Abstract
Human calciphylaxis reflects a form of severe tissue compromise attributable to a unique microangiopathy that combines features of vascular thrombotic occlusion with endoluminal calcification. While most frequently described in patients with renal failure, it is seen in other settings, such as multiple myeloma; polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome; cirrhosis; and rheumatoid arthritis. Although most commonly involving the skin, calciphylaxis can affect other organs including the heart and gastrointestinal tract, in which cases it falls under the appellation of systemic calciphylaxis. There are cases in which the main pathology is one of endovascular thrombosis of the vessels of the fat without discernible calcification or one manifesting a pseudoangiosarcomatous pattern, hence adding to the histomorphologic spectrum of calciphylaxis. A variety of factors contribute to this severe occlusive microangiopathy, including an underlying procoagulant state and ectopic neo-osteogenesis of the microvasculature through varied mechanisms, including increased osteopontin production by vascular smooth muscle or reduced synthesis of fetuin and GLA matrix protein, important inhibitors of ectopic neo-osteogenesis. Certain factors adversely affect outcome, including truncal and genital involvement and systemic forms of calciphylaxis. With a better understanding of its pathophysiology, more-effective therapies, such as sodium thiosulfate and biphosphanates to reduce reactive oxygen species and receptor activator of nuclear factor κβ-mediated nuclear factor κβ activity, respectively, are being developed.
Keywords: Calciphylaxis, Calcium deposition, Microangiopathy, Skin necrosis
Background
In 1961, Seyle et al coined the term calciphylaxis to characterize a sudden precipitous deposition of calcium in the soft tissue of animals under a unique set of sensitizing factors antedating the calcification (ie, hyperparathyroidism) in concert with subsequent triggers provoking calcium deposition (ie, trauma).1 Although Seyle published in excess of 60 experimental papers on the topic, the first reported case of calciphylaxis was in 1969 by Rees and Cole.2 In 1982 another case of calciphylaxis was described.3
While the body of earlier studies was clearly in the context of experimental calciphylaxis, the term calciphylaxis is now associated with a distinct syndromic complex in humans and one that is pathologically distinct from experimental calciphylaxis. The precipitous calcium deposition is really a vascular phenomenon as opposed to one of extravascular soft tissue calcification, the distribution pattern emphasized in experimental calciphylaxis. In the context of human calciphylaxis, its essence is one of tissue compromise attributable to a unique microangiopathy that combines features of vascular thrombotic occlusion with endoluminal calcification.4–7
Calciphylaxis has fallen under alternative appellations, including calcific uremic arteriolopathy and uremic small artery disease with medial wall calcification and intimal hyperplasia.8,9 However, these latter terms are not preferable in light of its development in clinical settings not limited to renal dysfunction. In particular, most earlier reports are in the context of its occurrence in uremic patients on chronic hemodialysis who at some point in their illness have hyperparathyroidism. There is an emerging body of literature describing calciphylaxis in diverse clinical settings unassociated with renal dysfunction, including cirrhosis; multiple myeloma; and polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome.10-20
Calciphylaxis remains a highly morbid and poorly understood disease of vascular calcification and tissue necrosis, most frequently of the skin. The condition affects 1% to 4% of the population with end-stage renal disease. The mortality rate has been reported to be as high as 60% to 80%, with sepsis and internal organ failure being the leading causes of death. Women are more likely to be affected, with the man-to-woman ratio being 1:3. Affected patients range in age from 6 months to 83 years.4-6,21 In this review we discuss the clinical and pathologic features of calciphylaxis, the proposed pathophysiological basis of the disorder, its treatment, and prognosis.
Clinical Presentation
Clinically, one observes grouped and painful ecchymoses that eventuate into large areas of infarction. The lesions are frequently symmetrical and most commonly involve the lower extremities; a more proximal lower extremity and truncal and genital distribution is associated with a worse prognosis (Figure 1).22-25 The clinical presentation can resemble other ischemic dermopathy syndromes, such as Coumadin skin necrosis and necrotizing fasciitis.26 This condition is not limited to the skin. Ischemia to extracutaneous organs has been described, including the gastrointestinal tract, where massive hemorrhage can occur. The heart can resemble stone where the vascular deposition creates an instant angiogram (ie, a “heart of stone”).27 The term systemic calciphylaxis is used for this multiorgan ischemic vasculopathy syndrome.28-33
Figure 1.
