Abstract
Introduction
Skin fissures are a common dermatologic condition caused by excessive dry skin, numerous systemic diseases, and backless shoe gear. They are defects in skin that fall into the category of damaged, partial-thickness skin wounds, as opposed to full-thickness wounds. Patients with heel fissures are at an increased risk for developing infection, which could cause more severe issues, especially in patients with diabetes and peripheral vascular disease.
Methods
Five patients from Temple Foot and Ankle Institute, Philadelphia, PA, with a total of 8 heel fissures and 2 hallux fissures, were studied. Patients were dispensed 9 vials of a cyanoacrylate liquid skin protectant (Marathon™, Medline Industries, Inc, Mundelein, IL) to be applied to the fissure every 3 days. Patients returned every 2 weeks for follow-up in clinic.
Results
The hallux fissures and 4 of the heel fissures went to complete closure after 2 weeks. There was an average decrease of 1.16 cm in length of the heel fissure dimensions after 2 weeks and an average decrease of 1.1 cm in length of the hallux fissures.
Conclusion
This novel skin protectant proved to be a comfortable, easy, and effective tool in aiding the resolution of pedal skin fissures.
Keywords: Partial-thickness skin damage, Skin, Skin fissure, Skin protectant
Introduction
Heel fissures are disruptions in the skin that can be caused by epidermis that has dried excessively, backless shoe gear, and numerous systemic diseases.1 Mechanical strain, caused by weight bearing by the affected area, can lead to deepening of the fissure, which increases discomfort and mobility. Diabetes and/or vascular compromise may lead to additional complications, such as cellulitis and ulcerations.1,2 Diabetic patients are often insensate and unaware of the dry skin condition and progressing fissures until the skin opens, resulting in a full-thickness ulcer formation. The current management for heel fissures includes use of skin-softening agents (emollients and keratolytics) and proper shoe gear. This study examined the use of a novel skin protectant based on an interesting polymer technology used in acute and long-term care settings. The product investigated in this study is a cyanoacrylate-based skin protectant that is used as a barrier against moisture and friction.3-5 The use of this product in the management of heel fissures, as described in this article, is of interest in the podiatric environment and builds on existing information on this subject.6
Cyanoacrylates
Cyanoacrylate skin protectant barriers are transparent, flexible film-forming products that adhere to the skin at a molecular level.7 These novel products have a high affinity for moisture and bond to surfaces that contain any degree of moisture, such as skin. On application to such a water-rich or water-containing surface, the monomeric substance present in the applicator immediately sets up a “chain reaction” to form a robust polymeric barrier, protecting the underlying intact or damaged skin from moisture, and friction-related breakdown.8,9 These protective films may also have the capacity to maintain the integrity of newly healed skin.10 Once set on the skin, the film is claimed to provide up to 3 days of wear, subsequently shedding during normal skin surface turnover, thus avoiding the need for daily reapplication. Skin protectants have long been available in the clinic; however, there are key differences between the traditional solvent-based protectants (commonly called skin preps) and newer materials such as the cyanoacrylates reported in this study. These newer-generation products contain no solvents; therefore there is no potential for solvent-related stinging. Additionally, because of the lack of solvent presence, most of the product remains on the skin, leading to a robustness of the deposited film not seen with solvent-containing products. Cyanoacrylate skin protectants can be used on at-risk skin to prevent and manage certain types of skin tears, friction-related breakdown of skin, and shear- and moisture-related assault on skin in at-risk patients.3-5 A more comprehensive list of appropriate body areas and skin conditions includes the following:
-
•
Bony prominences
-
•
Incontinence-related damaged skin
-
•
Inflamed, irritated skin
-
•
Periwound skin
-
•
Skin tears of type I and II (Payne-Martin Skin Tear Classification)11
-
•
Macerated skin
-
•
Delicate periwound skin in wounds under negative pressure therapy
-
•
Damaged skin around stomas
-
•
Diabetic or neuropathic feet
-
•
Skin around gastrostomy tubes to prevent damage from leaking fluid
-
•
Skin around IV and other tube insertion sites
-
•
Skin under and around nasal tubes
-
•
Perineal area
-
•
Sacrum and inner thighs
-
•
Fissures on heels and fingers
-
•
Healed but still fragile amputation sites
-
•
Skin under skin folds
-
•
Recently closed wounds
Preventive and protective uses of cyanoacrylate skin protectant in podiatric practice can be seen in Figures 1 through 4. Cyanoacrylates may also protect recently closed wounds (Figure 5).
