Abstract
With advantages such as design flexibility in modifying degradation, surface chemistry, and topography, synthetic bone–graft substitutes are increasingly demanded in orthopedic tissue engineering to meet various requirements in the growing numbers of cases of skeletal impairment worldwide. Using a combinatorial approach, we developed a series of biocompatible, hydrolytically degradable, elastomeric, bone–like biocomposites, comprising 60 wt% poly(2–hydroxyethyl methacrylate–co–methacrylic acid), poly(HEMA–co–MA), and 40 wt% bioceramic hydroxyapatite (HA). Hydrolytic degradation of the biocomposites is rendered by a degradable macromer/crosslinker, dimethacrylated poly(lactide–b–ethylene glycol–b–lactide), which first degrades to break up 3–D hydrogel networks, followed by dissolution of linear pHEMA macromolecules and bioceramic particles. Swelling and degradation were examined at Hank’s balanced salt solution at 37 °C in a 12–week period of time. The degradation is strongly modulated by altering the concentration of the co–monomer of methacrylic acid and of the macromer, and chain length/molecular weight of the macromer. 95% weight loss in mass is achieved after degradation for 12 weeks in a composition consisting of HEMA/MA/Macromer = 0/60/40, while 90% weight loss is seen after degradation only for 4 weeks in a composition composed of HEMA/MA/Macromer = 27/13/60 using a longer chain macromer. For compositions without a co–monomer, only about 14% is achieved in weight loss after 12–week degradation. These novel biomaterials offer numerous possibilities as drug delivery carriers and bone grafts particularly for low and medium load–bearing applications.
Keywords: pHEMA, hydrolytic degradation, macromer
1. Introduction
Polymers and bioceramics such as hydroxyapatite (HA), tricalcium phosphate (β–TCP), and bioglass may form biodegradable, biocompatible organic/inorganic composites that can be used as synthetic bone grafts. They are important to bone repair and regeneration, with structural and chemical properties that provide a biochemical environment to promote cellular responses and tissue integration [1,2]. To meet the requirements of these functions, the most desirable synthetic organic/inorganic hybrid materials should be biocompatible, porous (with diameters of at least 100 µm), and hydrolytically or enzymatically biodegradable within a specific time frame (e.g., 4–6 months) [3–7]. They should also be both osteoconductive and osteoinductive, so that the cells can bind to the hybrid biomaterials, proliferate, migrate, and differentiate. Further, stability of mechanical properties in the materials is essential for cell survival and ingrowth of new bone tissue. Otherwise, stress shielding, inflammation, and other discomforts may result from a mismatch between mechanical properties of hybrid materials and those of surrounding tissue.
There is a significant body of literature that describes methods for fabrication of porous synthetic organic/inorganic biocomposites for bone repair and regeneration [3, 4, 6–13]. However, development of porous, biomimetic hybrid materials that simultaneously meet all requirements of bone repair and replacement still remains a challenge [14–16]. Furthermore, porous hybrid materials generally have low mechanical properties restricting their load–bearing applications [17]. In some applications, the hybrid biomaterials can be dense or non–porous. An example is the fixation of a bone fracture, where a rod of the synthetic polymer/bioceramic is inserted and fixed between the fractured bone pieces, or the rod is inserted within the broken bone [18, 19]. In this case, dense material that is both biocompatible and biodegradable is required to bear loads. Demand is increasing for both porous and dense organic/inorganic biomaterials, as evidenced by the growing number of cases of skeletal impairment [20,21].
Recent reports have described the fabrication of non–degradable, dense, poly(2–hydroxyethyl methacrylate)/hydroxyapatite biocomposites (also known as FlexBone) based on in situ polymerization of 2–hydroxyethyl methacrylate in the presence of dispersed hydroxyapatite [22–24]. These newly formed biocomposites show elastomeric behavior. Because of the elastomeric nature of these biocomposites, they can be cut, bent, and machined into various shapes suitable for low and medium load bearing applications. However, these biomaterials are not degradable, limiting their usefulness in bone repair and replacement [6, 9, 13, 16, 25]. In certain applications, such as delivery of bone–morphogenetic proteins and antibiotics for bone repair, biomaterials are typically required to be biodegradable. To achieve biodegradability and biocompatibility of hydrogel/HA biocomposites, we recently focused our efforts on development of pHEMA–type hydrogel/HA systems [26]. PHEMA hydrogel is widely known to be biocompatible, but not biodegradable [27, 28]. Our design of degradable, elastomeric pHEMA/HA biocomposites was inspired by the observation that a degradable crosslinker (also termed a macromer here) disintegrates by hydrolytic degradation the three–dimensional hydrogel network. Without the use of enzymes, the network decomposes into small structural molecules and generates water–soluble linear pHEMA macromolecular chains. Generally, this will occur if the molecular weight of linear pHEMA is below 8–10k Da [29]. HA is a bioactive ceramic and also resembles the inorganic phase of human bone, and thus may be gradually resorbed by the human body. Mindful of those traits, we purposely synthesized macromers/crosslinkers of dimethacrylated triblock copolymers, poly(lactide–b–ethylene glycol–b–lactide)(PLA–b–PEG–b–PLA), since these materials are biocompatible and hydrolytically degradable, and their degradation has been well investigated [30–37]. Interestingly, these dimethacrylated triblock macromers were not used as the crosslinkers in the synthesis of degradable pHEMA hydrogels [38–43]; nor were they used in the fabrication of degradable biocomposites comprising pHEMA and hydroxyapatite or other bioactive ceramics. Only few biopolymer/bioceramics (or bioglass) systems were reported in literature showing significant degradation in 4–6 months for bone tissue engineering [4–7, 44–46]. Our broad objective was to develop a series of degradable, bone–like pHEMA/HA biomaterials (not degradable pHEMA hydrogel because the pure hydrogel is useless without bioceramics for bone regeneration) as bone grafts/implants with controlled degradation especially in 4–6 months, which is highly recommended by surgeons in clinical trials [47]. Since there are various parameters in combination to determine degradation of the hybrid biomaterials such as crosslink density and wettability, we used a combinatorial approach that tuned the molecular structures of hydrogels for low to medium load–bearing applications in bone repair and regeneration [48–52]. Here, we report in detail how crosslink density, chain length (molecular weight), hydrophilicity of the macromer/crosslinker, and a polar co–monomer influence swelling and hydrolytic degradation of biomaterials.
2. Experimental section
2.1 Materials
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) and used without purification unless otherwise specified.
2.2 Synthesis of the macromer/crosslinker and fabrication of the poly(HEMA–co–MA)/HA biocomposites
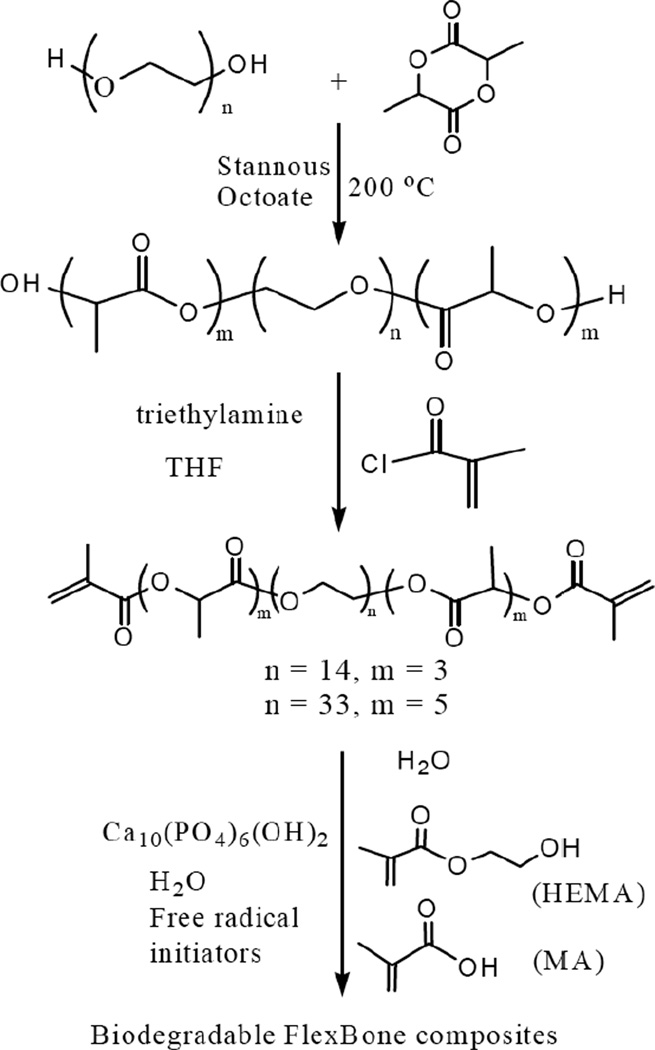
Synthesis of the macromer/crosslinkers has been previously described [30, 33, 36, 37], but here we used a revised process. Prior to synthesis of the macromer/crosslinker, a lactide–co–polyethylene glycol (PEG)–co–lactide triblock copolymer was first synthesized, as shown in Scheme 1.
