Abstract
This article focuses on the pathophysiology of phlebolymphedema, as well as proper diagnosis and treatment. It is hoped that this article will improve care of patients in wound care clinics and motivate wound care physicians to consider adding care of lymphedema patients to their clinical practice.
Keywords: Chronic venous insufficiency, Edema, Lymphedema, Phlebolymphedema
The correction and treatment of chronic swelling is inescapable in the wound care clinic, and it is impossible to do good wound care and not encounter patients with various forms of lymphedema. Yet most physicians have very little formal training or knowledge of treatment and diagnosis of lymphedema.
The most common form of lymphedema worldwide may be filarial infection, but the most common in the Western world is phlebolymphedema. Phlebolymphedema is a mixed-etiology swelling due to chronic venous insufficiency (CVI) and lymphatic insufficiency. Phlebolymphedema is most commonly due to inability of the lymphatic system to adequately drain the interstitial fluid that accumulates in severe chronic venous hypertension. Additionally, there are a host of contributing factors that can increase demand on the lymphatic system and lead to development of phlebolymphedema. Lymphatic insufficiency due to damage to the lymphatic system can also result in phlebolymphedema. Often multiple factors are at play, including systemic disease (eg, congestive heart failure, cirrhosis, or nephropathy), which compounds the problem and leads to inability of the lymphatic system to drain interstitial fluids and macromolecules. Thus, phlebolymphedema is due to insufficiency of the venous or lymphatic system (or both), in combination with possible systemic contributors, leading to accumulation of interstitial protein-rich fluid in the interstitial space.
This article focuses on the pathophysiology of phlebolymphedema, as well as proper diagnosis and treatment. It is hoped that this article will improve care of patients in wound care clinics and motivate wound care physicians to consider adding care of lymphedema patients to their clinical practice.
Incidence
Lymphedema is a lifelong condition for which no cure exists. An estimated 300 million people are affected by lymphedema worldwide. Filariasis is a paracytic infection and is the most common cause of lymphedema worldwide, affecting 100 million people.1 Phlebolymphedema, the most common form of lymphedema in the Western world, is a secondary lymphedema that develops in patients with CVI. Contributing factors to lower extremity swelling are increased survival of heart failure patients, numerous medications associated with edema, and increased incidence of obesity. With the aging of the baby boomers and the lack of education of caregivers in proper treatment of lymphedema, the incidence will almost certainly increase in upcoming decades. Lymphedema has been considered an “orphan” disease because it does not fall into any medical specialty. Consequently, few physicians are well versed in its pathophysiology or treatment.2
Pathophysiology of CVI
CVI is caused by dysfunction of the one-way valvular system in the perforators and deep venous plexus of the lower extremity. Valvular dysfunction results in backflow of venous blood to the superficial venous system. This loss of valvular function may result from age-related decrease in valvular competency, occult or previous deep venous thrombosis (DVT), surgery, or other etiologies. Incidence is approximately 6% to 7% of patients at 50 years of age, with an increase to >20% at age 70. CVI has a slight female preponderance.3 Up to 76% can be diagnosed by clinical presentation alone, but duplex ultrasound can also be used and is done routinely by some clinicians.4
Pathophysiology of Lymphedema
The problem of lymphedema starts at the capillary. Review of capillary physiology reminds us that at the precapillary end of the arteriole, the hydrostatic pressure drives fluid (called the ultrafiltrate) out of the capillary. Toward the venule side of the capillary, the oncotic pressure inside the capillary is the main force pulling fluid back into the capillary. About 10% of the ultrafiltrate is not reabsorbed back into the capillary. The ultrafiltrate also contains small amounts of proteins and cellular debris. It is the job of the lymphatic system to capture and drain this fluid, filter and concentrate it in the lymph nodes, and then return it to the vascular system. The actual flow of lymph fluid varies greatly in the human body. Lymph flow is based on demand and cardiovascular hemodynamics. It is not uncommon for lymph flow to vary more than 20-fold above baseline flow when the lymphatic system is at a maximum.5 Normally there is a large reserve capacity to the lymphatic. Normal healthy tissues lymphatics work at only 5% to 10% of capacity.6 Actual amount of lymph fluid returned through the thoracic duct is estimated to be 2 to 4 L daily. However, the total flow into the lymphatic system prior to concentration by lymph nodes produces a volume that is at least 8 L in a normal adult.7 The removal of proteins from the interstitium is an essential function. Without functional lymphatics, we would die in about 24 hours.5
Drainage into the lymphatic system starts in the lymphatic precollectors, which contain single-layer endothelial cells with anchoring filaments and valves. Stretch of these anchoring filaments opens clefts between cells to expand, allowing entry of interstitial fluid and macromolecules. From the lymphatic precollectors and collectors, lymph flows through lymphatic capillaries. Thereafter, it flows into the lymph nodes, which concentrate and filter the lymph. Eventually the lymph returns to the cardiovascular system via the thoracic duct.
