Abstract
Background
While there is extensive literature on the relationship between the P3 component of event-related potentials (ERPs) and risk for alcoholism, there are few published studies regarding other potentially important ERP components. One important candidate is the N4(00) component in the context of semantic processing, as abnormalities in this component have been reported for adult alcoholics.
Method
A semantic priming task was administered to non-alcohol dependent male offspring (18 to 25 years) of alcoholic fathers [high risk (HR) n=23] and non-alcoholic fathers [low risk (LR) n=28], to study whether the two groups differ in terms of the N4 component. Subjects were presented with 150 words and 150 non-words. Among the words, 50 words (primed) were preceded by their antonyms (prime, n=50), whereas the remaining 50 words were unprimed. For the analysis, N4 amplitude and latency, as well as behavioral measures for the primed and unprimed words were considered.
Results
A significant interaction effect was observed between semantic condition and group, where HR subjects did not show N4 attenuation for primed stimuli.
Conclusion
The lack of N4 attenuation to primed stimuli and/or inability to differentiate between primed and unprimed stimuli, without latency and reaction time being affected, suggest deficits in semantic priming, especially in semantic expectancy and/or post-lexical semantic processing in HR male offspring. Further, it indicates that it might be an electrophysiological endophenotype that reflects genetic vulnerability to develop alcoholism.
Keywords: Semantic priming, N4, alcoholism, high risk, endophenotype
1. Introduction
N4(00) is a negative component of the event related potential (ERP), occurring predominantly over the centroparietal scalp region and approximately 300 to 650 ms after the presentation of a word that is incongruent with its semantic context (Kutas and Hillyard, 1980; Bentin, 1989; Bentin et al., 1993; Hamberger et al., 1995; Gunter and Friederici, 1999; Nixon et al., 2002). In the classic experiment of Kutas and Hillyard (1980), N4 was elicited by the final anomalous word in sentences presented one word at a time (Kutas and Van Petten, 1988; Nixon et al., 2002). Though it is observed predominantly to semantic violations, a recent body of work has shown that N4 varies systematically with the processing of potentially meaningful stimuli at the level of meaning, where the amplitude is reduced by a variety of factors that increase these items predictability in their context (Kutas and Federmeier, 2000). Some of these factors are semantic congruity, antonyms, high frequency words and repetitions. Studies have shown that N4 reflects contextual integration (Brown and Hagoort, 1993). This view emphasizes the importance of the fit between the eliciting item and context-based information currently held in working memory. If there is a fit, integration will be easier and correspondingly the N4 is reduced. In addition, N4 also appears to vary inversely with the ease of accessing information from long-term memory. For example, the more the frequency of usage (or repetition) of a word, the smaller the N4 amplitude it will elicit (Fischler et al., 1983; Kutas and Federmeier, 2000). There are different strategies for eliciting N4 that have been reported in the literature. Unlike the earlier methods of presenting sentences with the last word being congruent or incongruent, the lexical decision task used in this study involves presentation of letter strings in sequence. The subject must decide whether the stimulus presented is a word or a non-word. Within this framework, the semantic priming task has been one of the most extensively used paradigms to observe the effect of priming on N4 (Bentin, 1989; Ganis et al., 1996). Classically, with respect to behavioral studies, semantic priming effect refers to the faster reaction time to the related targets than to the unrelated targets in a lexical decision task (Meyer and Schvaneveldt, 1971). Similarly, with regard to ERP tasks, semantic priming is observed in reduced N4 amplitude to the primed stimuli. A body of early work shows that, N4 amplitude is inversely related to the word’s ‘cloze probability’ (Kutas and Hillyard, 1984), i.e., the degree to which a particular word is the most likely completion for a sentence fragment (Taylor, 1953). For example, in the sentence, ‘I had coffee and omelet for breakfast’, the last word ‘breakfast’ has a greater degree of probability and/or association to complete the sentence, than the word ‘office’. Recently it has been shown that the N4 amplitude reduction observed to a primed stimulus, such as in antonym-pairs, is similar to the N4 amplitude reduction observed to congruent last words in sentences (Kutas and Federmeier, 2000). With respect to the semantic priming paradigm, a word preceded by an unrelated word (unprimed condition) produces a larger N4 in comparison to a word preceded by a related word (primed condition) (McCarthy and Nobre, 1993). For example, in the following two pairs of stimuli ‘North–Pencil’ and ‘Before–After’, the word ‘after’ elicits a smaller N4 compared to the word ‘pencil’. This is because the word ‘after’ is primed by the word ‘before’, while there is no priming for the word ‘pencil’.
There are different theories regarding what the N4 in a priming paradigm reflects. The often quoted mechanisms are those of Neely and Keefe (1989), who purport that these mechanisms are automatic spreading activation, expectancy and semantic matching. The latter two are generally referred to as controlled processes. According to the automatic spreading activation theory, presentation of a word is thought to activate the corresponding conceptual representation in the semantic network, and the activation automatically spreads to related nodes, thereby increasing their activation level and reducing the processing time of the related words (Collins and Loftus, 1975). Expectancy is the second mechanism used to explain the priming effect. It is assumed that based on the prime, subjects generate expectancy for a set of semantically related targets. Targets that are expected are recognized more quickly compared to targets that are not expected. This mechanism is said to be influenced by the instructions and the proportions of prime-target pairs (den Heyer et al., 1983; Neely and Keefe, 1989; Silva-Pereyra et al., 1999). In general, semantic matching is also referred to as a post-lexical priming mechanism. It refers to the strategy, where after the lexical and semantic access and activation of prime and target pair, but before the subject’s decision and response to the target is completed, the subject may match the target with the prime that preceded it. If it is related, a ‘word’ decision/response bias ensues, thereby facilitating responses to related word target; if it is unrelated, a ‘non-word’ decision/response bias follows (Neely and Keefe, 1989; Silva-Pereyra et al., 1999). The latter two mechanisms are referred to as controlled priming mechanisms. The relatively recent body of research suggests that the controlled priming mechanisms are generally believed to act more effectively at relatively long stimulus onset asynchrony (SOA) of greater than 500 ms, whereas automatic spreading activation is thought to be the mechanism that influences the priming effect at short SOAs (De Groot et al., 1986; Neely, 1991).
Nevertheless, compared to the earlier findings, more recent work has shown that semantic matching strategies can be active at SOAs as short as 150 ms (Koivisto, 1998) and automatic spreading activation can influence priming as long as 2000 ms (Deacon et al., 1999). In general, research evidence suggests that the N4 in the priming paradigm reflects different mechanisms, such as automatic spreading activation (Kutas and Hillyard, 1989; Kiefer and Spitzer, 2000; Deacon et al., 2000; Kiefer, 2002; Deacon et al., 2004), expectancy (Kutas et al., 1984; Silva-Pereyra et al., 1999) and semantic matching (Chwilla et al., 1998; Holcomb, 1993) depending on the paradigm used.