Scheme 1.
Synthesis of degradable macromers and poly(HEMA-co-MA)/HA biocomposites
PEG600 or PEG1500, lactide, and stannous octoate were added sequentially according to a molar ratio of 93:279.5:1 (in the case of PEG600) or 93:465:1 (in the case of PEG1500) into a reaction flask at 23 °C. This mixture was purged with dry nitrogen for 30 min, and then stirred at 110 °C under vacuum for another 30 min. Next, dry nitrogen was charged to replace the vacuum and the reaction continued at 180 °C under nitrogen protection for 6 h. After cooling to 23 °C, the resultant triblock copolymer was dissolved in dichloromethane and further precipitated in anhydrous diethyl ether. The triblock copolymer precipitate was separated and dried in a vacuum for subsequent synthesis of the macromer/crosslinker. During the synthesis of the macromer/crosslinker, the obtained lactide–co–PEG–co–lactide triblock copolymer and triethylamine (with a mole ratio of 1:6.94) were first dissolved in 230 mL dichloromethane in a reaction flask and then cooled in an ice bath to 0 °C. Subsequently, the mixture was purged with dry nitrogen for 30 min. The methacryloyl chloride, with the same molar ratio of triethylamine, was slowly added (dropwise, with stirring) into the flask and reacted for 9 h at 0 °C. The entire mixture was cooled to 23 °C and the reaction was maintained for an additional 14 h. The reaction mixture was filtered to remove triethylamine hydrochloride, and subsequently hexane was added for precipitation. The obtained crystalline solid (i.e., macromer) was further purified twice by dissolution and re–precipitation using dichloromethane and hexane. The macromer was dried at 50 °C for 5 h under vacuum.
Fabrication of the non–biodegradable pHEMA/HA composites was previously reported [23]; similar procedures were employed here to fabricate degradable pHEMA hydrogel/HA biomaterials (Scheme 1). The monomers (main monomer, co–monomer, and macromer/crosslinker with desired ratios, e.g., 20:20:60), ethanol, de–ionized water, ammonium persulfate (480 mg/mL water), sodium metabisulfite (180 mg/mL water) were mixed thoroughly at a ratio of 20:10:10:1:1 by mass. 40 wt.% HA (Trans–Tech, size 1–3 µm) relative to the total weight of all the components (i.e., 57.1 wt% relative to the total weight of monomers, macromere, and HA) was uniformly dispersed into the mixture and stirred at 5,000 rpm for 2 min using a homogenizer (Polytron PT 10–35). The resultant mixture was pumped into a syringe (20 mL, diameter of 18.32 mm) and capped. It was left overnight to gel at room temperature. As shown in Table 1, a series of hydrogel/HA materials with various formulations was fabricated using the process described above. These hydrogel/HA biomaterials were specifically labeled such as S–MA80–H0–Mac20 and L–MA0–H23–Mac77, where S represents the short chain of lactide/PEG600= 3, L the long chain of lactide/PEG1500 = 6, MA methacrylic acid, H HEMA, and Mac the macromer. The number right after MA, H or Mac represents the weight percentage of the associated component relative to the total weight (MA + H + Mac). For example, L–MA0–H23–Mac77 represents the biocomposite composed of 40 wt% HA and 60 wt% hydrogel which was synthesized using 0 wt% methacrylic acid, 23 wt% HEMA, and 77 wt% macromer based on the long chain of lactide/PEG1500 = 6.
Table 1.
Formulations of degradable 60% poly(HEMA-co-MA)/40% HA biomaterials
| Composition representation |
Precursor | Lactide/PEG | MA (%) | HEMA (%) | Macromer (%) |
|---|---|---|---|---|---|
| S-MA80-H0-Mac20 | PEG600 | 3 | 80 | 0 | 20 |
| S-MA60-H0-Mac40 | PEG600 | 3 | 60 | 0 | 40 |
| S-MA40-H0-Mac60 | PEG600 | 3 | 40 | 0 | 60 |
| S-MA20-H0-Mac80 | PEG600 | 3 | 20 | 0 | 80 |
| S-MA27-H13-Mac60 | PEG600 | 3 | 26.7 | 13.3 | 60 |
| S-MA20-H20-Mac60 | PEG600 | 3 | 20 | 20 | 60 |
| S-MA13-H27-Mac60 | PEG600 | 3 | 13.3 | 26.7 | 60 |
| S-MA0-H80-Mac20 | PEG600 | 3 | 0 | 80 | 20 |
| S-MA0-H60-Mac40 | PEG600 | 3 | 0 | 60 | 40 |
| S-MA0-H40-Mac60 | PEG600 | 3 | 0 | 40 | 60 |
| S-MA0-H20-Mac80 | PEG600 | 3 | 0 | 20 | 80 |
| L-MA0-H40-Mac60 | PEG1500 | 6 | 0 | 40 | 60 |
| L-MA0-H23-Mac77 | PEG1500 | 6 | 0 | 22.5 | 77.5 |
| L-MA13-H27-Mac60 | PEG1500 | 6 | 13.3 | 26.7 | 60 |
2.3 Characterization of degradable poly(HEMA–co–MA)/HA composites
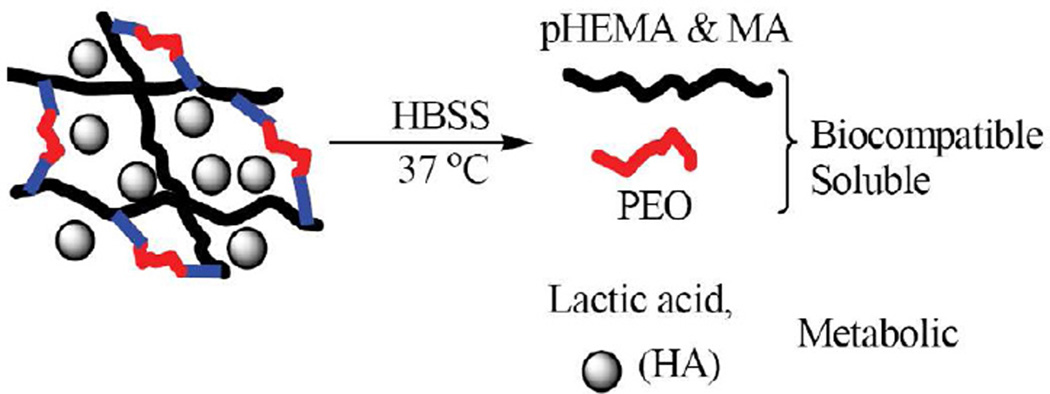
The synthesized macromers/crosslinkers were evaluated by FTIR and NMR. A Nicolet 6700 FTIR spectrometer was used to collect the spectra on the specimens made through KBr. 1H NMR spectra were collected using a Bruker Biospin Avance II 500 MHz NMR spectrometer. Quantitative analysis was conducted to obtain the compositional ratios between PLA and PEO blocks of the macromer based on the area under peaks. The obtained poly(HEMA–co–MA)/HA biocomposites were extensively characterized. Swelling and degradation were examined in HBSS at 37 °C and pH = 7.3 as shown in Scheme 2 [30].
Scheme 2.
