Abstract
The sequential flow of electrons in the respiratory chain, from a low reduction potential substrate to O2, is mediated by protein-bound redox cofactors. In mitochondria, hemes—together with flavin, iron–sulfur, and copper cofactors—mediate this multi-electron transfer. Hemes, in three different forms, are used as a protein-bound prosthetic group in succinate dehydrogenase (complex II), in bc1 complex (complex III) and in cytochrome c oxidase (complex IV). The exact function of heme b in complex II is still unclear, and lags behind in operational detail that is available for the hemes of complex III and IV. The two b hemes of complex III participate in the unique bifurcation of electron flow from the oxidation of ubiquinol, while heme c of the cytochrome c subunit, Cyt1, transfers these electrons to the peripheral cytochrome c. The unique heme a3, with CuB, form a catalytic site in complex IV that binds and reduces molecular oxygen. In addition to providing catalytic and electron transfer operations, hemes also serve a critical role in the assembly of these respiratory complexes, which is just beginning to be understood. In the absence of heme, the assembly of complex II is impaired, especially in mammalian cells. In complex III, a covalent attachment of the heme to apo-Cyt1 is a prerequisite for the complete assembly of bc1, whereas in complex IV, heme a is required for the proper folding of the Cox 1 subunit and subsequent assembly. In this review, we provide further details of the aforementioned processes with respect to the hemes of the mitochondrial respiratory complexes. This article is part of a Special Issue entitled: Cell Biology of Metals
Keywords: Heme, Succinate dehydrogenase, Cytochrome c oxidase, Ubiquinol cytochrome c oxidoreductase, Mitochondria, Respiration
1. Introduction
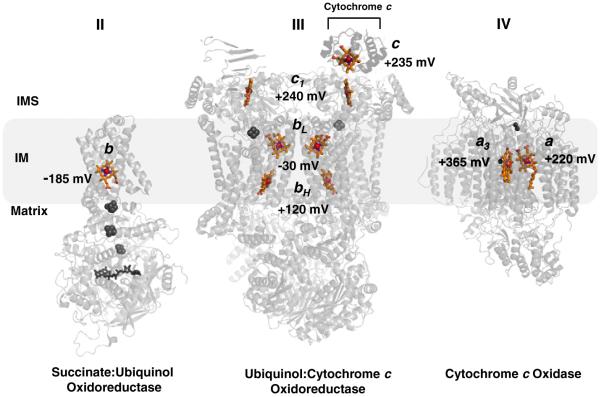
Insertion of a ferrous iron atom into the porphyrin macrocycle by the enzyme ferrochelatase creates heme, which is a common cofactor used for a multitude of biological reactions. Within a protein scaffold, heme can carry out O2 transport [1], nitric oxide and carbon monoxide sensing [2], oxygenase reactions [3], enzyme activity regulation [4,5], and electron transport [6]. The latter function is central in the mechanism associated with the driving force in oxidative phosphorylation. In eukaryotic cells, seven hemes of differing architecture (hemes b, c and a) are embedded in the proteins of the electron transport chain (ETC), and they are reduced and oxidized in generally increasing reduction potentials to transfer four electrons ultimately to reduce O2 (Fig. 1). These “cellular pigments,” or cytochromes, give rise to spectral absorption bands from heme b of complex II (succinate dehydrogenase), hemes b of complex III (bc1 complex), hemes a of complex IV (cytochrome c oxidase) and hemes c of complex III and the soluble electron mediator, cytochrome c. Complex I (NADH Dehydrogenase) contains flavin and iron–sulfur cofactors, but is devoid of hemes.
Fig. 1.
Distribution of hemes b, c, and a, with respective midpoint potentials, in respiratory complexes II, III, and IV in the mitochondria. Note the general trend of increasing redox potential from the b heme of Complex II to the a3 heme of Complex IV, the site of the terminal electron acceptor and O2 reduction. Other cofactors in the complexes are grayed for clarity: Complex II; FAD, [2Fe–2S], [4Fe–4S], [3Fe–4S]: Complex III; Rieske 2Fe–2S: Complex IV; binuclear CuA, CuB. Complex III is shown as a dimer with cytochrome c docked to one of the subunit. References used for midpoint potentials: Complex II [21]; Complex III [124]; Complex IV [125]. The figure was generated using PDB accession numbers: 1YQ3, 3CX5 and 2OCC.
