SUMMARY
Ca2+ entry signals are crucial in the control of smooth muscle contraction. Smooth muscle cells are unusual in containing plasma membrane (PM) Ca2+ entry channels that respond to voltage changes, receptor activation and Ca2+ store depletion.
Activation of these channel subtypes is highly coordinated. The TRPC6 channel, widely expressed in most smooth muscle cell types, is largely non-selective to cations and is activated by diacylglycerol arising from receptor-induced phosholipase C activation.
Receptor activation results largely in Na+ ion movement through TRPC6 channels, depolarization and subsequent activation of voltage-dependent L-type Ca2+ channels. The TRPC6 channels also appear to be activated by mechanical stretch, resulting again in depolarization and L-type Ca2+ channel activation. Such a coupling may be crucial in mediating the myogenic tone response in vascular smooth muscle.
The emptying of stores mediated by inositol 1,4,5-trisphosphate receptors triggers the endoplasmic reticulum (ER) Ca2+ sensing protein stromal-interacting molecule (STIM) 1 to translocate into defined ER–PM junctional areas in which coupling occurs to Orai proteins, which serve as highly Ca2+-selective low-conductance Ca2+ entry channels.
These ER-PM junctional domains may serve as crucial sites of interaction and integration between the function of store-operated, receptor-operated and voltage-operated Ca2+ channels. The STIM, Orai and TRPC channels represent highly promising new pharmacological targets through which such control may be induced.
Keywords: calcium channels, calcium signals, Orai, stromal-interacting molecule (STIM), TRPC channels
INTRODUCTION
Cytosolic Ca2+ signals control a vast number of cellular functions, ranging from short-term responses, such as contraction and secretion, to longer-term regulation of transcription, growth, cell division and apoptosis.1,2 In all muscle cells, including smooth muscle, cytoplasmic levels of free Ca2+ are the pre-eminent controlling factor for contraction.1-4 However, in smooth muscle cells, Ca2+ signals involve many components: rapid voltage-induced Ca2+ entry channels, slower receptor-induced Ca2+ entry, Ca2+ store release channels and still slower Ca2+ entry channels that become activated as the internal stores are depleted.5-9 These temporally distinct Ca2+ signalling events control not only smooth muscle contractile events, but also longer-term transcriptional and growth control of smooth muscle cells.10 In the present article, we consider the Ca2+ entry signals that are generated in smooth muscle cells in response to receptor activation. Two major types of channel proteins appear to be involved in receptor-induced Ca2+ entry signals. These are the canonical class of transient receptor potential (TRP) channels known as TRPC channels11 and the store-operated Ca2+ channels that we now know are mediated by the widely expressed Orai channel proteins.5,12
TRPC CHANNELS AND RECEPTOR-INDUCED Ca2+ ENTRY SIGNALS
Receptor-induced Ca2+ signals are crucial to the function of all cells1 and involve both the release of Ca2+ from stores and the entry of Ca2+ through plasma membrane channels.1,5,12 For several years, TRPC channels have been considered to play a major role in receptor-induced Ca2+ entry in smooth muscle and many other cell types.5,12-14 The TRPC channels appear to be activated in response to phospholipase C (PLC)-coupled receptors.5,12-17 Within the TRPC family, there are two structurally divided subgroups: (i) TRPC3, TRPC6 and TRPC7 channels; and (ii) TRPC1, TRPC4 and TRPC5 channels. One functional characteristic distinguishing these two subgroups is the ability of diacylglycerol (DAG) to activate TRPC3/6/7 channels but not the TRPC1/4/5 channels.5,15-19 As a product of receptor-induced PLC activation, DAG is an obvious mediator of TRPC channel activation. However, its role in the activation of endogenously expressed TRPC3/6/7 channels is not clear.6,16,17,20,21 The question of whether TRPC channels function as ‘store-operated’ channels (SOC) in smooth muscle and other tissues has been a highly contentious issue, with much evidence to support and negate such a role.5,22