Hydrolytic degradation process of the biocomposites comprising poly(HEMA-co-MA) and HA
The originally formed hydrogel/HA biomaterials were cut into cylindrical shape (1 cm thick × 0.7 cm in diameter). The cylindrical specimens of all compositions were freeze dried at –50 °C under vacuum at a FreeZone® 4.5 Liter freeze dry system (by Labconco Corporation, Kansas City, USA) to obtain the initial dry weight (mid). All the initially dried specimens (needed for a 12–week period of time) were then put in 10 mL HBSS solution for swelling and degradation. The swollen specimens were shaken twice daily. A swollen specimen was taken out at week 1, week 2, …, week 12, respectively for each composition to obtain the swollen weight (msw). A different specimen was used each week for swelling and degradation. The swollen specimens were then freeze dried at –50 °C under vacuum to obtain the final dry weight (mfd). On the basis of the obtained initial dry weight, swollen weight, and final dry weight, volumetric swelling ratio, Q, and percent mass loss were calculated as follows:
| (1) |
| (2) |
where q is mass swelling ratio, q = msw/mfd, ρcomposite is the density of biocomposites of pHEMA/HA and approximated to be 1.936 g/cm3, and ρsolvent is the density of the solvent of HBSS and equal to 1.0 g/cm3.
The morphology of selected degradable pHEMA/HA before and after degradation for eight weeks was assessed using an Hitachi Model S–4300SE/N scanning electron microscope operated at 20 kV. The signals of back–scattered electrons were collected for imaging to enhance contrast associated with the difference in the atomic number. To determine the amount of HA after degradation, a differential thermal/thermogravimetry analyzer (DTA–TGA, SDT 2960 simultaneous, TA Instruments) was used at a heating rate of 10 °C/min.
3. Results
3.1 Synthesis of the macromer/crosslinker and fabrication of the degradable poly(HEMA–co–MA)/hydroxyapatite biocomposites
Synthesis of the macromer/crosslinker is assessed by FTIR (see Figure S1) and 1H NMR. FTIR spectra show characteristic peaks of the macromer: 2900–3000 cm−1 (−CH of PLA blocks), 2840 cm−1 (−CH of PEO), 1756 cm−1 (−CO of PLA), and 947 and 843 cm−1 (characteristic of PEO). 1H NMR spectra show peaks in δ (ppm) at: 6.20 (s, 2H), 5.64 (s, 2H), 5.16 (m, 5.2H), 4.43 (m, 8.4H), 3.64 (m, 50H), 1.97 (s, 6H), and 1.53 (m, 16H) for PEG600–based macromer; for PEG1500–based macromer, 1H NMR spectra show peaks in δ (ppm) at: 6.20 (s, 2H), 5.64 (s, 2H), 5.16 (m, 10.6H), 4.43 (m, 8.2H), 3.64 (m, 128H), 1.97 (s, 6H), and 1.53 (m, 32H). Based on the areas under the peaks, the compositional information is obtained: 14mers of PEG block which is connected by two trimers of PLA block at the ends for PEG600–based macromer; for PEG1500–based macromer, there are 33mers of PEO block connected by two 5mers of PLA at the two ends. The molecular structures of the two macromers/crosslinkers are shown in Scheme 1. The average yield of the synthesis was 68%. To gain insight into the role that variations of molecular architecture play in influencing the swelling and degradation of the hydrogel/HA biocomposites, a combinatorial approach was employed to fabricate a series of poly(HEMA–co–MA)/HA materials, where one sub–series was composed of only MA and macromer (i.e., 0 % HEMA); another was composed of MA, macromer, and HEMA; and third was composed of only macromer and HEMA (i.e., 0% MA). Chain–length and relative hydrophilicity of the macromer/crosslinker were varied to obtain the effect of the crosslinker on swelling and hydrolytic degradation. Detailed formulations are listed in Table 1. In all the formulations, the concentration of hydroxyapatite is fixed at 40 wt% unless specified otherwise.
3.2 Swelling of poly(HEMA–co–MA)/HA biocomposites in HBSS
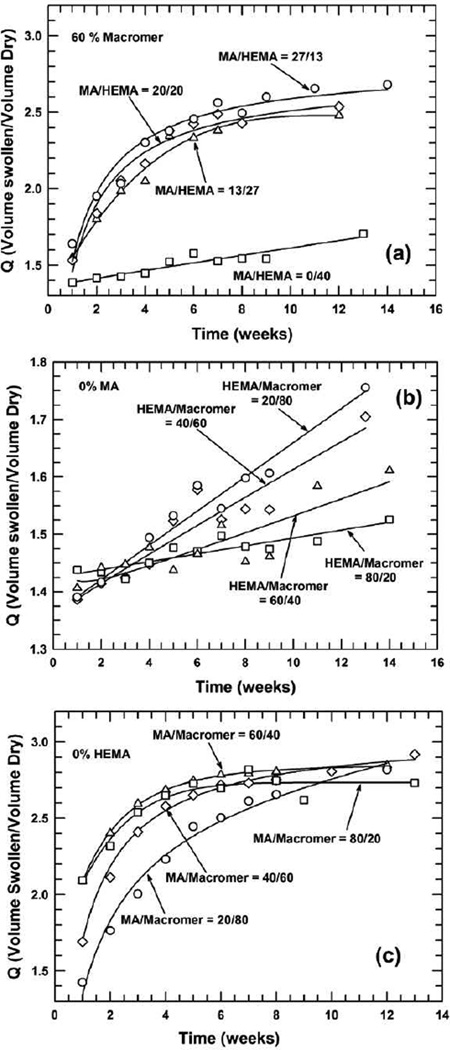
Volumetric swelling ratio is examined as a function of time among these pHEMA–based hydrogel/HA biomaterials. Figure 1 shows volumetric swelling ratio as a function of time for three different systems: the content of macromer/crosslinker was fixed as 60% with varying ratios of MA/HEMA (Figure 1a); 0% MA was applied but the ratio of HEMA/Macromer was varied (Figure 1b); and 0% HEMA was used but the ratio of MA/Macromer differed (Figure 1c).
Figure 1.
Volumetric swelling ratio as a function of time in poly(HEMA-co-MA)/HA biomaterials where: (a) 60 wt% macromer is fixed while the ratio of MA/HEMA is varied, (b) the ratio of HEMA/Macromer is varied without the presence of MA, and (c) the ratio of MA/Macromer differs without incorporation of HEMA. In all cases, the macromer is made from PEG600
For the system with a fixed 60% of macromer/crosslinker, the swelling ratio is observed to increase with time; the increase in the swelling ratio is linear for the composition of MA/HEMA=0/40, but follows a power–law and is much greater for other compositions, i.e. MA/HEMA=27/13, 20/20, and 13/27. The greater the MA content, the higher the gain in the swelling ratio of the hydrogel composite. The difference in trends is due primarily to the incorporation of the ionic co–monomer, methacrylic acid [53, 54]. Because MA is much more hydrophilic, it attracts water more readily, which promotes the swelling. The lowered pH as a result of incorporation of MA in the formulation and the use of HA result combinatively in relatively lower swelling ratios [55]. Also, these different trends demonstrate that two distinct swelling mechanisms arose in each case — one with and one without MA incorporation. For the system without MA (Figure 1(b)), the overall volumetric swelling ratio does not alter significantly, ranging from 20% to roughly 40% within the time frame examined. However, the swelling ratio monotonically increases with time; the higher content of macromer/crosslinker leads to increased swelling. This is unusual, as higher crosslinking densities typically result in reduced swelling because of the smaller mesh size of the 3–D network and less room for water contact. This unusual increase is attributed mainly to greater hydrophilicity of the PEG segment of the macromer/crosslinker than that of the pHEMA for attracting more water for degradation. It is believed that the hydrophilic nature of the PEG segment plays a much greater role in swelling than the effects of higher crosslinking density.
For the system shown in Figure 1(c) in which only MA and macromer were used in the synthesis, the swelling ratio increases significantly in the first seven weeks and then levels off for the compositions MA/Macromer=40/60,60/40, and 80/20, while it increases monotonically for the composition MA/Macromer=20/80. The magnitude of increase is directly related to the proportion of MA/Macromer; the initial increase was much greater for MA/Macromer=20/80 and MA/Macromer=40/60 than for MA/Macromer=60/40 or MA/Macromer=80/20. In comparing these three systems, volumetric swelling ratio was much greater for the system with a fixed 60% macromer and for the system with 0% HEMA but with various ratios of MA/Macromer; the swelling ratio was lower for the system composed of 0% MA and various ratios of HEMA/Macromer. This difference would determine the degree of hydrolytic degradation, as the continuous water contact is essential to degradation.
