Abstract
Background
Fever is a common presenting complaint to the emergency department (ED), and the evaluation of the febrile child remains a challenging task.
Objective
The aim of this study was to examine the relationship between secretory phospholipase A2 (sPLA2) and infection in febrile children.
Methods
A prospective convenience sample of children presenting with fever to an urban pediatric ED were studied. Blood and urine cultures, a complete blood count, and serum concentrations of sPLA2 were obtained, and patients were compared based on their final diagnosis of either a viral or bacterial infection.
Results
In the 76 patients enrolled, 60 were diagnosed with a viral infection, 14 with a bacterial infection, 1 with Kawasaki disease, and 1 with acute lymphoblastic leukemia. The difference in the serum concentration of sPLA2 in patients with viral infections (22 ± 34 ng/mL) versus those with bacterial infections (190 ± 179 ng/mL) was statistically significant (P < .0001). Receiver operator characteristic curve analysis revealed that sPLA2 was more accurate at predicting bacterial infection (area under the curve = 0.89) than the total white blood cell count (area under the curve = 0.71) and that a value of more than 20 ng/mL had a sensitivity of 93%, specificity of 67%, positive predictive value of 39%, and negative predictive value of 97%.
Conclusion
Secretory phospholipase A2 differs significantly in children with viral versus bacterial infection and seems to be a reliable screening test for bacterial infection in febrile children.
1. Introduction
Despite fever being one of the most common reasons why children are taken to emergency departments (EDs) for evaluation, the management of a febrile child without a clinically apparent source of infection still differs among practicing physicians. Fortunately, the prevalence of serious bacterial infections (SBIs) has significantly decreased with the implementation of universal vaccination programs [1,2]. However, because occult bacterial infections still occur as a result of vaccine failures and also by strains not covered by routine immunizations, it is unclear to what extent a febrile child needs to be evaluated [3,4]. Although most cases are likely to be caused by self-limiting viral infections, a significant percentage are bacterial in etiology including pneumonia, urinary tract infection, meningitis, bacterial gastroenteritis, osteomyelitis, and bacteremia, all of which may be associated with significant morbidity and mortality [5-7].
Because it is often difficult to clinically identify children with SBIs, physicians have resorted to laboratory studies to support making a diagnosis. Culture technique remains the criterion standard in detecting bacterial infection; however, the average time for a culture to become positive is 15 hours and may be as long as 24 to 48 hours [7]. This delay in making a definitive diagnosis may increase the risk of complication if a bacterial infection is left untreated. As a result, young children are often hospitalized for observation and/or started on empiric antibiotic therapy until culture results are available. In an effort to identify children at risk for bacterial infection, several diagnostic screening tests including total white blood cell count (WBC), C-reactive protein, and absolute neutrophil count have been studied as markers of systemic infection [8-11]. Although these test results can be obtained within a reasonable amount of time, because of their relatively low sensitivities and specificities, decisions based on their results can lead to unnecessary treatment and hospitalization. More recent studies have revealed that the inflammatory mediator procalcitonin has definite utility as a marker of systemic infection [12-20]. However, patient characteristics and clinical settings have varied greatly, making it difficult for physicians to implement its use in the ED. Consequently, several decision rules and predictive models have been developed to aid in the management of the febrile child [21,22]. However, despite these tools, clinicians have yet to come to a consensus on the evaluation of fever in the ED, and thus, there still remains a need to identify other potential predictors of SBI in febrile children.
Physiologically, a host's response to a bacterial infection results in the release of a number of inflammatory mediators. Despite extensive research on these compounds, few studies have examined their roles as predictors of bacterial infection in children. Type IIa secretory phospholipase A2 (sPLA2) is an enzyme that is generated and secreted by cells in response to a number of inflammatory stimuli [23]. In several studies, increased levels of sPLA2 have been shown to be associated with a number of diseases involving inflammation, including bacterial infection in adults, with high levels correlating with multiorgan system failure and death [24-29]. Because infections often result in varying degrees of inflammation, and sPLA2 plays an important role in the inflammatory process, we postulate that measuring sPLA2 levels in febrile children might help to differentiate those patients with viral versus bacterial infections.
The purposes of this study are to examine the relationship between sPLA2 and the infected febrile child and to evaluate its ability to discriminate between viral and bacterial infection.
