Abstract
STIM (stromal interaction molecule) and Orai, two recently identified protein families, mediate cellular Ca2+ signals through a remarkably dynamic interaction. STIM proteins are sensors of Ca2+ stored within the endoplasmic reticulum (ER). Orai proteins are highly selective plasma membrane (PM) channels that allow only Ca2+ ions to flow into cells. Although present in separate membranes, the two proteins undergo profound reorganization culminating in an exquisite pas de deux within small junctional regions between the ER and PM. Before these proteins can embrace, STIM undergoes an activation process triggered by depletion of Ca2+ stores. During its union with Orai, STIM induces the channel pore within Orai to open, allowing Ca2+ ions to flow through the PM and provide crucial intracellular signals. Recent studies on the activation of STIM and its coupling to Orai provide valuable new insights into the nature of the liaison between these two proteins and the intricate mechanism through which activation of Ca2+ signals occurs.
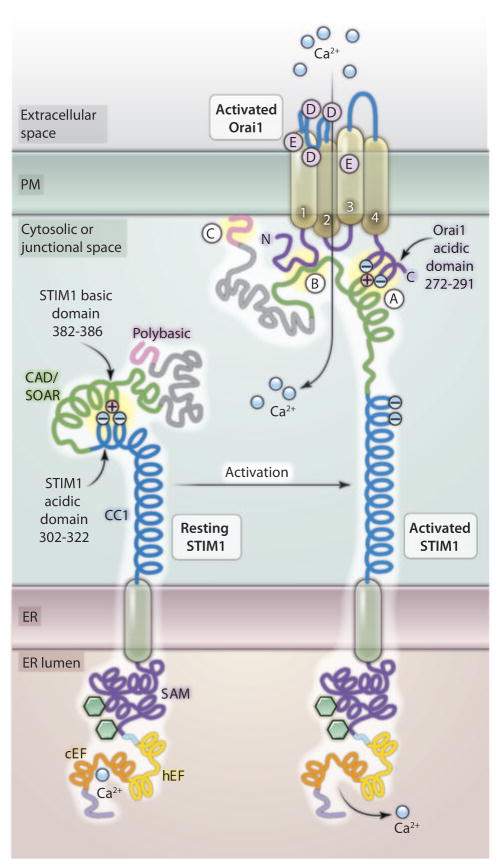
Ca2+ signals are crucial for controlling a spectrum of cellular functions, ranging from rapid responses such as secretion, neural excitation, and muscle contraction to longer-term responses such as transcription, cell division, and apoptosis (1, 2). The endoplasmic reticulum (ER) Ca2+ stores within cells are a crucial source of Ca2+ from which to generate signals. These signals are mediated by activation of Ca2+ release channels, after which it is essential to replenish the Ca2+ stored within the ER lumen. Luminal Ca2+ is also critical for maintaining the correct protein-folding environment inside the ER. Decreased Ca2+ within the ER triggers a process known as store-operated Ca2+ entry (SOCE), in which Ca2+ channels in the PM are activated to allow entry of Ca2+ from outside the cell (3–5). In addition to replenishing stores, the entering Ca2+ provides longer-term and spatially restricted Ca2+ signals in most cells (3–7) to control such processes as early gene transcription (8, 9). Although SOCE was originally described in 1986 (10), its molecular mediators were unknown until recently. The ER membrane proteins STIM1 and STIM2 (stromal interaction molecule 1 and 2) were the first proteins identified as required components of SOCE (11, 12); this discovery was followed a year later by identification of the family of tetra-membrane–spanning membrane proteins Orai1, Orai2, and Orai3 as additional essential components (13–15). The roles of these proteins are now well understood. STIM1 (and STIM2) functions as an ER Ca2+ sensor through a low-affinity EF-hand Ca2+ binding site (Fig. 1). In response to decreased ER Ca2+ concentrations, STIM1 aggregates and translocates to form discrete PM-associated junctions where it physically associates with and activates Orai1 or other Orai channels, which are highly Ca2+-selective channels located exclusively in the PM (3–7) (Fig. 1)
Fig. 1.