Figure 1.
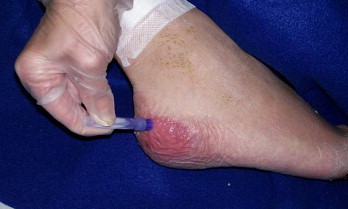
Use of cyanoacrylates to protect heel area from friction damage.
Figure 2.
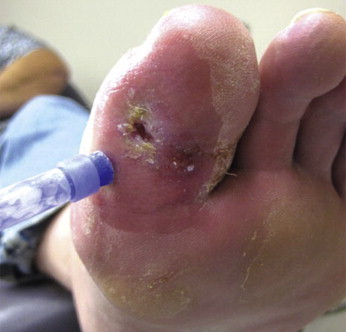
Use of cyanoacrylates to protect periwound skin from maceration from wound drainage
(Photo courtesy of Dr Lee Rogers).
Figure 3.
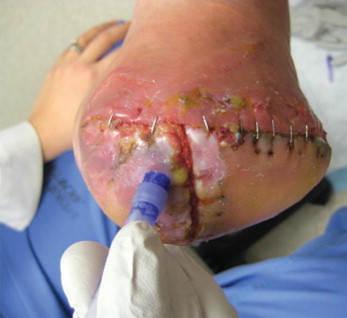
Application of cyanoacrylate barrier to protect periwound skin around a recently closed wound on the foot
(Photo courtesy of Dr Lee Rogers).
Figure 4.
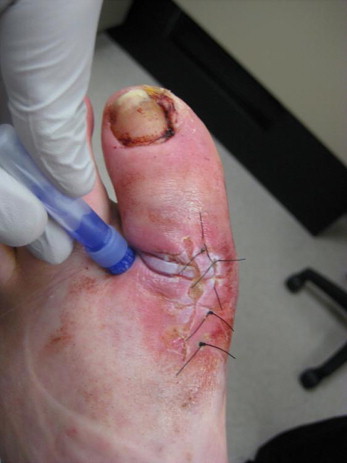
Postoperative application of cyanoacrylate skin protectant
(Photo courtesy of Dr Lee Rogers).
Figure 5.
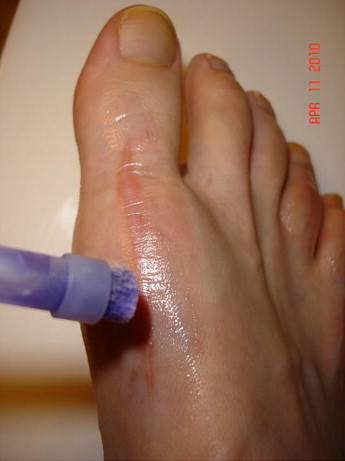
Postbunionectomy application on remodeling incision site (Photo compliments of Cynthia Ann Fleck).
Contrast Between Cyanoacrylates and Solvent-Based Polymer Barriers, the Current Standard of Care
A range of skin-protetcant or skin-prep products have been available to provide various degrees of skin protection under a range of circumstances. Skin protectants available to date have generally consisted of natural or synthetic polymers dispensed in a dissolved form in a solvent, frequently alcohol, which is known to “sting” when applied to skin that is damaged. After application and drying of the solvent, these first-generation products tend to form a clear, vapor-permeable, reasonably water-resistant coating that can protect skin from a certain degree of adhesive trauma, moisture, incontinence, and friction. When used around periwound skin, for example, to prevent dressing removal trauma, skin preps are usually applied with every dressing change because of the inherent transient nature of their bonding to skin. Most of these products are available in a small sachet, a sponge-tipped applicator, or a spray. No-sting or no-alcohol varieties are also available in this class of solvent-based products and are usually water- or synthetic silicone solvent–based. These products claim to have stingless application and are particularly helpful if the skin is denuded, damaged, or compromised and likely to sting. Some of these products have been deemed to be safe enough to allow application on neonatal skin.12 From available testing data, however, it appears that these protectants have nowhere near the strength one may expect from polymers such as cyanoacrylates. The reason for the difference in the robustness of protection attributed to these 2 classes of product is important to understand.
