Abstract
Collagen, which is produced by fibroblasts, is the most abundant protein in the human body. A natural structural protein, collagen is involved in all 3 phases of the wound-healing cascade. It stimulates cellular migration and contributes to new tissue development. Because of their chemotactic properties on wound fibroblasts, collagen dressings encourage the deposition and organization of newly formed collagen, creating an environment that fosters healing. Collagen-based biomaterials stimulate and recruit specific cells, such as macrophages and fibroblasts, along the healing cascade to enhance and influence wound healing. These biomaterials can provide moisture or absorption, depending on the delivery system. Collagen dressings are easy to apply and remove and are conformable. Collagen dressings are usually formulated with bovine, avian, or porcine collagen. Oxidized regenerated cellulose, a plant-based material, has been combined with collagen to produce a dressing capable of binding to and protecting growth factors by binding and inactivating matrix metalloproteinases in the wound environment. The increased understanding of the biochemical processes involved in chronic wound healing allows the design of wound care products aimed at correcting imbalances in the wound microenvironment. Traditional advanced wound care products tend to address the wound’s macroenvironment, including moist wound environment control, fluid management, and controlled transpiration of wound fluids. The newer class of biomaterials and wound-healing agents, such as collagen and growth factors, targets specific defects in the chronic wound environment. In vitro laboratory data point to the possibility that these agents benefit the wound healing process at a biochemical level. Considerable evidence has indicated that collagen-based dressings may be capable of stimulating healing by manipulating wound biochemistry.
Keywords: Collagen, Dressing, Fibroblasts, Matrix, Wound healing
Introduction
Collagen, which is produced by fibroblasts, is the most abundant protein in the human body. A natural structural protein, collagen is involved in all 3 phases of the wound-healing cascade. It stimulates cellular migration and contributes to new tissue development.1 Collagen dressings encourage the deposition and organization of newly formed collagen, creating an environment that fosters healing because of the dressings’ chemotactic properties on wound fibroblasts. Collagen-based biomaterials stimulate and recruit specific cells, such as macrophages and fibroblasts, along the healing cascade in order to enhance and influence wound healing. These biomaterials can provide moisture or absorption, depending on the delivery system. Collagen dressings are easy to apply and remove and are conformable. Collagen dressings are usually formulated with bovine, avian, or porcine collagen. Oxidized regenerated cellulose, a plant-based material, has been combined with collagen to produce a dressing capable of binding to and protecting growth factors by binding and inactivating matrix metalloproteinases in the wound environment.2
The increased understanding of the biochemical processes involved in chronic wound healing allows the design of wound care products aimed at correcting imbalances in the wound microenvironment. Traditional advanced wound care products tend to address the wound’s macroenvironment, including moist wound environment control, fluid management, and controlled transpiration of wound fluids. The newer class of biomaterials and wound-healing agents, such as collagen and growth factors, targets specific defects in the chronic wound environment. In vitro laboratory data point to the possibility that these agents benefit the wound-healing process at a biochemical level. Considerable evidence has indicated that collagen-based dressings may be capable of stimulating healing by manipulating wound biochemistry.3
Collagen Formation in the Human Body
Collagen, which constitutes 25% of the total protein mass of mammals,4,5 is in the bones, connective tissue, tendons, blood vessels, and skin. In the skin, collagen, in conjunction with other proteins, such as elastin, forms the basic flexible and pliable matrix that incorporates living dermal cells, blood vessels, sebaceous glands, and other components of the extracellular matrix (glycosaminoglycans, glycoproteins).4,5
Each collagen molecule in the dermal extracellular matrix is formed from 3 helical protein chains assembled into a super, triple-helical procollagen molecule inside a cell (fibroblast) and then secreted into the extracellular space (Figure 1). In the extracellular space, the chains undergo proteolytic processing, allowing them to spontaneously assemble into larger, more complex fibrils that are stabilized by covalent cross-links. The large, cross-linked bundles of collagen (Figure 1) provide the tensile strength of skin that supports all the specialized structures in skin. The maintenance of the various superstructures of collagen in the body preserves the natural functions of the organs that use collagen as a structural protein. Considerable scientific evidence demonstrates that the chemotactic properties of collagen depend on the retention of its native structure. Fibroblasts, for example, possess integrin receptors that recognize domains of intact, native collagen and fibronectin molecules.6
Figure 1.