3.3 Percent mass loss of poly(HEMA–co–MA)/HA biocomposites under hydrolytic degradation
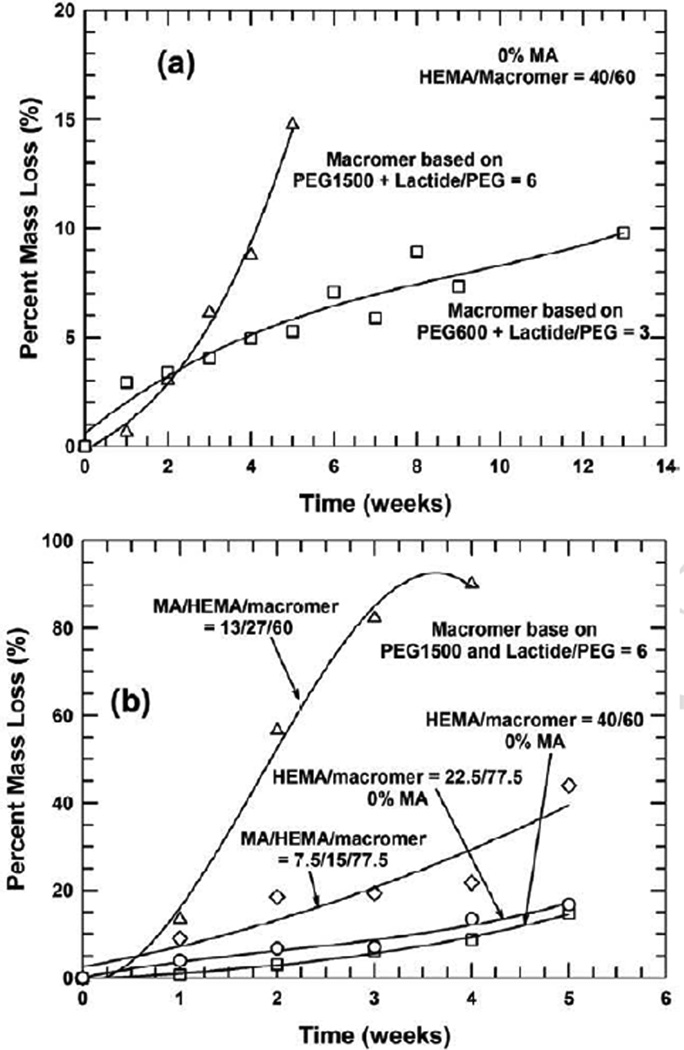
An essential part of this work was to explore the degradation behavior of the formed biocomposites. Figure 2 shows percent mass loss as a function of time for the pHEMA–based hydrogel/HA biomaterials.
Figure 2.
Percent mass loss as a function of degradation time in various poly(HEMA-co-MA)/HA biomaterials where: (a) the ratio of MA/HEMA is varied in the presence of fixed 60 wt% macromer, (b) the ratio of HEMA/Macromer is altered with 0% MA used and, (c) the ratio of MA/Macromer is altered without the presence of HEMA. In all cases, the macromer is made from PEG600
When the concentration of the macromer/crosslinker is maintained constant at 60 wt% (presumably a constant crosslinking density), incorporation of an ionic and hydrophilic co–monomer, methacrylic acid, into the backbone results in a much greater mass loss than is the case without the use of MA, as shown in Figure 2(a). This can be attributed to the ionic nature of carboxyl groups in the backbone of the macromolecular chains, which attracts more water for degradation (see Figure 1(a)). As shown in Figure 2(b), when only HEMA and the macromer were used (i.e., without the presence of the co–monomer, MA) in the synthesis of the hydrogel/HA biocomposite, the increase in the concentration of the crosslinker/macromer leads to greater hydrolytic degradation in the first six weeks; afterwards, the degradation appears to level off for the compositions of HEMA/Macromer=20/80 and 40/60, while progressive weight loss occurs for the compositions of HEMA/Macromer=40/60 and 20/80. Interestingly, accelerated degradation appears to emerge after 10 weeks. The degradation pattern shown in this system is complex, as a higher crosslinking density typically leads to slower degradation [56], because less water was absorbed in the 3–D networks. When higher crosslinker concentrations are used, higher crosslink densities are expected, and as a result less water would be absorbed for swelling. However, as the PEG block of the macromer/crosslinker, dimethacrylated PLA–b–PEG–b–PLA, is more hydrophilic than the monomer of HEMA, more water would be absorbed around the crosslinks, which facilitates the hydrolytic degradation of the crosslinker.
Here, the role of hydrophilicity of the PEG block seems to outweigh the effect of higher crosslink density. The observed accelerated degradation is abnormal, and may be ascribed to the “self–acceleration effect” as a result of the significantly increased release, during degradation, of carboxylic chain ends from the macromer/crosslinker [57–59]. The degradation of the hydrogel composites starts with the swelling of water, and the water subsequently attacks labile sites, such as ester bonds within the PLA blocks and ester bonds linking PEG and PLA blocks [37]. These ester bonds are cleaved to generate lactic acid, lactyllactic acid, and other oligo (lactic acid) that may be dissolved in water [57, 58, 60]. Higher contents of macromer/crosslinker (and thus higher crosslinking density) would impart more carboxylic chain ends, and these hydrophilic chain ends attract more water, causing more swelling and degradation. The “self–acceleration” effect interplays with crosslink density to determine the overall degradation behavior of the biomaterials.
As shown in Figure 2(c), the use of the ionic and hydrophilic monomer, methacrylic acid, and the macromer without HEMA, produces much more and complex degradation. Here, the traits of the macromer and the hydrophilic and ionic monomer, MA, intertwine in determining the degree of hydrolytic degradation. Influencing factors include MA’s strong solubility in water, crosslink density, and the self–acceleration effect that is triggered by the significantly released hydroxylic chain ends of the macromer; which factor dominates depends on the relative ratio of MA versus the macromer. Figure 2(c) also shows that the composition, MA/Macromer=80/20, exhibits greater percent mass loss in the first nine weeks than other compositions, MA/Macromer=60/40, 40/60, and 20/80. This may be due to its smallest crosslink density. For the compositions of MA/Macromer=60/40, 40/60, and 20/80 with increased crosslinking densities, however, a different degradation pattern emerges: degradation occurs relatively slowly in the initial four weeks, plateaus, and then takes off at a greatly accelerating rate because of the self–acceleration effect. Overall, the hydrophilic and ionic nature of the monomer plays a dominant role in the composition of MA/Macromer=80/20, while the “self–acceleration effect” is more important for the compositions of MA/Macromer=60/40, 40/60, and 20/80 after the seventh week. The lowered pH in the initial formulations with MA in Figures 2(a) and (c) may escalate overall degradation of biomaterials, because the acidity promotes the degradation of the hydrogel and also increases the solubility of HA.
3.4 Effect of chain length (molecular weight) and relative hydrophilicity of macromer/crosslinker on degradation
Tuning the molecular architecture of the macromer/crosslinker by altering the chain length and/or relative hydrophilicity is a useful strategy to modulate degradation. Figure 3 shows the effect of the macromer/crosslinker made from PEG1500 on degradation behavior.
Figure 3.