Molecular architecture of STIM-Orai interactions. In its resting state, acidic residues of the cytosolic coiled-coil domain (CC1) of STIM1 electrostatically bind to and mask the basic residues of the active site of the CAD/SOAR domain. The transition to the activated state of STIM1 involves conformational changes on both the luminal and cytosolic sides of the protein. Decreased ER luminal Ca2+ causes dissociation of Ca2+ from the tight complex formed by the canonical (cEF) and hidden (hEF) EF-hands and the sterile α motif (SAM) on the luminal side of STIM1. This causes interactions between STIM1 molecules (see Fig. 2) and alteration in the cytosolic domain of STIM1 such that the intramolecular electrostatic interactions between CC1 and CAD/SOAR are broken and the CAD/SOAR domain positive charges are free to interact with the acidic domain within the C-terminal domain of Orai1 and activate the channel (“A”). The STIM1 protein also appears to interact with the N-terminal domain of Orai1 (“B”), although the nature of this interaction is not known. In addition, the far C-terminal polybasic sequence of STIM1 interacts with the plasma membrane (“C”), likely with negatively charged phospholipids.
Although the roles of STIM1 and Orai1 are clear, the precise nature of their interaction remains a topic of intense investigation. Korzeniowski et al. (16) provide important information on the molecular mechanism of interaction between these two proteins. It has become clear that the cytosolic domain of STIM1 contains all the crucial machinery necessary for interaction with and activation of the Orai1 channel. Despite this, expression of just the cytosolic C-terminal portion of STIM1 (STIM-ct, amino acids 238 to 685) results in poor activation of Orai1 (17–19). However, the efficiency of Orai1 activation is greatly improved by expressing the much smaller CRAC activating domain (CAD; amino acids 342 to 448) (18) or STIM1 Orai activating region (SOAR; amino acids 344 to 442) (20) of the STIM1 protein. One implication of the greater effectiveness of the shortened protein is that there could be an inhibitory site somewhere within the cytosolic portion of STIM1, but outside the CAD/SOAR domain, perhaps a site that masks a segment key to the interaction with Orai1. Korzeniowski et al. reasoned that such an inhibitory site might resemble a region on the Orai1 protein that interacts with STIM1 (16). One region of Orai1 known to be important for STIM1 interaction is the short coiled-coil C-terminal cytoplasmic domain (Fig. 1) (3, 6, 7, 21). Indeed, a tight group of acidic residues between Glu272 and Asp291 in this region of Orai1 was identified by Calloway et al. (22) as being crucial for interactions with STIM1 following store depletion. Korzeniowski et al. (16) noticed that several of these acidic residues in Orai1 align well with a short sequence of acidic amino acids (Glu302 to Glu308) toward the C-terminal end of the first coiled-coil region (CC1) of the cytoplasmic domain of STIM1 (Fig. 1) and that there was another nearby group of four acidic residues (Glu318 to Glu322). Korzeniowski et al. created mutant forms of STIM1-ct and whole STIM1 in which the acidic residues in either or both of these short acidic sequences were changed to alanines. They found that the mutated STIM-ct was transformed from a poor activator of Ca2+ entry through Orai1 channels to one that constitutively activated much higher Ca2+ entry. Similarly, the mutated whole STIM1 molecule constitutively activated Orai1 and remained tightly clustered with Orai channels without store depletion. The “acid test” of the action of these acidic sequences came in experiments in which expression of a portion of STIM1 (amino acids 238 to 343) containing these acidic residues was shown to interfere with the ability of the highly active CAD/SOAR domain (amino acids 315 to 462) to activate Orai1 channels, strongly supporting the role of the acidic domains in the STIM1-CC1 segment in inhibiting STIM1 basal activity.
In the model proposed by both Korzeniowski et al. (16) and Calloway et al. (22), an electrostatic interaction between STIM1 and Orai is required for their coupling. The identification of a series of basic residues within the CAD/SOAR domain of STIM1 (amino acids 382 to 386), mutation of which blocks the action of STIM1, STIMct, or the active CAD/SOAR fragment from activating Orai1, provided strong support for this electrostatic interaction model. The importance of the 382 to 386 basic region of STIM1 was also highlighted in another recent paper (23). This study showed that despite mutation of both the Orai1 acidic sequence and the STIM1 basic sequence, the two proteins could still interact as revealed by FRET (Förster resonance energy transfer) analysis (23). This indicates that another region is involved in “gripping” between the two molecules, whereas the activating “union” of the proteins resulting in channel opening is mediated by the electrostatic interactions. Both sites are shown in Fig. 1. The elegant studies of Park et al. (18) revealed that interactions of the CAD sequence occur at both the N- and C-terminal cytoplasmic domains of Orai1, suggesting that the additional site likely involves the Orai1 N-terminal domain. This second site on the Orai1 N terminus is likely close to the first transmembrane-spanning domain because the first 73 N-terminal amino acids of Orai1 appear to be unnecessary for function (18). In addition, the polybasic residues at the far C-terminal tail of STIM1 interact with the PM itself (18, 24, 25) and can assist in the STIM-Orai docking process by directing STIM1 to interact with the PM, and there be available to trap and activate nearby Orai channels. This polybasic lysine-rich area is not involved in interactions with the acidic domain in STIM1 (23).
