful genetic tools that allow for rapid dissection of molecular disease mechanisms. Here, we describe the diet-dependent development of metabolic phenotypes in adult fruit flies. Depending on the specific type of nutrient, as well as its relative quantity in the diet, flies show weight gain and changes in the levels of storage macromolecules. Furthermore, the activity of insulin-signaling in the major metabolic organ of the fly, the fat body, decreases upon overfeeding. This decrease in insulin-signaling activity in overfed flies is moreover observed when flies are challenged with an acute food stimulus, suggesting that overfeeding leads to insulin resistance. Similar changes were observed in aging flies, with the development of the insulin resistance-like phenotype beginning at early middle ages. Taken together, these data demonstrate that imbalanced diet disrupts metabolic homeostasis in adult D. melanogaster and promotes insulin-resistant phenotypes. Therefore, the fly system may be a useful alternative tool in the investigation of molecular mechanisms of insulin resistance and the development of pharmacologic treatment options.
Keywords: Diabetes, Drosophila melanogaster, Insulin signaling, Insulin resistance, Diet
1. Introduction
The fruit fly Drosophila melanogaster has in the past been spectacularly successful in addressing fundamental biological problems, such as genetics or developmental biology [1]. In recent years, however, Drosophila has emerged as a viable model system for a variety of human diseases and medical conditions and as a promising alternative to expensive mammalian models. Drosophila is currently used to study immune [2], renal [3] and cardiac function [4], sleep [5], memory [6], neurodegenerative diseases [7], infectious diseases [8], aging [9], stem cells [10] and even aggression [11] and alcoholism [12].
The overall composition of the Drosophila neuroendocrine system resembles that of mammals. Cells similar in function to mammalian pancreatic α- and β-cells have been identified: The β-like cells exist as two clusters of seven cells each in the anterior portion of the fly brain [13]. These insulin-producing cells (IPC) are surrounded by cells that secrete the Drosophila ortholog of mammalian Neuropeptide Y, the small Neuropeptide F (sNPF), thus linking food intake to IPC function [14,15]. IPC respond to nutrients with the secretion of several insulin-like peptides into the fly blood, the hemolymph, near the fly heart [13]. Cells with α-cell-like function are situated in the ring gland, which surrounds the fly heart, and secrete adipokinetic hormone, the fruit fly equivalent of glucagon [16]. In addition, the mammalian prolactin-thyroid hormone axis may have its functional equivalent in the Drosophila ecdysteroid-juvenile hormone axis [17].
Molecular neuroendocrine signaling mechanisms are likewise conserved between flies and mammals. Drosophila has seven insulin-like peptides (ILP), four of which are secreted from the IPC [13]. dILPs bind to the single insulin receptor (InR) [18], which activates the insulin/insulin-like growth factor signaling pathway (IIS). InR stimulation results in the activation of the protein kinase Akt, which in turn modulates the activity of a variety of proteins and signaling pathways, e.g. the transcription factor Foxo [19], the TOR pathway that controls protein synthesis [20] and the cAMPpathway that governs metabolic activity [21].
This remarkable conservation in cellular architecture, inter- and intra-cellular signaling between flies and mammals has in recent years lead to an explosion of research into Drosophila neuroendocrine signaling mechanisms. It has moreover generated a lot of interest in utilizing the fly as a model system to study metabolism, physiology and the development of metabolic diseases, such as obesity and diabetes [22]. For example, Drosophila cardiac function is negatively affected by a high-fat diet, as a consequence of deregulated dTOR activity [23].
Recently, Drosophila has been used to study Type 1 diabetes. Ablation of IPC in Drosophila larvae results in a hyperglycemic phenotype [13] that persists into adulthood and is characterized by elevated hemolymph sugar levels [24].When IPC are partially ablated specifically in adults, flies similarly have elevated fasting glucose levels yet remain insulin-sensitive upon insulin injections [25].
The Drosophila system is well suited to complement more traditional mammalian model systems for metabolic research. It provides advantages regarding the low costs associated with fly work and the rapid generation time and short lifespan of flies, allowing for cost-effective and rapid testing of multiple experimental parameters. However, the greatest strengths of the Drosophila system lie in the powerful and well established genetic tools that allow for easy genetic manipulation. Drosophila allows tissue- and stage-specific expression experiments to be performed routinely and with remarkable ease. In addition, forward genetic screens can be performed using already available mutations or by performing large-scale mutagenesis. The combination of ease-of-use and powerful genetic tools make Drosophila a compelling model system to investigate complex metabolic diseases such as diabetes.
Therefore, we sought to utilize Drosophila as a model to study diet-induced disruption of metabolic homeostasis. We thus raised adult fruit flies on different dietary regimens and measured key metabolic parameters. Adult Drosophila show diet-dependent weight gain and signs of metabolic dysfunction, such as the development of a catabolic metabolic state and elevated levels of dILP mRNA. Importantly, overfed flies develop insulin resistance, a hallmark of type 2 diabetes. These phenotypes are dependent on the nutrient content of the fly diet and are aggravated by age. Therefore, Drosophila may serve as a convenient model to study metabolic diseases.
2. Materials and methods
2.1. Fly culture and strains
All flies were kept in a humidified (50%), temperature-controlled incubator with 12 hour on/off light cycle at 25 °C in vials containing standard cornmeal medium [26]. The PH-domain-GFP fusion expressing line tGPH was a kind gift from B. Edgar (Fred Hutchinson Cancer Research Center, Seattle, WA). All other lines were from the Bloomington Drosophila Stockcenter at Indiana University (Bloomington, IN).
All experiments were performed using female flies. Flies were raised on food containing the indicated amounts yeast extract or sucrose (w/V) (1%, 2%, 5%, 10%, 15%, 20%, or 30%, respectively), but no cornmeal. For ΔS experiments, yeast extract was kept constant at 15%, while varying the sucrose content [27], while for ΔY experiments sucrose was kept constant at 15% and yeast extract was varied. 'Starvation diet' therefore refers to diets that contain only 1% of the variable ingredient.