Alcohol dependence has been shown to adversely affect numerous cognitive functions, including semantic processing (Williams and Rundell, 1984; Maylor et al., 1987; Ji et al., 1999). Relatively old and new neurophysiological studies have found abnormalities, such as decreased P3 amplitude in target detection tasks (Pfefferbaum et al., 1991; Porjesz and Begleiter, 1985; Porjesz and Begleiter, 1987; Prabhu et al., 2001), and in Go/NoGo tasks (Cohen et al., 1997; Kamarajan et al., 2005), decreased N2 and N2-P3 complex (Realmuto et al., 1993), and delayed latency for the N2 in a visual discrimination task in abstinent alcoholics (Porjesz et al., 1987). Further, the decreased P3 amplitude persisted after extended abstinence (Porjesz and Begleiter, 1987; Fein and Chang, 2006). These abnormalities observed in ERP components reveal general cognitive impairment in alcoholics (For a detailed review see Porjesz and Begleiter, 2003).
N4 abnormalities in semantic priming tasks have been observed in alcoholics. For example, Ceballos et al. (2005) found significantly less negative N4 amplitude in alcohol dependents relative to non-dependent controls. Similarly, Nixon et al. (2002) obtained reduced N4 amplitude of the difference waveform between primed and unprimed words in alcoholics compared to community controls. In a very large study, in contrast with controls, alcohol dependent subjects exhibited large N4 amplitudes to both primed and unprimed words alike (Porjesz et al., 2002), showing no attenuation of N4 for primed words; this indicates that alcoholics exhibit a lack of differentiation in their N4 responses between primed and unprimed words, responding similarly to both.
Risk for alcoholism and cognitive functions
Alcoholism is a complex disorder attributed to the interaction of genetic and environmental factors. There is considerable evidence to demonstrate that genetic predisposition accounts for roughly half of the risk in the development of alcohol dependence (Schuckit, 2000; Hines et al., 2005). Twin studies have shown that the concordance rates of alcoholism in monozygotic twins are higher than that of fraternal twins (Prescott and Kendler, 1999) and that genetic risk factors are the same for women and men (Heath et al., 1997; Prescott et al., 1999). Similarly, studies of children of alcoholics who were adopted at an early age and reared by non-relatives showed six fold higher rates of alcohol abuse, regardless of their postnatal environment (Sigvardsson et al., 1996). These studies underscore the existence of predisposition or risk for alcoholism.
Risk status has been associated with deficits in cognitive functioning as revealed by electrophysiological and neuropsychological studies. These deficits may be associated with a predisposition for alcoholism and related disorders. In earlier neuropsychological studies, family history positive (FHP) men performed poorly compared to family history negative (FHN) men on language and memory tests (Hegedus et al., 1984; Tarter et al., 1984). With respect to electrophysiology, P3 amplitude reductions have been noted in children of alcoholics, thus indicating that the deficits associated with P3 are also associated with risk (Begleiter et al., 1984; Polich et al., 1994; Ramachandran et al., 1996). Using the lexical decision task/semantic priming paradigm that was used in the current study, Almasy et al. (2001) found significant heritability for N4 amplitude. Further, a significant reduction in N4 amplitude was observed in the FHP male offspring compared to FHN male offspring in a letter rhyming task (Schmidt and Neville, 1985). These studies indicate that the N4 deficits observed in alcoholics (Porjesz et al., 2002) may also be found in the HR individuals. This may be an index of vulnerability that predates alcohol dependence. Some of these electrophysiological findings observed have served as intermediate phenotypes or endophenotypes, which have been successfully used to study the genetic susceptibility to alcoholism (Begleiter and Porjesz, 1999). It is likely that this susceptibility may manifest as deficits in cognitive and linguistic functioning (Kuperman et al., 1995).
Hence, as previous work from our laboratory has demonstrated a lack of differentiation in N4 amplitude measures between primed and unprimed words in adult alcoholics, the present research was undertaken to study whether the deficits are already present in high risk offspring of alcoholics. Further, as the literature reports that the N4 predominantly occurs over centroparietal regions, we have included regional differences in the analysis.
2. Materials and methods
2.1. Subjects
Subjects were selected from the ongoing Collaborative Study on the Genetics of Alcoholism (COGA), a multi-site national consortium. The consortium is composed of six data collection centers, located at: State University of New York – Downstate Medical Center; University of Connecticut Health Center; Washington University School of Medicine in St. Louis; University of California at San Diego; University of Iowa; and Indiana University Medical School. Alcoholic families were recruited through probands from inpatient and outpatient treatment facilities, and they and their family members were interviewed with the Semi Structured Assessment of the Genetics of Alcoholism (SSAGA) to determine psychiatric diagnoses. A detailed description of the COGA recruitment and assessment procedures has been described previously (Begleiter et al., 1995; Bucholz et al., 1994; Hesselbrock et al., 1999). For comparison, and to obtain a representative sample of the general population, control families were recruited from HMOs, drivers’ license records, and dental clinics at each site. The control families were interviewed with the SSAGA, and underwent the same full protocol as COGA family members. The time period of the data collection was from 1993 to 2003.
Twenty-three high risk (HR) male offspring from the alcoholic families in COGA (Collaborative Study on the Genetics of Alcoholism) and 28 low risk (LR) male offspring from the control families who fulfilled the inclusion and exclusion criteria as mentioned below, were selected for the present study. All the subjects were in the age range of 18 to 25 years. They were categorized as LR if they were from control families and their parents were diagnosed negative for DSM IIIR alcohol dependence. Further, LR subjects could not be alcohol dependent or abusers and could not have a concurrent or past history of externalizing disorder (Attention Deficit Hyperactivity Disorder, Antisocial Personality Disorder and Conduct Disorder). Subjects assigned to the HR category were offspring of male alcoholics from COGA families whose mothers were not alcohol dependent (to rule out the confounding effects of Fetal Alcohol Syndrome or fetal alcohol effects), but who could have additional first degree relatives who were alcohol dependent. Further, HR subjects could not be alcohol dependent or abusers, but those with concurrent or past history of externalizing disorders were not excluded. In addition, subjects whose parents had any history of psychosis were not included. All the subjects who participated in the study were right-handed (table 1).
Table 1.
Sociodemographic and clinical characteristics of the sample
| Low risk | High Risk | |
|---|---|---|
| Number of subjects | 28 | 23 |
| Mean Age (S.D) | 20.82 (2.00) | 21.00 (1.95) |
| Average alcohol intake per month (no. of drinks) | 16.55 (18.24) | 34.51 (39.72) |
| % of cocaine dependence – DSM IIIR | 3.57 | 4.35 |
| % of marijuana dependence – DSM IIIR | 10.71 | 26.09 |
| % of stimulant dependence – DSM IIIR | 3.57 | 0 |
| % of opioid dependence – DSM IIIR | 0 | 0 |
| % of lifetime depression – DSM IIIR | 3.57 | 0 |
For the ERP protocol, subjects with hepatic encephalopathy/cirrhosis of the liver, multiple sclerosis, stroke, seizures, head injury, any other history of neurological, psychiatric disorders and neurosurgical procedures were excluded. Subjects who were taking medication that affects/influences brain functioning, who tested positive for HIV, had uncorrected vision and/or hearing deficits, or had used any psychoactive substances in the past 5 days were also excluded. Prior to neurophysiological assessment, breath analyzers were used to exclude subjects who tested positive for the presence of alcohol.
Each center obtained separate Institutional Review Board approval for the COGA research and written informed consent was obtained from each subject prior to participation.