Interstitial fluid pressure and degree of activity of the lymphatic pump are the main factors driving flow through the lymphatic system. The lymphatic pump is driven by several factors. These factors are contraction of muscles in the body portion, movement of body parts, arterial pulsations, compression by objects outside the body, and the lymphangion micropump. The micropump is located throughout the lymphatic capillary and collecting system. The lymphangion micropumps are crude pumps of the lymphatic system that have their own contractile activity and bicuspid valves, which prevents lymphatic backflow.5 The lymphangions contain actomyosin filaments and have their own rhythmic contractility and action potentials. This system is similar to the gastrointestinal system. The rate of lymphatic peristalsis varies greatly, from 1 to 30 pulsations per minute. The lymphangions have a series of one-way valves, allowing flow of fluid only one way. The degree of lymphatic flow varies widely. During exercise or periods of high drainage, flow may increase 10-fold to 30-fold. During periods of rest, however, lymphatic flow may be very sluggish. The effectiveness of lymphangion pump action on lymphatic flow is debated, but it is likely that the lymphangion and lymphatic contraction play some role in determining lymph flow rates.
In summary, the lymphatic system is a dynamic system that handles capillary ultrafiltrate with great variance depending on the need. The lymphatic system has limits in volume of fluid it can handle. The lymphatic system contains fragile vessels that can be damaged easily from infection, trauma, tissue inflammation, or radiation. Damage to lymphatic vessels leads to development of lymphedema with a swollen extremity or body portion.
Use of diuretics for patients with lymphedema will merely increase concentration of proteins and macromolecules in the interstitial space and speed the inflammatory process that leads to irreversible skin and soft tissue changes and increased risk of cellulitis. Therefore, use of diuretics solely for reduction of swelling in patients with lymphedema is contraindicated.
Development of Phlebolymphedema (Secondary Lymphedema in Venous Insufficiency)
To understand how patients with venous hypertension develop secondary lymphedema, we need to review venous pathophysiology. In the recumbent position, arterial pressure may be approximately 100 mm Hg and venous pressure approximately 8 mm Hg.8 Standing will increase pressures in the veins of the leg because of hydrostatic pressure increase. The degree of pressure increase is directly proportional to a person's height and the vertical distance from the heart to the bottom of the feet. Thus, for an average person, both arterial and venous pressures increase by approximately 100 mm Hg when the person is standing. Standing results in passive hyperemia in the blood capillaries because of elevated hydrostatic pressures and decreased oncotic pressure. Thus, reabsorption of ultrafiltrated fluid does not occur. During walking, venous pressure in the foot may decrease to ∼25 mm Hg because of the calf muscle's action on the deep venous system augmenting venous return. This pressure is considered the normal ambulatory venous hypertension and is much higher than the 8 mm Hg seen when a person is supine.8
In patients with acute DVT, blockage of venous vessels results in massive hyperemia due to increased venous hypertension. This results in accumulation of interstitial fluid and subfascial edema and increased compartment pressure. It is the job of the lymphatic system to drain this interstitial fluid. However, the lymphatic system can become overwhelmed and cause further accumulation of interstitial fluid. The accumulation of lymph fluid in the interstitium leads to a proinflammatory state that can cause tissue fibrosis. DVT often organizes at the pocket of the valve and grows from there. Acute inflammation results in long-term destruction of the valve leaflets. Patients with significant DVT will present with subfascial edema, which clinically has a very firm and almost tense feel on exam. Subfascial edema can be differentiated from lipodermatosclerotic changes in that it affects deeper tissues, whereas lipodermatosclerosis (LDS) affects the dermal skin layer.
In postphlebotic syndrome, organized DVT is replaced with connective tissue with variable levels of recanalization, resulting in long-term venous hypertension.8 In severe cases, ambulation can result in arterial inflow that overwhelms the outflow capacity of the veins. This produces even higher pressures during ambulation than during standing. This venous claudication sign results in pain when walking. Venous claudication pain is different than that seen in patients with peripheral arterial disease, in that it continues until limb elevation relieves the pain by reducing the elevated venous pressure. As the DVT becomes chronic, secondary varicose veins develop, relieving venous hypertension when the patient is in the recumbent position. The patient, however, maintains ambulatory venous hypertension caused by valvular insufficiency due to dilatations in the valvular annulus in remaining functional venous valves.
The venous hypertension and passive hyperemia start a cascade of events that leads to tissue fibrosis can eventually lead to. The interendothelial junctions, widened already because of leukocyte diapedesis, stretch further.8 The widened junctions produce diapedesis of red blood cells, which leads to the classic hemosiderosis seen in CVI. This greater permeability of blood capillaries leads to further extravasation of proteins. The proteins, with their associated oncotic pressures, are now in the interstitial space. Thus, simple compression will only concentrate the proteins further by removing some of the water, but the remaining proteins will hold onto water molecules. This explains why treatment of lymphedema with compression alone is not highly successful at reducing limb volumes. In order to be successful, treatment must help push the protein-rich fluid back into remaining functional lymphatics. Proper removal of the proteins requires Manual Lymph Drainage, which effectively disposes the proteins by pushing them into the lymphatic system, where they are disposed of. We will discuss this in more later in the article.
The proteins and proinflammatory cytokines can lead to tissue fibrosis, a complicated process that is called lipodermatosclerosis. In is interesting that many dermatology articles discussing the process of LDS fail completely to mention the lymphatic system and focus on skin and dermatopathology, whereas some lymphedema texts portray all LDS to be due to insufficiency of the lymphatics to handle the hyperemia and increased ultrafiltrate load.
The presence of proteins in the interstitial space creates a proinflammatory state. Accumulation of these proteins leads to inflammatory processes as the body tries to eliminate them.