The TRPC1 channel, which is highly expressed in many smooth muscle cell types, has been particularly implicated as an SOC.23 However, from knockout studies in mice, smooth muscle tissue from TRPC1-deficient mice appears to have normal vascular function and normal Ca2+ entry responses to store emptying. Hence, at present the real physiological role of TRPC1 channels in smooth muscle is still unknown. The TRPC6 channel is also highly expressed in a number of different smooth muscle cell types24-26 and there have been studies indicating that it plays a role in receptor-induced Ca2+ signalling in smooth muscle.24-28 In primary portal vein myocytes, current closely corresponding to overexpressed TRPC6 channels was reduced by treatment with TRPC6 antisense oligonucleotides.24 Downregulation of TRPC6 by antisense sequences in pulmonary vascular smooth muscle cells resulted in a reduction of store-operated Ca2+ entry.25 Using the clonal A7r5 aortic-derived smooth muscle cell line, Jung et al.26 described a TRPC6-like current activated by the permeant DAG analogue oleoyl-2-acetyl-sn-glycerol (OAG). In this case, the current was enhanced by PLC-coupled receptor activation but was not modified by Ca2+ store depletion.26 In recent studies using TRPC6-knockout mice, a phenotype of increased arterial blood pressure, augmented arterial tone and enhanced agonist- and DAG-induced current in smooth muscle was observed.15-17,29 Although such knockout would be expected to prevent rather than augment the Ca2+-mediated responses, there appeared to be an overcompensatory increase in the expression of the closely related TRPC3 channel.15-17,29
TRPC CHANNEL COUPLING TO VOLTAGE-DEPENDENT Ca2+ CHANNELS IN SMOOTH MUSCLE
Whereas knockout of TRPC6 in the whole animal is complicated by the upregulation of related proteins, TRPC6 RNA interference (RNAi) was achieved in cultured cells without significant compensation by other channels. Thus, in our studies,28 targeted RNAi was combined with a rigorous assessment of both message and protein to provide information on the presence and function of endogenously expressed TRPC6 channels in A7r5 smooth muscle cells. When TRPC6 was knocked down, the expression levels of each of the other TRPC channels was not altered. Thus, unlike the whole-animal TRPC6 knockout approach, the knockdown was not compensated for by expression of even closely related TRPC channels, such as TRPC3 or TRPC7.28 TRPC6 knockdown experiments revealed that an OAG-activated non-selective cation current with a current–voltage relationship close to that of known TRPC6 channels was substantially reduced.28 This reduction in current mirrored the reduction in TRPC6 protein. However, the corresponding TRPC6-mediated OAG-dependent entry of Ca2+ was not significantly altered by TRPC6 knockdown. Yet, the OAG-induced Ca2+ entry was almost completely inhibited by L-type Ca2+ channel blockers, indicating Ca2+ was entering through L-type voltage-dependent Ca2+ channels. However, pharmacological characterization of this current revealed TRPC6-like characteristics. Thus, it was sensitive to inhibitors of Src kinase and it was strongly inhibited by activation of protein kinase C (PKC), which is known to inhibit TRPC channels.19,30 The explanation for these results is that the TRPC6 channel, as a non-selective cation channel, is predominantly mediating the entry of Na+ as opposed to Ca2+ ions, resulting in depolarization and the opening of L-type Ca2+ channels. Hence, the TRPC6 channel is a mediator between PLC-generated DAG and the activation of Ca2+ entry through L-type Ca2+ channels. A scheme depicting this signalling process is shown in Fig. 1. Calculations reveal that even 90% reduction of TRPC6 channels would still allow depolarization sufficient to activate L-type Ca2+ channels. Thus, under conditions of RNAi resulting in approximately 90% reduction of TRPC6 protein and current carried by TRPC6 channels, there was still substantial depolarization-mediated activation of Ca2+ entry through L-type Ca2+ channels.28 The function of TRPC channels mediating depolarization and activation of L-type Ca2+ channels has also been indicated in other studies. Thus, in cerebral arteries, TRPC6 antisense treatment reduced pressure-induced depolarization and arterial constriction, suggesting that TRPC6 channels are activated as a result of pressure and may play an important role in the control of myogenic tone.31 Recently, the TRPC3 channel, which is also expressed in cerebral arteries, was shown to mediate purinergic receptor-induced depolarization and contraction.32 Thus, members of the TRPC3/6/7 subfamily of non-selective cation channels may play an important role in the control of smooth muscle cell membrane potential to effect control over voltage-operated Ca2+ entry and muscle contraction.