2.2. Determination of metabolite levels
Flies were raised on the indicated food at a density of 25 males and 25 females each per vial. At the indicated ages, flies were anesthetized, weighed in groups of maximally 10 flies, and transferred to chilled microcentrifuge tubes containing buffer A (20 mM HEPESKOH pH7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, protease inhibitors, 0.5 mM PMSF) and homogenized using a motorized micropestle. After centrifugation, supernatants were used for determination of metabolite levels. Glucose was determined by using the glucose detection kit (SIGMA). Trehalose and glycogen were converted into glucose through addition of trehalase (SIGMA, 0.2 U/ml) or amyloglucosidase (SIGMA, 0.1 U/ml), respectively, followed by glucose determination. Assays were measured using at least three biological replicates on a BioTek Synergy2 96-well plate reader in at least three technical replicates.
2.3. Fluorescent microscopy
For tGPH sub-cellular localization, adult fat body tissue was dissected into PBS from the abdomen of female flies, as described [28] and visualized using a Zeiss Axiovision Z1 fluorescent microscope with ApoTome™ optics (Zeiss). Flies were dissected and visualized by one experimenter, while the resulting staining was analyzed by another experimenter after data on all food conditions was collected. The complete set of experiments was repeated twice by different experimenters. We furthermore verified membrane staining by performing co-staining experiments with octadecyl rhodamine (data not shown), a dye that specifically stains membranes. Cells were considered IIS-inactive, if diffuse cytosolic staining was observed and IIS-active when plasma membrane staining was present.
For each food condition at least five individual flies were used. For measurements of insulin resistance, flies were starved on wet filter papers to avoid desiccation for 6 h and re-fed for 5 min with the food they were raised on in liquid form. Feeding was verified by visual observation of proboscis extension.
2.4. Fat measurements
Nuclear magnetic resonance (NMR) was conducted using a specially designed nuclear magnetic resonance (NMR) instrument (Bruker Minispec MQ10, Billerica, MA), which provides a read out of fat mass, lean tissue mass, and free fluid mass. Approximately 2000 flies per dietary condition (0.9–1.0 g) were placed in the reader and the individual readings of fat mass, lean tissue, and free fluid mass were obtained according to manufactures instructions.
2.5. Quantitative PCR
Total mRNA was isolated from at least 75 10-day old females using Trizol (Invitrogen) and further purified using the RNeasy kit (Qiagen). cDNA was generated with 0.5 µg total mRNA in a 10 µl reaction using the iScript cDNA synthesis kit (BioRAD). 0.8 µl of the iScript reaction was used as QPCR template. QPCR was performed as described [29] on a BioRAD CFX96 RealTime PCR System using the ABI SYBR-Green PCR master mix following the manufacturer’s instructions. Each QPCR reaction was performed using four biological replicates in triplicate each.
2.6. Statistics
Statistical analyses, including 1-way and 2-way ANOVA, were performed using the Prism suit of biostatistical software (GraphPad, San Diego).
3. Results
In order to determine whether flies develop diabetes-like metabolic changes, flies were raised on different diets with varying nutrient content, ranging from diets that lead to undernutrition to diets that cause overfeeding. Normal fly food consists of a carbohydrate source, sucrose, and a protein source, yeast extract. Fly diets were prepared in which each component was varied independently to investigate the effects of the different macronutrients on fly health, while keeping the other component constant at 15% (w/V). The 1% diets thus represent starvation diets, the 5% diets are slight underfeeding diets while 15% is slight overfeeding, and 30% constitutes overfeeding. At defined ages, flies were analyzed for metabolic benchmarks (glucose, glycogen, and trehalose).
3.1. Effects of a high sucrose diet on fly metabolism
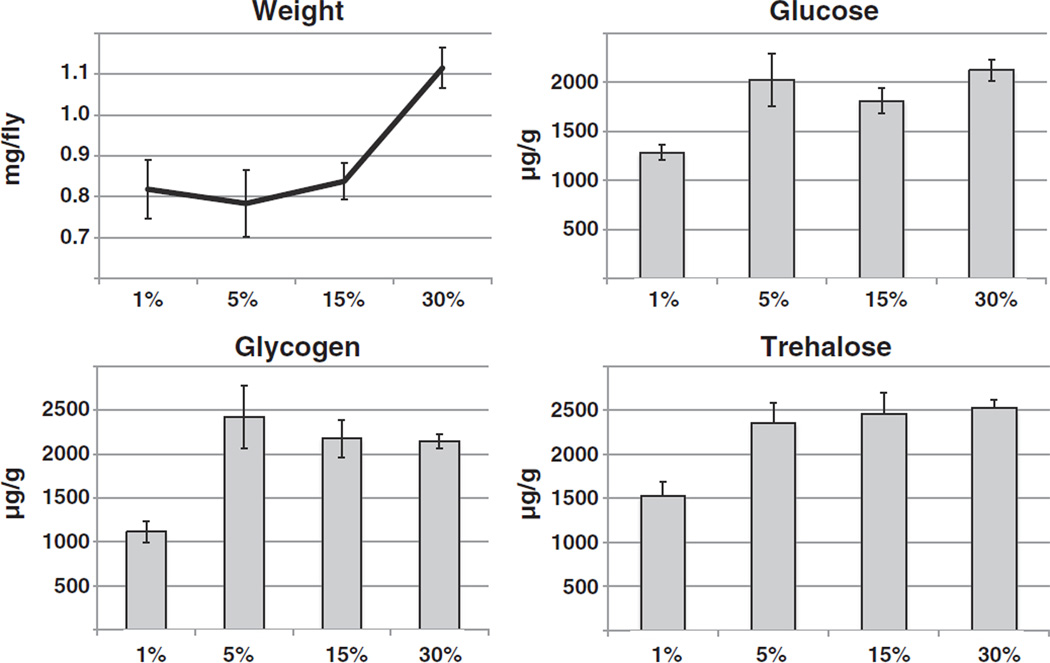
To determine the effects of sucrose on Drosophila metabolism, flies were raised on food containing 15% (w/V) yeast extract and varying amounts of sucrose (ΔS diet). At ten days of age, flies were weighed, harvested and their total glucose, glycogen and trehalose levels determined as described [30]. Significant increases in weight were only observed on the overfeeding diet, while the levels of carbohydrates remained constant on all food types, except for the decreased levels observed on the starvation diet (Fig. 1).