2. Methods
A prospective study was conducted on a convenience sample of febrile children presenting to an urban pediatric ED between October 2004 and August 2005. All children 3 months or older with a measured temperature of 38.5°C or higher and those younger than 3 months with a measured temperature of 38.0°C or higher were candidates for enrollment. Those with a clinically apparent infection, a previous diagnosis of an acute or chronic inflammatory disease, a history of antibiotic use within 7 days before presentation or who had received a vaccination within 2 days before presentation were excluded. The decision to evaluate each patient for occult bacterial infection was made by an ED physician, and those requiring both blood and urine collection for evaluation were enrolled into the study. The study protocol was reviewed and approved by the institutional review board, and informed consent was obtained for all patients.
Patient data including age, sex, maximum temperature, duration of fever, antibiotic use/duration of treatment, and length of hospital stay were documented for all patients enrolled. Blood and urine specimens were obtained before the administration of any antibiotics. Blood was collected using sterile technique for a complete blood count with differential WBC count, blood culture, and serum sPLA2 analysis. Urine specimens were obtained by urethral catheterization using sterile technique for urinalysis, microscopy, and urine culture. The differential WBC and urinalysis were determined by hospital laboratory technicians using automated equipment and microscopy. All blood and urine specimens were cultured and monitored for a total of 5 days. A positive blood culture was defined as the isolation of a single bacterial pathogen, and a positive urine culture as growth more than 100 000 colony forming units/mL of a single urinary tract pathogen. Serum sPLA2 concentrations were measured directly by an Enzyme Immunometric Assay outlined by Cayman Chemical (Ann Arbor, Mich).
2.1. Statistical analysis
The primary outcome measure was culture proven bacterial infection. Patients with negative blood and urine cultures were presumed to have a viral infection. Student t tests were performed to test for differences between the infection groups for each of the demographic variables. To examine the relationship between sPLA2 and bacterial infection, a nonparametric Wilcoxon test was used comparing patients with viral versus bacterial infection. Receiver operator characteristic (ROC) curves were constructed for both sPLA2 and the total WBC count to assess their ability to discriminate between viral and bacterial infection. A c2 test was used to compare the correlated ROC curve areas. The sensitivities, specificities, and predictive values for the test at various cut points were calculated, and an optimal cutoff was determined. A significance level of .05 was used for all statistical tests.
3. Results
3.1. Characteristics of study subjects
Eighty-one children were initially enrolled in the study; however, 5 were excluded because an insufficient amount of either blood or urine was collected for complete analysis. In the 76 patients studied, 60 (79%) had a presumed viral infection, 12 (16%) had a urinary tract infection, 2 (3%) had pneumococcal bacteremia, 1 had Kawasaki disease (KD), and 1 had acute lymphoblastic leukemia as their final diagnosis. The patients with KD and leukemia were excluded from further analysis based on their final diagnosis.
Patient characteristics are summarized in Table 1. The median age was 7 months, with a range of 2 weeks to 14 years and an interquartile range of 13.75 months. There was no difference in sex, mean temperature, duration of fever, or length of hospitalization between patients with viral versus bacterial infection. Twenty-eight percent of patients with presumed viral infections were hospitalized, compared with 71% of patients with bacterial infection.
Table 1.
A comparison of the patients studied
| Viral (n = 60) | Bacterial (n = 14) | P * | |
|---|---|---|---|
| Age (mo), median (SD) | 8 (31.0) | 5 (18.8) | .149 |
| Sex (% male) | 50 | 57 | .769 |
| Maximum temperature (°C) (SD) | 39.4 (0.8) | 39.2 (0.4) | .316 |
| Duration of fever (d) (SD) | 2.1 (1.7) | 2.1 (1.2) | .980 |
| Hosp admission (%) | 28 | 71 | .0003 |
| Length of hospital stay (d) (SD) | 2.1 (0.5) | 2.3 (2.1) | .823 |
| WBC mean (SD) | 11.9 (5.5) | 16.4 (5.4) | .009 |
| sPLA2 mean (SD) | 21.6 (33.6) | 189.5 (178.5) | <.0001 |
P values based on Student t test, Wilcoxon, or c2 test.