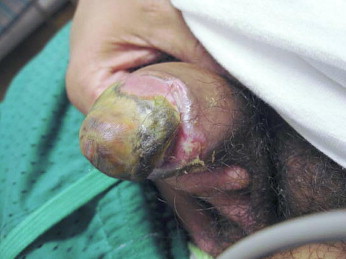
Penile calciphylaxis is very rare but has a mortality rate of 69% within 6 months. There is extensive cutaneous necrosis attributable to the striking microvascular changes integral to calciphylaxis.
While no one laboratory finding is specific for the diagnosis, in those patients with underlying renal failure, elevations in phosphate levels, the calcium-phosphate product, and parathyroid hormone levels can be demonstrated at some point in the patient’s clinical course, albeit not specifically cotemporaneous with the development of calciphylaxis.7
Pathology
The dominant pathology is localized to the subcutaneous fat, whereby one observes mural calcific and fibrous expansion of the intima with luminal thrombosis involving capillaries, venules, arterioles, and small arteries of the subcutaneous fat (Figures 2 and 3). There is variable extension into the overlying dermis, although the dominant changes in the dermis are those reflective of ischemia. In the capillaries of the fat, vascular thrombosis may antedate overt calcification (Figure 4). In addition, there is calcific mummification of the endothelium lining the capillaries and the venules (Figure 5). Such vessels, while showing “stone” endothelium, may be relatively devoid of thrombus. The fully evolved and prototypic microangiopathy is one exhibiting calcified endothelium and vascular thrombosis. Typically there is attendant ischemic change in the adjacent tissue. There can also be a true calcifying interstitial lobular panniculitis somewhat analogous to the extravascular calcification described in Seyle’s original animal model (Figure 6). The vascular changes are best described as a form of calcific thrombogenic microangiopathy.4 The diameter of the affected vessels ranges from 30 to 600 microns, with the average size being approximately 100 microns (Figure 7).34
Figure 2.
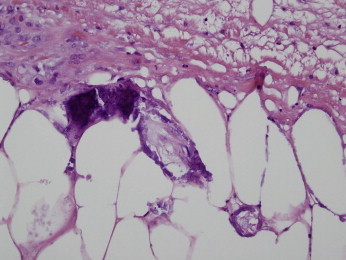
A 57-year-old woman presents with a right hip eschar in the setting of underlying renal failure. The findings are typical for calciphylaxis. In this photomicrograph, the capillaries and venules show an obliterative occlusive calcific microangiopathy. Note the striking calcium deposition within the wall, as well as within the vascular lumens. The endothelial cells have a calcified appearance. (Hematoxylin and Eosin 400x)
Figure 3.
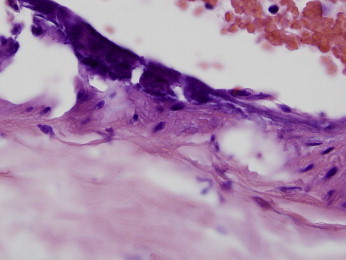
A larger-caliber artery shows calcification. Note how the calcification involves the endothelium as well as the subendothelial intima. (Hematoxylin and Eosin 100x)
Figure 4.
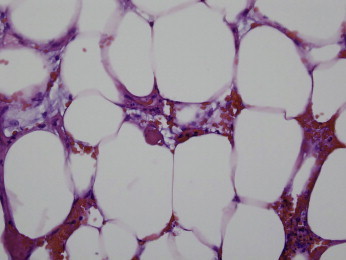
One observes a thrombogenic vasculopathy without discernible calcification. There are cases of calciphylaxis whereby this may be the dominant histopathology. Calciphylaxis is the only condition that we are aware of that can evoke this pattern of pauci-inflammatory thrombosis localized to the subcutaneous fat. In conditions that have skin necrosis, there is usually involvement of the overlying dermis. (Hematoxylin and Eosin 400x)
Figure 5.
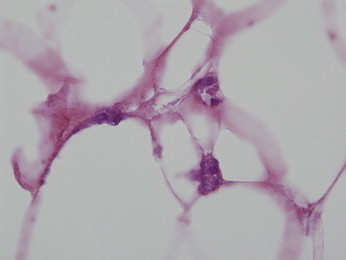
The endothelial cells exhibit a stone-like pattern of calcification. (Hematoxylin and Eosin 100x)
Figure 6.