Breaks in the skin can lead to introduction of bacteria, which propagate quickly through the protein-rich interstitial space. Some experts think these proteins may serve as a substrate for microorganisms, extending and intensifying infections. Other experts feel it is the inflammatory process and resultant altered immune system that contributes to higher rate and severity of cellulitis in lymphedema patients. These infections can lead to increased scarring and trauma to the fragile lymphatic collectors, and can cause further damage to the lymphatic system. Damage to the lymphatic system further decreases the body's ability to drain lymphedema fluid. Thus, repeated infections can cause stairstep type damage to remaining lymphatics and result in a clinical downward spiral of worsening lymphedema with each additional infection.
If the lymphatic system cannot handle the interstitial fluid load, then fluid and proteins start to accumulate. These proteins have their own oncotic pressures and hold water molecules with them, creating a swollen extremity. This is sometimes referred to as a dynamic insufficiency because the fluid load has exceeded the lymphatic maximum transport capacity and leads to clinical development of phlebolymphedema.9 A mechanical insufficiency develops when the lymphatic system is damaged and has reduced transport capacity. Mechanical insufficiency can occur from trauma, surgery, radiation, damaged lymphatics from previous infections, and so forth. Often, the phlebolymphedema is due to a combination of both dynamic insufficiency and mechanical insufficiency. Systemic factors contributing to the swelling can increase the dynamic insufficiency, contributing to development of phlebolymphedema.
If the lymphatic system becomes damaged (mechanical insufficiency) or the ultrafiltrate load becomes greater than the ability of the lymphatic system to drain it (dynamic insufficiency), proteins and macromolecules start to accumulate in the interstitium. These proteins have their own oncotic pressure, which creates clinical lymphedema. Additionally, the presence of these proteins induces an inflammatory cascade leading to the irreversible changes seen in late stages of lymphedema. The rate of inflammation varies greatly among individuals and is not fully understood. Thus, while all patients progress toward irreversible changes, some patients progress much faster than others.
Stasis Dermatitis
Stasis dermatitis is a common inflammatory skin disease that occurs on the lower extremities of patients with CVI. It is usually the first cutaneous sequela of CVI, but it can be a precursor to venous ulceration and lipodermatosclerosis. Stasis dermatitis is a direct consequence of venous hypertension. Increased hydrostatic pressure is transmitted to the dermal microcirculation, leading to increased permeability of the capillaries and fibrinogen leakage. The fibrinogen leakage and decreased fibrinogen activity are thought to cause fibrin cuffs. This results in tissue hypoxia and cell damage.6,10 Clinically, the patient typically presents with insidious onset of pruritis, followed by development of reddish-brown skin discolorization and hyperpigmentation. These deep dermal deposits of hemosiderin are from degraded extravasated erythrocytes and are called hemosiderosis. As stasis dermatitis progresses, scaling eczematous patches can occur. The medial ankle is most commonly affected. When it extends circumferentially, it is referred to as stocking erythroderma. Other skin changes include atrophic patches. Patients with stasis dermatitis have a higher risk of developing allergic contact dermatitis from topical treatments. The most frequent contact allergens include topical antibiotics such as neomycin and bacitracin.
Treatment of stasis dermatitis includes compression, along with bland topical emollients to maximize epidermal moisture. One inexpensive occlusive moisturizer is white petrolatum. FDA approved medications for stasis dermatitis include topical nonsteroidal calcineurin inhibitors, tacrolismus and pimecrolismus. Use of high-potency steroids is not recommended because of increased absorption, risk of steroid-induced cutaneous atrophy, and possible tachyphylaxis.10
Lipodermatosclerosis (LDS)
LDS is a skin change in the lower legs that is a type of panniculitis (inflammation of the subcutaneous fat). Affected patients often present with skin induration due to dermal fibrosis and increased pigmentation.11 The leg can develop into the classic “inverted champagne bottle” or “bowling pin” appearance (see Figure 1). LDS can be an acute or chronic condition. Acute LDS (ALDS) can mimic cellulitis, presenting as painful inflammation of the leg with redness, tenderness, and increased warmth.12,13 LDS occurs when there are changes in the deep dermis and subcutis, resulting in a constricting panniculitis. The pathology of the disease is quite variable.14 Fibrosis can be seen in the dermal layers. The pathology shows thick-walled capillaries surrounded by fibrin, siderophages (macrophages that have ingested iron), and fibrosis. Lobular and septal panniculitis can occur in the subcutaneous fat layers, mediated by lymphocytes. Later this progresses to ischemic necrosis of the fat with cystic spaces.
Figure 1.
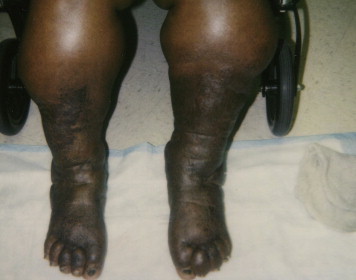
Classic Champagne Bottle Change of Phlebolymphedema (Chronic Venous Insufficiency With Secondary Lymphedema Development and Lipodermatosclerosis).
ALDS presents as extremely painful and inflamed, red or purple poorly demarcated plaques, often with indurated or edematous skin. It is mostly associated with CVI, but up to 30% may occur without underlying CVI.12
Chronic LDS produces pain and localized thickening and can cause atrophie blanche (seen as small white scarred areas). The exact mechanism of tissue fibrosis is still not fully understood, but there is clear evidence to show leukocyte activation and upregulation of proinflammatory cytokines. In chronic LDS, there may be low-grade injury to the endothelium by neutrophils and monocytes. This chronic inflammatory process results in enhanced cytokine release and cell proliferation. Macrophages release transforming growth factor beta (TGF-Beta) that in turn stimulates fibroblasts to synthesize more collagen and connective tissue proteins.15
Biopsies of patients with LDS shows pathology with characteristic evidence of venous stasis – superficial dermis, dilated vessels, panniculus with thickening and fibrosis of the septa, and lipophagic change with adipocyte necrosis in fat lobules. Lipomembranous change is typical, with feathery eosinophilic pseudomembranes staining strongly periodic acid–Schiff positive.15Biopsy for diagnosis of LDS is actively discouraged because of slow healing of biopsy sites and should be a clinical diagnosis made on physical exam in the absence of infection.