Percent mass loss as a function of degradation time in poly(HEMA-co-MA)/HA biocomposites crosslinked by a macromer made from PEG1500 with a greater chain length/higher molecular weight: (a) comparison of the macromers made from PEG600 and PEG1500 respectively in the system comprising HEMA/Macromer=40/60 only, (b) comparison between the systems of MA/HEMA/Macomer=0/40/60, MA/HEMA/Macromer= 13/27/60, MA/HEMA/Macromer=7.5/15/77.5, and HEMA/Macromer=22.5/77.5
As shown in Figure 3(a), the macromer/crosslinker with a longer and more hydrophilic macromolecular chain made from PEG1500 leads to much more mass loss under the similar crosslink density — after three weeks — than does the counterpart based on PEG600. This is attributed to more (degradable) lactide blocks available for hydrolysis and longer and more hydrophilic chains of PEG that can attract more water for degradation. When this longer and more hydrophilic macromer/crosslinker was used, introduction of the ionic co–monomer, MA, significantly promotes the degradation shown in Figure 3(b). In addition, the higher content of MA leads to much more mass loss. Without using the co–monomer MA, percent mass loss reaches only about 16 %. However, with 7.5% MA incorporated, 44% mass loss is achieved after five weeks. Substantial (90%) mass loss is observed after only four weeks when 13% MA is incorporated. The significant mass loss may be ascribed primarily to more hydrophilic nature of the macromer and the “self–acceleration effect” resulting from the release of more carboxylic groups during degradation. Similar to the cases of Figures 2(a) and (c), the original acidity of the compositions as a result of incorporation of MA may act as an additional promoter for the overall degradation of biocomposites.
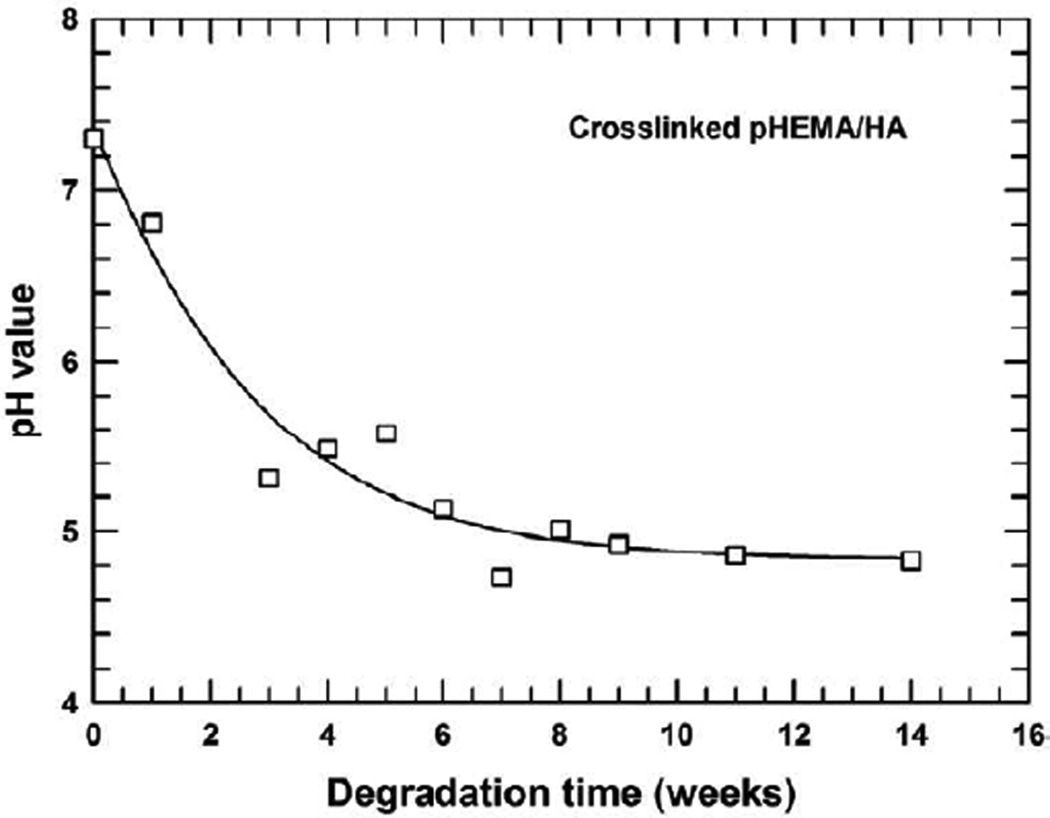
3.5 Evolution of pH value of HBSS during degradation for crosslinked pHEMA/HA
During the degradation, the macromer/crosslinker is attacked by water prior to hydrolysis. Hydrolysis of the lactide of the crosslinker produces acidic substances, which will lower the pH of the solution. The variation of pH for the HBSS was monitored on a selected biocomposite composed of 40 wt% HA and 60 wt% poly(HEMA) hydrogel to avoid potential effects of the co–monomer of MA. Figure 4 shows the evolution of pH of the HBSS during degradation for the crosslinked pHEMA hydrogel/HA, where the hydrogel is composed of 60% HEMA and 40% PEG600–based macromer only (i.e., S–M0–H40–Mac60 as shown in Table 1). The pH is seen to decrease considerably in the first six weeks and then to level off around 4.85. This decrease in pH during degradation has a positive effect, not only to the pHEMA hydrogel, but also to hydroxyapatite, as acidity would accelerate degradation of the hydrogel and significantly increase the solubility of the HA.
Figure 4.
Change of pH as a function of degradation time in the pHEMA/HA system comprising HEMA/Macromer=60/40 with the macromer made from PEG600
3.6 Comparison of morphology before and after eight–week degradation for polyMA/HA biocomposites
The hydrolytic degradation is further examined on selected compositions using scanning electron microscopy as shown in Figures 5 and 6. Here, surfaces and cross–sections of the polyMA/HA composites are both examined on two compositions: MA/Macromer=80/20 (i.e., s–MA80–H0–Mac20, shown in Table 1) and MA/Macromer=20/80 (i.e., s–MA20–H0–Mac80, also shown in Table 1) without HEMA incorporated, respectively. These two compositions were purposely chosen to show the effects of the ratio of MA/Macromer on degradation, which may compare with what is observed in Figure 2(c). After eight weeks in HBSS, the big chunks of crosslinked hydrogel composites were reduced to smaller pieces on the surfaces of both compositions, implying that significant degradation had occurred. For the cross–section, the composition of MA/Macromer=80/20 (Figure 5) appeared to degrade much more than did the composition of MA/Macromer=20/80 (Figure 6). The observed difference in degradation here agrees with what is observed in mass loss as shown in Figure 2(c); the former lost about 56% of its weight, while the latter degraded about 28%. Apparently, this difference was primarily the result of the higher crosslink density and lower concentration of the ionic and hydrophilic monomer, MA, which cause less water to contact the hydrogel composites during degradation. Similar trends are observed in other compositions.
Figure 5.
Comparison of morphology revealed by SEM photomicrographs before and after degradation for eight weeks in the composition of HEMA/MA/Macromer = 0/80/20 with the macromer based on PEG600. Before degradation: (a) surface, and (b) cross-section; after degradation for eight weeks: (c) surface, and (d) cross-section. Scale bar = 10 µm
Figure 6.
Comparison of morphology revealed by SEM photomicrographs before and after degradation for eight weeks in the composition of HEMA/MA/Macromer = 0/20/80 with the macromer based on PEG600. Before degradation: (a) surface, and (b) cross-section; after degradation for eight weeks: (c) surface, and (d) cross-section. Scale bar = 10 µm
4. Discussion
Novel, degradable, and rubbery biocomposites, comprised of hydrogels and hydroxyapatite, are increasingly desirable materials for bone–tissue engineering. This work demonstrates an approach for synthesis of degradable pHEMA/HA biomaterials at controlled rates by increasing the hydrophilicity of the backbone of the hydrogel (through addition of an ionic co–monomer of methacrylic acid); increasing the relative hydrophilicity of the degradable macromer/crosslinker; and increasing the chain length and thus molecular weight of the macromere. Our design rationale has been shown to work in pHEMA/HA systems. However, there are a number of important caveats. While linear pHEMA usually dissolves in water, it is not soluble when its molecular weight exceeds 8–10k Da. Therefore, caution must be taken in the design of degradable pHEMA hydrogel biocomposites with hydroxyapatite to ensure that its molecular weight is below this threshold. Furthermore, since the pHEMA hydrogel/HA is obtained in situ, the molecular weight of linear pHEMA is impossible to be easily determined. However, an observation of fast degradation, (i.e., ~15% weight loss in five weeks, as shown in Figure 4(a)) indicates that the molecular weight of the linear pHEMA must be lower than 8–10k Da.