Fig. 1.

Receptor-induced TRPC6 activation via diacylglycerol (DAG) results in Na+ entry, membrane depolarization and activation of Ca2+ entry though L-type voltage-activated Ca2+ channels. GPCR, G-Protein-coupled receptor; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-trisphosphate.
MECHANICAL ACTIVATION OF TRPC CHANNELS AND THE MYOGENIC TONE RESPONSE
From a number of studies, it seems that TRPC6 channels are expressed to a large extent in cells responding to hydrostatic pressure changes, including vascular smooth muscle cells and glomerular podocytes, in which the TRPC6 channels have been suggested to mediate pressure-induced responses.33,34 As noted above, TRPC6 channels have been implicated in generating myogenic tone in arteries31,33 and increased expression of TRPC6 channels may be connected with smooth muscle proliferation in patients with idiopathic pulmonary arterial hypertension.35 In addition, TRPC6 channels have been shown to be essential for proper function of podocytes that are exposed to hydrostatic pressure driving glomerular ultrafiltration in the kidney.34 In view of the connections between TRPC6 channels and pressure regulation, Spassova et al.36 investigated the role of mechanical stretch on TRPC6 channel function. The results indicated that the TRPC6 channels may function as a direct sensor of mechanically and osmotically induced membrane stretch. Experiments revealed that the TRPC6 channels could be activated by both hypo-osmotic-induced swelling of cells and by direct application of pressure pulses to isolated membrane patches.36 The stretch-induced activation of TRPC6 channels was blocked by the tarantula peptide GsMTx-4, a specific peptide inhibitor of mechanosensitive channels that is thought to disturb the lipid–channel boundary.37,38 As described above, the TRPC6 channel is also activated by receptor-induced PLC activation, an effect mediated by DAG through a PKC-independent mechanism.18,19 However, the mechanical activation of TRPC6 channels is independent of PLC activation and, hence, likely through direct sensing of membrane lipid stretch. Interestingly, the GsMTx-4 peptide blocked both receptor- and DAG-induced TRPC6 activation.36 The effects of the peptide on both stretch- and DAG-mediated TRPC6 activation suggest that both chemical lipid-sensing and mechanical lipid-sensing by the channel could have a common molecular basis. Considering that TRPC6 channels are highly expressed in vascular smooth muscle cells, the results from this study suggested that mechanical activation of TRPC6 channels could play an important role in controlling myogenic tone.36 As described above, studies indicate that TRPC6 channels in vascular smooth muscle may control myogenic tone in response to intravascular pressure in small arteries.31 Previously, it was thought that the TRPC6 channel was activated indirectly, perhaps as a result of PLC-coupled stretch receptors.31 The results from Spassova et al.36 reveal that TRPC6 channels may be the direct sensors of mechanical stretch, as well as sensors of increased DAG levels. As shown in Fig. 2, the opening of TRPC6 channels is depicted as resulting from mechanically induced stretching of the membrane that would result from increased vascular pressure. In this case, the passage of Na+ ions results in depolarization of the membrane and the subsequent activation of L-type voltage-activated Ca2+ channels in a manner similar to that depicted in Fig. 1 resulting from receptor activation. Entry of Ca2+ through L-type Ca2+ channels would result in an influx of Ca2+ ions sufficient to induce smooth muscle contraction. In this way, the mechanical activation of TRPC6 channels would be the key step in the activation of contraction mediating the myogenic tone response of vascular tissue. The myogenic response is the acute reaction of blood vessel walls to an alteration in pressure and is characterized by a pressure-induced depolarization leading to activation of L-type Ca2+ channels.39,40 This response is crucially important in the development of resting vascular tone and the control of blood flow, especially through small arteries.31 We hypothesize that the high expression of TRPC6 channels in vascular smooth muscle would be the basis for the myogenic tone response.
Fig. 2.