2.2. Data recording
All six sites used the identical experimental paradigm and electrophysiological software and hardware. An internal consistency study by Kuperman et al. (1995) showed that the data from all six laboratory locations were consistent with each other for this paradigm. Subjects were seated on a comfortable chair in a sound-attenuated temperature-regulated and dimly lit booth (Industrial Acoustics Company; Bronx, NY). An Electro-Cap (Electro-Cap International Inc.; Eaton, OH) with 19 leads based on the International 10–20 system (Jasper, 1958) was placed on the scalp of each subject. A forehead electrode served as the ground and the nose electrode served as the common reference. Electrode impedance was maintained below 5 kΩ. For eye movement artifact correction, both vertical and horizontal electro-ocular (EOG) activities were recorded with the electrodes placed supraorbitally and at the outer canthus of the left eye. Amplifier gain was set at 10,000 times on Sensorium EPA-2 Electrophysiology amplifiers (Charlotte, VT), with a high pass filter of 0.02 Hz and low pass filter of 50 Hz and digitized on a Concurrent 5550 computer (Concurrent Computer Corp. Atlanta, GA). The sampling rate was 256 Hz with sampling beginning 187 msec prior to and continuing for 1413 msec after the stimulus onset. Online artifact rejection was used whenever the voltages exceeded ± 73.3 µV. Offline analysis was performed at SUNY Downstate Medical Center, New York. Data were further processed using an 8 Hz low pass digital filter and epochs including 200 ms prior to the stimulus and 800 ms after the stimulus onset were extracted. The average waveforms of each subject were inspected and the records of subjects who had anomalous and artifact contaminated wave forms were removed from further analysis. The trials in which the response time exceeded 1000 msec were excluded from all analyses. Further, only the correct response trials were included. In order to avoid the variation in the number of correct trials, the minimum and maximum numbers of correct trials were taken as 20 and 35 respectively. The mean number of accepted trials for the final analysis was 27 for both primed and unprimed words in the HR group, and 30 for primed and 31 for unprimed in the LR group.
2.3. Lexical Decision Task
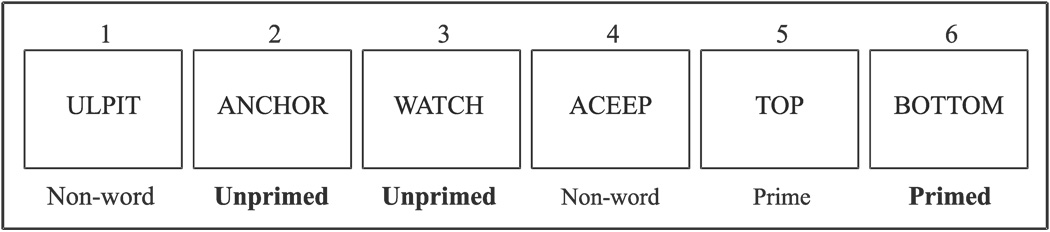
The experiment used for the study was a lexical decision task that required subjects to indicate whether a stimulus was a word or a non-word by pressing a button with the index finger of one hand or the opposite hand, respectively. The hand used for the button press was counter-balanced across subjects. Subjects were instructed to respond as quickly and as accurately as possible. Within the above framework, the present study involved a semantic priming paradigm. The subjects were sequentially presented with a partially randomized list of 150 words and 150 non-words with a uniform inter-stimulus interval of 1600 ms. The exposure time for each stimulus was 150 msec. Among the 150 words, 50 words (primed) were always preceded by their antonyms (prime: n = 50), whereas the remaining 50 words (unprimed) were unrelated and sometimes preceded and/or were followed by the non-words. The remaining 150 stimuli consisted of non-words comprising jumbled letters (Figure 1). The prime-antonym pairs (prime and primed words) were always preceded and followed by a non-word.
Figure 1.
An example of the stimuli and the order of presentation
Electrophysiological data recorded to the second word of the prime-antonym pair (n = 50; primed) and the unrelated word (word not preceded by its antonym; n = 50; unprimed) were averaged to produce mean waveforms of primed and unprimed words for each subject. The N4 component was selected as the largest negative peak between 300 to 500 ms and occurring before the large positive component after the stimulus onset in averaged waveforms for each subject and condition; its peak amplitude and latency values were extracted using a semi-automatic peak-picking program. This study focused on assessing differences that were previously noted in alcoholics namely, between primed and unprimed words.
Word length was the same for primed and unprimed conditions and averaged 4.5 letters. Non-word length also averaged 4.5 letters and consisted of pronounceable combinations of letters. Both word and non-word were of 2.5 cm in height and were of white color presented over a black background. Word familiarity, using the scale of Toglia et al. (1978), for both the primed and unprimed words, averaged 6.3 on a scale of 1 (unfamiliar) to 7 (very familiar). A standardized spelling list Forbes, 1968 placed the average grade level of the primed and unprimed words in the 3rd grade and ranged from 2nd to 7th grade. Imaginability of the words ranged from 215 to 641 on the scale of 100 to 700 with the average of 484.31. The average concreteness of the words was 447.36 with the range from 242 to 624. Both imaginability and concreteness scores were obtained online from MRC Psycholinguistic Database (Refer http://www.psy.uwa.edu.au/mrcdatabase/mrc2.html). Written frequency according to Kucera-Francis frequency count averaged 456.80 and ranged from one to 21,341 (Kucera and Francis, 1967). The parts of speech of the words included noun, verb, adjective, adverb and preposition; some words fell into more than one category.
2.4. Statistical analysis
Data were analyzed using SPSS version 12.0. Of the 19 channels, data from nine electrodes were selected and grouped into three regions, viz. frontal (Fz, F3, F4), central (Cz, C3, C4), and parietal (Pz, P3, P4). Subject and task related variables such as age, average alcohol intake (number of drinks) per month for the last six months, number of correct responses, and reaction time were analyzed for group differences using student’s t test and paired samples t test. As a primary analysis, repeated measures ANCOVAs were used separately for amplitude and latency, with groups as the between-subjects variable. Semantic condition (primed and unprimed) and scalp region (frontal, central, and parietal) were entered as the within-subjects factors with average alcohol intake per month for the last six months as covariate. As a secondary analysis, group differences were examined using separate repeated measures ANCOVAs for different semantic conditions (primed-amplitude, primed-latency, unprimed-amplitude, and unprimed-latency). For all the secondary analyses, scalp region and electrode position were entered as within-subjects factors and average alcohol intake per month for the last six months as covariate. Greenhouse-Geisser correction was employed when required. Unless otherwise mentioned the results were non-significant and mean values indicated are covariate-adjusted.
3. Results
3.1. Demographic and clinical variables
Table 1 lists the demographic and clinical characteristics of the HR and LR samples. The groups showed no significant difference in their age (t value = −0.32, p = .75). Alcohol intake per month did not show any significant correlation with any of the dependent variables. However, the groups differed significantly in average alcohol intake per month for the past six months (t value = −2.14, p = .038). Therefore, to rule out any possible influence of alcohol consumption, average alcohol intake per month for the past six months was used as covariate. The covariate did not show any significant main or interaction effect in any of the analyses. The groups were not matched on substance use and other Axis I conditions, since the reported rates for some co-occurring conditions, such as substance use in subjects with family history of alcoholism, are elevated over normal population levels (Sher et al., 1991; Cuijpers et al., 1999). This was also observed in the current study where more HR subjects had marijuana dependence compared to LR subjects. Marijuana dependence could not be included as a covariate in the statistical analysis as the data was a categorical variable. Therefore, we plotted scatterplots using the amplitude as the dependent variable and the marijuana dependence and non-marihuana dependence as the independent variable. We found that marijuana dependent subjects were randomly distributed across the non-dependent subjects.