Clinical Examination and Diagnosis
The most common finding of patients with phlebolymphedema is swelling of the lower extremity. To help differentiate phlebolymphedema from other types of edema, there are several important clinical findings.
Kaposi-Stemmer's Sign
Classic diagnosis of lymphedema in the swollen extremity is by the Kaposi-Stemmer sign (see Figure 2), named for Kaposi, who first described it in 1887, and then Stemmer, who described it in 1976, after which it came into wider clinical use.2,16 The Kaposi-Stemmer sign assesses the ability to pinch a fold of skin, classically done at the base of the second toe. A negative Kaposi-Stemmer sign occurs when the skin easily tents. A positive Kaposi-Stemmer sign occurs when the skin on the dorsum of the second toe base cannot be pinched as a fold between the fingers. The Kaposi-Stemmer sign, while described for the toes, can be done anywhere on the body to assist in the diagnosis of lymphedema. If the skin does not easily tent, this is indicative of lymph proteins and fluid preventing redistribution of the interstitial fluids and thus preventing easy tenting. A positive Kaposi-Stemmer's sign is considered diagnostic for lymphedema. If the Kaposi-Stemmer sign is negative, however, it is still possible the patient has lymphedema. Reasons for a negative Kaposi-Stemmer sign in lymphedema can be due to diurnal variation or regionally functional lymphatics.
Figure 2.
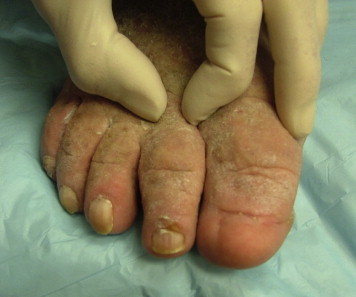
Kaposi-Stemmer Sign: Inability to Tent Skin Between Fingers Is Diagnostic of Lymphedema.
“Sausage Toes”
The dorsum of the foot is classically involved in lymphedema, and a patient may develop large, swollen, sausage-appearing toes. The dorsum of the foot may develop a large shelf-like hump because of accumulation of lymph in the interstitial tissues. Figure 2 shows advanced sausage toe appearance with papillomas. Figure 3shows early sausage toe changes because of lymph accumulation and dorsal foot swelling which is characteristic of patients with lymphedema.
Figure 3.
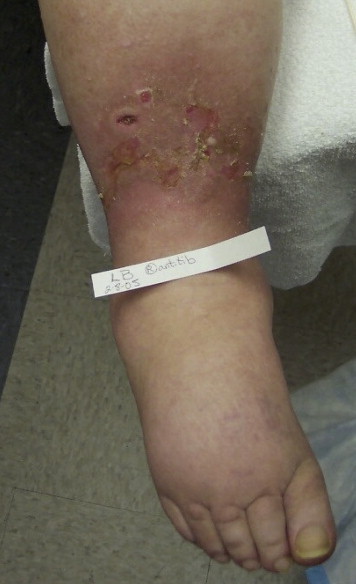
Phlebolymphedema Patient With Venous Ulceration With Associated Lipodermatosclerosis of Periwound Area. Note Slight Sausage Toe Changes and Dorsal Foot Swelling That Are Characteristic of Lymphedema.
Lipodermatosclerotic Changes
If the Kaposi-Stemmer sign is negative, it is still possible that the patient may have lymphedema. Chronic skin inflammatory changes of LDS that are seen in lymphedema include dermal fibrosis (see lower shin of Figure 3) and fine papillomatosis, which can feel like sandpaper (also called hyperkeratosis—see skin changes of foot of Figure 2) or can in late stages become larger and more verrucous appearing, as shown in the patient with elephantiasis (Figure 4). Nodular epidermal fibrosis (also referred to as nodular fibrosis) is also a manifestation of LDS in patients with lymphedema (Figures 5A and 5B).
Figure 4.
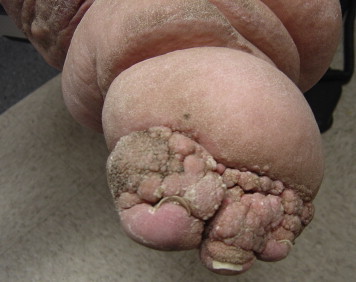
Severe Elephantiasis of Chronic Lymphedema With Papillomatosis, Lipodermatosclerosis, and Fibrosis of Dermal Skin.
Figure 5.
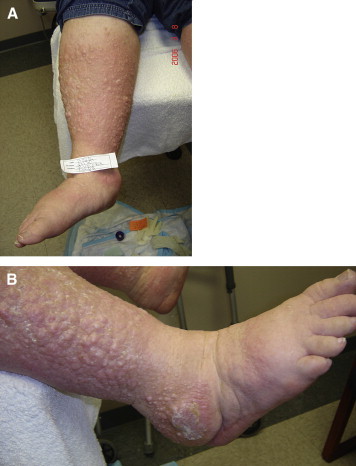
A, Phlebolymphedema Patient With Extensive Nodular Fibrosis Changes of Advanced Lipodermatosclerosis. B, Close-up of Patient of Figure 5A: Phlebolymphedema With Extensive Nodular Fibrosis Changes of Advanced Lipodermatosclerosis.