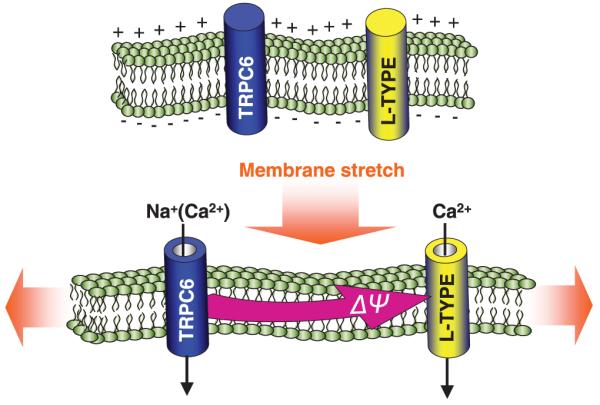
TRPC6 channels are sensors of membrane stretch. Channel opening allows Na+ entry, smooth muscle cell depolarization and the activation of activation of L-type voltage-activated Ca2+ channels. Such coupling between stretch and activation of Ca2+ entry signals may be crucial in mediating the myogenic tone response of vascular smooth muscle.
STORE-OPERATED CA2+ ENTRY: NEW PARADIGMS FOR Ca2+ CONTROL IN SMOOTH MUSCLE
In smooth muscle, as in other tissues, receptor-induced Ca2+ signals involve two closely coupled components, relatively fast, inositol 1,4,5-trisphosphate-mediated Ca2+ release from ER stores, followed by Ca2+ entry through SOCs.5,12,41,42 The activation of SOCs is key to mediating longer-term cytosolic Ca2+ signals and replenishing intracellular stores.5,12,42 In many cell types, SOCs carry a highly Ca2+-selective, non-voltage-gated, inwardly rectifying current termed the Ca2+ release activated Ca2+ current, or ICRAC.5,12,41 It is clear that similar types of store-operated currents exist in smooth muscle cells. Despite intense study, molecular characterization of SOCs and the activation process for store-operated Ca2+ entry remained elusive.5,12 Recent high-throughput RNAi screens revealed that stromal-interacting molecule (STIM) 1 is required for store-operated Ca2+ entry43,44 and conductance through CRAC channels.45 It is likely that STIM1 is the ‘sensor’ of Ca2+ within ER Ca2+ stores,44,46 translocating in response to store depletion into localized areas of the ER or ‘puncta’ close to the PM.44 More recently, store-operated Ca2+ entry and the function of CRAC channels have been shown to require the PM four-transmembrane spanning protein Orai1 (or CRACM1).47-49 This revelation came from a combination of elegant studies including genome-wide RNAi screening47-49 and modified linkage analysis identifying an Orai1 mutation as the cause of severe combined immune deficiency, which results in ablated T cell Ca2+ entry.47 Although expression of wild-type Orai1 restored CRAC to normal levels,47 the exact role of Orai1 in CRAC activation was not established initially. However, subsequent studies established that expression of STIM1 and Orai1 in combination results in an enormous gain in function of store-operated Ca2+ entry and CRAC channel activity.49-52 This has more recently been followed by studies showing that point mutations in Orai1 can alter the Ca2+ selectivity of the CRAC channel.53-55 It is now clear that the STIM and Orai proteins function together to mediate the store-operated Ca2+ signalling pathway; that is, to recognize and transduce the store-dependent signal and mediate entry of Ca2+ across the PM.