STIM1 therefore has three possible sites to interact with Orai1 or the PM: one involved in an electrostatic activating interaction with the Orai1 C terminus (“A” in Fig. 1); a distinct site through which it interacts with the Orai1 N terminus (“B” in Fig. 1); and the polybasic region, which mediates a direct interaction with the PM (“C” in Fig. 1), perhaps involving acidic phospholipids. From the work of Korzeniowski et al. (16), it appears that activation of STIM1 following store depletion involves the dissociation of inhibitory electrostatic interactions between the CC1 and CAD/SOAR regions of STIM1, resulting in exposure of the CAD/SOAR acidic domain. This allows the CAD/SOAR acidic domain to interact with the basic sequences on the Orai1 C terminus to activate the channel. In the STIM1 resting state, these acidic residues would remain hidden, suggesting that STIM1 has an autoinhibitory intramolecular regulatory mechanism.
Although the formation of junctions between the ER and PM would seem like a structurally complex process, the interaction between STIM and Orai appears to require no additional components. Indeed, Korzeniowski et al. (16) showed that a STIM1-ct domain directed to the surface of mitochondria in Orai1-expressing cells, rather than to the ER, could still interact with and activate Orai channels. The ability of STIM1 to form PM junctions and actively couple to Orai from a distinct organelle with few if any similar proteins suggests that STIM and Orai function independently of other proteins, a conclusion supported by recent elegant yeast expression studies (26).
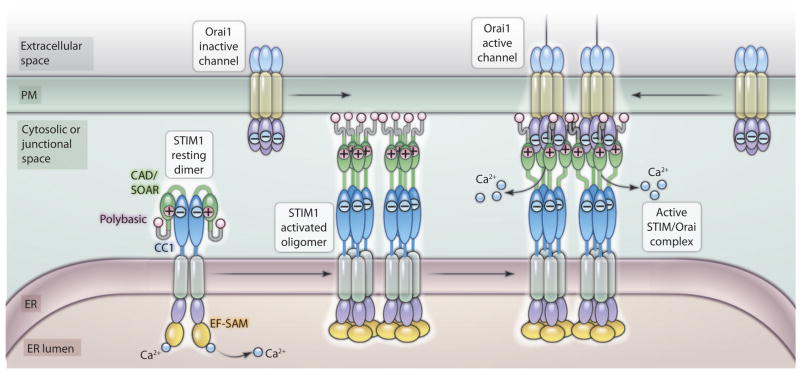
Just how STIM1 activation occurs in response to decreased Ca2+ within the ER remains unclear. We provide a model depicting this process in Fig. 2. The triggering of STIM1 to activate Orai channels involves a complex process of aggregation that depends on both its luminal Ca2+-sensing N-terminal domain and its C-terminal cytoplasmic domains. The STIM1 N terminus has a tight complex of domains, including tandem EF-hand sequences (one of which binds Ca2+) that can sense small decreases in luminal Ca2+ (3–5) and a tightly associated SAM domain. The combined EF-hand and SAM domain (EF-SAM) undergoes a conformational change after Ca2+ dissociation, leading to association between adjacent STIM1 molecules (27). Covington et al. provided important insights into the interactions within and between STIM1 molecules during activation (28). They found that, in store-replete cells, STIM1 may exist as a dimer held together predominantly by interactions between the CC1 regions on the cytosolic side of the ER membrane (Fig. 2, STIM1 resting dimer). Notably, Korzeniowski et al. (16) made artificial dimers of STIM-ct that had almost no Orai1-coupling activity, suggesting that, when STIM1 exists as a dimer, the intramolecular electrostatic interactions between CC1 and CAD/SOAR may prevent the CAD/SOAR and the polybasic regions from being available for interaction. Upon store depletion, Ca2+ dissociation from the EF-SAM domain results in this domain changing conformation and self-associating so that STIM1 forms tetrameric or larger oligomers (27, 28). Covington et al. (28) found that the CAD/SOAR region was also crucial to this intermolecular interaction. The model in Fig. 2 shows the activated STIM1 oligomer as having undergone a transition in which dissociation between the CC1 and CAD/SOAR domains has occurred, allowing exposure of both the polybasic and CAD/SOAR regions so that they are available for interactions. The activated STIM1 oligomer can interact with the PM and form junctions in the absence of Orai1 channels as a result of interactions of the polybasic STIM1 C terminus with the PM (18). The STIM1 proteins are then able to tether and trap Orai1 channels that are diffusing in the membrane, and the electrostatic interaction between the available acidic domains in CAD/SOAR and basic domains in the C terminus of Orai1 results in formation of the activated channel complex (Figs. 1 and 2). The stoichiometry between STIM1 and Orai subunits is not certain; however, because the expressed CAD domain appears predominantly tetrameric (18) and it has been postulated that activated STIM1 proteins may exist as tetramers (28), it is likely that single STIM proteins may interact with each of the four Orai1 subunits that make up a single Orai1 channel (Fig. 2). Recent evidence, however, suggests that maximal opening of a single Orai1 channel may require up to eight STIM1 proteins (29)