3.2. sPLA2 and Infection
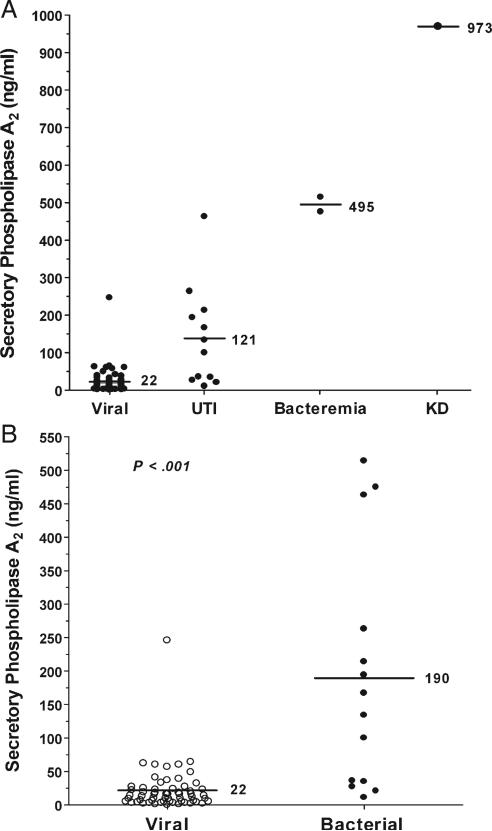
Serum concentrations of sPLA2 for the study population are shown in Fig. 1. We found a significant increase in the serum concentration of sPLA2 in the patients with urinary tract infection (121 ± 133 ng/mL) and bacteremia (495 ± 27 ng/mL) compared with those with presumed viral infection (22 ± 34 ng/mL). When patients with bacterial infection were compared with those with presumed viral infection, we found that there was a statistically significant difference in the serum concentrations of sPLA2 between the 2 groups (P < .001).
Fig. 1.
Mean serum concentrations of sPLA2 in the study population (A) and in patients with presumed viral (B; n = 60) versus proven bacterial infection (n = 14). The study population (A) included patients ultimately diagnosed with a presumed viral infection (viral; n = 60), urinary tract infection (UTI; n = 12), pneumococcal bacteremia (Bacteremia; n = 2), and KD (n = 1). Serum concentrations of sPLA2 are higher in patients with bacterial infection than in those with viral infection (P < .001). The highest sPLA2 value is identified in a patient with KD.
Of note, the highest concentration of sPLA2 measured was in a patient with KD (973 ng/mL). Subsequently, an sPLA2 concentration of 840 ng/mL was measured in one other febrile patient with a presumptive diagnosis of KD. Because this patient was not enrolled as part of the original study, the data observed were not included in any of the analyses.
3.3. Sensitivity, specificity, and predictive value analysis
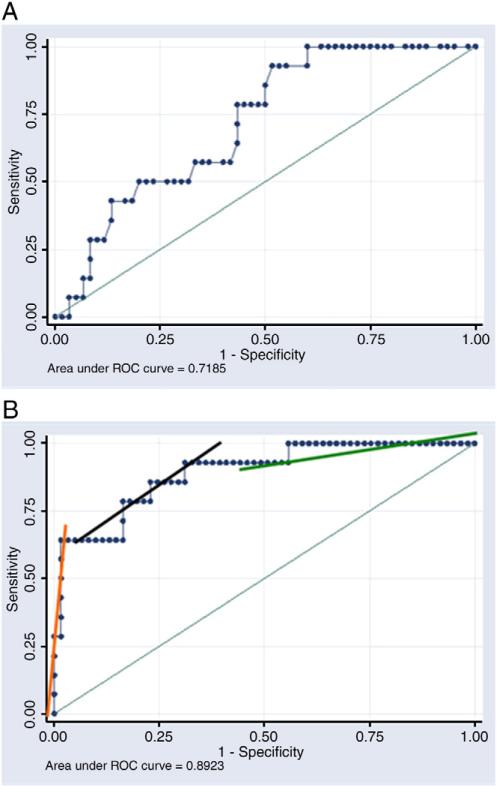
The diagnostic properties of sPLA2 and WBC count were analyzed using ROC curves (Fig. 2). The area under the curve (AUC) for sPLA2 was 0.892 (95% confidence interval [CI], 79.8-98.5) compared with 0.718 (95% CI, 60.4-86.2) for WBC (P = .079). Based on the ROC analysis, sensitivities, specificities, and positive and negative predictive values were calculated for sPLA2 at various concentrations (Table 2).