3.2. Behavioral measures
The groups did not show any significant differences in the number of correct responses either to the primed t = −1.45; p = .153) or unprimed words (t = −1.83; p = .074). Similarly, no significant group differences between HR and LR groups in reaction time (RT) were observed either for primed (t = −0.44; p = .665) or for unprimed (t = 0.11; p = .913) word conditions. Further, analysis showed a significantly higher number of correct responses for primed words compared to unprimed words in both HR (t = 3.48, p = .002) and LR (t = 3.54, p = .001) group. Similarly, significantly shorter RTs for primed words compared to unprimed words were observed in both HR (t = −2.74; p = .012) and LR (t = −4.61; p = .00009) groups.
3.3. ERP findings
3.3.1. Amplitude
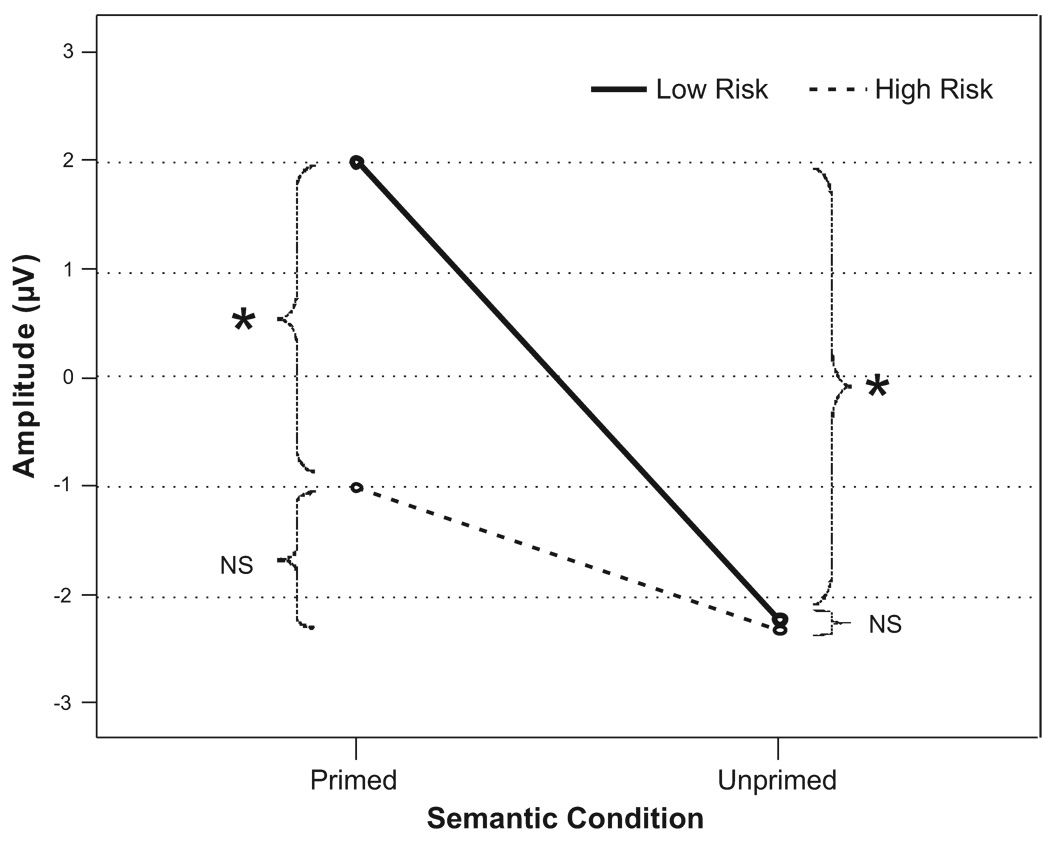
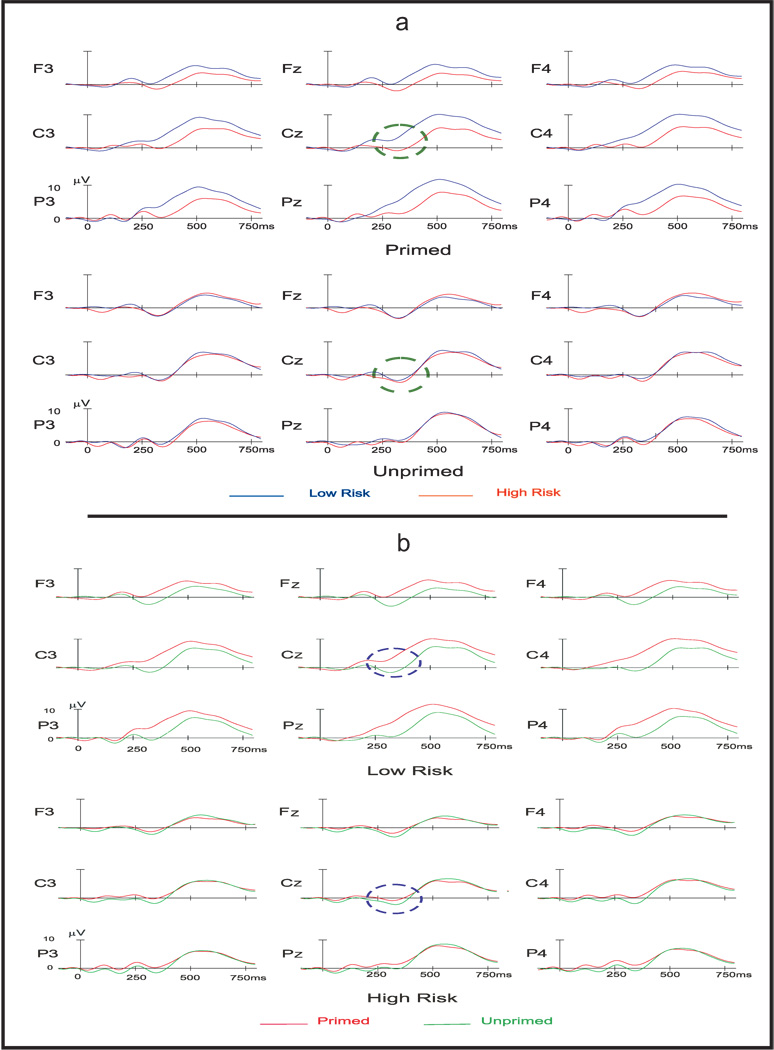
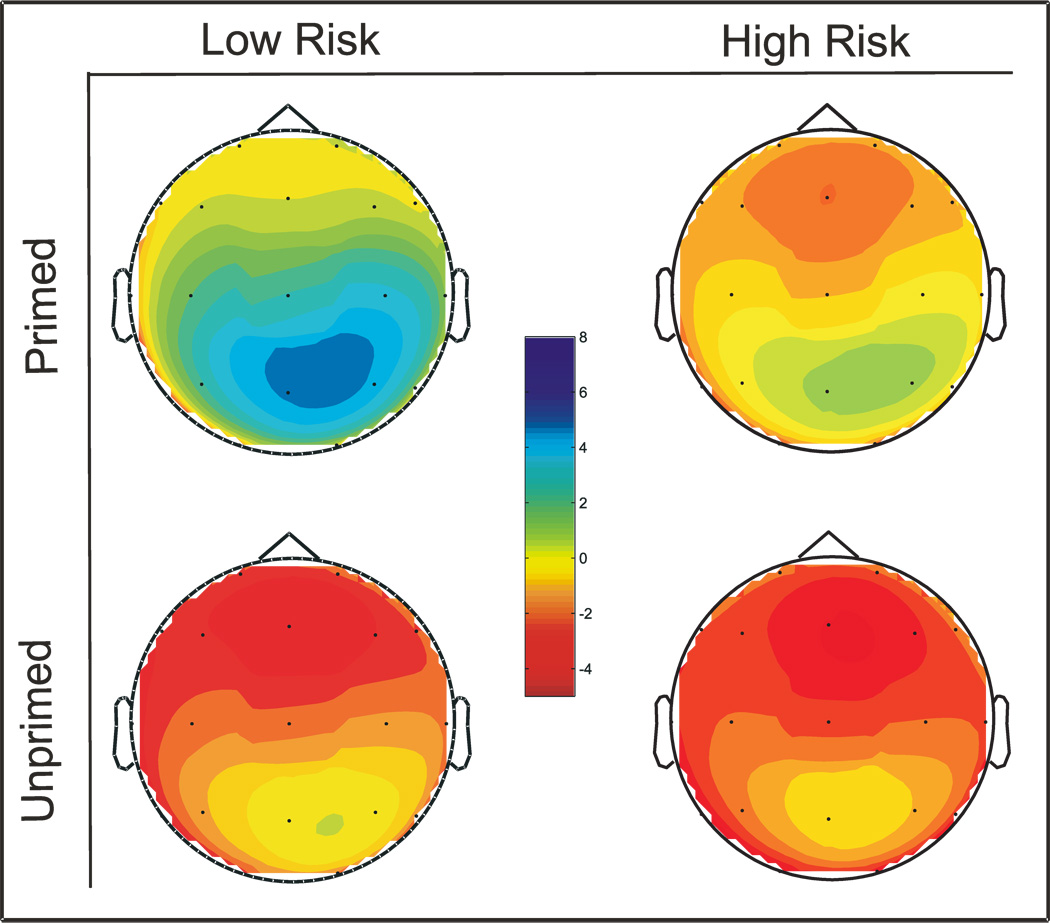
The mean peak N4 amplitude for the LR group was 2.00 µV (SE = .92) and −2.23 µV (SE = .68) for the primed and unprimed words respectively. Mean peak amplitude for the HR group was −1.01 µV (SE = .98) and −2.33 µV (SE = .76) for the primed and unprimed words, respectively. Repeated measures ANCOVA of the amplitude measure showed a main effect for the semantic condition and significant interaction effect between semantic condition and group (table 2). Both groups as a whole had more negative amplitude for the unprimed word compared to primed word. HR subjects exhibited a more negative N4 for primed word compared to LR subjects. For the unprimed condition, the groups did not differ on N4 (Figure 2 & 4a). Significant differences between primed and unprimed words were observed in the LR, but not in HR group (Figure 2 & 4b). These differences are also clearly depicted in the topographical representation (Figure 3). In the secondary analysis where the primed and unprimed conditions were analyzed separately, a significant main effect for group was observed in the primed condition (F = 4.65; p = .036), but not in the unprimed condition (F = 0.10; p = .921), substantiating the results obtained in the primary analysis.
Table 2.
Results of the primary analysis: repeated measures ANCOVA for amplitude and latency measures
| Effect | Amplitude | Latency | ||
|---|---|---|---|---|
| F | Sig. | F | Sig. | |
| Group | 2.23 | .142 | 1.24 | .270 |
| Semantic condition | 16.51 | .0002*** | 0.35 | .852 |
| Scalp region | 3.01 | .059 | 3.10 | .054 |
| Group x Semantic condition | 4.91 | .032* | 0.68 | .414 |
| Group x Scalp region | 0.85 | .436 | 2.23 | .119 |
| Semantic condition x Scalp region | 2.92 | .064 | 2.39 | .103 |
p < .05
p < .001
Figure 2.