Lymphedema Rubra
Figures 6A and 6B show a patient with lymphedema rubra. Lymphedema rubra is the blanching redness of affected skin that can mimic and be mistaken for cellulitis. Often patients present with a history of repeated treatment for cellulitis and long-term treatment with antibiotics, when in fact there was never an infection to start with. These patients typically have a history of no fever and no resolution of the redness with antibiotic treatment. Lymphedema rubra represents inflammatory hyperemia due to the proinflammatory changes from high protein content fluid in the interstitium. This sign is indicative of early lipodermatosclerotic inflammatory process. These patients can have warmth to the area due to increased blood flow from the proinflammatory state, which can cause confusion for the inexperienced examiner.
Figure 6.
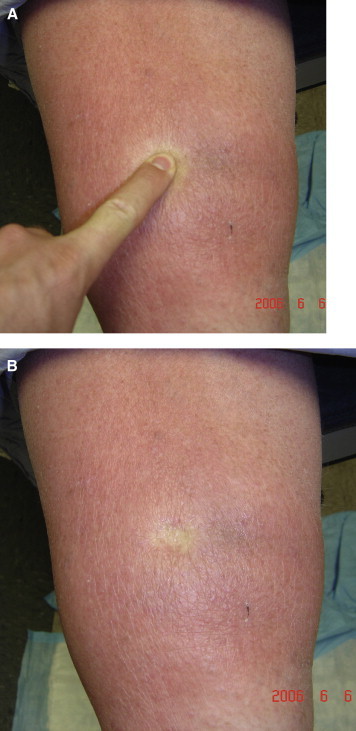
A, Demonstration of Lymphedema Rubra in Patient With Phlebolymphedema. B, Demonstration of Lymphedema Rubra in Patient With Phlebolymphedema: Photo Taken Directly After Finger Release.
It is important for the astute physician to be able to delineate lymphedema rubra from true cellulitis. Proper history is important. Lymphedema rubra patients will have lack of fever, lack of progression of the erythema, and chronicity of symptoms and may report history of past treatment for “multiple infections” that were misdiagnosed as cellulitis.
According to Foldi, all lipodermatosclerotic processes in patients with CVI indicate malfunctioning or inadequate lymphatics.8
Although the diagnosis of lymphedema is typically by history and examination, in difficult cases the diagnosis can be confirmed by lymphoscintigraphy. This process will show delayed lymph drainage in patients with lymphedema. This is often not an appropriate test for phlebolymphedema patients, however, as most of these patients have phlebolymphedema due to dynamic insufficiency, and their lymphatics are working at maximum capacity but are still overwhelmed. Patients with dynamic insufficiency phlebolymphedema have severe venous hypertension and can have lymph transit speeds that are above normal.8 The disease is not always defined by the speed of lymph transport but is often a matter of inadequate ability of remaining lymph collectors to handle the high volume of ultrafiltrate seen in CVI. The venous hypertension may be overwhelming the lymphatic system, resulting in excessive accumulation of fluid in the interstitium. Hence, lymphoscintigraphy may give false negative results in patients with phlebolymphedema and is therefore not a reliable test for the diagnosis of patients with dynamic insufficiency-related phlebolymphedema.
LDS and ALDS often improve and soften over time with reduction of swelling via compression. This compression can be achieved with multilayer or short-stretch bandaging. If there is clinical evidence of lymphedema, manual lymph drainage and short-stretch bandaging are recommended.
Medical treatment for ALDS has been documented successfully using stanozolol 2 mg twice a day for an 8-week course.17,18 Stanozolol has a low ratio of androgenic effect and can in some cases greatly reduce pain in 3 to 4 weeks. It has been used successfully in patients unable to tolerate compression because of pain from their ALDS. Side effects are reportedly few, although weekly blood pressure monitoring and lipid profiles every 3 to 4 weeks are recommended. Stanozolol is no longer available on the U.S. market.
Mixed Edemas
Mixed-etiology edema can have a myriad of contributing factors, and it is not uncommon for patients to have multiple contributing factors underlying their lower extremity swelling. Thus, there are many factors other than CVI that can cause chronic lower extremity edema, and in some cases the severity of these other causes can lead to secondary development of lymphedema. Table 1 above outlines the main systemic contributors to lower extremity swelling, and they are discussed in the next paragraphs.
Table 1.