For efficient long-range electron transfer, and to generate a proton gradient across the inner membrane of mitochondria or plasma membrane of bacteria, nature has evolved highly specialized modifications to heme to fine tune the electron relay system. For instance, heme a is present only in terminal oxidases. It is synthesized from heme b (protoheme) —the product of ferrochelatase—by two sequential chemical modifications using two specialized enzymes: heme o synthase (HOS) and heme a synthase (HAS). HOS converts one of the vinyl carbon of the macrocycle into a hydrophobic hydroxyfarnesyl tail (heme o) followed by a HAS catalyzed conversion of one of the methyl carbon of the macrocycle to a formyl group (heme a). The latter methyl group oxidation results in a marked increase in the mid-point potential (~+180 mV) of heme a relative to heme o [7,8]. The farnesyl tail serves a structural role in stabilizing, via hydrophobic interactions, the helical structure of the Cox1 subunit of cytochrome c oxidase (CcO) [9]. These two heme a prosthetic groups are necessary for electron transfer and O2 reduction activities in CcO.
Protein scaffolds are also used to provide a modification to the heme macrocycle. Proteins bind hemes via the combination of three interactions: (i) the interaction between an axial ligand and the heme iron; (ii) hydrophobic interactions between the protein and the porphyrin macrocycle; and (iii) polar interactions between the protein and the propionic acids of the macrocycle [7]. To strengthen this heme-protein interaction, heme b can be covalently attached to the protein backbone, which defines the heme as c-type. Proteins with c-type hemes (cytochromes c) contain a CxxCH heme-binding motif where two cysteine (rarely one cysteine) residues form thioether linkages to the vinyl carbons of the heme macrocycle, and the His residue binds to the axial position of the heme iron.
The above specialization is not necessarily to provide tuning of the redox potential of the heme as previously believed; the potential can more easily be tuned over a wide range using only the protein environment [10,11]. The c-type modification does add structural stability of the heme-protein complex over its cytochrome b globin counterpart [12]. In addition to being a cofactor in the soluble cytochrome c found in the inter-membrane space (IMS) of mitochondria, covalently bound heme is also a cofactor in the cytochrome c1 subunit of the bc1 complex. It is interesting to note that both the non-covalently linked b and a hemes of the respiratory chain reside in the relatively protected environments within the inner membrane (IM) and the matrix, whereas both c hemes of cytochrome c1 and cytochrome c reside in the more oxidizing intermembrane space, analogous to the harsher environment of the periplasm in bacteria where a stable heme linkage might be advantageous.
In this review, we take a hemocentric view of the mitochondrial respiratory chain. We describe the heme cofactors from structural and functional perspectives, and also the assembly of heme cofactor centers within respiratory complexes II, III, and IV. We focus on the respiratory complexes from the yeast Saccharomyces cerevisiae with information from bacterial and mammalian systems when appropriate for comparisons and when yeast information is lacking.
2. The heme moiety of succinate dehydrogenase (SDH)
2.1. Overview
Succinate dehydrogenase (SDH, complex II), also known as succinate:quinone oxidoreductase in bacterial systems (SQR), is a heterotetrameric, integral membrane–protein complex (~120 kDa) that catalyzes the FAD-dependent, two-electron oxidation of succinate to fumarate, coupled with the reduction of ubiquinone to ubiquinol [13–16]. It is one of the eight enzymes of the citric acid cycle. It is also part of the aerobic respiratory chain, as it transfers reducing equivalents to the bc1 complex in the form of quinol. SDH is the only enzyme that couples the Kreb’s cycle to the respiratory electron transport chain, although unlike the other respiratory complexes, there is no proton transfer across the membrane coupled with its activity. The closely related menaquinol:fumarate oxidoreductase (QFR) catalyzes the reverse reaction in bacteria during anaerobic respiration, and due to the structural and sequence similarities between SQR and QFR, both enzymes are believed to have evolved from a common ancestor [15,17].