Amplitude for the primed and unprimed condition for the two groups. Note that the N4 is positive to the primed word in LR subjects. The star symbol represents significant difference and NS represents No significant difference.
Figure 4.
Top panel (a) represents ERP average waveforms of LR and HR groups for both primed and unprimed semantic conditions (Green dashed circle indicates N4 component at Cz for both primed and unprimed semantic conditions). Bottom panel (b) represents ERP average waveforms of primed and unprimed semantic conditions for LR and HR subjects (Blue dashed circle indicates N4 component at Cz for both groups).
Figure 3.
Scalp topography of N4 amplitude for primed and unprimed words in LR and HR groups. The colors towards dark blue represent positivity and colors towards red represent negativity. For primed words, LR subjects show more attenuation (dark blue) whereas HR subjects show less attenuation (green). Both the group performed similarly for unprimed words.
3.3.2. Latency
The mean N4 latency for the LR group was 320.39 ms (SE = 6.43) and 329.87 ms (SE = 6.23) for the primed and unprimed words, respectively. For the HR group, it was 333.51 ms (SE = 7.13) and 336.29 ms (SE = 6.90) for the primed and unprimed words, respectively. Statistical analysis showed no significant main and/or interaction effects for the latency measures (table 2).
4. Discussion
Previous results in our laboratory in adults using the same lexical decision paradigm as in the present study have demonstrated that, in contrast with controls, adult alcoholics fail to show reduction in N4 amplitude to the primed stimulus compared to the unprimed stimulus (Porjesz et al., 2002). Given these results, the current research was carried out to examine whether the nonalcoholic male offspring of alcoholics have similar impairment as alcoholics in semantic processing as measured through N4 in the same lexical decision paradigm, to determine whether these deficits antecede the development of alcoholism.
We report here that, while LR subjects showed significant N4 attenuation for the primed condition compared to the unprimed condition, this was not observed in HR offspring of alcoholics (Figure 2 & 4). This clear differentiation in the processing of primed and unprimed words demonstrates the priming effect in low risk subjects and the lack of the priming effect in high risk subjects. These results are similar to results obtained for adult male alcoholics using the same paradigm in our laboratory (Porjesz et al., 2002). This demonstrates that risk for alcoholism is associated with lack of N4 attenuation and priming effect. While the HR and LR groups were significantly different for the primed condition, in the unprimed condition, there was no significant difference between the two groups in N4 amplitude (Figure 3 & 4a).