STIM1 IN THE ER MEMBRANE IS THE INTRALUMINAL Ca2+ SENSOR
It is clear that smooth muscle is just one of many tissues in which the STIM1 protein acts as the ER Ca2+ sensor to activate SOCs in the PM. RNAi-based screens have consistently identified STIM proteins as essential for SOC activation.43,44 However, the screening also revealed no effect on SOC activation of the knockdown of many other gene products, including TRP channel members,43,44 arguing against the role of TRP channels as SOC mediators. Despite this, recent evidence has indicated potentially important links between TRPC channels and STIM1.56,57 It is clear that suppressed STIM1 expression prevents SOC-mediated Ca2+ entry and CRAC channels activation.43-45 The STIM1 protein is the ‘sensor’ of ER Ca2+,44,46 this function being mediated via the EF-hand Ca2+-binding domain on the N-terminal ER luminal portion of STIM1. Decreased ER Ca2+ results in a profound intracellular redistribution of STIM1 from a uniform ER pattern to spatially discrete areas termed puncta.44-46,58 Mutation of the EF-hand in STIM1 to reduce its Ca2+ affinity results in the highly selective CRAC channel being fully and constitutively active, without any release of luminal Ca2+.45 The EF-hand-mutated STIM protein is already distributed in puncta, exactly mimicking the store-depleted mode.44 This indicates that the EF-hand is, indeed, the luminal Ca2+ sensor for SOCs. The single unpaired EF-hand has low Ca2+ affinity ideally suited to sense the high levels of Ca2+ within ER (0.5–2.0 mmol/L range). Stathopulos et al.59 examined the folding and interaction of the N-terminal segment of STIM1 containing the EF-hand and sterile α-motif (SAM) domains, revealing that Ca2+ bound to this fragment with a Kd of approximately 0.4 mmol/L. The N-terminus undergoes a conformational change upon Ca2+ binding and, when Ca2+ dissociates, the molecule is able to undergo multimerization.
In smooth muscle as well as other tissues, an ER–PM ‘interactional’ model best explains activation of SOCs, the STIM1 protein in the ER interacting directly with components in the PM.45,58 It is clear that SOC activation in smooth muscle involves functional STIM1 proteins,60-62 which appear to translocate and activate SOCs the same as in other tissues. Total internal reflection microscopy (TIRF) measurements63 indicate that aggregated STIM1 in the ER approaches as close as 10–20 nm to the PM, likely interacting directly with PM proteins. This model is compatible with the ‘conformational coupling’ model for SOC activation originally proposed by Irvine64 and Berridge65 and supported by evidence that close interactions, but not ER–PM fusion, are involved in SOC activation.5,12,65-67 Even though STIM1 distribution is profoundly altered by store emptying, the overall distribution of ER markers under the same store-emptying conditions is not significantly changed.44 Although a significant proportion of the STIM1-formed junctions at the PM are pre-existing, approximately one-third may be formed de novo following store depletion.63 The process of STIM1 aggregation and approach towards the PM is a totally reversible phenomenon, reinforcing the theory that channel activation results from a reversible interaction between ER and PM components, as opposed to a fusion or insertional event.
STORE-OPERATED CHANNEL MOIETIES: THE ORAI PROTEINS
Store-operated channel function was shown to require another protein, the plasma membrane four-transmembrane-spanning protein Orai1 or CRACM1.47-49 This revelation came from genome-wide RNAi screening47-49 and modified linkage analysis identifying an Orai1 mutation as the cause of a rare combined immune deficiency that results in ablated T-cell Ca2+ entry.47 The naturally occurring R91W mutation in Orai1 led to elimination of ICRAC,47 as did Orai1 knockdown.47-49 From recent work, it is now clear that the Orai1 protein fulfills all the criteria of being the SOC moiety itself.49-55 The Orai1 protein is expressed largely in the PM and, from labelling studies, both the N- and C-termini of the tetraspanning membrane protein exist within the cytoplasm.47,54 There are three closely related and widely expressed Orai genes (Orai1, Orai2 and Orai3).47 These are widely distributed among tissues47-49 and all three Orai proteins appear to be expressed in airway smooth muscle.68 The combined expression of STIM1 and Orai1 resulting in a huge gain of SOC function indicates that the two proteins are likely sufficient to mediate the operation of SOCs.49-52 The function of Orai1 was examined by expressing it in HEK293 cell lines stably expressing STIM1.51 Although significant store-operated Ca2+ entry can be observed in HEK293 cells, the level of endogenous CRAC channel activity in these cells is extremely low. Despite its necessity in store-operated Ca2+ entry,47,48 Orai1 expressed in vector-control cells strongly suppressed Ca2+ entry.51 This strong dominant negative effect likely reflects a coupling stoichiometry of more than one STIM1 protein per Orai1 protein.7,51 Dramatically, Orai1 coexpressed in STIM1-expressing cells resulted in a massive and rapid increase in store-operated Ca2+ entry.51 The huge increase in store-operated Ca2+ entry was rapidly blocked by application of 50 μmol/L 2-aminoethoxydiphenyl borate (2-APB), typical of known store-operated Ca2+ entry and CRAC channel function.69,70 The scheme shown in Fig. 3 represents the simple model in which, after store-depletion, aggregated STIM1 within the near-PM puncta, interacts directly via its cytoplasmic C-terminus with the Orai1 protein, activating the channel function of the latter. Yeromin et al.53 provide strong coimmunoprecipitation data to show that the Orai1 and STIM1 proteins interact and, more significantly, that store emptying results in increased interaction between the proteins.