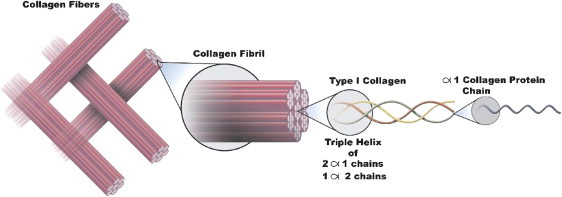
Organized Fiber Bundles Constituting Collagen.
The Role of Collagen and Use in Chronic Wounds
In the chronic wound, the deposition of de novo collagen is delayed or prevented by numerous factors.7 Recruitment of fibroblasts, the cells dominant in the proliferative phase of normal wound healing, is retarded.8 In addition, the expression of the collagen gene in fibroblasts is suppressed.9 Environmental factors also affect the collagen level in the chronic wound bed. Among these factors are 2 classes of enzymes, whose levels are known to be elevated in chronic wounds: the matrix metalloproteinases (MMPs)10 and elastase.2 MMPs are implicated in proteolytic degradation of native intact collagen and partially degraded fragments of collagens. The dynamic creation and destruction of collagen components are normal events in acute wound healing, and MMPs also have a role to play in normal metabolic processes in the skin. However, in the chronic wound, MMP levels are abnormally elevated.11-14 Moreover, a key set of regulating enzymes, the tissue inhibitors of matrix metalloproteinases, are found at lower-than-normal levels.15
The elevated ratio of MMPs to tissue inhibitors of MMPs leads to excessive extracellular matrix degradation.16 Elastase is also involved in the healing process. The primary substrate of the enzyme is the extracellular matrix protein elastin, which contributes to the elasticity of dermal tissue. Elastase activity is high in the chronic wound. Elastase acts to convert pro-MMPs (the natural precursor of MMPs) to active MMPs.17 In doing so, elastase contributes heavily to the MMP load in the chronic wound.18 Elastase, being a relatively nonspecific protease, can also bind to native collagen and degrade it.19 Results from laboratory experiments demonstrate that the affinity of elastase for the triple helix domain of native collagen is substantial; thus, dressings containing native collagen will act as a substrate magnet for elastase, as well as MMPs. In summary, the chronic wound is characterized by both decreased collagen deposition and increased collagen breakdown. The complex vicious cycle of wound chronicity is shown in Figure 2 in a very simplified and schematic manner. Elastase is shown to play a key role in perpetuating the vicious cycle. This figure makes clear that removing elastase essentially removes the hub of this vicious cycle, potentially terminating the chronic state of the wound.
Figure 2.
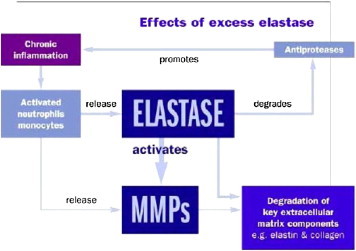
Effect of Elastase on Chronic Wounds. MMPs, matrix metalloproteinases.
Collagen’s Effect on the Brutal Cycle of Chronic Wounds
It is reasonable to speculate on the biochemical reasons that collagen dressings can potentially promote wound healing. For one, collagen can seriously reduce elastase levels in a wound environment, disrupting the cycle of chronicity shown in Figure 2. In addition, the MMP-binding activity of collagen is benevolently disruptive to the biochemically stalled chronic wound. This MMP-binding activity is easily detected in in vitro assays of all collagen-based products.20 Several collagen-derived materials have been used in recent years as wound dressings. Products based on extensively and aggressively processed collagen sources would tend to have a preponderance of denatured collagen, defined as collagen that has largely lost its triple helical basic unit structure. Some of the newer products have perhaps been produced through newer, gentler extraction or purification technologies. Such new-generation products tend to possess higher quantities of the native triple helix structural unit characteristic of the collagen in living organisms. Further evidence demonstrates numerous advantages to the retention of the native structure of collagen.21 For example, native collagen allows for more efficient angiogenesis22 and greater fibroblast chemotaxis. In addition to collagen’s molecular properties, its gross microstructure at cellular dimensional levels may be important. Fibroblasts and macrophages function best when anchored in a 3-dimensional architectural structure.23,24 Collagen-based substrates with a microstructure whose internal porosity and scale allow the infiltration and anchorage of these key cells may allow the promotion of natural wound healing. The microstructure in a recently introduced collagen is shown in Figure 3.
Figure 3.
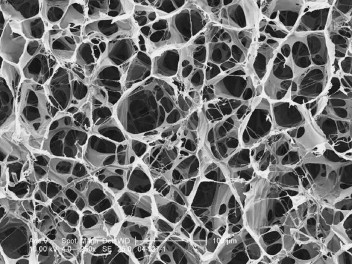
Microstructure in a Second-Generation Collagen Product.