Fig. 2.
Receiver operator characteristic curves for WBC (A) and sPLA2 (B). Secretory phospholipase A2 was a more accurate biomarker of bacterial infection than the WBC count. Three distinct regions are identified as demonstrated by the 3 lines on the curve in B. The slope of the middle section approximates the value of 1, which means that sPLA2 values in this region do very little to alter the pretest probability. The other 2 sections have very high and very low slopes, and values in these ranges alter the pretest probability substantially. Extrapolating from the ROC curve, sPLA2 performs well above the value of 100 ng/mL and below the value of 20 ng/mL.
Table 2.
Sensitivities, specificities, and predictive values for sPLA2
| sPLA2 (ng/mL) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| 20 | 93 (66-100) | 67 (53-78) | 39 (23-58) | 97 (87-100) |
| 30 | 79 (49-95) | 80 (67-89) | 47 (27-69) | 94 (84-99) |
| 40 | 64 (35-87) | 87 (87-94) | 53 (28-77) | 91 (81-97) |
| 50 | 64 (35-87) | 90 (79-96) | 60 (32-84) | 92 (81-97) |
| 100 | 64 (35-87) | 98 (91-100) | 90 (55-100) | 92 (83-97) |
PPV indicates positive predictive value; NPV, negative predictive value. Optimum diagnostic values, derived from the ROC curve, are shown in bold.
4. Discussion
Although an extensive body of research has been done on several markers of inflammation, the development of improved methods to determine the presence of bacterial infection in children remains a critically unmet need. In the present study, we demonstrated that among febrile children presenting to our pediatric ED, serum concentrations of sPLA2 differ significantly between those with presumed viral infection and those with known bacterial infection. These results support our hypothesis that an increased serum concentration of sPLA2 is associated with a bacterial infection and may be a useful screening tool.
To evaluate the ability of sPLA2 at predicting bacterial infection, an ROC curve was constructed. The AUC represents the overall accuracy of a test, and as a rule, values between 0.7 and 0.8 have minimal accuracy, values between 0.8 and 0.9 have good accuracy, and values greater than 0.9 have excellent accuracy [10]. When sPLA2 was compared with the total WBC count, the most commonly used diagnostic screening test for bacterial infection, it proved to be a more accurate test at determining bacterial infection, with an AUC = 0.892 (95% CI, 79.8-98.5), compared with the WBC count, AUC = 0.718 (95% CI, 60.4-86.2).
In examining the sPLA2 ROC curve further, we identified 3 distinct regions as demonstrated by the 3 lines on the curve in Fig. 2B. The slope of the middle section approximates the value of 1, which means that sPLA2 values in this region do very little to alter the pretest probability. The other 2 sections have very high and very low slopes, and values in these ranges alter the pretest probability substantially. Extrapolating from the ROC curve, we found that the test performs well above the value of 100 ng/mL and below the value of 20 ng/mL. Although neither of these points represent the optimal combination of sensitivity and specificity, they are still clinically useful. An sPLA2 value less than 20 ng/mL essentially “rules out” bacterial infection with a high negative predictive value. This is of significant importance with regard to the emergence of multidrug resistant bacteria, risk of allergic reactions, and the financial costs associated with empiric antibiotic use [30,31]. Conversely, an sPLA2 value greater than 100 ng/mL would be concerning for the presence of a bacterial infection, having a relatively high positive predictive value. In such cases, empiric antibiotic use far outweighs the risk of missing a SBI.
The main limitation to this study was its small sample size. Consequently, we were unable to control for potential confounding variables such as age, anti-inflammatory use, or varying practice patterns among physicians. It is possible that the immune system of an infant might not be able to mount the same systemic response to an infection as that of an older child, thereby affecting the production of various inflammatory mediators such as sPLA2. Also, the effect of anti-inflammatory medications on sPLA2 production is not known. Furthermore, because attending physician practices differ, not all patients with fever have both blood and urine collected for analysis, thereby excluding them from enrollment and possibly creating bias toward those patients who were either very young or ill-appearing. However, despite these limitations, we believe that these results are promising and need to be tested in a separate prospective validation study.