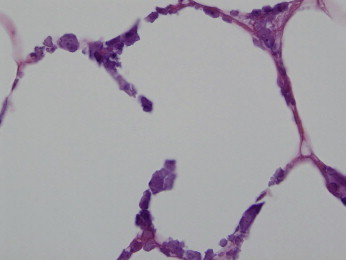
Although the dominant localization of calcification is within the vasculature, there is evidence of extravascular calcification. The interstitial spaces of the fat show calcium deposits defining a form of lobular calcific panniculitis. (Hematoxylin and Eosin 400x)
Figure 7.
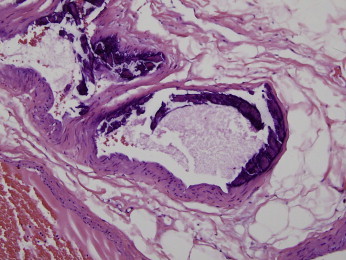
The largest caliber of vessel is in the 500-micron range. Note this larger vessel shows an intimal pattern of calcification with involvement of the endothelium. (Hematoxylin and Eosin 200x)
While the aforesaid features define the classic pathologic changes encountered in calciphylaxis, it should be emphasized that there is a morphologic spectrum. We have encountered cases wherein the main abnormality is in the context of a thrombogenic microangiopathy localized to the subcutis without concomitant or discernible calcification; a von Kossa stain might demonstrate an incipient stippled pattern of microvascular calcification (Figure 8). Such cases differ from other severe ischemic dermopathy syndromes such as Coumadin skin necrosis because of the lack of dermal involvement. There also are cases associated with striking neovascularization imparting an almost pseudoangiosarcomatous morphology.35 The increased vascularity may also reflect an effect of osteopontin, since it has been shown that osteopontin promotes angiogenesis.
Figure 8.
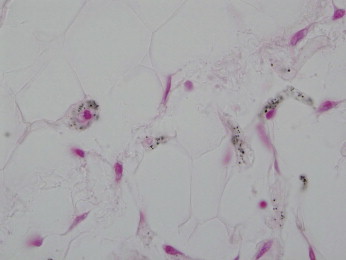
The microvasculature of the fat shows a stippling of calcium not discernible through routine light microscopic assessment but only visible on the von Kossa stain. (Von Kossa stain 100x)
Although calciphylaxis is a form of vascular calcification, vascular calcification per se should not be equated with calciphylaxis. For example, Monckeberg’s medial calcific sclerosis is an innocuous form of dystrophic calcification affecting the media of small and medium arteries; the calcium deposits assume a quiescent pattern of deposition in the wall of the vessel without encroachment on the intima. True endoluminal calcification is not observed, and vascular compromise does not occur (Figure 9).36
Figure 9.
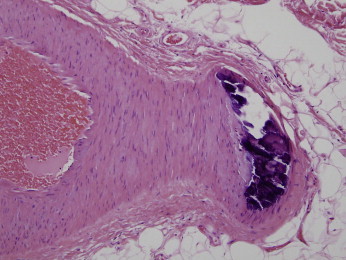
This is a classic lesion of Monckeberg’s medial wall calcific atherosclerosis. Unlike calciphylaxis, the calcium deposition is within the medial wall and is unassociated with vascular compromise. (Hematoxylin and Eosin 200x)
Pathophysiology
Given the striking vascular thrombosis and the occurrence of cases in which the dominant finding morphologically is one of a pauci-inflammatory thrombogenic vasculopathy without calcium deposition, we feel that a procoagulant state is likely integral to the pathogenesis of calciphylaxis. A very recent review of the literature found a strong association between hypercoagulability and calciphylaxis. In cases in which the levels of protein C and S were reported, 38% of the patients had decreased protein C levels, and 43% had decreased levels of protein S. From a review of case reports, three cases of improvement of skin lesions with low-molecular-weight heparin treatment and a fourth case of healing of skin lesions with tissue plasminogen activator treatment were found.37 Calciphylaxis was also found in a patient with antiphospholipid antibody syndrome. In addition, a patient with cryofibrinogenemia had clinical and histologic findings consistent with possible calciphylaxis indicative that a hyperviscosity state could also define a potential risk factor.