Fig. 3.
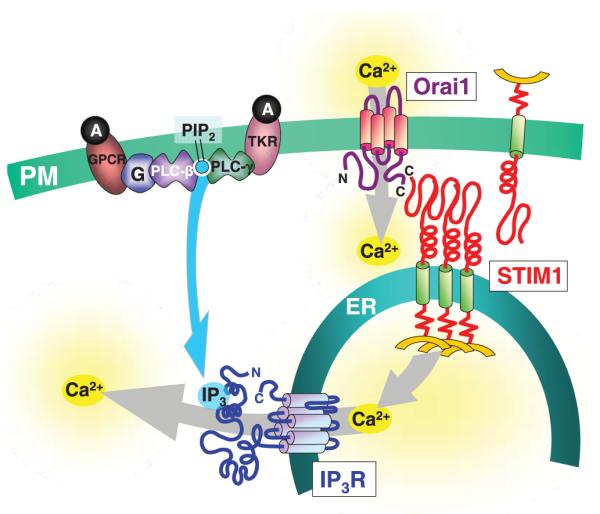
Scheme showing coupling between endoplasmic reticulum (ER) stores and Ca2+ entry mechanisms mediated by receptor-induced signalling pathways in smooth muscle and other cell types. G-Protein-coupled receptors (GPCR) or tyrosine kinase-coupled receptors (TKR) activated by agonists (A) are shown to activate either phospholipase C (PLC)-β or PLC-γ and the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) to release the head group inositol 1,4,5-trisphophate (IP3) into the cytosol. Inositol 1,4,5-trisphophate receptors (IP3R) on the ER membrane are activated to release Ca2+ from stores. The lowered luminal Ca2+ causes dissociation of Ca2+ bound to the low-affinity EF-hand Ca2+-binding site on the N-terminus of stromal-interacting molecule (STIM) 1. This Ca2+ dissociation causes STIM1 molecules to aggregate and be translocated to regions of the ER in close proximity to the plasma membrane (PM) and to interact directly with the Orai1 protein in the PM, which is the highly Ca2+-selective store-operated channel moiety.
Examination of the Orai1 molecule revealed a small, highly conserved region extending from the end of the first transmembrane domain into the first extracellular loop between the first and second transmembrane domains. In Orai1, this nine amino acid region is residues 106–114 and is closely conserved in the three mammalian Orai proteins, as well as in the Drosophila protein.53,54 This is a highly acidic region with a glutamate (position 106), glutamine (position 108) and three aspartate residues (positions 110, 112, 114), strongly suggesting the pore entrance. The E106A mutant had a powerful dominant negative effect such that its expression, even in normal HEK293 cells, resulted in no SOC-mediated Ca2+ entry.55 Importantly, the E106 residue has been shown to be critical for defining the Ca2+ selectivity of CRAC channel function.53-55 Hence, the subtle E106D mutation, in which the acidic residue is reduced in size, results in a markedly reduced cation selectivity of the CRAC channel, allowing monovalent cations as well as divalent cations to move through the channel.53,54
INTEGRATION OF STIM, ORAI AND TRPC CHANNELS IN CONTROL OF SMOOTH MUSCLE Ca2+ ENTRY SIGNALS
From the above discussion, it is clear that both TRPC and STIM-operated Orai channels are important in mediating Ca2+ entry signals in smooth muscle cells, as well as in many other cell types. Smooth muscle cells also use voltage-activated Ca2+ channels; hence, they represent an interesting hybrid between excitable and non-excitable cells. We have seen that the TRPC channels provide a crucial mediating link between receptors and the activation of L-type channels by permitting cation entry to depolarize the cell. The TRPC channels may also provide a critical link between the sensing of contraction and the activation of voltage changes to mediate myogenic tone responses in vascular tissue. Answers to the question of the occurrence and role of interactions between TRPC channels and the STIM1- and Orai1-mediated store-coupling process are beginning to emerge. There is a huge literature on the store-operating role of many of the TRPC channel family members,5,12 but this interaction could not really be addressed until the discovery of the SOC machinery: the STIM and Orai proteins. Recent information indicates that the STIM proteins can interact with and functionally connect to TRPC channels.23,56,57,71 The TRPC channels are also reported to physically and functionally interact with Orai1 channels.72,73 Indeed, both STIM1 and Orai1 appear to be associated within complexes that also contain the TRPC1 channel.23,57 This suggests that the coupling domains between ER and PM, defined by STIM1-mediated junctions, may contain multiple proteins and may represent organized loci in which the activation of multiple channels is coordinated.