The two electrons abstracted from the dehydrogenation of succinate are channeled sequentially into the ETC, from the flavin adenine dinucleotide (FAD) to the membrane-entrapped ubiquinone (UQ), through the cofactors [2Fe–2S], [4Fe–4S], [3Fe–4S] and possibly heme b. This long-range, near-linear electron conduit extends over 40 Å from the soluble catalytic domain of SDH to the membrane-anchoring domain of the enzyme [18]. The participation of heme b as part of the electron transfer chain has not been clearly established, so the functional significance of heme within SDH is unclear (see Section 2.4).
Although a few exceptions exist, SDH is in general comprised of four distinct polypeptides designated Sdh1–4 in yeast (SDHA-D in bacteria and humans). Sdh1 is a large ~70-kDa flavoprotein (Fp) with a mono/dinucleotide-binding Rossmann fold [19] that contains a buried, covalently attached FAD via a histidine residue. The three distinct iron–sulfur clusters in the ~27-kDa Sdh2 act as an electron wire connecting the FAD to the ubiquinone-binding site. The Sdh1–Sdh2 catalytic dimer is oriented exclusively on the matrix side of the IM in eukaryotes and cytosolic side of the plasma membrane in bacteria. Two hydrophobic membrane-spanning subunits (Sdh3 and Sdh4) anchor the catalytic dimer to the membrane and, due to their relevance to heme, are discussed in detail below.
2.2. Presence of heme within the SDH transmembrane anchor domain
The small membrane-spanning domains, Sdh3 (SdhC, ~15 kDa) and Sdh4 (SdhD, ~14 kDa), together form a classical six-helix bundle. SDH crystal structures from Escherichia coli, porcine, and avian have confirmed the presence of a six-coordinate bis-His heme b located at the inter-subunit interface to form what is referred to as a cytochrome b domain. Heme propionate groups interact with nearby charged Arg and His residues [18,20] with further stabilization by hydrophobic interactions between the porphyrin group and four of the six helices [20]. The heme lies spatially close to the UQ-binding cavity located near the matrix-lipid interface comprised of Sdh2, Sdh3 and Sdh4 [18] and proximal to the [3Fe–4S] center (UQp). A second UQ site, distal to the 3Fe–4S cluster (Uqd), has been suspected in mitochondrial SDH [21]. The crystal structure of porcine SDH provided further evidence of this second site based on the presence of a Q site inhibitor, and that this second site is located close to the IMS side of the IM [20]. This structure places the heme in between the two UQ-binding sites and poses interesting speculative scenarios regarding the electron flux in the transmembrane (TM) domain. For instance, the two electrons from the oxidation of succinate could flow sequentially to the Qp site, and when ubiquinone reduction becomes rate limiting for electron flux, the excess electrons could then be transferred to the Qd site mediated by heme b. This heme pathway may assist in the coordination of succinate and UQ turnover rates.
The superfamily of SQR, and the related QFR proteins, can be classified into five subfamilies (type A to E) based on variations in the membrane-anchoring subunits [15,21]. A single group associated with Archaea (Type E) lacks the typical Sdh3–Sdh4 association to the membrane and likely lacks heme. QFR enzymes from the Type D group also lack heme, although they contain the two distinct hydrophobic domains in which heme would reside. However, all SQR proteins that contain the distinct membrane anchors contain one to two hemes b [15]. Thus, the presence of the heme between the two TM domains is highly conserved, suggesting functional importance. Curiously, though, investigations into functionally characterizing this heme have brought into question the physiological importance of this redox center and whether the heme is required for assembly of SDH [22–24].
2.3. The Physical and Thermodynamic Barriers to Electron Transfer to Heme b
The chain of FAD-[2Fe–2S]-[4Fe–4S]-[3Fe–4S]-UQ are all positioned within a relatively short edge-to-edge distance (10–14 Å) between each pair of cofactors that allows electrons to tunnel rapidly between them [25]. Heme b lies curiously outside this linear chain; the UQ is positioned between the [3Fe–4S] cluster and the heme, resulting in what appears to be a branching of the electronic path at the [3Fe–4S] cluster (Fig. 2). Furthermore, the edge to edge distance between the [3Fe–4S] cluster and heme b (11.4 Å) is greater than the distance to UQ (7.6 Å) [18,20]. Although this spatial position of heme b does not necessarily indicate directional specificity, the notion that the heme is not involved is further substantiated by the redox potentials of the three cofactors in question. The potential of heme b (−185 mV), is considerably lower than that of the [3Fe–4S] cluster (+60 mV) and of ubiquinone reduction (+113 mV) that would indicate an uphill electron transfer [21]. Thus, both in structural and thermodynamic terms, a sound, albeit theoretical, argument can be made that the heme may not be part of the electronic pathway from FAD to UQ.