While renal disease is a common problem, the rarity of calciphylaxis leaves many questions as to why certain patients develop it. Occasionally there are “triggers” which are thought to induce this syndrome. In the study by Mazhar et al, women were at a 6-fold higher risk of developing calciphylaxis. A reduced risk of developing calciphylaxis was associated with higher levels of serum albumin during the year prior to diagnosis. There was a 3.51-fold increase in the risk of calciphylaxis associated with each 1 mg/dL increase in the mean serum phosphate during the year prior to diagnosis. At the time of diagnosis of calciphylaxis, for each 10 IU/L increment in alkaline phosphatase, the risk of calciphylaxis increased by 19%. Factors that could not consistently be linked with the development of calciphylaxis included body mass index, diabetes, blood pressure, aluminum, and higher dosages of erythropoietin and iron dextran.38
Ectopic production of osteopontin by smooth muscle cells within the vessel wall is likely a very important factor. When smooth muscle cells are exposed to high levels of calcium and/or phosphorus, conversion to an osteogenic phenotype occurs. Osteopontin production is a feature of the osteogenic phenotype. One study showed that only those vessels in which smooth muscle cells expressed osteopontin exhibited calcification.39,40 Studies have shown that the bone-associated protein osteopontin was produced at a higher level by smooth muscle cells when cultured vascular smooth muscle cells were incubated with uremic serum.39 The role of osteopontin in the pathogenesis of calciphylaxis may potentially explain why this condition develops in settings other than renal failure. As already alluded to, calciphylaxis can occur in the setting of inflammatory bowel disease, rheumatoid arthritis, cirrhosis, and myeloma.41 Interestingly, these aforesaid conditions are associated with increased levels of osteopontin.42-44
Tumor necrosis factor α (TNF-α) induces an osteogenic phenotype in human vascular smooth-muscle cells. Interleukin (IL) 1, IL-6, and other cytokines appear to contribute to vascular calcification, independent of TNF-α. In addition, TNF-α, IL-1, and IL-6 levels are increased in patients with liver disease.6 Patients with liver disease have reduced levels of the hepatic-derived fetuin-A and matrix GLA protein (a vitamin K–dependent protein) which are both inhibitors of extraosseous calcification.45 Hence, alternative mechanisms leading to calciphylaxis unrelated to uremia and renal failure could create a microenvironmental milieu conducive to vascular calcification.
Treatment
Treatment options range from hyperbaric oxygen therapy to basic wound care. However, the most crucial aspect in treatment of this disease is successful clinical management of the underlying disease process, namely, renal failure in the majority of cases. A multidisciplinary approach involving several specialists is important, both in understanding the scope of the disease process and in creating a comprehensive treatment plan.
Treatment for calciphylaxis is still experimental. Theoretically, the vascular calcification that causes the ulcerations is reversible with aggressive therapy, although this has not been found to be the case clinically.21 More aggressive measures such as limb amputation may be required. In certain patients, parathyroidectomy has been shown to be beneficial, with patients reporting significant wound healing within months of the surgical procedure.46 Dietary changes such as a reduction in phosphate intake may be useful. In most studies, hyperbaric oxygen as an approach to treatment has had variable results.47 Arch-Ferrer et al observed iron deposition only in affected microvasculature.48 Although no direct correlation could be made, the study linked iron replacement therapy and exposure to oral or intravenous iron prior to the diagnosis of calciphylaxis.
The use of intravenous sodium thiosulfate for chelating calcium and possibly iron has been a treatment that has increased in use very recently.49 The pathogenetic basis of its efficacy is complex but likely relates to its effect on reducing reactive oxygen species, as well as vasodilatory properties through the generation of glutathione and hydrogen sulfide. The reduction of reactive oxygen species is followed by a downregultion in the activation of nuclear factor κβ (NF-κβ) and subsequent downstream cytokines such as IL-1 and IL-6, resulting in reduced inflammation. There also ensues an increase in the synthesis of fetuin-A and matrix GLA protein, both inhibitors of extraosseous calcification, as mentioned earlier.45
Phosphate binders, a low-phosphate diet, low calcium dialysate, and vitamin D analogs all have been used for tight control of hyperparathyroidism. Aggressive wound care with dressing changes, debridement, and negative pressure wound therapy is necessary. Antibiotics for secondary infections, hyperbaric oxygenation, and application of allogenic skin substitutes are further treatment options for the wounds.46-49 Prompt diagnosis with definitive histologic diagnosis is important since many comorbid conditions may present with lesions that appear similar and hence delay appropriate treatment.