Causes and Contributors to Lower-Extremity Swelling
|
Certain items in the table deserve comment. Rate of peripheral edema with nonsteroidal anti-inflammatory drugs is estimated at 5%. Rate of peripheral edema with calcium channel blockers is dose dependent. The actual rate is difficult to determine because it is different for different types of calcium channel blockers and is dose dependent, but rates of 5% to 70% have been reported.19 Lyrica is a common cause of lower-extremity peripheral edema, and dose dependent rates as high as 17% to 27% have been reported.20 Brain Natriuretic Peptide (BNP) level is good to rule out congestive heart failure (CHF) because of its high sensitivity of 90%.21
Obesity is a common cause of venous hypertension and a risk factor for CVI, lipodermatosclerosis, and phlebolymphedema. There is an almost linear relationship between increased abdominal girth and increased abdominal pressure. Abdominal obesity increases intraabdominal pressure that is transmitted through the vena cava and common iliacs directly to the femoral vein, where it can thus be exerted on the superficial and deep venous systems. A large pedunculated pannus may also impair flow of superficial lymph. Thus, any abdominal obesity can worsen CVI and increase risk of development of phlebolymphedema.22
Pulmonary hypertension is an often underdiagnosed etiology of lower extremity swelling. In one study of 45 patients with lower extremity swelling evaluated by their primary physician, 71% were felt to have CVI. After complete workup, final diagnosis was pulmonary hypertension (>40 mm Hg) in 20% and borderline pulmonary hypertension (31-40 mm Hg) in 22%. Therefore, consider echo in patients older than 45 with leg edema of unclear etiology in order to evaluate for pulmonary hypertension.23
Calf muscle dysfunction is an often overlooked cause of venous hypertension. Muscle atrophy or arthropathy of the ankle joint reduces calf muscle pump. This can greatly impair healing rates of venous ulcerations.
Treatment of Phlebolymphedema
Treatment of the patient with significant phlebolymphedema is the same as treatment of any patient with diagnosis of lymphedema. In very mild cases of phlebolymphedema that have mostly a venous component, multilayer compression wraps or short-stretch compression bandages can often be used, and formal lymphedema treatment may not be necessary. In these mild cases, the bandages help augment lymphatic flow sufficiently. As the patient ambulates against bandages, the high subbandage pressure can help promote lymph flow through the dermis. It is often recommended to bandage the toes to prevent retrograde accumulation of lymph fluid to the toes and distal forefoot. This can be done with specialized very-low-compression toe bandages. For patients with any significant degree of phlebolymphedema, the standard of care is complete decongestive therapy (CDT).8
Complete Decongestive Therapy (CDT)
The majority of patients with phlebolymphedema will require a course of CDT. CDT consists of an acute phase to reduce the lymphedema, followed by a lifelong maintenance phase to prevent recurrence.24 Phase I CDT consists of treatments 2 to 5 times weekly for approximately 4 weeks. Treatments consist of manual lymph drainage (MLD), skin care, exercise, and compression bandaging with short-stretch compression bandages. Often, limb measurements are made weekly, and therapy is stopped after patient reduces to baseline or no longer makes progress. After phase I reduction of lymphedema, patient starts phase II CDT. Phase II CDT consists of lifelong daily compression, compression at night if needed, skin care, and exercise. Patients are often taught self-MLD, which they can continue themselves at home. Some experts do not advocate teaching self-massage to their patients.
It is recommended that phase I CDT treatment be performed by a certified lymphedema therapist, or a therapist who has undergone advanced training in MLD.25 Such training involves 135 hours of training as established by the Lymphology Association of North America. The National Lymphedema Network has a position paper on treatment of lymphedema, as well as training of lymphedema therapists.24,25 It is recommended the therapist undergo advanced training by a program that will lead to certification.
Manual Lymph Drainage (MLD)
MLD is a superficial technique that stimulates lymphatic vessels and helps open and force fluid into the lymphatic system. MLD may be done in such a manner as to direct lymphatic flow out of congested areas and into functional lymphatics proximally.26 MLD works by a gentle push-pull mechanism on the anchoring filaments of the lymphatic collectors, which opens the clefts. The movements of MLD force fluid into the lymphatic system and stimulate lymphatic peristaltic action in remaining functional lymphatics.27
Short-Stretch Bandaging
Lymphedema bandaging is accomplished with reusable short-stretch bandages, which have limited extensibility (30%-70% maximum extensibility), compared with other bandages. The bandages are wrapped in multiple layers, typically in a figure-8 configuration. The padding consists of layers of foam, viscose-cotton, or polyester padding. Pressures in the short-stretch bandages are designed to be low when the limb is inactive, which is often called the resting pressure. With an increase in muscle contraction during limb activation, the muscle expands against the semirigid bandages. This muscle activation increases pressure in the limb, creating a higher pressure, which is often called the working pressure. Although this increase is seen to the greatest extent in the calf muscle, the same phenomonon occurs with other bandaged muscular areas, such as the forearm. Short-stretch bandages work by creating a cycling between working and resting pressures that creates an internal muscle pump and encourages movement of congested lymph fluid out of the affected limb by stimulating lymph transport.28
High-density foam or chipped foam is often used in conjunction with compression bandaging to pad bony and tendinous prominences, as well as to help reduce tissue fibrosis of LDS. Use of foam and compression over time is generally accepted by most lymphedema therapists as being helpful to reduce tissue fibrosis. It is supported by a series of cases studies, but large random trials are lacking. Skin lobules should be padded, lifted, and compressed to facilitate decompression and can shrink considerably over time with adequate compression. Many therapists use channeled foam, which mimics flow of the lymphatics. The channeled foam creates areas of high pressure where the foam pushes on the skin and channels of low pressure areas where the foam presses less against the skin. The channeled foam is thought to work by pushing lymph from the high-pressure areas and allowing it to drain proximally through the low-pressure areas. While these types of therapies have widespread use in the United States and are felt by many therapists to be beneficial, rigidly controlled clinical studies are lacking.