Fig. 2.
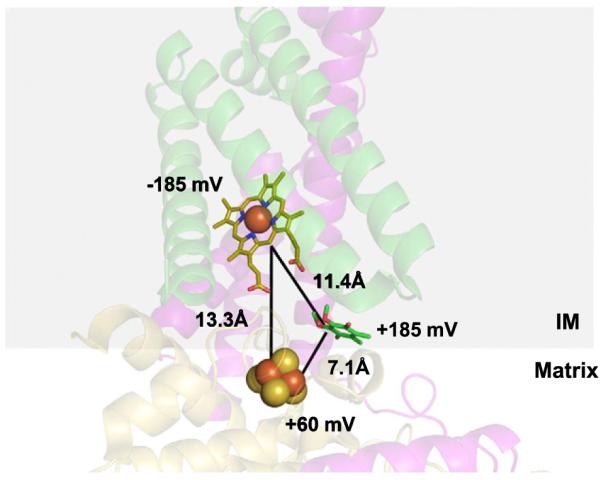
Structural and thermodynamic barriers to electron transfer from the 3Fe–4S cluster to heme b during quinone reduction in complex II. The transfer of an electron from the 3Fe–4S cluster (yellow and red spheres) to a lower potential heme b (yellow stick) would involve an “uphill” transfer, whereas a transfer to the higher potential quinone (green stick) would be more favorable thermodynamically. Furthermore, electron transfer to heme b involves a longer distance relative to the distance to the quinone. Subunit 2; yellow: Subunit 3; purple: Subunit 4; green. PDB accession number: 1YQ3 was used to generate the figure.
A caveat to the above argument is the presumption that each redox center is discrete and operates independently of neighboring redox centers. However, with close edge to edge distances, deviations from Nernstian behavior might be likely and that allosteric electronic interactions between the redox centers could exist [26,27]. Thus, under physiological conditions, the reduction of one redox center could influence the potential of the neighboring center to a more favorable value for reduction. Additionally, and as mentioned in the previous section, the presence of a second distal UQ binding site places the heme between the two UQ-binding sites. This raises the possibility that the heme could function to deliver electrons to the second Qd site.
2.4. The functional role of heme in SDH
Much of the investigations into the function of the heme in SDH have utilized E. coli SQR as a model system due to its high protein expression levels and easy genetic manipulations. Comparison of the crystal structures of E. coli [18] and mitochondrial SQRs [20] showed that the heme-binding His residues are conserved, lending validation to use of the E. coli model system. An exception to this conservation is found in S. cerevisiae, where one of the His ligands is replaced by a Tyr, which has led to an interesting debate regarding the presence of the heme in yeast.
The literature regarding the functional aspect of the heme in SDH spans three decades. While true that the heme is not involved in catalysis of succinate or fumarate, the exact nature of its function in electron transfer is still unclear. Heme might have a structural role in stabilizing the two TM subunits; however, the QFR from E. coli lacks heme with no effect on its stability [28]. Some SQRs and QFRs contain two hemes of high and low potentials, reminiscent of the bc1 complex hemes bH and bL hemes involved in trans-membrane electron transfer (see Section 3). Heme could be involved in the prevention of reactive oxygen species (ROS) formation during electron transfer from FAD to UQ by acting as a capacitor in times of high electron flux [18]. The presence of the heme center may prevent ROS formation with reverse electron flow from ubiquinol, minimizing the level of semiquinone.
It is clear that SDH contains a bound heme moiety; thus, the major question relates to the function of the heme center. Below, we highlight some studies focused on the functional aspects of the heme in SDH from bacteria, yeast, and mammals.