With respect to N4 latency, both HR and LR groups did not differ with each other in either of the semantic conditions. Other studies, with semantic processing paradigms, have shown similar lack of significant differences in N4 latency between alcoholics and controls (Nixon et al., 2002; Ceballos et al., 2005). Moreover, within each group, there were no significant differences in N4 latency between primed and unprimed words. The lack of N4 latency differences between the two groups is consistent with the conclusion that the observed deficits are not linked to a general slowing of cognitive processes related to semantic interpretation (Grillon et al., 1991; Nixon et al., 2002).
In terms of behavioral measures, subjects in both the HR and LR groups showed significantly better performance for the primed words compared to the unprimed words with respect to number of correct responses and RT. This is in accordance with the findings observed in the literature (Meyer and Schvaneveldt, 1971; Osterhout and Holcomb, 1995). However, both groups did not differ significantly from each other in RT, either in primed or in unprimed word conditions.
In the current study, the stimuli were presented sequentially and the subjects had to respond to each stimulus. This paradigm is expected to reduce post-lexical strategies on the semantic priming effect (McNamara and Altarriba, 1988; Silva-Pereyra et al., 1999). However, unlike those studies, the current study had antonym-pairs as compared to related pairs. Therefore, it can be said that ‘expectancy mechanism’ has more influence on semantic priming in the present study, where the subjects had a possibility to generate expectancy for the potential second-word of the antonym-pair. In addition, as the SOA was 1750 ms in the current study, it is assumed that controlled processes are relatively more involved with the priming effect rather than automatic spreading activation. Thus, it appears that expectancy for the second word of the antonym-pair was not (adequately) generated in HR subjects, or that these individuals could not process the inherent semantic relatedness of the antonym-pairs, and thus failed to match the semantic context to that of the antonyms. This indicates deficits in semantic expectancy and post-lexical semantic processing in high risk subjects. Similar deficits have been reported; e.g., in a neuropsychological study, Drejer et al. (1985) found that HR adolescent sons of alcoholic fathers have poorer vocabulary and categorizing ability in comparison with sons of nonalcoholic fathers. Further, Ji et al. (1999) found no significant amplitude difference between category matching and nonmatching process in adult alcoholics, indicating deficient processing of sample stimuli in dealing with processing of matching stimuli.
Regional and hemispheric differences have been observed in the scalp distribution of N4 (Curran et al., 1993). Studies have shown that N4 was maximally negative at the centroparietal region and exhibited larger amplitude at locations in the right hemisphere (Kutas and Hillyard, 1982; Kutas and Van Petten, 1988; Hill et al., 2002; Franklin et al., 2007). These topographic effects were robust across differences in presentation rate as well as in proportion of congruous to incongruous sentences (Kutas and Van Petten, 1988). In contrast to the above findings, we have observed predominantly more negative N4 at frontocentral regions in the left hemisphere for both primed and unprimed word conditions (Figure 3). This may be due to the different semantic priming paradigm adopted in this study that involved antonym-pairs. However, there were no statistically significant differential priming effects with respect to the brain regions and hemispheres. In general, the nature and objectives of this study were more clinical, and aimed at studying whether HR subjects have deficits in semantic priming, rather than to study the differential contribution of automatic spreading activation or controlled processes on semantic priming or the differential activation pattern with respect to the scalp region and hemisphere.
Several studies have already highlighted the genetic predisposition to develop alcoholism in family history positive young offspring (Goodwin, 1979; Cotton, 1979; Begleiter et al., 1984; Schmidt and Neville, 1985; Porjesz and Begleiter, 1990; Kamarajan et al., 2005). The heritability of P3 amplitude has been reported to be high for both visual (Porjesz et al., 1998; Almasy et al., 1999 and auditory P3 O'Connor et al., 1994; Almasy et al., 1999). Many of the electrophysiological indices have served as endophenotypes for alcoholism (Porjesz et al., 2005; Frederick and Iacono, 2006). It is important that these indices demonstrate good heritability. In this regard, a large scale genomewide linkage study using the same semantic priming task showed significant heritabilities for N4 and P3 amplitude in response to primed and unprimed words. As for the P3 component, the N4 component too showed significant genetic correlations, indicating shared genetic influences. Suggestive evidence of linkage for N4 amplitudes has been noted in several chromosomal regions (Almasy et al., 1999; Almasy et al., 2001). Studies have shown decreased P3 amplitude in alcohol dependent individuals (Porjesz and Begleiter, 1985), that persisted after long abstinence (Porjesz and Begleiter, 1987), and in young alcohol-naïve children of alcoholic probands (Begleiter et al., 1984; Polich et al., 1994). P3 amplitude is thought to be related to stimulus significance and the reduced amplitude in alcoholics and their offspring implies difficulty in discriminating stimulus relevance (Porjesz and Begleiter, 1996). In a semantic priming study, impairment for associated targets during alcohol intake in subjects with positive parental history compared to negative parental history for alcoholism was observed (Sayette et al., 2001). Similarly, adult male alcoholics (Porjesz et al., 2002) and HR male subjects in the current study, showed lack of priming effect as well as lack of discrimination between the primed and unprimed stimuli. Based on these results, it appears that the inability to discriminate between primed and unprimed words and lack of priming effect seen in alcoholics is already present in high risk offspring of alcoholics prior to alcohol dependence. The results suggest that the lack of N4 attenuation to the primed word in HR subjects might reflect another aspect of a vulnerability to alcohol dependence that may have genetic underpinnings. Therefore, the lack of N4 attenuation to the primed stimuli found in nonalcoholic sons of alcoholics may be an electrophysiological endophenotype that characterizes the genetic vulnerability to alcohol dependence.
The results of the study should be viewed with caution because of a few limitations. Six subjects in the HR group and 3 subjects in the LR group had marijuana dependence, and one from each group had cocaine dependence. However, visual analysis of the wave pattern of these subjects did not show any gross deviation from others in the same group. Analysis of the data without including these subjects still yielded similar results. In addition, it has been shown that the presence of comorbid marijuana and cocaine dependence did not have any effect on N4 of the alcoholic subjects in a similar semantic priming task (Ceballos et al., 2005). On the other hand, subjects in both groups were not matched on any measure of intelligence. However, this might not be a major limitation considering the reaction time and latency measures did not differ between the two groups. Another, important factor to consider is that the high risk subjects, despite having a parent who is alcohol dependent, were not alcohol dependent themselves, though they may have the genetic risk to develop alcoholism and/or related disorders. Alcoholism is a complex disorder that is caused by multiple factors, genetic and environmental, and an interaction of both. Not all high risk offspring of alcoholic parents become alcohol dependent, and even if they carry a genetic predisposition, genetic risk alone is not sufficient to cause alcohol dependence (Grant, 1998; Grant and Dawson, 1997; Hingson et al., 2006; Liu et al., 2004a; Liu et al., 2004b). The clinical outcome can be due to several factors, including genetic, familial, social and the interaction of all of these factors; some offspring in high risk families even carry protective factors, preventing them from becoming alcohol dependent themselves (Volkow et al., 2006; Kuehn, 2006). In the future, further studies are required to explore the relationship between the reduced P3 amplitude found in alcoholics and their nonalcoholic offspring, and the lack of N4 differentiation between primed and unprimed stimuli. Similarly, studies are required to identify the protective factors that shield some subjects from high risk families from developing alcoholism.