As many as 34% of patients with end-stage renal disease have concomitant peripheral vascular disease, and therefore a distinction between ischemic ulcers and the wounds of calciphylaxis must be established.50 Wounds suggestive of calciphylaxis are those that are located proximally, with intact peripheral pulses, no neuropathy, but associated with pain and subcutaneous nodules.50 Identification of arterial occlusive disease with revascularization improves wound healing, and therefore patients diagnosed with calciphylaxis should be evaluated for underlying peripheral vascular disease that may be corrected.51 Therefore, early recognition of the disease and appropriate medical therapy, wound debridement, and correction of underlying vascular disease lead to improved wound healing and limb salvage.26
Parathyroidectomy has consistently been a controversial treatment option, primarily with regard to the extent of the excision. The surgery definitively removes parathyroid hormone and therefore removes the sensitizing agent, leading to decreased calcium-to-phosphate ratios. When the procedure is performed by experienced surgeons, morbidity is low and the cure rates are high.46 Parathyroidectomy has been shown to improve survival in some studies but not in others. Lal et al found no difference in overall survival.51 However, there was a positive correlation with improved survival between those who underwent parathyroidectomy and operative debridement of wounds. A significantly improved survival rate has been shown with those who underwent operative debridement alone. It is recommended that patients undergo total parathyroidectomy without parathyroid tissue implantation, completely eliminating the secretion of parathyroid hormone and allowing for medical treatment of hypoparathyroidism.46 With this treatment option, Girotto et al showed resolution of pain and improved healing of wounds, along with improved mortality. A literature review indicated a significant increase in survival rates with those who underwent parathyroidectomy compared with those who did not undergo surgery.46
Alteration in NF-κβ also plays a key role by its effects of soft tissue mineralization. The receptor activator of NF-κB (RANK) and its ligand (RANKL) are essential for normal bone development, osteoclast differentiation, and bone mineral resorption. Osteoprotegerin is a dummy antagonist of RANKL. RANK, RANKL, and osteoprotegerin are expressed by vascular smooth-muscle cells and endothelial cells. Increased NF-κβ activity causes extraosseous mineral deposition, including involvement of vessels. Inhibition of RANK-mediated NF-κβ activity may prevent vascular calcification. Patients treated with biphosphanates that inhibit RANK-mediated NF-κβ activity may show a significant improvement.6
Prognosis
The prognosis varies according to the site, with proximal involvement (ie, trunk, shoulders, buttocks, and thighs) being associated with considerable morbidity and mortality, while distal lower-extremity skin necrosis is only infrequently associated with death (Figures 10 and 11). A 6-month mortality rate of up to 80% has been reported in those with proximal disease (thighs, abdomen wall, and buttocks). Penile calciphylaxis is very rare but has a mortality rate of 69% within 6 months.
Figure 10.
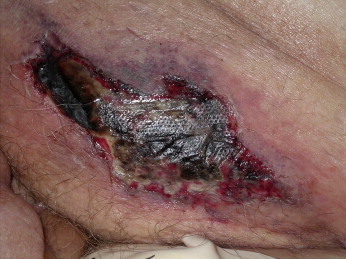
77 year old male on dialysis developed a striking suprapubic sharply demarcated deep ulcer. This type of genital/proximal calciphylaxis is associated with a worse prognosis.
Figure 11.
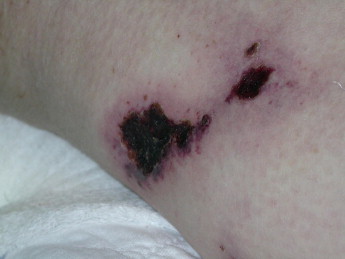
55 year old female with renal disease developed deep well demarcated ulcers on the thigh which was preceded an area of violaceous induration.