Hydrolytic degradation of poly(HEMA–co–MA)/HA biocomposites is rather complex, because there are a variety of variables that may play certain roles in combinatorially determining the degradation. These variables partly include crosslink density, hydrophilicity/hydrophobicity (i.e., wettability), the swollen water content, surface charges and topography, specimen thickness, the pH and ionic strength of the solution, and interaction between the hydrogel and HA. In addition, the relative concentration of the components during polymerization is also important. Because of “self–acceleration effect” which results from carboxylic ends released during degradation, the overall degradation kinetics is complicated. Very often, more than two variables are involved. The macromers/crosslinkers, we synthesized in this work, are far less hydrophilic than those reported in the literature [30, 34], because PEG used here have much lower molecular weights. This difference may potentially cause immiscibility between the components during mixing prior to polymerization (i.e., gelling), which in return might result in polymerization induced phase separation, i.e., pore structures, particularly when HEMA, MA, and Macromer are all used in the formulation of pHEMA hydrogels. Based on what is shown in Figures 5 and 6 where only MA and Macromer were employed in the formulation, clearly, there are no porous structures from initial polymerization and subsequent swelling and degradation, regardless of the concentration of the macromer. For other compositions composed of either MA/HEMA/Macromer or HEMA/Macromer, it is possible but not likely for polymerization induced phase separation to occur, because the percent mass loss would have been much higher if the phase separation had occurred.
Generally, a higher concentration (presumably higher crosslink density) of the macromer/crosslinker leads to less mass loss; this is true for the initial degradation irrespective of compositions, but it is not always the case for compositions with MA/Macromer and is totally the opposite for the compositions containing HEMA/Macromer at a late phase of the 12–week period of degradation. Apparently, the role of crosslink density coupled with other variables. Regardless of the composition, a greater volumetric swelling ratio generally leads to greater mass loss as might be expected. The obtained swelling ratio, ranging from 1.3–3.0, is beneficial for these biocomposites to serve as bone grafts/implants [61, 62], because a big swelling ratio may result in increased localized compression to the surrounding tissue and lowered mechanical properties.
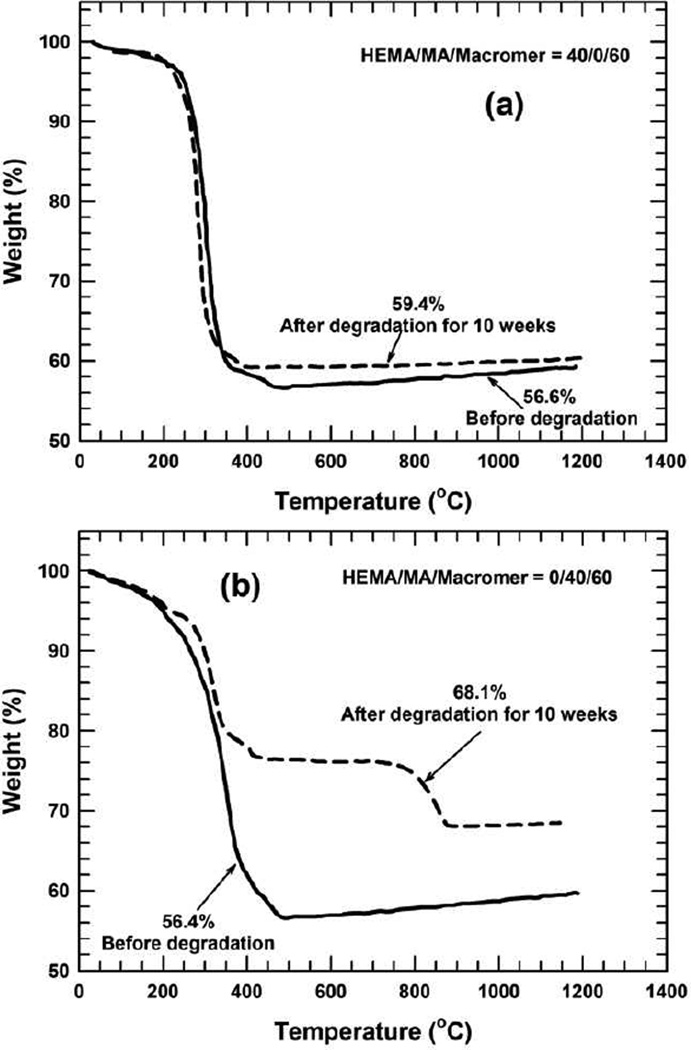
Incorporation of hydroxyapatite in the formulation complicates the degradation kinetics of the crosslinked pHEMA hydrogel, as HA itself has a very low solubility [63] (solubility product = 14.4 ± 0.3 at 25 °C) and controls the degradation of the pHEMA to some degree. It has been claimed that hydroxyapatite is biodegradable. However, it has also been reported that scaffolds composed of a polymer and hydroxyapatite still did not decompose and were resorbed entirely after seven years [18, 19]. A recent study by our group — examining the degradation of 3–D porous HA scaffolds by solid freeform printing in simulated body fluids — showed no degradation of scaffolds for two weeks. To the contrary, they gained weight. The degradation of HA in vitro differs from that in vivo. Interestingly, the pHEMA/HA biomaterials formed here show remarkable degradability (Figures 2 and 3). This can be partly explained by a sharp drop in pH during degradation (Figure 4), which substantially enhances the solubility of hydroxyapatite, which is known to have a higher solubility in an acidic environment [64]. The solubility of hydroxyapatite increases by nearly two orders of magnitude when the pH decreases from 7.4 to 4.8 [64]. In addition, as the degradation progresses, the decomposition of the macromer/crosslinker and disintegration of the crosslinked pHEMA macromolecules facilitates the dispersion of mobile hydroxyapaite particles, thereby increasing the diffusion coefficient, which consequently accelerates degradation. While the pHEMA hydrogel influences HA degradation, HA may also promote degradation of the pHEMA hydrogel in three ways [65]. First, hydroxyapatite exerts a buffering effect on the pH of the surrounding solution, partially blocking the autocatalytic effect of the acidic end groups of PLA. Without the buffering effect, the pH of the saline solution would be approximately 3.0 as a result degradation of the PLA segments of the macromere [66]. Second, incorporation of hydroxyapatite into the pHEMA hydrogel promotes water absorption for degradation as a result of significantly increased surface areas. Third, incorporation of the HA introduces surface topography (e.g., micro roughness) that may partially promote water contact and help form tenacious bonding of the biocomposite with the surrounding bone tissue in vivo. The inter–relationships between the pHEMA hydrogel and hydroxyapatite complicate the overall degradation kinetics of biocomposites. In the course of degradation, it is clinically important to know the degradation rate of the structural components of the pHEMA/HA biomaterials, because hydrogels of pHEMA and HA may not necessarily degrade at the same rate. We purposely selected two compositions to demonstrate the significant role of the co–monomer of MA in accelerating the dissolution of HA. Figure 7 compares thermogravimetric analysis between two compositions: HEMA/MA/Macromer=40/0/60 (Figure 7(a)) and HEMA/MA/Macromer=0/40/60 (Figure 7(b)) before and after degradation for ten weeks.
Figure 7.
Thermogravimetric analysis of the pHEMA/HA biomaterials before and after degradation for 10 weeks, where HEMA/MA/Macromer = 40/0/60 is employed: (a) before degradation, (b) after degradation for 10 weeks, and HEMA/MA/Macromer =0/40/60 is formulated: (a) before degradation and (b) after degradation for 10 weeks. The macromer is based on PEG600
Before the degradation, the two compositions have nearly the same ratio of hydrogel/HA (i.e., 43.5/56.5); however, after degradation for 10 weeks, this ratio alters only a little bit for HEMA/MA/Macromer=40/0/60, (i.e., ~41/59), but changes substantially to 32/68 for the HEMA/MA/Macromer=0/40/60. This indicates that the hydrogel and HA degrade nearly at the same rate in the former, while the hydrogel degrades much more than does HA in the latter. In experiments with these crosslinked poly(HEMA–co–HA)/HA biocomposites, it is presumed that the decomposition of the crosslinking molecule (i.e., dimethacrylated PLA–b–PEG–b–PLA) plays a vital role in degradation, which subsequently motivates the dissolution of the linear poly(HEMA–co–MA), poly(HEMA), or polyMA chains in addition to HA, causing degradation of the overall crosslinked hydrogel composites.