Long-stretch bandages such as Ace-type bandages have high extension of approximately 300% stretch (300% extensibility). Long-stretch bandages typically lose approximately 50% of their compression in 6 hours and do not recreate the important cycling between working and resting pressures. These long-stretch bandages lack a bandage “lock-out” or end-stretch, as seen in short-stretch bandages. Short-stretch bandages create a flexible nonelastic shell when applied to a limb and maximally augment the calf muscle pump. Long-stretch bandages are discouraged for management of any acute or chronic edema for the following reasons: (1) They lose compression rapidly over time and so become subtherapeutic, and (2) they are not applied at or near bandage lock-out, so they offer very little augmentation of calf muscle function. Therefore, high extension (long-stretch) bandages should play no role in the management of edema and are not recommended to be used because of their ineffectiveness in edema reduction and maintenance.
Exercise
Exercise is a very important component of CDT and works by improving local muscle pumping action to raise efficiency of lymph transport.24 Increased negative thoracic pressure with exercise can also improve lymphatic flow into the thorax as lymph fluid follows the pressure gradient to lower pressure areas. Exercise is especially useful in conjunction with short-stretch bandages and garments. Exercise can be done as ambulation or can be nearly as effective with toe up-down exercises, which significantly augment the calf muscle pump. For treatment of patients with lymphedema involving the arm, exercise is still an effective and important component of reducing swelling through activation of forearm musculature.
Skin Care
Skin care is an important component of CDT. Goals of skin care are to reduce dermal colonization by bacteria and fungi.29 Since lymphedema patients have large skin folds and may have lymphostatic ulcers with high drainage rates, it is imperative to use good periwound skin barriers. Zinc based products are often recommended to prevent periwound maceration and breakdown. Furthermore, meticulous skin care between skin folds is necessary to prevent breakdown. Olivamine containing products have been reported to be anti-inflammatory. Dimethicone barriers are useful to reduce and prevent dermatitis reactions. Pruritis and dermatitis can be treated with triamcinolone cream during dressing changes. Products containing balsam of Peru are useful for periwound barriers as well as shallow ulcerations with low drainage.
Compression Garments
After phase I CDT reduction of lymphedema, a patient will need maintenance compression garments to prevent edema reaccumulation. Typical phlebolymphedema patients will need at least 30 to 40 mm Hg compression. For most patients with significant lymphedema, flat-knit compression stockings are preferable to circular knit compression stockings. Flat-knit stockings are usually custom made to fit measurements and stretch less than circular knit stockings, providing firmer compression to prevent limb swelling. Therefore, for patients with severe swelling, flat-knit garments are recommended over circular-knit garments. For patients who cannot use compression stockings because of difficulties donning and doffing, or for patients with atypical limb geometry, Velcro binder type products are recommended.29 Chipped foam garments such as the Jovi-Pak use channeled foam following lymphatic channels, can be useful adjuncts for reducing tissue fibrosis, and are often used for nighttime compression. Other nighttime garments used as an alternative to bandaging include the ReidSleeve.
Pneumatic Pumps
Pneumatic pumps are not components of CDT but can be useful adjuncts.24 In general, advanced pumps with higher numbers of chambers and an ability to preclear truncal areas are thought by many experts to be more effective than pumps with fewer chambers. Preclearance of lymphatics refers to a process of decompressing lymphatics proximal to the affected area. By decompression of proximal lymphatics before the pump is used on the affected area, more fluid can be removed from the affected areas without overfilling the proximal lymphatics. Pumps should be used in conjunction with recommendations from qualified lymphedema therapists and should not be considered a panacea or an alternative to MLD. CDT is the standard of care for treatment of lymphedema. Pneumatic pumps are not a component of CDT but can be used adjunctively. Use of pneumatic pumps does not replace the need for CDT or replace the need for long-term daily maintenance compression.
Summary
Phlebolymphedema can be due to insufficiency of the venous or the lymphatic system or both. Most cases of phlebolymphedema are due to an overloaded of the venous system that overwhelms the lymphatic systems ability to remove interstitial fluid. Systemic and contributing factors can play a significant role in causing or exacerbating phlebolymphedema and should be corrected when possible. Use of a certified lymphedema therapist in the clinic or in close conjunction with wound care treatment is tantamount to providing quality care. The standard of care for all patients with clinically significant lymphedema should be CDT by a qualified therapist, including MLD, skin care, exercise, and short-stretch compression. The goal of CDT should be to control all the swelling all the time because anytime there is accumulation of proinflammatory lymph fluid in the interstitium, there is increased risk to the patient of infection and long-term LDS processes.
Additional Resources to Learn More About Lymphedema
-
1.
National Lymphedema Network: http://www.lymphnet.org
-
2.
Földi's Textbook of Lymphology, chapter 11: Chronic Venous Insufficiency (CVI) and Venous-Lymphostatic Insufficiency)
-
3.
International Society of Lymphology 2009 Consensus Document
-
4.
Wound Care Practice, 2nd ed., chapter 15: Lymphedema, an Epidemic Hidden in Plain Sight
-
5.
Lymphology Association of North America: http://www.clt-lana.org
Footnotes
Conflict of interest: The author reports no conflicts of interest. The author is a board member of the National Lymphedema Network and the founder and CEO of Farrow Medical Innovations.