Fig. 1.
Effects of a high sucrose diet on Drosophila metabolism. Following eclosion, adult flies were raised for ten days on a diet of yeast extract (15%, all contents w/V) and differing amounts of sucrose as indicated. Flies were harvested, weighed and their glucose, glycogen and trehalose levels determined in whole body extracts in at least triplicate. Flies show weight gain with increased food content (1-way ANOVA p < 0.0001; with post-test for linear trend p < 0.0001), but no changes in stored carbohydrates when on non-starvation diets. Shown is a representative of two independent experiments.
3.2. Effects of a high protein diet on fly metabolism
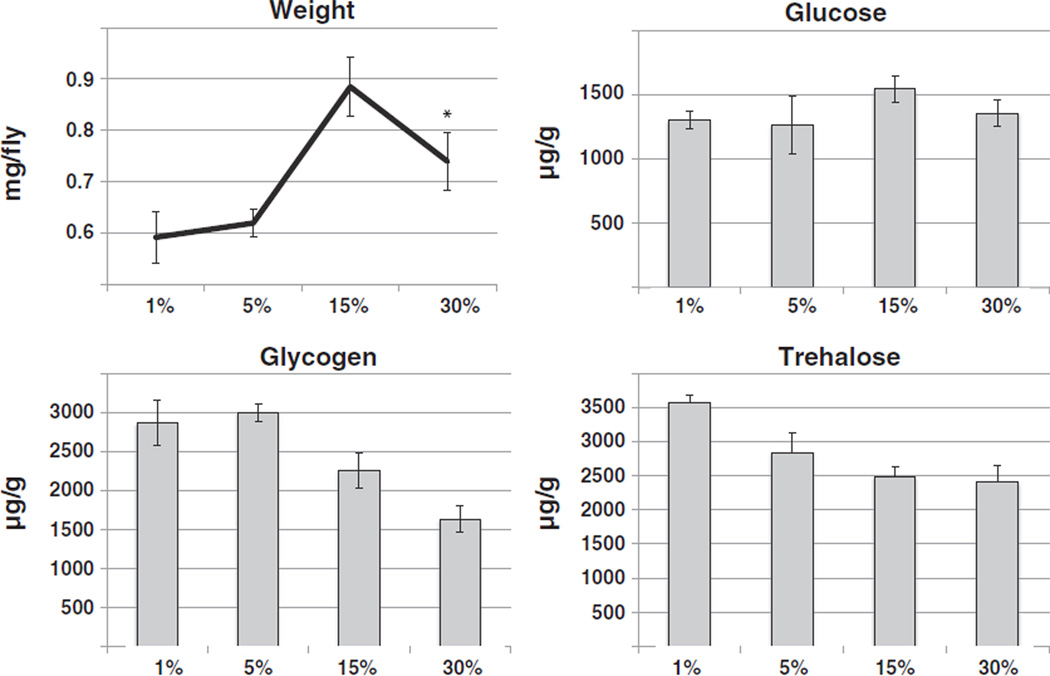
Similarly to the experiments described above, the influence of varying the amounts of yeast extract was assessed, while keeping sucrose levels constant at 15% (ΔY diet). Interestingly, flies had maximum weight when raised on slight overfeeding food (15%), but showed a weight drop from that maximum under heavy overfeeding (30%) conditions. Glucose levels stayed constant on all food conditions. In contrast, levels of stored carbohydrates, in the form of glycogen and trehalose, decreased significantly with increasing yeast extract content (Fig. 2). Together, these data suggest that a high sugar diet promotes weight gain, without drastically altering fly metabolism. In contrast, a very high protein diet promotes a catabolic state, characterized by weight loss and decreasing storage molecules.
Fig. 2.
Effects of a high protein diet on Drosophila metabolism. Following eclosion, adult flies were raised for ten days on a diet of sucrose (15%, all contents w/V) and differing amounts of yeast extract as indicated. Flies were harvested, weighed and their glucose, glycogen and trehalose levels determined in whole body extracts in at least triplicate. Flies show weight gain with increased food content when on non-starvation diets (1-way ANOVA p < 0.0001; with post-test for linear trend p = 0.0023; asterisk: t-test for the weight drop between 15% and 30%: p = 0.0038). Glycogen and trehalose levels decline with increasing food content (1-way ANOVA with post-test for linear trend: p < 0.0001 and p = 0.0108, respectively), but no changes in glucose levels are observed. Shown is a representative of two independent experiments.
3.3. Effects of varying diets on fat content and ovary size
In order to investigate the nature of the observed weight gain, we measured the influence of the various diets on fat storage levels. Flies raised on the ΔS diet showed a trend towards increased fat tissue content with increasing dietary sucrose that did not quite reach statistical significance (Supplemental Fig. 1A). In contrast, flies raised on the ΔY diet trended to maximal fat content at intermediate yeast extract levels, and decreased fat levels on the most severe overfeeding diet. On both diets, muscle tissue content did not change. These data show a similar pattern to the weight gain pattern observed on the ΔS and ΔY diets, respectively, and are consistent with measurements of TAG levels by Skorupa et al. [31].