2.4.1. Effect of heme on the assembly and activity of SDH in bacteria
Initial hints of coupling between heme and the assembly of SDH was described using heme-deficient Bacillus subtilis strains where abrogation of heme synthesis resulted in a decrease in membrane-bound SDH [29]. Parallel to this decrease, Sdh1 (presumed to be associated with Sdh2) was found in the cytosolic fraction in an increased amount. This led to the conclusion that the cytochrome b subunits cannot anchor the Sdh1–Sdh2 complex in the absence of heme.
Corroborating these early observations, E. coli cells that lack the gene for either of the two TM subunits form only the catalytic Sdh1–Sdh2 heterodimer and its succinate oxidase activity is found only in the cytoplasmic fraction [30,31]. Expression of all four subunits in a heme-deficient, ferrochelatase mutant results in the same phenotype where the catalytic SQR was found only in the cytoplasmic fraction, suggesting heme is required for the proper insertion of SQR into the membranes. The two TM subunits could not be found in the membranes when heme was not present [31]. When heme is presented to cells lacking ferrochelatase, SQR is assembled and active within the membrane.
Subsequent studies, conducted to analyze the role of heme in SDH, have employed the generation of site-directed mutants of the heme-binding ligands [22–24,32,33]. Anaerobic expression of SQR E. coli histidyl mutants, SDHC H84L or SDHD H71Q, from a plasmid showed that heme was retained in an altered six-coordinated environment, or as a penta-coordinated high-spin heme, respectively, as determined by EPR [33]. The enzyme was shown to be catalytically active, but with lowered activities and lowered substrate affinity. Contrary to these results, the same mutants when expressed aerobically showed that the heme was lost in SDH, but the enzyme assembled and was catalytically active [32].
Substitution of the same histidyl ligands to tyrosine residues (SDHC H84Y or SDHD H71Y), either as a single or double mutant, appears to cause a loss of the heme in SDH in E. coli cells grown under a microaerobic condition. The most convincing case was demonstrated using enriched, overexpressed SQR mutants in E. coli where the loss of the cofactor was confirmed via optical and EPR spectra of the solubilized membranes [23,24]. Cells harboring either of these mutations grew in succinate-supplemented medium with a doubling time similar to WT cells. According to the authors, the loss of the heme did not cause an assembly defect, based partly on the fact that all four subunits were visible by SDS-PAGE analysis. The mutant enzyme was compromised in catalytic activity and substrate affinity as measured by quinone reduction. The substitution of the His ligand with a Cys residue induces a penta-coordinated high-spin heme that can bind CO, indicating that the Cys residue is unable to provide the sixth coordination site to heme b [24]. This result has implications to the yeast SDH, which is believed by some to bind heme using a Cys residue [34].
2.4.2. Effect of heme on the assembly and activity of SDH in eukaryotes
A phylogenetic analysis of SDH from S. cerevisiae places the enzyme in the type C subgroup of SQR/FRD superfamily of proteins based on the sequence of either of the catalytic subunits [15]. All type C enzymes possess two transmembrane subunits with a single inter-subunit heme b. Thus, the prediction based on this in silico analysis is that SDH in yeast is a single heme-containing enzyme. However, it has been suggested that yeast SDH may lack heme altogether, perhaps giving way to evolutionary pressure to dispense the heme from a possible lack of need [24].
Before any SQR/FRD structural information was available, biochemical evidence indicated the presence of a SDH-specific heme in a ratio of ~1 mol of heme per mol of FAD in yeast membranes [34]. The heme was termed “fumarate-oxidizable heme” and corresponds to an absorbance peak at 562 nm that appears in a difference spectrum after the addition of fumarate to dithionite-reduced mitochondrial membranes. The 562 nm heme peak is not present in mutants lacking either Sdh3 or Sdh4. The reliability of using the fumarate-oxidizable spectrum as an indicator of a SDH-associated heme has been questioned; however, due to contributions from the more abundant cytochromes b from complex III having an absorption at 563–564 nm [24]. The absence of the heme 562 nm peak in a Sdh3 or Sdh4 deletion; however, appears to be suggestive of a non-complex III dependent peak.