Lastly, an interesting finding was that the highest recorded values of sPLA2 were seen in patients with KD. This is not surprising because KD is a known systemic inflammatory condition. However, the marked increase raises the possibility of sPLA2 as a potential marker for KD. Although this question certainly cannot be answered in this study, our observation of the high levels of sPLA2 in 2 patients with KD (973 and 840 ng/mL) warrants further investigation.
In conclusion, increased levels of sPLA2 have been associated with a number of disease processes involving inflammation, and in this study, we have shown another example of its potential use in clinical practice. We demonstrated that serum concentrations of sPLA2 are higher in patients with bacterial infection than in those with presumed viral infection. It is also a more accurate predictor of bacterial infection than the WBC and may be a useful tool, either alone or in conjunction with other markers, in identifying febrile children at risk for bacterial disease. Although sPLA2 can be measured using rapid and inexpensive commercially available kits, unfortunately, the assay is not routinely available in most clinical laboratories. We argue that given its potential role in patient care, its availability should be considered.
Acknowledgments
We would like to thank the Emergency Department and PCRC staff at Children's Hospital & Research Center Oakland, as well as Drs Lee Learman, Kathleen Chang, and Michael Ansari for their support and Mark Hudes, Meredith Milet, and Ginny Gildengorin for their statistical assistance. We would also like to acknowledge the generous donation from The Harleen Family to support the Jonathan Grisham Pediatric Emergency Medicine Fellowship at Children's Hospital & Research Center Oakland.
Footnotes
Sources of support: Pediatric Clinical Research Center at Children's Hospital & Research Center Oakland grant M01-RR01271 (awarded to K. M.M.). National Institute of Health grants 5 U54 HL-070583, HL-66355 (awarded to F.A.K.). National Institute of Health grant HL-04386-05 (awarded to C.R.M.).
Presentations: The work described in this manuscript was presented at the Pediatric Academic Societies Meeting as an Emergency Medicine Platform Session Presentation on April 29, 2006, San Francisco, Calif.
References
- 1.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004;113:443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 3.Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. Changes in Streptococcus pneumoniae serotypes causing invasive disease with non-universal vaccination coverage of the seven-valent conjugate vaccine. Clin Microbiol Infect. 2008;14(9):835–43. doi: 10.1111/j.1469-0691.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee GM, Harper MB. Risk of bacteremia for febrile young children in the post-Haemophilus influenzae type b era. Arch Pediatr Adolesc Med. 1998;152(7):624–8. doi: 10.1001/archpedi.152.7.624. [DOI] [PubMed] [Google Scholar]
- 5.Bachur R, Perry H, Harper MB. Occult pneumonias: empiric chest radiographs in febrile children with leukocytosis. Ann Emerg Med. 1999;33(2):166–73. doi: 10.1016/s0196-0644(99)70390-2. [DOI] [PubMed] [Google Scholar]
- 6.Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102(2):e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 7.Alpern ER, Alessandrini EA, Bell LM, Shaw KN, McGowan KL. Occult bacteremia from a pediatric emergency department: current prevalence, time to detection, and outcome. Pediatrics. 2000;106(3):505–11. doi: 10.1542/peds.106.3.505. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao AL, Baker MD. Fever in the new millennium: a review of recent studies of markers of serious bacterial infection in febrile children. Curr Opin Pediatr. 2005;17(1):56–61. doi: 10.1097/01.mop.0000151781.13635.70. [DOI] [PubMed] [Google Scholar]
- 9.Bonsu BK, Chb M, Harper MB. Identifying febrile young infants with bacteremia: is the peripheral white blood cell count an accurate screen? Ann Emerg Med. 2003;42(2):216–25. doi: 10.1067/mem.2003.299. [DOI] [PubMed] [Google Scholar]