Since high calorie food promotes fertility [32], we next investigated how diet affects the size of ovaries. Flies raised on ΔS diets had similar sized ovaries, independent of levels of dietary sucrose. On the other hand, flies raised on the 1% or 5% ΔY diets showed small ovaries barely containing any eggs, while flies raised on the higher ΔY diets had well-developed ovaries with many eggs (Supplemental Fig. 1B).
3.4. Age influences stored carbohydrate levels
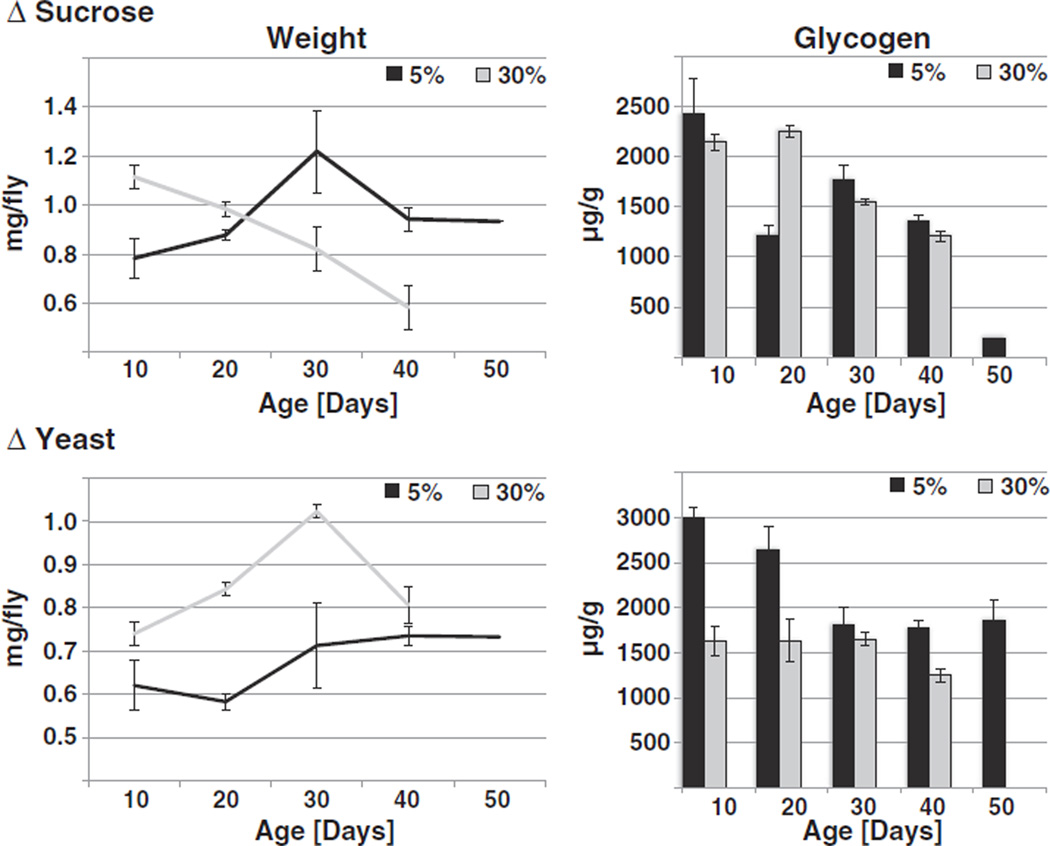
Next, flies were aged under the same conditions as described above, and samples were taken every ten days over the lifespan of the animals. Flies raised on 5% sucrose steadily gained weight as they aged, while flies raised on 30% sucrose lost weight. The reverse situation was observed when yeast extract was varied: Flies on 5% yeast extract only slightly increased their weight with age, while overfed flies gained weight until ~30 days, after which their weight dropped (Fig. 3). As overfed flies on either diet regimen have shortened lifespans [31], we therefore did not obtain enough surviving flies for analysis at age 50 days.
Fig. 3.
Effects of age on Drosophila metabolism. Flies were raised on ΔS food (top panels) or ΔY food (lower panels). At the indicated ages, flies were weighed and their glycogen levels determined in triplicate. Glycogen levels decrease with age in both ΔS and ΔY treatment groups (2-way ANOVA, p < 0.0001 for the effect of age on glucose levels for either diet). On ΔS diets, overfeeding only had a small effect on glycogen levels (2-way ANOVA, p = 0.0559 for the comparison of age-dependent variation between different food levels), while overfeeding on a ΔY diet significantly reduced glycogen levels compared to underfeeding diets (2-way ANOVA, p < 0.0001). Flies raised on a slight underfeeding diet (5%) are in black, flies on an overfeeding diet (30%) are in grey. Shown are representatives of two independent experiments.
Levels of glucose stayed almost constant (Supplemental Fig. 2), while glycogen and trehalose declined with age, regardless of feeding regimen (Fig. 3 and data not shown). Interestingly, either overfeeding regimen accelerated this decrease in storage molecules (Fig. 3). These data indicate that aging leads to an increase in catabolic activity, which is accelerated by overfeeding.