Native Collagen vs Denatured Collagen in Wound Healing
It is reasonable to expect that some of the benefits that native collagen may bestow on a wound dressing may be lost if the collagen was to be largely denatured in the manufacturing process. From the point of view of nomenclature, such denatured collagen may be more accurately described as gelatin, and it appears that in the field of wound dressings, the name collagen is somewhat loosely applied because gelatin-based dressings are sometimes marketed as collagen dressings. Aside from the problem with nomenclature, gelatin or gelatin-like, highly processed collagen would appear to be unable to elicit some of the biological responses native collagen nature has programmed into cells involved in wound healing.
Development From Procollagen Chains to Complex Fiber Bundles
Formation of procollagen chains in the primary structure (amino acid sequence) is a unique process. Collagen molecules consist of 3 protein chains, each of which is a long, rodlike, left-handed helix (similar to a spiral staircase). Glycine, the smallest of the amino acids, is the third amino acid in the chains; such spacing allows the procollagen chains to form their characteristic spiral helix structure. Three procollagen protein chains spontaneously assemble into a right-handed triple helix, forming a single procollagen molecule. The helix is maintained in this critical conformation by interchain hydrogen bonding. Procollagen molecules are secreted into the extracellular space where propeptide sequences are removed by specific enzymes, generating active collagen molecules that can assemble into higher-ordered structures. The formation of organized fiber bundles is an intricate process. Many triple-helix collagen molecules assemble in a laterally staggered, repeating pattern in head-to-tail alignment and become chemically cross-linked with each other to form stable fibrils that are visible in electron microscopy. Collagen fibrils assemble into larger fibers that further associate into complex superstructures of organized fiber bundles.4 See Figures 1, 2, and 3.
New generations of collagen-based dressings have tended to combine properties of stimulation of wound healing with absorption of moderate to high levels of wound drainage. Such dual functionality is possible by adding alginate to the product, for example, although some pure collagen dressings seem absorbent enough to be described as “absorbent.” Many pure collagen or denatured collagen dressings combine collagen with silver to decrease the bioburden in the dressing. Some new products in the collagen dressing category seem to present a remarkably pure form of collagen, which could conceivably lead to less giant cell type of reaction in the wound bed due to the lack of non-biodegradable materials (such as plant-based or marine-based cellulose derivatives). Denatured or degraded collagen (more accurately described as gelatin), especially when mixed with noncollagenous substances, is expected to degrade faster than a native collagen dressing in a wound environment because of its inherently modified and processed nature. Crosslinking through the use of chemical crosslinking reagents is a conceivable method to improve the degradation rate of gelatin (denatured collagen) products. From a biochemical cellular response perspective, such artificial chemical methods do not improve, and may actually deteriorate, the nature of a material that is meant to be as close in chemical structure to its natural source as possible. The presence of a minimally degraded native collagen is expected to allow a collagen dressing to last longer (compared with denatured dressings) in the wound bed because of the longer time that wound proteinases take in digesting a native protein vs a denatured protein. Such a property may lead to a higher longevity of the dressing in the wound bed, associated of course with a longer period of biological action in the wound bed. The resulting reduced frequency in dressing changes may lessen trauma associated with dressing changes and also be more cost-effective.
When choosing a collagen dressing, consider the type of collagen it contains, the way the dressing has been manufactured, and how the dressing works to provide a reduction of proteolytic enzymes and a scaffold for healing. Collagen dressings can provide anti-infective, anti-inflammatory, antifibrotic, and analgesic properties, as well as promote angiogenesis, returning the body to its normal state and function and providing a foundation for wound healing.
Footnotes
Conflict of interest: Cynthia Fleck is an employee of Medline Industries, Inc. Richard Simman has no financial interest with any company mentioned here.