In addition, systemic calciphylaxis is associated with higher mortality (Figure 1).28 In one study, factors associated with poorer survival included female gender, increased weight, and the need for vascular procedures. Improved survival was associated with operative debridement. Parathyroidectomy alone did not emerge as a determinant of patient survival.51
Conclusion
Calciphylaxis represents a unique calcific thrombogenic vasculopathy not limited to renal failure. Its basis is complex; however, an underlying procoagulant state and elevations in osteopontin and reactive oxygen species, along with a reduction in hepatic proteins to inhibit extravascular calcification, are likely common pathogenetic triggers. Rather than a single intervention such as parathyroidectomy, a multidisciplinary approach involving early diagnosis, aggressive medical management, operative debridement, and parathyroidectomy may improve survival in calciphylaxis.51
Footnotes
Conflict of interest: The authors report no conflicts of interest.
References
- 1.Seyle H. Calciphylaxis. Allerg Asthma. 1961;7:241–249. [PubMed] [Google Scholar]
- 2.Rees J.K., Coles G.A. Calciphylaxis in man. Br Med J. 1969;2(5658):670–672. doi: 10.1136/bmj.2.5658.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richens G, Piepkorn MW, Krueger GG. Calcifying panniculitis associated with renal failure: a case of Selye’s calciphylaxis in man. J Am Acad Dermatol. 1982l;6(4 Pt 1):537-539. [DOI] [PubMed]
- 4.Wilmer W.A., Magro C.M. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15(3):172–186. doi: 10.1046/j.1525-139x.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhambri A., Del Rosso J.Q. Calciphylaxis: a review. J Clin Aesthetic Dermatol. 2008;1(2):38–41. [PMC free article] [PubMed] [Google Scholar]
- 6.Weenig R.H. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol. 2008;58(3):458–471. doi: 10.1016/j.jaad.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Arseculeratne G., Evans A.T., Morley S.M. Calciphylaxis—a topical overview. J Eur Acad Dermatol Venereol. 2006;20(5):493–502. doi: 10.1111/j.1468-3083.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers N.M., Coates P.T. Calcific uraemic arteriolopathy: an update. Curr Opin Nephrol Hypertens. 2008;17(6):629–634. doi: 10.1097/MNH.0b013e32830f4566. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer K.M., Francis D.M., Hill P.A., Murphy B.F. Calcific uraemic arteriolopathy: local treatment and hyperbaric oxygen therapy. Nephrol Dial Transplant. 2002;17(6):1148–1149. doi: 10.1093/ndt/17.6.1148. [DOI] [PubMed] [Google Scholar]
- 10.Kolli S., Douglas J., Doumit E., Guglin M. Cardiomyopathy and calciphylaxis in a patient with normal renal function: a case report. Congest Heart Fail. 2010;16(2):71–72. doi: 10.1111/j.1751-7133.2009.00121.x. [DOI] [PubMed] [Google Scholar]
- 11.Almafragi A., Vandorpe J., Dujardin K. Calciphylaxis in a cardiac patient without renal disease. Acta Cardiol. 2009;64(1):91–93. doi: 10.2143/AC.64.1.2034368. [DOI] [PubMed] [Google Scholar]
- 12.Hineno A., Kinoshita T., Kinoshita M. Calciphylaxis as a catastrophic complication in a patient with POEMS syndrome. Case Rep Neurol. 2009;1(1):47–53. doi: 10.1159/000259906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couto F.M., Chen H., Blank R.D., Drezner M.K. Calciphylaxis in the absence of end-stage renal disease. Endocr Pract. 2006;12(4):406–410. doi: 10.4158/EP.12.4.406. [DOI] [PubMed] [Google Scholar]
- 14.De Roma I., Filotico R., Cea M., Procaccio P., Perosa F. Calciphylaxis in a patient with POEMS syndrome without renal failure and/or hyperparathyroidism: a case report. Ann Ital Med Int. 2004;19(4):283–287. [PubMed] [Google Scholar]
- 15.Korkmaz C., Dündar E., Zubaroğlu I. Calciphylaxis in a patient with rheumatoid arthritis without renal failure and hyperparathyroidism: the possible role of long-term steroid use and protein S deficiency. Clin Rheumatol. 2002;21(1):66–69. doi: 10.1007/s100670200016. [DOI] [PubMed] [Google Scholar]