3.5. Overfeeding diminishes insulin-signaling
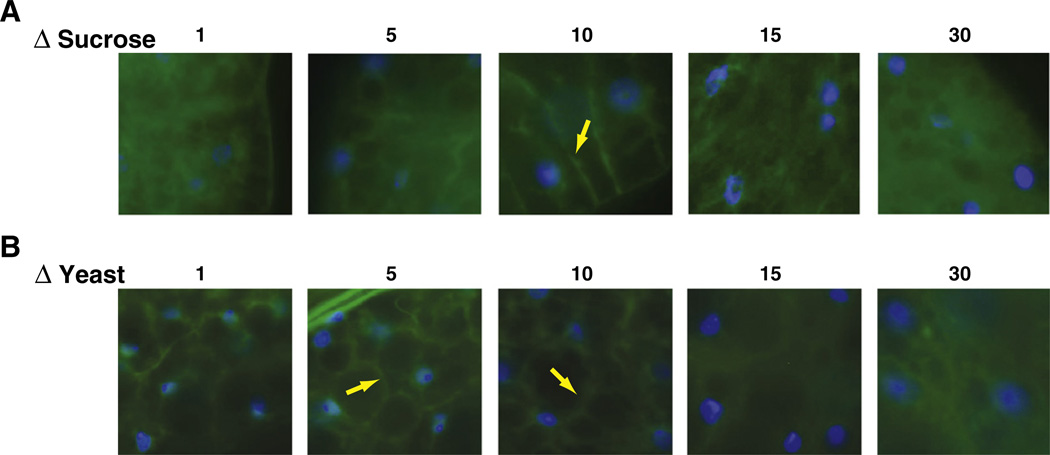
One of the most important hallmarks of Type 2 diabetes is the development of cellular insulin resistance, that is, the inability of cells to respond to an insulin signal. To measure insulin/insulin-like growth factor signaling (IIS) activity, we utilized a pleckstrinhomology domain-GFP fusion protein (tGPH), which is localized in the plasma membrane upon insulin-dependent activation of PI3K. If IIS is inactive, the tGPH reporter remains cytosolic [33]. Using this assay, we have recently shown that PI3K activity in the fat body of flies is highest at intermediate calorie levels, while either under- or overfeeding lowers PI3K activity [34]. However, in these experiments, both sucrose and yeast extract content were varied concomitantly. Therefore, we investigated IIS status in the fat body, an organ that combines functionalities of liver and adipose tissue, in flies fed either of our ΔS or ΔY diet regimens. Flies fed the ΔS diet showed strongest plasma membrane staining at the intermediate sucrose concentrations of 10% sucrose. Higher sucrose concentrations led to a drastic increase in cytosolic staining, while lower concentrations showed a modest increase in cytosolic staining (Fig. 4A). Similar results were obtained using the ΔY diet, with the maximum plasma membrane staining shifted to slightly lower concentrations (5% yeast extract, Fig. 4B).
Fig. 4.
Insulin-signaling activity as a function of food content. Flies expressing the tGPH reporter construct were raised for ten days on ΔS diets (top panels) or ΔY diets (lower panels). Dissected fat bodies were evaluated for GFP staining in the membrane. Strongest membrane GFP staining is observed at intermediate food concentrations and declines both with low and high nutrient content (blue: DAPI; green: GFP; yellow arrows depict tGPH staining in the plasma membrane).
3.6. Insulin-mRNA levels vary with dietary content
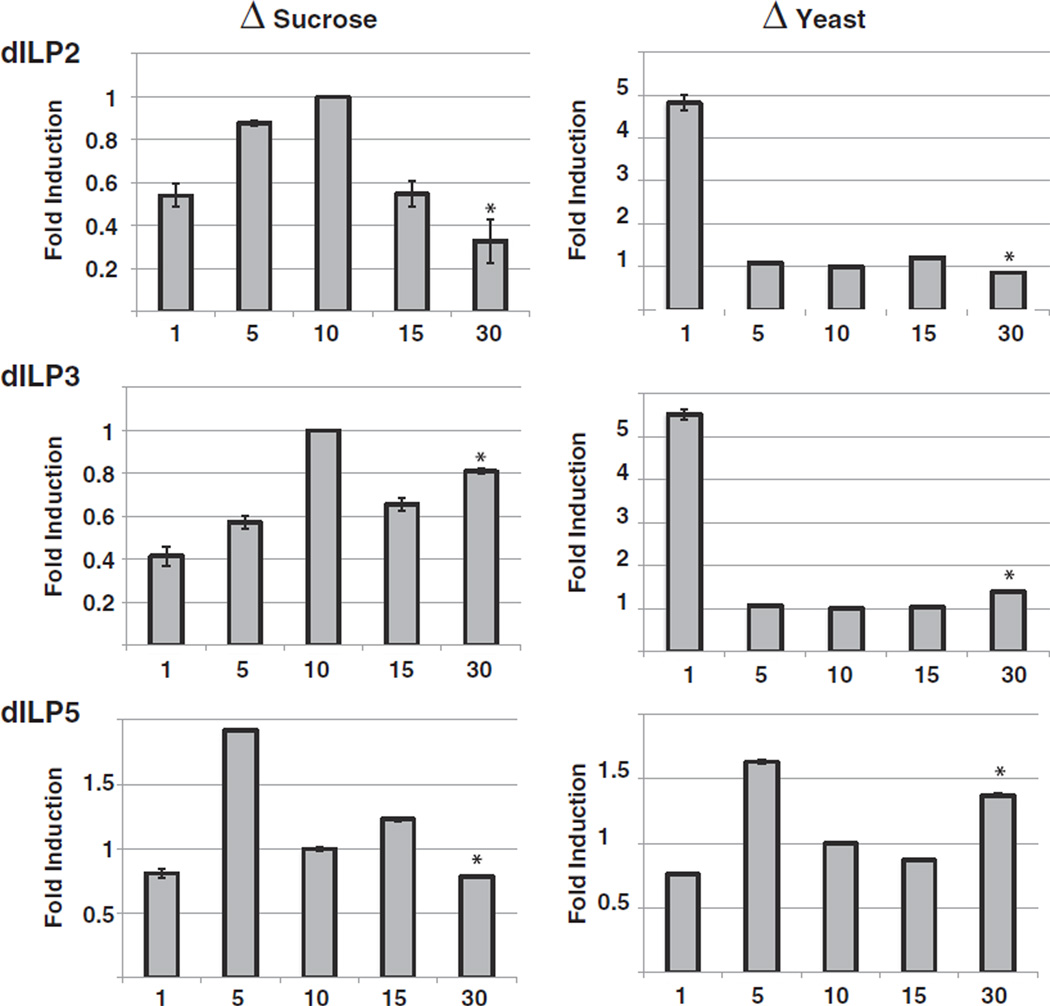
In order to determine whether reduced insulin-signaling was associated with altered Drosophila insulin-like peptide (dILP) levels, we performed QPCR analysis of the mRNA levels of the dILPs secreted from insulin-producing cells (IPC) under different dietary conditions. When yeast content was kept constant (ΔS diet), dILP2 and dILP3 were most highly expressed at intermediate sucrose levels (Fig. 5), corresponding to the highest insulin-signaling activity. Similar results were observed with dILP5, which showed highest levels at slightly lower sucrose concentrations. In contrast, on ΔY diets, dILP2 and 3 were greatly elevated on starvation conditions (Fig. 5). Interestingly, dILP3 and dILP5 were elevated at the high end of the ΔY diet spectrum and showed lowest levels at intermediate food concentrations (Fig. 5). These data suggest that low yeast extract levels induce dILP expression to facilitate cellular nutrient uptake. Moreover, the elevated levels of dILPs on the high ΔY diets suggest a compensatory response to diminished insulin-signaling.