References
- 1.Ayello E.A., Baranoski S., Kerstein M.D., Cuddigan J. Wound treatment options. In: Baranoski S., Ayello E.A., editors. Wound Care Essentials: Practice Principles. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. p. 138. [Google Scholar]
- 2.Cullen B., Smith R., McCullough E. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16–25. doi: 10.1046/j.1524-475x.2002.10703.x. [DOI] [PubMed] [Google Scholar]
- 3.Fleck C.A., Chakravarthy D. Understanding the mechanisms of collagen dressings. Advin Skin Wound Care. 2007;20(5):256–259. doi: 10.1097/01.ASW.0000269310.00145.e2. [DOI] [PubMed] [Google Scholar]
- 4.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Water P. 4th ed. Garland Science; New York, NY: 2002. Molecular Biology of the Cell. [Google Scholar]
- 5.Lodish H., Berk A., Zipurski L., Matsudaira P., Baltimore D., Damell J. 4th ed. H.W. Freeman; New York, NY: 2000. Molecular Cell Biology. [Google Scholar]
- 6.Greiling D., Clark R. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110(Pt 7):861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 7.Herrick S.E., Ireland G.W., Simon D., McCollum C.N., Ferguson M.W. Venous ulcer fibroblasts compared with normal fibroblasts show differences in collagen but not fibronectin production under both normal and hypoxic conditions. J Invest Dermatol. 1996;106:187–193. doi: 10.1111/1523-1747.ep12329920. [DOI] [PubMed] [Google Scholar]
- 8.Falanga V. Chronic wounds: pathophysiologic and experimental considerations. J Invest Dermatol. 1993;100:721–725. doi: 10.1111/1523-1747.ep12472373. [DOI] [PubMed] [Google Scholar]
- 9.Hansen S.L., Young D.M., Boudreau N.J. HoxD3 expression and collagen synthesis in diabetic fibroblasts. Wound Repair Regen. 2003;11:474. doi: 10.1046/j.1524-475x.2003.11615.x. [DOI] [PubMed] [Google Scholar]
- 10.Yagar D., Nwomeh B. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433–441. doi: 10.1046/j.1524-475x.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Wysocki A.B., Staiano-Coico L., Ginnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 ad MMP-9. J Invest Dermatol. 1993;101:64–65. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 12.Schultz G.S., Mast B.A. Molecular analysis of the environment of healing and chronic wounds: cytokines, proteases, and growth factors. Wounds. 1998;10(6 Suppl F):1F–9F. [Google Scholar]
- 13.Kirsner R., Katz M., Eaglstein W., Falanga V. The biology of wound fluid. Wounds. 1993;3:122–128. [Google Scholar]
- 14.Palolahti M., Lauharanta L., Stephens R.W., Kuusela P., Vaheri A. Proteolytic activity in leg ulcer exudates. Exp Dermatol. 1993;2:9–37. doi: 10.1111/j.1600-0625.1993.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig G.P., Robson M.C., Liu R., Kuhn M.A., Muir D.F., Schultz G.S. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely co-related with healing of pressure ulcers. Wound Repair Regen. 2003;10:26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 16.Trengrove N.J., Stacey M.C., Macauley S. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–452. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y.K., Liu X.D., Skold C.M. Synergistic neutrophil elastase-cytokine interaction degrades collagen in three-dimensional culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L868–L878. doi: 10.1152/ajplung.2001.281.4.L868. [DOI] [PubMed] [Google Scholar]
- 18.Konig M., Peschen M., Vanscheidt W. Molecular biology of chronic wounds. Curr Probl Dermatol. 1999;27:8–12. doi: 10.1159/000060629. [DOI] [PubMed] [Google Scholar]
- 19.Kafienah W., Buttle D.J., Burnett D., Hollander A.P. Cleavage of native type I collagen by human neurophil elastase. Biochem J. 1998;330(Pt 2):897–902. doi: 10.1042/bj3300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey A. Perspective article: the fate of collagen implants in tissue defects. Wound Repair Regen. 2000;8:5–12. doi: 10.1046/j.1524-475x.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 21.Horch R.E., Debus M., Wagner G., Stark G.B. Cultured human keratinocytes on Type I collagen membranes to reconstitute the epidermis. Tissue Eng. 2000;6:53–67. doi: 10.1089/107632700320892. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney S.M., DiLullo G., Slater S. Angiogenesis in collagen I requires alpha2beta1ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J Biol Chem. 2003;278:30516–30524. doi: 10.1074/jbc.M304237200. [DOI] [PubMed] [Google Scholar]
- 23.Lamme E.N., Van Leeuwen R.T., Jonker A., Marle J., Middelkoop E. Living skin substitutes: survival and function of fibroblasts seeded in a dermal substitute in experimental wounds. J Invest Dermatol. 1998;111:989–995. doi: 10.1046/j.1523-1747.1998.00459.x. [DOI] [PubMed] [Google Scholar]
- 24.Griffith L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann N Y Acad Sci. 2002;961:83–95. doi: 10.1111/j.1749-6632.2002.tb03056.x. [DOI] [PubMed] [Google Scholar]