The solvent-containing skin-prep products that have been used for many years to protect skin generally contain a natural or synthetic polymeric substance dissolved in a solvent that may be organic (alcohol or silicone based) or simply water. These formulations are analogous to a thin coat of paint because a large part of the applied product (ie, the solvent part) is lost via evaporation.
It is interesting to observe the microscopic characteristics of these products after application to skin, whether of human or of animal origin. In Figure 6, undesirable gaps are clearly visible in the bonding of a solvent-based barrier to the underlying skin.
Figure 6.
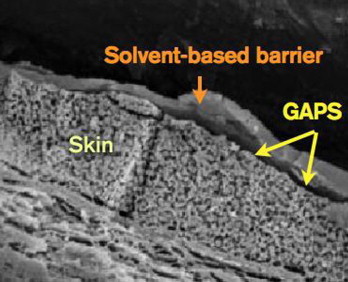
Photomicrograph of a solvent-based barrier deposited on skin (Note the existence of undesirable “gaps” in the interface, which reduce the robustness of skin protection afforded by solvent based barriers.)
In contrast, cyanoacrylate (monomer) liquid skin protectants chemically bond to the skin, with single-coat coverage being generally enough to protect underlying skin without any apparent gap that will tend to decrease the bond strength, as shown in Figure 7. This photograph shows the intimate bonding of a cyanoacrylate-based barrier to the skin. (Pig skin was used to generate Figures 6 and 7).
Figure 7.
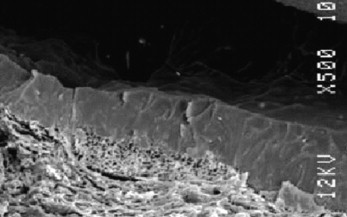
Photomicrograph of cyanoacrylate bonded to skin (Note the intimate level of bonding to the underlying skin.)
This chemically induced molecular-level integration with the epidermis (Figure 7) as the cyanoacrylate polymerizes strongly supports the natural integrity of the skin. The higher resistance to both friction and damage from corrosive liquids that has been reported is understandable in the context of this fundamental chemical difference between solvent-based preps and cyanoacrylates. The cyanoacrylate polymers have the added benefit that they do not dissolve in water and thus the resulting film on skin is designed to be unusually resistant to bodily fluids.
Methods
The study described here represents a pilot evaluation of the product on pedal skin fissures with no inclusion of a control group. The product applicator used in this study on heel and foot fissures contained 0.5 g, which is designed to cover approximately a 10.2 × 10.2 cm2 (4 × 4 sq in) area.
We evaluated 5 patients with a total of 8 heel fissures and 2 hallux fissures. Patient inclusion criteria included fissure(s) on their feet and agreement to follow up in the clinic every 2 weeks during a 1-month period. Pain or discomfort in and around the fissure was also part of the inclusion criteria. Exclusion criteria included infection, purulent drainage, or active serosanguinous drainage. All patients had heel fissures except 1, who had fissures of the hallux bilaterally. All the heel fissures and hallux fissures were not symptomatic of infection by clinical history and examination. One patient had failed treatment of her heel fissure using moisturizing skin barrier foam daily for a period of 4 weeks prior to using the liquid skin protectant. Patient demographics are listed in Table 1.
Table 1.
Patient Demographics
| Patients (N) | 5 |
| Female vs male | 4 to 1 |
| Fissure on heel | 6 |
| Fissure on toe | 2 |
| Patients with diabetes mellitus | 1 |
| Patients with psoriasis | 1 |
| Patients with atopic eczema | 1 |
All fissures were cleansed with sterile saline, and surrounding hyperkeratotic skin was debrided. After debridement, each heel fissure was measured and photographed. At the first visit, the cyanoacrylate liquid skin protectant was applied to the heel fissure and surrounding area according to the directions supplied by the manufacturer. The product was allowed to dry before patients donned their socks and shoes. Patients were educated on home application of the product, and all patient questions were answered. Patients were then supplied with 9 vials of the cyanoacrylate liquid skin protectant (Marathon™, Medline Industries, Mundelein, IL), to be used over a 2-week period, and a product use diary. Patients returned to clinic every 2 weeks. At subsequent visits, the fissures were measured and evaluated for signs of infection and healing rate. Photographs of the fissures were also taken at the follow-up appointments. Patients were instructed to bring their study material and diaries with them at follow-up visits to ensure compliance (Table 2).