Fig. 5.
Effects of diet on dILP abundance. Flies were raised on ΔS diets (left panels) or ΔY diets (right panels) for ten days and their dILP levels determined by QPCR. Levels of dILP2, dILP3 and dILP5 were normalized against levels of the housekeeping gene rp49. Normalized dILP levels were compared to dILP levels of flies raised on 10% diets. Shown is a representative of two independent experiments (error bars represent the standard deviation of five biological replicates; asterisks denote p < 0.0001 for the comparisons of 30% foods to 10% control food).
3.7. Development of insulin resistance due to overfeeding and age
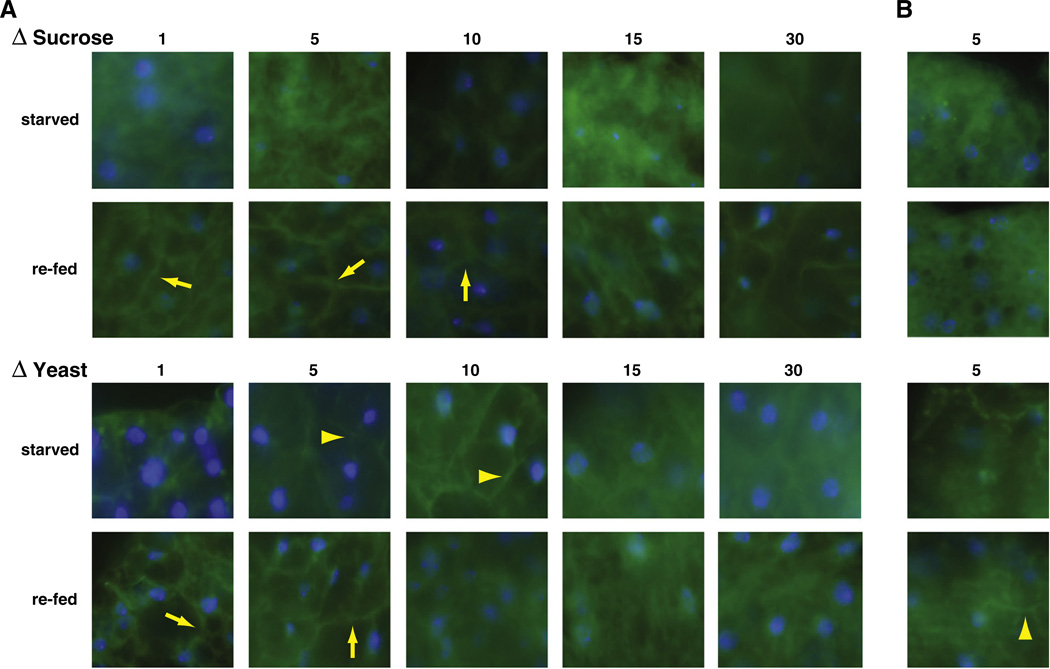
These results suggest that overfeeding may lower IIS activity, which may indicate the development of insulin resistance. To further test this possibility, we used the PI3K assay to test whether overfed flies were able to respond to an acute food stimulus with activation of IIS. Therefore, flies were raised under the same conditions as above. After ten days, flies were starved for 6 h to decrease IIS, and then re-fed for 5 min with their original food source. Using this starvation regimen, IIS was effectively shut down under most food conditions. Some residual plasma membrane staining was observed on 5% and 10% ΔY diets. Re-feeding led to strong plasma membrane staining at low to intermediate food concentrations (Fig. 6A). These data are similar to what is seen in the previous assay testing constitutive IIS activity. At higher food concentrations, however, cytosolic staining increased, indicating that under these conditions fat body cells cannot respond to nutrients with the activation of PI3K anymore.
Fig. 6.
Insulin-responsiveness as a function of food content and age. (A) Flies expressing the tGPH reporter construct were raised for ten days on food with differing sucrose content (top panels) or differing yeast extract content (lower panels), starved overnight and re-fed. Starved flies show almost no plasma membrane staining, except flies fed 5% and 10% yeast food. Re-feeding flies raised on low nutrient food leads to strong plasma membrane staining, while flies raised on high nutrient food do not respond. (B) Older flies raised on 5% sucrose food do not respond to sucrose re-feeding with plasma membrane staining, while flies raised on 5% yeast food retain residual IIS activity (blue: DAPI; green: GFP; yellow arrows depict tGPH staining in the plasma membrane, yellow triangles depict weak plasma membrane staining).
Next, we performed the re-feeding assay using older flies, to test whether age may also influence the development of insulin resistance in flies. As shown in Fig. 6B, even the food conditions that show maximum response at younger ages (5%), elicited only a reduced response to acute re-feeding in older flies, while flies aged on richer food lost all responsiveness (data not shown). These data suggest that overfeeding and aging lead to the development of insulin resistance in flies.