- 16.Pollock B., Cunliffe W.J., Merchant W.J. Calciphylaxis in the absence of renal failure. Clin Exp Dermatol. 2000;25(5):389–392. doi: 10.1046/j.1365-2230.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 17.Barri Y.M., Graves G.S., Knochel J.P. Calciphylaxis in a patient with Crohn’s disease in the absence of end-stage renal disease. Am J Kidney Dis. 1997;29(5):773–776. doi: 10.1016/s0272-6386(97)90133-5. [DOI] [PubMed] [Google Scholar]
- 18.Fader D.J., Kang S. Calciphylaxis without renal failure. Arch Dermatol. 1996;132(7):837–838. [PubMed] [Google Scholar]
- 19.Kolli S., Douglas J., Doumit E., Guglin M. Cardiomyopathy and calciphylaxis in a patient with normal renal function: a case report. Congest Heart Fail. 2010;16(2):71–72. doi: 10.1111/j.1751-7133.2009.00121.x. [DOI] [PubMed] [Google Scholar]
- 20.Almafragi A., Vandorpe J., Dujardin K. Calciphylaxis in a cardiac patient without renal disease. Acta Cardiol. 2009;64(1):91–93. doi: 10.2143/AC.64.1.2034368. [DOI] [PubMed] [Google Scholar]
- 21.Naik B.J., Lynch D.J., Slavcheva E.G., Beissner R.S. Calciphylaxis: medical and surgical management of chronic extensive wounds in a renal dialysis population. Plast Reconstr Surg. 2004;113(1):304–312. doi: 10.1097/01.PRS.0000095955.75346.6E. [DOI] [PubMed] [Google Scholar]
- 22.Bleyer A.J., Choi M., Igwemezie B., de la Torre E., White W.L. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32(3):376–381. doi: 10.1053/ajkd.1998.v32.pm9740152. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu G., Gini M.B., Ranade A., Djebali D., Smith S. Penile calciphylaxis: a life-threatening condition successfully treated with sodium thiosulfate. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181e3b0f2. Jul 10. [DOI] [PubMed] [Google Scholar]
- 24.Ruggian J.C., Maesaka J.K., Fishbane S. Proximal calciphylaxis in four insulin-requiring diabetic hemodialysis patients. Am J Kidney Dis. 1996;28(3):409–414. doi: 10.1016/s0272-6386(96)90499-0. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal M.M., Singh S.K., Mandal A.K. Penile gangrene in diabetes mellitus with renal failure: a poor prognostic sign of systemic vascular calciphylaxis. Indian J Urol. 2007;23(2):208–210. doi: 10.4103/0970-1591.32081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milas M., Bush R.L., Lin P. Calciphylaxis and non-healing wounds: the role of the vascular surgeon in a multidisciplinary treatment. J Vasc Surg. 2003;37(3):501–507. doi: 10.1067/mva.2003.70. [DOI] [PubMed] [Google Scholar]
- 27.Tom C.W., Talreja D.R. Heart of stone. Mayo Clin Proc. 2006;81(3):335. doi: 10.4065/81.3.335. [DOI] [PubMed] [Google Scholar]
- 28.Suryadevara M., Schurman S.J., Landas S.K. Systemic calciphylaxis. Pediatr Blood Cancer. 2008;51(4):548–550. doi: 10.1002/pbc.21631. [DOI] [PubMed] [Google Scholar]
- 29.Li Y.J., Tian Y.C., Chen Y.C. Fulminant pulmonary calciphylaxis and metastatic calcification causing acute respiratory failure in a uremic patient. Am J Kidney Dis. 2006;47(4):e47–53. doi: 10.1053/j.ajkd.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Rivera-Nieves J., Bamias G., Alfert J., Bickston S.J., Moskaluk C.A., Cominelli F. Intestinal ischemia and peripheral gangrene in a patient with chronic renal failure. Gastroenterology. 2002;122(2):495–499. doi: 10.1053/gast.2002.31387. [DOI] [PubMed] [Google Scholar]
- 31.Katsamakis G., Lukovits T.G., Gorelick P.B. Calcific cerebral embolism in systemic calciphylaxis. Neurology. 1998;51(1):295–297. doi: 10.1212/wnl.51.1.295. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K. Systemic calciphylaxis. Intern Med. 1995;34(9):822–823. doi: 10.2169/internalmedicine.34.822. [DOI] [PubMed] [Google Scholar]
- 33.Lowry L.R., Tschen J.A., Wolf J.E., Yen A. Calcifying panniculitis and systemic calciphylaxis in an end-stage renal patient. Cutis. 1993;51(4):245–247. [PubMed] [Google Scholar]