Table 2.
Treatment Protocol for Each Visit
| Visit number Day of visit |
1 Day 1 |
2 Day 15 |
3 Day 22 |
|---|---|---|---|
| Inclusion–exclusion criteria applied | x | ||
| Information and informed consent obtained | x | ||
| Physical examination performed | x | x | x |
| Fissure cleansed and length, width, and depth measured | x | x | x |
| Study medication dispensed | x | ||
| Adverse events of treatment examined | x | x | |
| Photo taken | x | x | x |
Results
Two of the heel fissures were lost to follow-up, leaving a total of 6 heel fissures and 2 hallux fissures. All of the fissures reached the study end point. Fissure size data are listed in Table 3. After 2 weeks there was an average 1.16-cm decrease in length of the heel fissures (Figures 8A, 9A, and 11A) and an average 1.1-cm decrease in length of the hallux fissures (Figure 10A). In addition, the depth of the heel fissures decreased by an average of 0.12 cm and the depth of the hallux fissures by 0.1 cm. The hallux fissures and 4 of the heel fissures were completely healed after 2 weeks. Of the other 2 heel fissures, 1 completely healed in depth and decreased in length by 1.5 cm, and the other decreased in length by 0.3 cm after a period of 4 weeks. Patients reported an application of the study product once every 3 days on average. Figures 8A, 8B, 9A, 9B, 10A, 10B, 11A, and 11B are before-and-after images, respectively, of the heel and hallux fissures.
Table 3.
Fissure Size Data
| Fissure Characteristic | Average Size (cm) |
|---|---|
| Size of heel fissures at visit 1 | 1.65 |
| Size of heel fissures at visit 2 | 0.45 |
| Size of hallux fissures at visit 1 | 1.15 |
| Size of hallux fissures at visit 2 | 0.0 |
Figure 8.
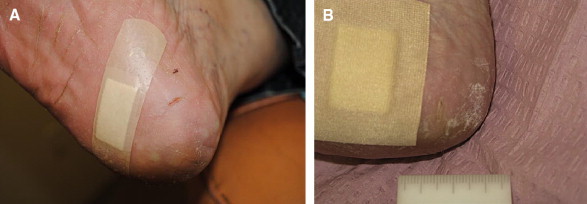
A, Heel fissure measuring 0.4 cm in length. B, Same fissure completely healed after 2 weeks with use of the cyanoacrylate.
Figure 9.
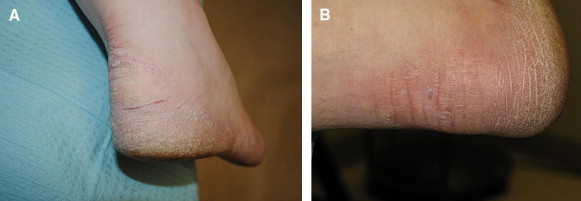
A, Patient with chronic psoriasis and heel fissures. B, Same patient at 2-week follow-up with resolution of fissures.
Figure 11.
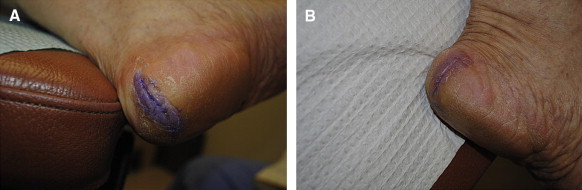
A, Heel fissure measuring 3.0 cm in length on the first day of cyanoacrylate use. B, Same heel fissure after 2 weeks with use of cyanoacrylate.
Figure 10.
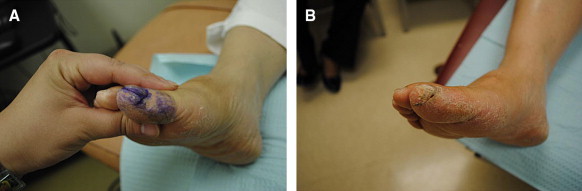
A, Hallux fissure in figure 2A measures 1.2 cm in length. B, Same hallux fissure resolved after 2 weeks with use of the cyanoacrylate.