4. Discussion
Traditionally, the fruit fly D. melanogaster has been a model system for investigating questions in genetics and developmental biology. The powerful genetic tools and the low costs of the Drosophila system are important factors in efforts to establish Drosophila as an alternative and complementary model system to rodent models of human metabolic diseases. For example, in a fly model for cardiac function, researchers are able to measure heart rate and rhythm, systolic and diastolic amplitudes and heart fibrillation [4]. These measures of fly heart function are influenced by metabolic signaling pathways, such as IIS or the TOR pathway [35].
The similarities in neuroendocrine architecture and signaling mechanisms between flies and mammals suggest that Drosophila might furthermore function as a convenient model system for studying disruption of metabolic homeostasis. Drosophila exposed to high fat diets accumulate fat and show signs of cardiac dysfunction. These symptoms are regulated by the TOR pathway [23]. Recently, Drosophila has been used to model Type 1 diabetes. Ablation of the IPCs, which are similar in function to pancreatic β-cells, in Drosophila larvae results in a hyperglycemic phenotype [13] that persists into adulthood and is characterized by elevated hemolymph sugar levels [24]. When IPC are partially ablated specifically in adults, a similar phenotype is observed. These IPC ablated flies have elevated fasting glucose levels yet remain insulin-sensitive upon insulin injections [25].
It has been shown previously that diet composition affects metabolic homeostasis and longevity in fruit flies. Diets high in carbohydrates were associated with increased fat content at middle ages, while diets high in yeast extract counteracted this effect [31]. In an effort to investigate how diet composition may lead to the disruption of metabolic homeostasis, we explored dietary influences on both carbohydrate and fat storage in adult fruit flies. A diet rich in carbohydrates leads to fat accumulation and weight gain, no other gross metabolic abnormalities, but also suppressed fertility (Supplemental Fig. 3A). In contrast, increasing amounts of yeast extract leads to increases in ovary size and fertility (Supplemental Fig. 3A). Interestingly, on the highest concentrations, the ΔY diet results in moderate weight loss. Moreover, in the most extreme ΔY flies we observe a decrease in the levels of storage molecules, reduced fertility and insulin resistance (this report), as well as shortened lifespan (data not shown). It has been suggested that high yeast extract levels negatively regulate feeding behavior [31]. However, we did not observe significant feeding rate differences (Supplemental Fig. 3B) between the different types of diets. This therefore suggests that high yeast extract levels may lead to severe disruption of metabolic homeostasis and the induction of a catabolic state. The catabolic state is aggravated with age and develops in aging ΔS flies as well. This disruption of metabolic homeostasis is accompanied by profound detrimental effects on fly health and physiology.
This situation is reminiscent of what is often seen with ketogenic diets (KD). KD are high fat/high protein and low carbohydrate diets that are frequently used for weight loss regimens or the treatment of epilepsy [36]. Using these diets, metabolism adjusts to use stored fats as the major source of energy, which are broken down by the HMG-CoA pathway into ketone bodies for use in the TCA cycle. Our high yeast extract diets additionally contain high levels of sucrose at 15%, while the yeast extract used to prepare the diets only contains ~1% fat. Therefore, these diets may not be classic KD. Nonetheless, our data suggest that the high ΔY diets may alter fly metabolism in a similar way. It will be interesting to measure the levels of ketone bodies in these overfed flies to more precisely define a possible ketogenic effect of our diets.
Disruption of metabolic homeostasis often leads to the development of insulin resistance, which is a hallmark of type 2 diabetes. Therefore, we measured constitutive and inducible IIS activity in flies raised on the different dietary regimes. Underfed flies on low ΔS diets have low constitutive IIS activity in fat body cells, which could be stimulated by acute re-feeding, suggesting that the fat body in underfed flies remains competent to respond to an insulin signal.
In contrast, IIS activity is compromised in overfed flies, as both ΔS and ΔY overfeeding conditions show a decrease in constitutive IIS activity. Importantly, food-inducible IIS is similarly impaired upon overfeeding. Therefore, these data suggest that the fat body in overfed flies loses the ability to respond to an insulin signal and thus shows signs consistent with an insulin resistant phenotype. Interestingly, aging flies start losing IIS at relatively young ages (~30 days) and are completely insulin resistant at middle ages (~40–50 days, data not shown). This age-dependent loss of IIS activity is not due to IPC loss, however, as we observe no decline in the number of IPCs with age (data not shown).
Nor can the lack of IIS activity in overfed or aging flies simply be explained by reduced dILP levels. On ΔS diets, dILP2 and 3 mRNA levels are strongly downregulated on overfed conditions, while dILP5 levels are only slightly lowered. High ΔY diets in contrast upregulate dILP3 and 5, while dILP2 is slightly downregulated, suggesting antagonistic effects of dietary sucrose and yeast extract. The upregulation of dILP3 and 5 on ΔY diets is not associated with increased IIS, but its reduction. This situation is reminiscent of hyperinsulinemia in type 2 diabetes and suggests a compensatory effect on dILP production. It has been suggested that dILP3 and 5 control growth and developmental timing [37], while dILP2 may be important in the regulation of adult fly longevity. In adult flies, reduction of dILP2 levels have been observed in a variety of long-lived genetic interventions [28,38,39], while our data indicates that dILP 3 and 5 play a role in the regulation of adult metabolic homeostasis. However, specific knockdown of each of the dILPs does not generate severe phenotypes [37], suggesting at least partial redundancy between the individual dILPs.
Interestingly, while both ΔS and ΔY overfeeding conditions resulted in an insulin resistance-like phenotype, the accompanying metabolic abnormalities manifested themselves slightly differently: ΔS diets led to weight gain and elevated glycogen levels, while flies raised on high ΔY diets showed a decrease in glycogen and a weight drop at the most extreme overfeeding condition, indicative of a catabolic state.