- 10.Isaacman DJ, Burke BL. Utility of the serum C-reactive protein for detection of occult bacterial infection in children. Arch Pediatr Adolesc Med. 2002;156(9):905–9. doi: 10.1001/archpedi.156.9.905. [DOI] [PubMed] [Google Scholar]
- 11.Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics. 2001;108(6):1275–9. doi: 10.1542/peds.108.6.1275. [DOI] [PubMed] [Google Scholar]
- 12.Gendrel D, Bohuon C. Procalcitonin as a marker of bacterial infection. Pediatr Infect Dis J. 2000;19(8):679–87. doi: 10.1097/00006454-200008000-00001. [quiz 688] [DOI] [PubMed] [Google Scholar]
- 13.Andreola B, Bressan S, Callegaro S, Liverani A, Plebani M, Da Dalt L. Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J. 2007;26(8):672–7. doi: 10.1097/INF.0b013e31806215e3. [DOI] [PubMed] [Google Scholar]
- 14.Gendrel D. Use of procalcitonin at the pediatric emergency room. Arch Pediatr. 2004;11(6):582–4. doi: 10.1016/j.arcped.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 15.van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4(10):620–30. doi: 10.1016/S1473-3099(04)01146-6. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez Lopez A, Luaces Cubells C, Garcia Garcia JJ, Fernandez Pou J. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J. 2003;22(10):895–903. doi: 10.1097/01.inf.0000091360.11784.21. [DOI] [PubMed] [Google Scholar]
- 17.Galetto-Lacour A, Zamora SA, Gervaix A. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referral center. Pediatrics. 2003;112(5):1054–60. doi: 10.1542/peds.112.5.1054. [DOI] [PubMed] [Google Scholar]
- 18.Lorrot M, Moulin F, Coste J, et al. Procalcitonin in pediatric emergencies: comparison with C-reactive protein, interleukin-6 and interferon alpha in the differentiation between bacterial and viral infections. Presse Med. 2000;29(3):128–34. [PubMed] [Google Scholar]
- 19.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics. 2008;122(4):701–10. doi: 10.1542/peds.2007-3503. [DOI] [PubMed] [Google Scholar]
- 20.Gendrel D, Bohuon C. Procalcitonin in pediatrics for differentiation of bacterial and viral infections. Intensive Care Med. 2000;26(Suppl 2):S178–81. doi: 10.1007/BF02900734. [DOI] [PubMed] [Google Scholar]
- 21.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108(2):311–6. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
- 22.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36(6):602–14. doi: 10.1067/mem.2000.110820. [DOI] [PubMed] [Google Scholar]
- 23.Pruzanski W, Vadas P. Phospholipase A2—a mediator between proximal and distal effectors of inflammation. Immunol Today. 1991;12(5):143–6. doi: 10.1016/S0167-5699(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 24.Ballas SK, Files B, Luchtman-Jones L, et al. Secretory phospholipase A2 levels in patients with sickle cell disease and acute chest syndrome. Hemoglobin. 2006;30(2):165–70. doi: 10.1080/03630260600642260. [DOI] [PubMed] [Google Scholar]
- 25.Rintala EM, Aittoniemi J, Laine S, Nevalainen TJ, Nikoskelainen J. Early identification of bacteremia by biochemical markers of systemic inflammation. Scand J Clin Lab Invest. 2001;61(7):523–30. doi: 10.1080/003655101753218283. [DOI] [PubMed] [Google Scholar]
- 26.Rintala EM, Nevalainen TJ. Group II phospholipase A2 in sera of febrile patients with microbiologically or clinically documented infections. Clin Infect Dis. 1993;17(5):864–70. doi: 10.1093/clinids/17.5.864. [DOI] [PubMed] [Google Scholar]
- 27.Partrick DA, Moore EE, Silliman CC, Barnett CC, Kuypers FA. Secretory phospholipase A2 activity correlates with postinjury multiple organ failure. Crit Care Med. 2001;29(5):989–93. doi: 10.1097/00003246-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Kuypers FA, Styles LA. The role of secretory phospholipase A2 in acute chest syndrome. Cell Mol Biol (Noisy-le-grand) 2004;50(1):87–94. [PubMed] [Google Scholar]
- 29.Neidlinger NA, Hirvela ER, Skinner RA, Larkin SK, Harken AH, Kuypers FA. Postinjury serum secretory phospholipase A2 correlates with hypoxemia and clinical status at 72 hours. J Am Coll Surg. 2005;200(2):173–8. doi: 10.1016/j.jamcollsurg.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96(3):1152–6. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child. 1983;137(12):1146–9. doi: 10.1001/archpedi.1983.02140380006003. [DOI] [PubMed] [Google Scholar]