Discussion
Heel fissures are a common dermatologic condition that has been given relatively little attention in the literature. Keratolytic treatment of heel fissures typically takes several weeks to achieve any improvement, as well as twice daily application by patients, a burden on those with reduced mobility. Debridement of fissures is also a common practice and can be painful for the patient. Patients with extensive heel fissures, especially those with psoriasis and diabetes, can have difficulty in ambulation due to the pain and deformity, as well as an increased chance of infection at the site.
Patients in our study reported that the cyanoacrylate-based liquid skin protectant was very simple to use and apply with the applicator vial–type unit dose. Patient compliance was high because of the need to apply the product only once every 3 days. The only complaint about the product was the purple stain that accumulates after each application, but this color was found to be eliminated with epidermal turnover and had no lasting effect. This coloring of the treated skin was deemed to be actually beneficial because some of the patients or their caregivers used the presence or absence of color to judge when to reapply the product. One patient preferred covering this area with a simple bandage to hide the purple color of the treated area.
Although the sample in this study was small, a significant improvement in the size of the fissures studied was noted after only 2 weeks of using the cyanoacrylate liquid skin protectant. After just 4 applications, a majority of the fissures in this study had resolved. The reason for this quick resolution may be as simple as the robust protection from friction and environmental factors to the fissure area, because once “set,” the cyanoacrylate polymer has no residual activity in directly promoting skin damage repair. Patient compliance with protocol was perhaps enhanced by the ease of application, a decrease in the discomfort associated with the heel fissures over time, and the rapid emergence of a reasonably cosmetically acceptable result. The cyanoacrylate liquid skin protectant proved to be a novel, useful, and rapid method of treatment for painful skin fissures of the foot and the heel and may be a valuable tool in practice of podiatric medicine.
Footnotes
Conflict of interest: Debashish Chakravarthy and Cynthia Fleck disclose that they are employed by Medline Industries, Inc.
References
- 1.Ignatoff W. Heel fissures and their management. J Nat Assoc Chirop. 1952;42(2):23–31. [PubMed] [Google Scholar]
- 2.Omura E., Rye B. Dermatologic disorders of the foot. Clin Sports Med. 1994;13(4):835. [PubMed] [Google Scholar]
- 3.Milne CT: The role of cyanoacrylates in the prevention of superficial tissue injury. Presentation at: Symposium on Advanced Wound Care. April 2008; San Diego, CA.
- 4.Milne CT, Saucier D, Trevellini C, Smith J: Evaluation of a cyanoacrylate protectant to manage peristomal skin irritation under ostomy skin barrier wafers. Presentation at: Clinical Symposium on Advanced Wound Care. September 2010; Orlando, FL.
- 5.Milne CT, Valk D, Mamrosh M. Evaluation of a cyanoacrylate protectant to manage skin tears in the acute care population. Presentation at: Symposium on Advanced Wound Care. April 2010; Orlando, FL. [PubMed]
- 6.Adelstein S., Bray J., Schram A. Superglue for the treatment of heel fissures. J Am Podiatr Med Assoc. 2008;89(8):434–435. doi: 10.7547/87507315-89-8-434. [DOI] [PubMed] [Google Scholar]
- 7.Coover H.W., McIntire J.M. Cyanoacrylate adhesives. In: Skeist I., editor. Handbook of Adhesives. 2nd ed. Van Nostrand Reinhold Co; New York: 1977. pp. 569–580. [Google Scholar]
- 8.Gary Lee Grove, Charles Zerweck, Danielle Fendrick. Study to compare the wash-off resistance of two barrier films exposed to synthetic urine. cybderDERM clinical studies. November 2008; Broomall, PA.
- 9.Gary Lee Grove, Charles Zerweck, Danielle Fendrick. Abrasion Test. cybderDERM clinical studies. November 2008; Broomall, PA.
- 10.Fleck C.A. From the other side of the bedrail. Healthy Skin. 2010;8(2):47–49. [Google Scholar]
- 11.Payne R.L., Martin M.L. Defining and classifying skin tears: need for a common language. Ostomy Wound Manage. 1993;39(5):16–26. [PubMed] [Google Scholar]
- 12.Visscher M. Update on the use of topical agents in neonates. Newborn & Infant Nurs Rev. 2009;9(1):31–47. [Google Scholar]