References
- 1.Szuba A., Shin W.S., Strauss H.W., Rockson S. The third circulation: radionuclide lymphoscintography in the evaluation of lymphedema. J Nucl Med. 2003;44(1):43–57. [PubMed] [Google Scholar]
- 2.Brenner E., Putz D., Moriggl B. Stemmer's (Kaposi-Stemmer-) sign—30 years later. Phlebologie. 2007;36(6):320–324. [Google Scholar]
- 3.Weismann K., Krakauer R., Wanscher B. Prevalence of skin diseases in old age. Acta Derm Venereol. 1980;60(4):352–353. [PubMed] [Google Scholar]
- 4.Valencia I.C., Falabella A., Kirsner R.S., Eaglstein W.H. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401–421. doi: 10.1067/mjd.2001.111633. [DOI] [PubMed] [Google Scholar]
- 5.Guyton A.C. 8th ed. WB Saunders; Philadelphia, PA: 1991. Textbook of Medical Physiology. [Google Scholar]
- 6.Földi M., Földi E., Kubik S. Textbook of Lymphology for Physicians and Lymphedema Therapists. Urban and Fisher; Munich, Germany: 2003. Deficiency and insufficiency of the lymphatic system. p. 209–215. [Google Scholar]
- 7.Kirkman R., Sawdon M. Capillary dynamics and interstitial fluid lymphatic system. Anaesth Intensive Care Med. 2004;6(1):179–183. [Google Scholar]
- 8.Földi E., Földi M. Földi's Textbook of Lymphology. 2nd ed. Elsevier; Munich, Germany: 2006. Chronic venous insufficiency and venous-lymphostatic insufficiency. p. 434–447. [Google Scholar]
- 9.Pillar N. Phlebolymphedema/chronic venous lymphatic insufficiency: an introduction to strategies for detection, differentiation, and treatment. Phlebology. 2009;24:51–55. doi: 10.1258/phleb.2009.009003. [DOI] [PubMed] [Google Scholar]
- 10.Flugman SL, Clark RA. Stasis dermatitis. Available at: http://emedicine.medscape.com/article/1084813-overview. Accessed April 26, 2010.
- 11.Falanga V., Moosa H.H., Nemeth A.J., Alstadt S.P., Eaglstein W.H. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol. 1987;123(5):620–623. [PubMed] [Google Scholar]
- 12.Bruce A.J., Bennett D.D., Lohse C.M., Rooke T.W., Davis M.D.P. Lipodermatosclerosis: review of cases evaluated at the Mayo Clinic. J Am Acad Dermatol. 2002;46(2):187–192. doi: 10.1067/mjd.2002.119101. [DOI] [PubMed] [Google Scholar]
- 13.Phelps R.G., Shoji T. Update on panniculitis. Mount Sinai J Med. 2001;68(4-5):262–267. [PubMed] [Google Scholar]
- 14.Kirsner R.S., Pardes J.B., Eaglstein W.H. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol. 1993;28(4):623–627. doi: 10.1016/0190-9622(93)70085-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith P.C. The causes of skin damage and leg ulceration in chronic venous disease. Int J Lower Extr Wounds. 2006;5:160–168. doi: 10.1177/1534734606292429. [DOI] [PubMed] [Google Scholar]
- 16.Stemmer R. Ein klinisches Zeichen zur gruhund differential diagnose des Lyphodems. Vasa. 1976;5:261–262. [PubMed] [Google Scholar]
- 17.Vesic S., Vukovic J., Medenica L.J., Pavlovic M.D. Acute lipodermatosclerosis: an open clinical trial of stanozolol in patients unable to sustain compression therapy. Derm Online J. 2008;14(2):1. [PubMed] [Google Scholar]
- 18.Helfman T., Falanga V. Stanozolol as a novel therapeutic agent in dermatology. J Am Acad Dermatol. 1995;33(2 Pt 1):254–258. doi: 10.1016/0190-9622(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 19.Sica DA. Calcium channel blocker-related peripheral edema: can it be resolved? Available at: http://www.medscape.com/viewarticle/460070_1. Accessed April 26, 2010.
- 20.Frampton J.E., Scott L.J. Pregabalin in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–2820. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- 21.Maisel A.S., Krishnaswamy P., Nowak R.M. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 22.Goldman M.P. Lipodermatosclerosis: review of cases evaluated at the Mayo Clinic. J Am Acad Dermatol. 2002;46:187–192. doi: 10.1067/mjd.2002.119101. [DOI] [PubMed] [Google Scholar]
- 23.Blankfield R.P., Finkelhor R.S., Alexander J.J. Etiology and diagnosis of bilateral leg edema in primary care. Am J Med. 1998;105:192–197. doi: 10.1016/s0002-9343(98)00235-6. [DOI] [PubMed] [Google Scholar]
- 24.Position statement of the National Lymphedema Network. Topic: Treatment. Review date 9/30/2009. Available at: http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Accessed April 26, 2010.
- 25.Position statement of the National Lymphedema Network. Topic: Training of lymphedema therapists. Approved Date: 11/10/2005. Available at: http://www.lymphnet.org/pdfDocs/nlntraining.pdf. Accessed April 26, 2010.
- 26.Diagnosis and treatment of peripheral lymphedema: 2009 consensus document of the International Society of Lymphology. Lymphology. 2009;42:51–60. [PubMed] [Google Scholar]
- 27.Wittlinger G., Wittlinger H. 7th ed. Georg Thieme Verlag; Stuttgart, Germany: 2004. Textbook of Dr. Vodder's manual lymph drainage. [Google Scholar]
- 28.Földi E. The lymhpedema chaos: a lancet. Ann Plast Surg. 1989;22:505–515. doi: 10.1097/00000637-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Fife C.E. Lymphedema, an epidemic hidden in plain sight. In: Joiner J.T., Lasky K., editors. Wound Care Practice. 2nd ed. Best; Flagstaff, AZ: 2007. pp. 379–399. [Google Scholar]