In exploring swelling and degradation of the pHEMA/HA biomaterials, the hydrophilicity of the backbone of the hydrogel (through incorporation of an ionic co–monomer of methacrylic acid) and macromer/crosslinker, the concentration of the co–monomer and macromer/crosslinker (and thus crosslink density), and chain length (molecular weight) of the macromer/crosslinker are all needed to be considered simultaneously. Swelling occurs prior to degradation, and also is an important step towards hydrolytic degradation. Swelling and subsequent degradation may be appreciated in terms of rubber–elasticity theory [67]. The volumetric swelling ratio, Qv, the average molecular weight between crosslinks, , crosslink density, νe, mesh size, ξ, and elastic modulus, G', have the following scaling relationships [56, 60, 68, 69]:
| (3) |
| (4) |
| (5) |
where ρp is the density of the polymer and may be estimated from the Flory–Rehner equation [69,70]. To maximize degradation, swelling is typically needed to be maximized. Accordingly, crosslink density should be minimized. However, the trends shown in Figures 3(b) and 3(c) do not seem to support this scaling relationship. In fact, because of the unavailability of exact crosslink density, it is unclear whether or not the scaling relationship applies here. In Figures 2(b) and 2(c), when the content of macromer/crosslinker is high (e.g., HEMA/Macromer=20/80 or MA/Macromer=20/80), the macromer/crosslinker may play two significant roles: it may act not only a crosslinking agent, but also a main monomer due to cyclization effect in the presence of solvents, in addition to the existing main monomer, either HEMA (Figure 2(b)) or MA (Figure 2(c)). It is likely that the crosslink density is still high in these cases. When, in addition to acting as a crosslinker, the macromer participates in the polymerization as an additional main monomer, the increased hydrophilicity from PEG blocks would significantly attract more water for swelling. That may be what has occurred in Figure 2(b), but it does not occur in Figure 2(c), because the methacrylic acid is more hydrophilic than the macromer. The participation of the macromer as an additional main monomer is critical for degradation, as the degradation may start not only with the decomposition of the crosslinks but also with the backbone of the hydrogel, enabling substantial improvement in degradation. At a similar crosslink density, the increase of chain length (molecular weight) of the macromer/crosslinker leads to a much greater mesh size of the networks, significantly facilitating attraction of more water for swelling, which consequently enhances the hydrolytic degradation. The hydrophilicity of PEG blocks would also allow them to attract more water for swelling and degradation.
As demonstrated here, a series of elastomeric pHEMA/HA biocomposites were synthesized using degradable macromers/crosslinkers based on dimethacrylated triblock copolymers of PLA–b–PEG–b–PLA. The degradability may be carefully controlled by incorporating an ionic and hydrophilic co–monomer of methacrylic acid, or altering the chemical architectures of the macromer/crosslinker and relative concentrations, or both. Our work lays a solid foundation for the fabrication of clinically useful elastomeric biopolymers/hydroxyapatite materials with expectancy degradation time of 4–6 months. Our findings demonstrate that our design rationale and strategies are capable of creating novel organic–inorganic biomaterials suitable for bone tissue engineering, particularly where biodegradability, biocompatibility, and mechanical properties would have to be considered simultaneously. Our rationale and strategies open up a path for the design of useful soft hydrogel materials and the incorporation of a bioactive inorganic material (e.g., HA) to obtain useful biocomposites as synthetic bone grafts for bone repair and regeneration. This rationale and these strategies are important to bone tissue engineering. They provide a viable solution for the creation of degradable and dense (i.e., non–porous) biomaterials. _ENREF_55 The rationale and strategies described here also have important implications for bone–tissue engineering in that they demonstrate that biomimetic porous inorganic–organic biomaterials can be created through the incorporation of a biomimetic co–monomer for cell adhesion [14]. Overall, the rationale and strategies open a vital new avenue for the generation of complex organic–inorganic hybrid biomaterials.
5. Conclusions
With a combinatorial approach, we have synthesized a series of degradable pHEMA/HA biocomposites with a range of degradation rates using macromers/crosslinkers based on a dimethacrylated triblock copolymer, PLA–b–PEG–b–PLA. The degradation behavior has been significantly varied by incorporating a co–monomer, methacrylic acid, or altering the concentration of the macromer/crosslinker (and thus crosslink density) and its chain length/molecular weight of the macromer coupled with relative hydrophilicity, or both. These variables are often coupled in the formulation, which makes the degradation behavior more complex.
Our findings on degradation rates indicate that, at a similar crosslink density, the incorporation of the co–monomer enhances the degradation dramatically. The more the co–monomer is used, the more mass loss is accrued by the biocomposites. When the concentration of macromer/crosslinker (and thus cross–density) is a variable in either the pHEMA/HA or polyMA/HA system, the trends of degradation are rather complex; in the case of pHEMA/HA (without MA), the overall effect of macromer/crosslinker concentration is negligible (variation between 9–13%) within the tested time frame. In the case of polyMA/HA, self–acceleration of degradation is observed in the compositions of MA/Macromer=60/40, 40/60, and 20/80 after seven to eight weeks, while the degradation in MA/Macromer=80/20 occurs in a uniformly progressive fashion. The polyMA/HA system degrades much more than does the pHEMA/Macromer system, i.e., 50–90 wt% for the former versus 9–13 wt% for the latter. In all cases, the incorporation of the ionic co–monomer of MA enhances the degradation tremendously.
Alternation of chain length (molecular weight), which is coupled with the variation of hydrophilicity of sub–segments (i.e., PEG block) of the macromer, strongly influences the degradation; our findings show that longer (and thus higher molecular weight) and more hydrophilic macromolecular chains of the macromer based on PEG1500 significantly promote the degradation compared with its counterpart based on PEG600. Overall, the degradation of the pHEMA/HA biomaterials has been shown to vary substantially with the macromer (molecular architectures and molecular weight) and the co–monomer of methacrylic acid. The strategies and approaches in this work are important for the development of biodegradable, biocompatible, and clinically useful organic–inorganic hybrid biomaterials that have numerous low to medium load–bearing applications in bone tissue engineering, particularly for rubbery hybrid materials with the degradation range of 4–6 months required in clinical trials.
6. Supplementary data
Supporting Information. Comparison of FTIR spectra between PEG600, triblock copolymer of PLA–b–PEG–b–PLA, and the macromer based on PEG600 (Figure S1).