As discussed above, the extreme ΔY diet phenotype may be due to a possible ketogenic effect of these diets, in addition to a phenotype resembling dILP3 and 5 hyperinsulinemia and cellular insulin resistance. In contrast, extreme ΔS diets lead to accumulation of storage molecules, insulin resistance, but no hyperinsulinemia. These symptoms are aggravated with age. Interestingly, our data clearly demonstrate the previously noted antagonistic effects (Skorupa et al. [31]) of the two main Drosophila dietary components. The disruption of metabolic homeostasis by severe nutritional imbalance thus produces fly phenotypes that, at least in part, appear to resemble metabolic phenotypes observed in humans, such type 2 diabetes or ketosis. These phenotypes can alternatively be generated by targeted disruption of genes responsible for homeostatic maintenance, such as the Drosophila glucose receptor BOSS. Fly larvae lacking BOSS have elevated fatty acid content and increased hemolymph glucose levels. Moreover, IIS activity is downregulated [40].While the metabolic status of adult BOSS null flies has not yet been determined, these symptoms very closely resemble the symptoms of our overfed flies, suggesting that BOSS plays a crucial part in glucose homeostasis and the development of metabolic symptoms as a consequence of overfeeding or aging.
The Drosophila system of diet-induced homeostatic disruption is well suited to complement more traditional mammalian model systems for metabolic research. It provides advantages due to the low costs associated with fly work and the rapid generation time of flies, and thus allows for rapid and cost-effective testing of multiple interventions. However, the greatest strengths of Drosophila system lie in the easy genetic manipulation and its genetic versatility, allowing rapid screens for novel genetic and pharmacologic interventions that modulate metabolic disruption.
Supplementary Material
Acknowledgements
The authors would like to thank B. Edgar for the kind gift of fly stocks. We also thank Chris Thephachanh for technical assistance. This work was supported NIA AG029723 to JHB and Hamilton Undergraduate Research Awards to CC and KHC. JHB is a recipient of the Ralph. E. Powe Junior Faculty Enhancement Award.
Abbreviations
- IIS
insulin/insulin-like growth factor signaling
- IPC
insulin-producing cells
- dILP
Drosophila insulin-like peptide
Footnotes
Author contributions
SNSM designed and performed experiments and analyzed the data; CC, KHC, SOF and SK performed experiments, JNK analyzed the data and JHB designed and performed experiments, analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbadis.2012.04.012.
References
- 1.Bendiner E. Of peas, fruit flies, and the birth of a science. Hosp. Pract. (Off. Ed.) 1987;22:116–120. 123-119, 132-113. [PubMed] [Google Scholar]
- 2.Kim T, Kim YJ. Overview of innate immunity in Drosophila. J. Biochem. Mol. Biol. 2005;38:121–127. doi: 10.5483/bmbrep.2005.38.2.121. [DOI] [PubMed] [Google Scholar]
- 3.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am. J. Physiol. Renal Physiol. 2010;299:F1237–F1244. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 2009;25:262–269. doi: 10.1016/j.tig.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog. Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu. Rev. Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- 8.Dionne MS, Schneider DS. Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis. Model. Mech. 2008;1:43–49. doi: 10.1242/dmm.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helfand SL, Rogina B. From genes to aging in Drosophila. Adv. Genet. 2003;49:67–109. doi: 10.1016/s0065-2660(03)01002-2. [DOI] [PubMed] [Google Scholar]
- 10.Pearson J, Lopez-Onieva L, Rojas-Rios P, Gonzalez-Reyes A. Recent advances in Drosophila stem cell biology. Int. J. Dev. Biol. 2009;53:1329–1339. doi: 10.1387/ijdb.072431jp. [DOI] [PubMed] [Google Scholar]
- 11.Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:e154. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devineni AV, Heberlein U. Addiction-like behavior in Drosophila. Commun. Integr. Biol. 2010;3:357–359. doi: 10.4161/cib.3.4.11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 14.Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat. Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- 15.Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J. Biol. Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 17.Flatt T, Moroz LL, Tatar M, Heyland A. Comparing thyroid and insect hormone signaling. Integr. Comp. Biol. 2006;46:777–794. doi: 10.1093/icb/icl034. [DOI] [PubMed] [Google Scholar]
- 18.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 19.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 20.Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 2009;41:1006–1010. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Mattila J, Bremer A, Ahonen L, Kostiainen R, Puig O. Drosophila FoxO regulates organism size and stress resistance through an adenylate cyclase. Mol. Cell. Biol. 2009;29:5357–5365. doi: 10.1128/MCB.00302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2010;2:523–526. doi: 10.18632/aging.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haselton A, Sharmin E, Schrader J, Sah M, Poon P, Fridell YW. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle. 2010;9:3063–3071. doi: 10.4161/cc.9.15.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. Lond. B Biol. Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 28.Bauer JH, Chang C, Morris SN, Hozier S, Andersen S, Waitzman JS, Helfand SL. Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13355–13360. doi: 10.1073/pnas.0706121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer JH, Morris SN, Chang C, Flatt T, Wood JG, Helfand SL. dSir2 and Dmp53 interact to mediate aspects of CR-dependent life span extension in D. melanogaster. Impact: Aging. 2009;1:38–48. doi: 10.18632/aging.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Blanco A, Fridell YW, Helfand SL. Involvement of Drosophila uncoupling protein 5 in metabolism and aging. Genetics. 2005;172:1699–1710. doi: 10.1534/genetics.105.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 33.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 34.Bauer JH, Chang C, Bae G, Morris SN, Helfand SL. Dominant-negative Dmp53 extends life span through the dTOR pathway in D. melanogaster. Mech. Ageing Dev. 2010;131:193–201. doi: 10.1016/j.mad.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balietti M, Casoli T, Di Stefano G, Giorgetti B, Aicardi G, Fattoretti P. Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev. 2010;9:273–279. doi: 10.1016/j.arr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 39.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Kohyama-Koganeya A, Kim YJ, Miura M, Hirabayashi Y. A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15328–15333. doi: 10.1073/pnas.0807833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.