- 34.Essary L.R., Wick M.R. Cutaneous calciphylaxis: an underrecognized clinicopathologic entity. Am J Clin Pathol. 2000;113(2):280–287. doi: 10.1309/AGLF-X21H-Y37W-50KL. [DOI] [PubMed] [Google Scholar]
- 35.Prinz Vavricka B.M., Barry C., Victor T., Guitart J. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31(7):653–657. doi: 10.1097/DAD.0b013e3181a59ba9. [DOI] [PubMed] [Google Scholar]
- 36.Lachman A.S., Spray T.L., Kerwin D.M., Shugoll G.I., Roberts W.C. Medial calcinosis of Mönckeberg: a review of the problem and a description of a patient with involvement of peripheral, visceral and coronary arteries. Am J Med. 1977;63(4):615–622. doi: 10.1016/0002-9343(77)90207-8. [DOI] [PubMed] [Google Scholar]
- 37.Harris R.J., Cropley T.G. Possible role of hypercoagulability in calciphylaxis: review of the literature. J Am Acad Dermatol. 2011;64(2):405–412. doi: 10.1016/j.jaad.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Mazhar A.R., Johnson R.J., Gillen D. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60(1):324–332. doi: 10.1046/j.1523-1755.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen N.X., O’Neill K.D., Duan D., Moe S.M. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62(5):1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed S., O’Neill K.D., Hood A.F., Evan A.P., Moe S.M. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37(6):1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 41.Vaskuring V., Vaskuring J. The development of the pathophysiological concept of calciphylaxis in experiment and clinic. Pathophysiology. 2001;7(4):231–244. doi: 10.1016/s0928-4680(00)00064-x. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y., Abe M., Hiasa M. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007;13(3):816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- 43.Robbiani D.F., Colon K., Ely S., Ely S., Chesi M., Bergsagel P.L. Osteopontin dysregulation and lytic bone lesions in multiple myeloma. Hematol Oncol. 2007;25(1):16–20. doi: 10.1002/hon.803. [DOI] [PubMed] [Google Scholar]
- 44.Colla S., Morandi F., Lazzaretti M. Human myeloma cells express the bone regulating gene Runx2/Cbfa1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia. 2005;19(12):2166–2176. doi: 10.1038/sj.leu.2403976. [DOI] [PubMed] [Google Scholar]
- 45.Hayden M.R., Tyagi S.C., Kolb L., Sowers J.R., Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4(1):4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girotto J.A., Harmon J.W., Ratner L.E., Nicol T.L., Wong L., Chen H. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130(4):645–650. doi: 10.1067/msy.2001.117101. discussion 650–651. [DOI] [PubMed] [Google Scholar]
- 47.Alikadic N., Kovac D., Krasna M. Review of calciphylaxis and treatment of a severe case after kidney transplantation with iloprost in combination with hyperbaric oxygen and cultured autologous fibrin-based skin substitutes. Clin Transplant. 2009;23(6):968–974. doi: 10.1111/j.1399-0012.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 48.Arch-Ferrer J.E., Beenken S.W., Rue L.W., Bland K.I., Diethelm A.G. Therapy for calciphylaxis, an outcome analysis. Surgery. 2003;134(6):941–944. doi: 10.1016/j.surg.2003.07.001. discussion 944–945. [DOI] [PubMed] [Google Scholar]
- 49.Cicone J.S., Petronis J.B., Embert C.D., Spector D.A. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43(6):1104–1108. doi: 10.1053/j.ajkd.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Fischer A.H., Morris D.J. Pathogenesis of calciphylaxis: study of three cases with literature review. Hum Pathol. 1995;26(10):1055–1064. doi: 10.1016/0046-8177(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 51.Lal G., Nowell A.G., Liao J., Sugg S.L., Weigel R.J., Howe J.R. Determinants of survival in patients with calciphylaxis: a multivariate analysis. Surgery. 2009;146(6):1028–1034. doi: 10.1016/j.surg.2009.09.022. [DOI] [PubMed] [Google Scholar]