Conclusion
The current findings support the earlier results of lack of N4 attenuation to primed words and/or inability to differentiate between the primed and unprimed words in alcoholics (Ji et al., 1999; Porjesz et al., 2002). However, as the earlier studies were conducted on alcoholic subjects, and the current study was conducted on subjects who are at HR to develop alcoholism, this suggests that the deficits were already present before the development of alcoholism. This indicates that similar to alcoholics, the HR subjects have deficits in semantic strategies especially in semantic expectancy and post-lexical semantic processing. These deficits in young male nonalcoholic offspring of alcoholics may represent an electrophysiological endophenotype that characterizes a genetic vulnerability to develop alcoholism and cognitive impairments. Follow up studies with this paradigm are underway in adolescents and young adults as part of the COGA prospective study.
Appendix
The Collaborative Study on the Genetics of Alcoholism (COGA), Co-Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes nine different centers where data collection, analysis, and storage take place. The nine sites and Principal Investigators and Co-Investigators are: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice); University of California at San Diego (M. Schuckit); Howard University (R. Taylor); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy). Max Guo serves as the NIAAA Staff Collaborator. This national collaborative study is supported by the NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
In memory of Henri Begleiter and Theodore Reich, Principal and Co-Principal Investigators of COGA since its inception; we are indebted to their leadership in the establishment and nurturing of COGA, and acknowledge with great admiration for their seminal scientific contributions to the field.
The superlative assistance of Ashwini K Pandey, Yongqiang Tang, Chamion Thomas, Tracy Crippen, Eric Talbert, Glenn Murawski, and Carlene Haynes is gratefully acknowledged.
References
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O'Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88(4):383–390. [PubMed] [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Goate A, Edenberg HJ, Chorlian DB, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Foroud T, Rice JP, Reich T, Begleiter H. Genetics of event-related brain potentials in response to a semantic priming paradigm in families with a history of alcoholism. Am J Hum Genet. 2001;68(1):128–135. doi: 10.1086/316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23(7):1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock VM, Porjesz B, Li TK, Schuckit MA, Edenberg HJ, Rice JP. The collaborative study on the genetics of alcoholism. Alcohol Health Res World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Bentin S. Electrophysiological studies of visual word perception, lexical organization, and semantic processing: a tutorial review. Lang Speech. 1989;32:205–220. doi: 10.1177/002383098903200302. [DOI] [PubMed] [Google Scholar]
- Bentin S, Kutas M, Hillyard SA. Electrophysiological evidence for task effects on semantic priming in auditory word processing. Psychophysiology. 1993;30(2):161–169. doi: 10.1111/j.1469-8986.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- Brown C, Hagoort P. The processing nature of the N400: evidence from masked priming. J Cogn Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Houston RJ, Smith ND, Bauer LO, Taylor RE. N400 as an index of semantic expectancies: differential effects of alcohol and cocaine dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):936–943. doi: 10.1016/j.pnpbp.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Hagoort P, Brown CM. The mechanism underlying backward priming in a lexical decision task: Spreading activation versus semantic matching. The Quarterly Journal of Experimental Psychology. 1998;51A(3):531–560. [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997;21(8):1398–1406. [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–428. [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. J Stud Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Langendoen Y, Bijl RV. Psychiatric disorders in adult children of problem drinkers: prevalence, first onset and comparison with other risk factors. Addiction. 1999;94(10):1489–1498. doi: 10.1046/j.1360-0443.1999.941014895.x. [DOI] [PubMed] [Google Scholar]
- Curran T, Tucker DM, Kutas M, Posner MI. Topography of the N400: brain electrical activity reflecting semantic expectancy. Electroencephalogr Clin Neurophysiol. 1993;88(3):188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- De Groot AMB, Thomassen AJWM, Hudson PTW. Primed lexical decision: The effect of varying the stimulus-onset asynchrony of prime and target. Acta Psychologica. 1986;61:17–36. [Google Scholar]
- Deacon D, Grose-Fifer J, Yang CM, Stanick V, Hewitt S, Dynowska A. Evidence for a new conceptualization of semantic representation in the left and right cerebral hemispheres. Cortex. 2004;40(3):467–478. doi: 10.1016/s0010-9452(08)70140-0. [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang C, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: evidence that the N400 does not reflect a post-lexical process. Brain Res Cogn Brain Res. 2000;9(2):137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Deacon D, Uhm TJ, Ritter W, Hewitt S, Dynowska A. The lifetime of automatic semantic priming effects may exceed two seconds. Brain Res Cogn Brain Res. 1999;7(4):465–472. doi: 10.1016/s0926-6410(98)00034-2. [DOI] [PubMed] [Google Scholar]
- den Heyer K, Briand K, Dannenbring GL. Strategic factors in a lexical-decision task: evidence for automatic and attention-driven processes. Mem Cognit. 1983;11(4):374–381. doi: 10.3758/bf03202452. [DOI] [PubMed] [Google Scholar]
- Drejer K, Theilgaard A, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: neuropsychological assessment. Alcohol Clin Exp Res. 1985;9(6):498–502. doi: 10.1111/j.1530-0277.1985.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30(12):2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler I, Bloom PA, Childers DG, Roucos SE, Perry NW., Jr Brain potentials related to stages of sentence verification. Psychophysiology. 1983;20(4):400–409. doi: 10.1111/j.1469-8986.1983.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Forbes CT. Graded and Classified Spelling Lists for Teachers Grades 2–8. Cambridge, MA: Educators Publishing Service; 1968. [Google Scholar]
- Franklin MS, Dien J, Neely JH, Huber E, Waterson LD. Semantic priming modulates the N400, N300, and N400RP. Clin Neurophysiol. 2007;118(5):1053–1068. doi: 10.1016/j.clinph.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Frederick JA, Iacono WG. Beyond the DSM: Defining endophenotypes for genetic studies of substance abuse. Current Psychiatry Reports. 2006;8:144–150. doi: 10.1007/s11920-006-0014-2. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kutas M, Sereno MI. The search for "common sense": an electrophysiological study of the comprehension of words and pictures in reading. J Cogn Neurosci. 1996;8:89–106. doi: 10.1162/jocn.1996.8.2.89. [DOI] [PubMed] [Google Scholar]