In smooth muscle, it is clear that STIM and Orai proteins are present and function the same as in other tissues.60-62,68 Such junctional sites in smooth muscle, and the channel interactions occurring within them, are likely to be of extreme importance in controlling and coordinating Ca2+ entry signals in response to receptors, contraction, voltage changes and the state of filling of ER stores. The location and role of the three different Orai channel proteins, the two different STIM proteins and the six different TRPC channels present in smooth muscle is just beginning to be understood. Certainly, the nature of store-activated junctional domains, the proteins that are contained within them and the functional inter-membrane interactions are crucial areas to be investigated in the control of Ca2+ entry signals in smooth muscle.
Finally, the STIM signalling process that senses the content of Ca2+ within the ER of smooth muscle and its relationship with the function of smooth muscle remains an important and unresolved area of study. Generally, ER in smooth muscle is referred to as ‘sarcoplasmic reticulum’ to signify its similarity in function to the extensive Ca2+-sequestering organelles of cardiac and skeletal muscle. However, the ER in smooth muscle generally is structurally and functionally more akin to that in non-muscle cells. Indeed, the presence and function of STIM proteins in smooth muscle ER can be considered evidence for this view. The STIM-mediated sensing of Ca2+ in smooth muscle ER, control of Ca2+ content within the ER and the activation of coupling with the PM all remain crucial areas of investigation in determining new means to control smooth muscle cell contraction to counteract serious vascular diseases. The STIM, Orai and TRPC channels represent highly promising new pharmacological targets through which such control may be induced.
Footnotes
Presented at the Experimental Biology Symposium: Multiple Calcium Channels in the Vasculature: Regulation of Arterial Tone, Washington, 28 April–2 May 2007.
REFERENCES
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 4.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 2000;278:C235–56. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 5.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002;4:E263–72. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 6.Gill DL, Patterson RL. Toward a consensus on the operation of receptor-induced calcium entry signals. Sci. STKE. 2004;243:Pe39. doi: 10.1126/stke.2432004pe39. [DOI] [PubMed] [Google Scholar]
- 7.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–82. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins: A new paradigm in inter-organelle communication. Biochim. Biophys. Acta. 2006;1763:1161–8. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Gill DL, Spassova MA, Soboloff J. Calcium entry signals: Trickles and torrents. Science. 2006;313:183–4. doi: 10.1126/science.1130811. [DOI] [PubMed] [Google Scholar]
- 10.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ. Res. 2006;98:868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 12.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 13.Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005:Re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 14.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 15.Freichel M, Vennekens R, Olausson J, et al. Functional role of TRPC proteins in vivo: Lessons from TRPC-deficient mouse models. Biochem. Biophys. Res. Commun. 2004;322:1352–8. doi: 10.1016/j.bbrc.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich A, Schnitzler M, Kalwa H, Storch U, Gudermann T. Functional characterization and physiological relevance of the TRPC3/6/7 subfamily of cation channels. Naunyn Schmiedebergs Arch. Pharmacol. 2005;371:257–65. doi: 10.1007/s00210-005-1052-8. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich A, Mederos YS, Gollasch M, et al. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell Biol. 2005;25:6980–9. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRP6 and TRP3 channels by diacylglycerol. Nature. 1999;397:259–63. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 2003;278:29, 031–40. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- 20.Gudermann T, Hofmann T, Schnitzler M, Dietrich A. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis. Found. Symp. 2004;258:103–18. [PubMed] [Google Scholar]
- 21.Gudermann T, Schnitzler MM, Dietrich A. Receptor-operated calcium entry – More than esoteric terminology? Sci. STKE. 2004;243:Pe35. doi: 10.1126/stke.2432004pe35. [DOI] [PubMed] [Google Scholar]
- 22.Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J. Physiol. 2007;583:25–36. doi: 10.1113/jphysiol.2007.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: The link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–23. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Inoue R, Okada T, Onoue H, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325–32. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, Sweeney M, Zhang S, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am. J. Physiol. Cell Physiol. 2003;284:C316–30. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- 26.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am. J. Physiol. Cell Physiol. 2002;282:C347–59. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 27.Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J. Physiol. 2003;552:789–95. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soboloff J, Spassova MA, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J. Biol. Chem. 2005;280(39):786–94. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich A, Mederos Y, Schnitzler Y, et al. TRPC6 deficient mice develop an elevated blood-pressure and an early onset of the myogenic tone in cerebral arteries. Naunyn Schmiedebergs Arch. Pharmacol. 2005;369(Suppl. 1):R62. Abstract. [Google Scholar]