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) Grant No. 1 R01 DE015633.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LG, Naughton G. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Burg KJL, Porter S, Kellam JF. Biomaterials. 2000;21(23):2347–2359. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 4.Hollinger JO. Bone tissue engineering. Boca Raton: CRC Press; 2005. [Google Scholar]
- 5.Liu XH, Ma PX. Ann Biomed Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 6.Salgado AJ, Coutinho OP, Reis RL. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 7.Rezwan K, Chen Q, Blaker J, Boccaccini A. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal CM, Ray RB. J Biomed Mater Res. 2001;55(2):141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Calandrelli L, Laurienzo P, Oliva A. Biodegradable composites for bone regeneration. New York: Nova Science Publishers; 2010. [Google Scholar]
- 10.Hutmacher DW. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 11.Hutmacher DW, Schantz JT, Lam CX, Tan KC, Lim TC. J Tissue Eng Regen Med. 2007;1(4):245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 12.Jia X, Kiick KL. Macromol Biosci. 2009;9(2):140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose FR, Oreffo RO. Biochem Biophys Res Commun. 2002;292(1):1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 14.Song J, Malathong V, Bertozzi CR. J Am Chem Soc. 2005;127(10):3366–3372. doi: 10.1021/ja043776z. [DOI] [PubMed] [Google Scholar]
- 15.Brandl F, Sommer F, Goepferich A. Biomaterials. 2007;28(2):134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Cuddihy MJ, Kotov NA. Tissue Eng PT B-Rev. 2008;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 17.Wagoner Johnson AJ, Herschler BA. Acta Biomater. 2011;7(1):16–30. doi: 10.1016/j.actbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa S, Ishii S, Tamura J, Furukawa T, Neo M, Matsusue Y, Shikinami Y, Okuno M, Nakamura T. Biomaterials. 2006;27(8):1327–1332. doi: 10.1016/j.biomaterials.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Shikinami Y, Matsusue Y, Nakamura T. Biomaterials. 2005;26(27):5542–5551. doi: 10.1016/j.biomaterials.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Rockville, MD: Department of Health, Human Services, Public Health Service, Office of the Surgeon General; 2004. Bone health, Osteoporosis: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 21.Cummings SR, Melton LJ. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Saiz E, Bertozzi CR. J Eur Ceram Soc. 2003;23(15):2905–2919. [Google Scholar]
- 23.Song J, Xu J, Filion T, Saiz E, Tomsia AP, Lian JB, Stein GS, Ayers DC, Bertozzi CR. J Biomed Mater Res A. 2009;89(4):1098–1107. doi: 10.1002/jbm.a.32110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filion TM, Li X, Mason-Savas A, Kreider JM, Goldstein SA, Ayers DC, Song J. Tissue Eng PT A. 2011;17(3–4):503–511. doi: 10.1089/ten.tea.2010.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese S, Elisseeff JH. Adv Polym Sci. 2006;203:95–144. [Google Scholar]
- 26.Huang JJ, Liu G, Song C, Saiz E, Tomsia AP. Chem Mater. 2012;24(7):1331–1337. [Google Scholar]
- 27.Wichterle O, Lim D. Nature. 1960;185(4706):117–118. [Google Scholar]
- 28.Montheard J-P, Chatzopoulos M, Chappard D. J Macromol Sci R M C. 1992;C32(1):1–34. [Google Scholar]
- 29.Ratner BD, Atzet S. Hydrogels for healing. In: Barbucci R, editor. Hydrogels : biological properties, applications. Milan New York: Springer; 2009. pp. 43–51. [Google Scholar]
- 30.Metters AT, Anseth KS, Bowman CN. Polymer. 2000;41(11):3993–4004. [Google Scholar]
- 31.Metters AT, Anseth KS, Bowman CN. J Phys Chem B. 2001;105(34):8069–8076. [Google Scholar]
- 32.Metters AT, Bowman CN, Anseth KS. J Phys Chem B. 2000;104(30):7043–7049. [Google Scholar]
- 33.Sawhney AS, Pathak CP, Hubbell JA. Macromolecules. 1993;26(4):581–587. [Google Scholar]
- 34.Shah NM, Pool MD, Metters AT. Biomacromolecules. 2006;7(11):3171–3177. doi: 10.1021/bm060339z. [DOI] [PubMed] [Google Scholar]
- 35.Li SM, Molina I, Martinez MB, Vert M. J Mater Sci Mater Med. 2002;13(1):81–86. doi: 10.1023/a:1013651022431. [DOI] [PubMed] [Google Scholar]
- 36.Li SM, Rashkov I, Espartero JL, Manolova N, Vert M. Macromolecules. 1996;29(1):57–62. [Google Scholar]
- 37.Rashkov I, Manolova N, Li SM, Espartero JL, Vert M. Macromolecules. 1996;29(1):50–56. [Google Scholar]
- 38.Bolgen N, Yang Y, Korkusuz P, Guzel E, El Haj AJ, Piskin E. Tissue Eng PT A. 2008;14(10):1743–1750. doi: 10.1089/ten.tea.2007.0277. [DOI] [PubMed] [Google Scholar]
- 39.He B, Wan E, Chan-Park MB. Chem Mater. 2006;18(17):3946–3955. [Google Scholar]
- 40.Khelfallah NS, Decher G, Mesini PJ. Biointerphases. 2007;2(4):131–135. doi: 10.1116/1.2799034. [DOI] [PubMed] [Google Scholar]
- 41.Khelfallah NS, Decher G, Mésini PJ. Macromol Rapid Comm. 2006;27(13):1004–1008. [Google Scholar]
- 42.Lim DW, Choi SH, Park TG. Macromol Rapid Comm. 2000;21(8):464–471. [Google Scholar]
- 43.Van Thienen TG, Lucas B, Flesch FM, van Nostrum CF, Demeester J, De Smedt SC. Macromolecules. 2005;38(20):8503–8511. [Google Scholar]
- 44.Lickorish D, Guan L, Davies JE. Biomaterials. 2007;28(8):1495–1502. doi: 10.1016/j.biomaterials.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. J Biomed Mater Res. 1998;43(4):422–427. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Schieker M, Seitz H, Drosse I, Seitz S, Mutschler W. Eur J Trauma. 2006;32(2):114–124. [Google Scholar]
- 47.Friedlaender GE, Mankin HJ, Goldberg VM American Academy of Orthopaedic Surgeons. Bone grafts, bone graft substitutes. 1st ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. [Google Scholar]
- 48.Brocchini S. Adv Drug Deliv Rev. 2001;53(1):123–130. doi: 10.1016/s0169-409x(01)00224-1. [DOI] [PubMed] [Google Scholar]
- 49.Brocchini S, James K, Tangpasuthadol V, Kohn J. J Am Chem Soc. 1997;119(19):4553–4554. [Google Scholar]
- 50.Brocchini S, James K, Tangpasuthadol V, Kohn J. J Biomed Mater Res. 1998;42(1):66–75. doi: 10.1002/(sici)1097-4636(199810)42:1<66::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 51.Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho SW, Mitalipova M, Pyzocha N, Rojas F, Van Vliet KJ, Davies MC, Alexander MR, Langer R, Jaenisch R, Anderson DG. Nat Mater. 2010;9(9):768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon CG, Jr, Lin-Gibson S. Adv Mater. 2011;23(3):369–387. doi: 10.1002/adma.201001763. [DOI] [PubMed] [Google Scholar]
- 53.Yarimkaya S, Basan H. J Macromol Sci A. 2007;44(9):939–946. [Google Scholar]
- 54.Am Ende MT, Peppas NA. J Appl Polym Sci. 1996;59(4):673–685. [Google Scholar]
- 55.Khare AR, Peppas NA. Biomaterials. 1995;16(7):559–567. doi: 10.1016/0142-9612(95)91130-q. [DOI] [PubMed] [Google Scholar]
- 56.Peppas NA. Hydrogels in medicine, pharmacy. Boca Raton, Fla.: CRC Press; 1986. [Google Scholar]
- 57.Kenley RA, Lee MO, Mahoney TR, Sanders LM. Macromolecules. 1987;20(10):2398–2403. [Google Scholar]
- 58.Tamada JA, Langer R. Proc Natl Acad Sci USA. 1993;90(2):552–556. doi: 10.1073/pnas.90.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang M-H, Li S, Coudane J, Vert M. Macromol Chem Phys. 2003;204(16):1994–2001. [Google Scholar]
- 60.Peppas NA, Moynihan HJ, Lucht LM. J Biomed Mater Res. 1985;19(4):397–411. doi: 10.1002/jbm.820190405. [DOI] [PubMed] [Google Scholar]
- 61.Lee KY, Alsberg E, Mooney DJ. J Biomed Mater Res. 2001;56(2):228–233. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Shin H, Ruhe PQ, Mikos AG, Jansen JA. Biomaterials. 2003;24(19):3201–3211. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 63.Denhollander W, Patka P, Klein C, Heidendal GAK. Biomaterials. 1991;12(6):569–573. doi: 10.1016/0142-9612(91)90053-d. [DOI] [PubMed] [Google Scholar]
- 64.Brown PW, Constantz B. Hydroxyapatite, related materials. Boca Raton: CRC; 1994. [Google Scholar]
- 65.Boccaccini AR, Gough JE. Tissue engineering using ceramics, polymers. Cambridge, England: Woodhead Publishing Limited; 2007. p. 604. [Google Scholar]
- 66.Agrawal CM, Athanasiou KA. J Biomed Mater Res. 1997;38(2):105–114. doi: 10.1002/(sici)1097-4636(199722)38:2<105::aid-jbm4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 67.Anseth KS, Bowman CN, BrannonPeppas L. Biomaterials. 1996;17(17):1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 68.Flory PJ. J Chem Phys. 1950;18:108–111. [Google Scholar]
- 69.Flory PJ. Principles of polymer chemistry. Ithaca: Cornell University Press; 1953. [Google Scholar]
- 70.Flory PJ, Rehner J., Jr J Chem Phys. 1943;11:512–520. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.