Fig. 2.
Store-operated oligomerization of STIM1 and formation of the active STIM/Orai complex. In its “resting” state, STIM1 may exist as a dimer maintained by interactions between the first coiled-coil (CC1) segment of STIM1. In the dimeric state, electrostatic interactions between basic residues in the CAD/SOAR domain and acidic residues within STIM1-CC1 mask the CAD/SOAR domain, making it incapable of interacting with Orai1. Dissociation of Ca2+ from the luminal side of STIM1 causes oligomerization of STIM1 through the EF-SAM domains. Although initiated within the ER lumen, STIM1 oligomer formation also involves interactions between CAD/SOAR domains, which may release this domain from its association with CC1. This “activated” oligomer of STIM1 interacts with the PM by virtue of exposed C-terminal polybasic domains and forms ER-PM junctions. The activated oligomeric STIM1 can then tether and trap Orai1 channels diffusing in the PM. At the same time, an electrostatic interaction between the available acidic domains in CAD/SOAR and basic domains in the C terminus of Orai1 results in formation of the activated channel complex, thereby allowing Ca2+ entry.
Remarkable progress has been made in understanding the molecular mechanisms of coupling between STIM and Orai proteins. The activation process for STIM proteins, the dynamic and completely reversible process by which STIM proteins translocate into ER-PM junctions, and the intricate molecular interactions between STIM and Orai proteins during channel activation remain ongoing areas of investigation. The molecular machinery mediating SOCE, which exists almost universally among cell types, represents a potentially important yet largely unexplored pharmacological target.
Acknowledgments
Funding: Supported by NIH grant AI058173 and the Novartis Institutes for Biomedical Research.
References and Notes
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: Dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth JT, Hwang SY, Tomita T, Dehaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahrner M, Muik M, Derler I, Schindl R, Fritsch R, Frischauf I, Romanin C. Mechanistic view on domains mediating STIM1-Orai coupling. Immunol Rev. 2009;231:99–112. doi: 10.1111/j.1600-065X.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 7.Várnai P, Hunyady L, Balla T. STIM and Orai: The long-awaited constituents of store-operated calcium entry. Trends Pharmacol Sci. 2009;30:118–128. doi: 10.1016/j.tips.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 9.Mancarella S, Wang Y, Gill DL. Calcium signals: STIM dynamics mediate spatially unique oscillations. Curr Biol. 2009;19:R950–R952. doi: 10.1016/j.cub.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 14.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korzeniowski MK, Manjarrés IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 18.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Deng X, Zhou Y, Hendron E, Mancarella S, Ritchie MF, Tang XD, Baba Y, Kurosaki T, Mori Y, Soboloff J, Gill DL. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci USA. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 22.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/ CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calloway N, Holowka D, Baird B. A basic sequence in STIM1 promotes Ca2+ influx by interacting with the C-terminal acidic coiled coil of Orai1. Biochemistry. 2010;49:1067–1071. doi: 10.1021/bi901936q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell. 2010;21:1897–1907. doi: 10.1091/mbc.E10-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2010 doi: 10.1038/cr.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]