- 30.Venkatachalam K, Zheng F, Gill DL. Control of TRPC and store-operated channels by protein kinase C. Novartis Found. Symp. 2004;258:172–85. [PubMed] [Google Scholar]
- 31.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002;90:248–50. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 32.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2055–61. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- 33.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J. Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005;37:739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Fantozzi I, Remillard CV, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl Acad. Sci. USA. 2004;101(13):861–6. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl Acad. Sci. USA. 2006;103(16):586–91. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–40. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 38.Suchyna TM, Johnson JH, Hamer K, et al. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 2000;115:583–98. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 40.Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J. Physiol. 2000;527:139–48. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parekh AB, Penner R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–30. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 42.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J. Cell Sci. 2001;114:2223–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 43.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc. Natl Acad. Sci. USA. 2006;103:4040–5. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 48.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang SL, Yeromin AV, Zhang XH, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl Acad. Sci. USA. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peinelt C, Vig M, Koomoa DL, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 2006;281(20):661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 52.Mercer JC, Dehaven WI, Smyth JT, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 2006;281(24):979–90. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 55.Vig M, Beck A, Billingsley JM, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang GN, Zeng W, Kim JY, et al. STIM1 carboxyl-terminus activates native SOC, I (crac) and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 57.Ong HL, Cheng KT, Liu X, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx: Evidence for similarities in store-operated and caclium release-activated calcium channel components. J. Biol. Chem. 2007;282:9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soboloff J, Spassova MA, Hewavitharana T, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr. Biol. 2006;16:1465–70. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 59.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281(35):855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 60.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir. Res. 2006;7:119–27. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietrich A, Kalwa H, Storch U, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch. 2007;455:465–77. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi Y, Watanabe H, Murakami M, et al. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2007;361:934–40. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 63.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irvine RF. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates: A possible mechanism. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 65.Berridge MJ. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patterson RL, van Rossum DB, Gill DL. Store operated Ca2+ entry: Evidence for a secretion-like coupling model. Cell. 1999;98:487–99. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 67.Berridge MJ. Conformational coupling: A physiological calcium entry mechanism. Sci. STKE. 2004;243:Pe33. doi: 10.1126/stke.2432004pe33. [DOI] [PubMed] [Google Scholar]
- 68.Peel SE, Liu B, Hall IP. ORAI and store operated calcium influx in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2008;38:744–9. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma HT, Venkatachalam K, Parys JB, Gill DL. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2004;277:6915–22. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 70.Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez J, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J. Biol. Chem. 2006;281(28):254–64. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 72.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl Acad. Sci. USA. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao Y, Erxleben C, Abramowitz J, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl Acad. Sci. USA. 2008;105:2895–900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]