- Goodwin DS. Alcoholism and heredity: A review and hypothesis. Arch Gen Psychiatry. 1979;36:57–61. doi: 10.1001/archpsyc.1979.01780010063006. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22(2):144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biol Psychiatry. 1991;29(5):467–480. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Friederici AD. Concerning the automaticity of syntactic processing. Psychophysiology. 1999;36(1):126–137. doi: 10.1017/s004857729997155x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Friedman D, Ritter W, Rosen J. Event-related potential and behavioral correlates of semantic processing in Alzheimer's patients and normal controls. Brain Lang. 1995;48(1):33–68. doi: 10.1006/brln.1995.1002. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol. Med. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hegedus AM, Alterman AI, Tarter RE. Learning achievement in sons of alcoholics. Alcohol Clin Exp Res. 1984;8(3):330–333. doi: 10.1111/j.1530-0277.1984.tb05522.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hill H, Strube M, Roesch-Ely D, Weisbrod M. Automatic vs. controlled processes in semantic priming--differentiation by event-related potentials. Int J Psychophysiol. 2002;44(3):197–218. doi: 10.1016/s0167-8760(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B. Alcoholism: the dissection for endophenotypes. Dialogues Clin Neurosci. 2005;7(2):153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2006;118(3):e755–e763. doi: 10.1542/peds.2006-0223. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ. Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology. 1993;30(1):47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. EEG Journal. 1958;10:371–375. [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H. Event-related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol Psychiatry. 1999;45(4):494–507. doi: 10.1016/s0006-3223(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005;69(3):353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. The N400 is modulated by unconsciously perceived masked words: further evidence for an automatic spreading activation account of N400 priming effects. Cognitive Brain Research. 2002;13(1):27–39. doi: 10.1016/s0926-6410(01)00085-4. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Spitzer M. Time course of conscious and unconscious semantic brain activation. Neuroreport. 2000;11(11):2401–2407. doi: 10.1097/00001756-200008030-00013. [DOI] [PubMed] [Google Scholar]
- Koivisto M. Categorical priming in the cerebral hemispheres: automatic in the left hemisphere, postlexical in the right hemisphere? Neuropsychologia. 1998;36(7):661–668. doi: 10.1016/s0028-3932(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Kucera, Francis WN. Computational Analysis of Present-Day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Kuehn BM. Protective factors may prevent alcoholism. Journal of the American Medical Association. 2006;296(15):1828–1829. doi: 10.1001/jama.296.15.1828. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Porjesz B, Arndt S, Bauer L, Begleiter H, Cizadlo T, O'Connor S, Rohrbaugh J. Multi-center N400 ERP consistency using a primed and unprimed word paradigm. Electroencephalogr Clin Neurophysiol. 1995;94(6):462–470. doi: 10.1016/0013-4694(94)00312-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. The lateral distribution of event-related potentials during sentence processing. Neuropsychologia. 1982;20(5):579–590. doi: 10.1016/0028-3932(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307(5947):161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. An electrophysiological probe of incidental semantic association. Journal of Cognitive Neuroscience. 1989;1(1):38–49. doi: 10.1162/jocn.1989.1.1.38. [DOI] [PubMed] [Google Scholar]
- Kutas M, Lindamood T, Hillyard SA. Word expectancy and event-related brain potentials during sentence processing. In: Kornblum S, Requin J, editors. Preparatory states and processes. Hillsdale, NJ: Lawrence Erlbaum; 1984. pp. 217–238. [Google Scholar]
- Kutas M, Van Petten C. Event-related potential studies of language. In: Ackles PK, Jennings JR, Coles GH, editors. Advances in psychophysiology. Greenwich, CT: JAI Press; 1988. pp. 138–187. [Google Scholar]
- Liu IC, Blacker DL, Xu R, Fitzmaurice G, Lyons MJ, Tsuang MT. Genetic and environmental contributions to the development of alcohol dependence in male twins. Arch Gen Psychiatry. 2004a;61(9):897–903. doi: 10.1001/archpsyc.61.9.897. [DOI] [PubMed] [Google Scholar]
- Liu IC, Blacker DL, Xu R, Fitzmaurice G, Tsuang MT, Lyons MJ. Genetic and environmental contributions to age of onset of alcohol dependence symptoms in male twins. Addiction. 2004b;99(11):1403–1409. doi: 10.1111/j.1360-0443.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PM, Kingstone A. Effects of alcohol on word categorization and recognition memory. Br J Psychol. 1987;78:233–239. doi: 10.1111/j.2044-8295.1987.tb02242.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC. Modulation of semantic processing by spatial selective attention. Electroencephalogr Clin Neurophysiol. 1993;88(3):210–219. doi: 10.1016/0168-5597(93)90005-a. [DOI] [PubMed] [Google Scholar]
- McNamara T, Altarriba J. Depth of spreading activation revisited: Semantic mediated priming occurs in lexical decisions. Journal of Memory and Language. 1988;27:545–559. [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J Exp Psychol. 1971;90(2):227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Neely JH, Keefe DE. Semantic context effects on visual word processing: A hybrid prospective-retrospective processing theory. In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 24. New York: Academic Press; 1989. pp. 207–248. [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian JC, Li TK. Heritable features of the auditory oddball event-related potential: peaks, latencies, morphology and topography. Electroencephalogr Clin Neurophysiol. 1994;92(2):115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related potentials and language comprehension. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-Related Brain Potentials and Cognition. New York: Oxford Univresity Press; 1995. pp. 171–215. [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15(5):839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115(1):55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RD, Van Thiel D, editors. Alcohol and the Brain. New York: Plenum Press; 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butters N, Nathan P, editors. Neuropsychology of alcoholism. Implications for diagnosis and treatment. New York: Plenum Press; 1987. pp. 45–63. [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7(5):465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. Alcohol and alcoholism. Vol. 2. New York: Oxford University Press; 1996. pp. 207–247. [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27(2):153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. The N2 component of the event-related brain potential in abstinent alcoholics. Electroencephalogr Clin Neurophysiol. 1987;66(2):121–131. doi: 10.1016/0013-4694(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O'Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22(6):1317–1323. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61(1–2):229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcohol Clin Exp Res. 2001;25(4):531–539. [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23(7):1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156(1):34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res. 1996;20(1):9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Realmuto G, Begleiter H, Odencrantz J, Porjesz B. Event-related potential evidence of dysfunction in automatic processing in abstinent alcoholics. Biol Psychiatry. 1993;33(8–9):594–601. doi: 10.1016/0006-3223(93)90097-w. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Perrott MA, Wertz JM. Parental alcoholism and the effects of alcohol on mediated semantic priming. Exp Clin Psychopharmacol. 2001;9(4):409–417. [PubMed] [Google Scholar]
- Schmidt AL, Neville HJ. Language processing in men at risk for alcoholism: an event-related potential study. Alcohol. 1985;2(3):529–533. doi: 10.1016/0741-8329(85)90129-6. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics of the risk for alcoholism. Am J Addict. 2000;9(2):103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100(4):427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53(8):681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Silva-Pereyra J, Harmony T, Villanueva G, Fernandez T, Rodriguez M, Galan L, Diaz-Comas L, Bernal J, Fernandez-Bouzas A, Marosi E, Reyes A. N400 and lexical decisions: automatic or controlled processing? Clin Neurophysiol. 1999;110(5):813–824. doi: 10.1016/s1388-2457(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Hegedus AM, Goldstein G, Shelly C, Alterman AI. Adolescent sons of alcoholics: neuropsychological and personality characteristics. Alcohol Clin Exp Res. 1984;8(2):216–222. doi: 10.1111/j.1530-0277.1984.tb05842.x. [DOI] [PubMed] [Google Scholar]
- Taylor WL. 'Cloze procedure': a new tool for measuring readability. Journalism Quarterly. 1953;30:415–433. [Google Scholar]
- Toglia MP, Battig WF, Barrow K, Cartwright DS, Posnansky CJ, Pellegrino JW, Moore TJ, Camilli GA. Handbook of Semantic Word Norms. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH. Effect of alcohol on recall and recognition as functions of processing levels. J Stud Alcohol. 1984;45(1):10–15. doi: 10.15288/jsa.1984.45.10. [DOI] [PubMed] [Google Scholar]