Mutation of either of the identified residues to alanines (Sdh3 H106A or Sdh4 C78A) causes a decrease in 562 nm heme content in mitochondrial membranes as measured by fumarate-oxidizable difference absorbance spectra [34]. It also led to a shift in the λ max from 562 to 560 nm. Both results suggest partial loss of the heme; the remaining heme existed in an altered environment. The steady-state level of assembled SDH and thus total SDH activity was reduced in both mutants. The stability of the SDH complex may be decreased from the loss of the heme. These results mirror those seen with the E. coli system: the amount of assembled enzyme decreases by about half, with parallel decreases in specific activity. A subsequent follow-up yeast study showed that mutation of both Sdh3 H106A and Sdh4 C78A results in a complete absence of the cytochrome b 562 nm peak in the fumarate-oxidizable absorption spectrum of the mitochondrial membranes [22]. Covalent FAD levels decreased ~30% and catalytic activity of the membranes decreased ~53%.
In mammalian cells, mutations harboring SDHC H127A and SDHC H127Y were constructed in Sdh3 to mimic the mutations mentioned above made in yeast and in E. coli, respectively [35]. However, unlike the effects in yeast and in E. coli, a change in the heme-binding ligand in SDHC led to the conclusion that heme is indispensable for SDH assembly in mammalian cells. For both SDHC H127A and SDHC H127Y transient reconstitutions in HeLa cells with downregulated endogenous SDHC, steady-state levels of SDHC were significantly decreased. Concomitant decreases in enzyme activities of about 50% were also observed [35]. Stable reconstitution of the same SDHC mutants in SDHC negative cells caused an even more dramatic effect with a near complete loss of SDHC and SDHD steady-state protein levels and near complete loss of SDH activities. These results would suggest that either SDH assembly was completely impaired, or that the assembled complex is rapidly degraded resulting from destabilization in the absence of the heme.
2.5. Dual function of Sdh3
The heme in yeast SDH (or its absence) has become even more intriguing recently—the Sdh3–Sdh4 heme-containing domain has been identified in another, separate mitochondrial protein complex. In S. cerevisiae, the Sdh3 subunit forms a heterodimer with Tim18 as part of the TIM22 channel-forming, protein translocase complex [36]. This translocase functions to import nuclear-encoded carrier family proteins destined to the mitochondria [37,38]. Sdh3 and Tim18 likely form a membrane anchor complex very similar to the Sdh3/Sdh4 membrane anchor in SDH. Using structural prediction based on pig and chicken complex II structures as templates, the overall fold of Tim18 shares an identical three-transmembrane helical bundle fold to that of Sdh4, and the predicted Sdh4–Tim18 structures can be superimposed with relatively small deviations. Remarkably, the quinone-binding site of Sdh3–Sdh4 is conserved in Sdh3–Tim18, both in terms of structure and the residues involved in forming the quinone ligands [24,36]. The atypical tyrosine residue in yeast Sdh4 that replaces the conserved heme-binding His residue is also present in Tim18, suggesting that this Tyr residue could be critical for function both in Sdh4 and in Tim18. One major unanswered question is whether the Sdh3–Tim18 complex contains a heme at the interface of the heterodimer utilizing this Tyr residue, and if so, whether this heme is merely a structural moiety or has an additional functional role.
3. Hemes of cytochrome bc1 complex
3.1. Overview
The ubiquinol:cytochrome c oxidoreductase (cytochrome bc1, complex III) is a central component of the respiratory and photosynthetic electron transport chains involved in oxidative phosphorylation. In eukaryotes, the complex exists as a dimer, embedded in the IM of mitochondria. In many bacteria, bc1 complexes are present in the plasma membrane, and the bc1 complex equivalent, the b6f complex, is located in the thylakoid membrane of cyanobacteria and plant chloroplasts. The function of the bc1 complex is to mediate transfer of electrons to the diffusible soluble cytochrome c via the oxidation of membrane-localized quinol, which can be generated by SDH, as described above. This redox-dependent reaction is coupled to translocation of protons across the lipid bilayer in which the complex resides.
All bc1 complexes contain three protein subunits with redox prosthetic groups known as the core subunits: a di-heme cytochrome b (Cob) containing both the high-potential bH (b562) and the lower-potential bL (b565); the c-type heme c1 associated with the Cyt1 subunit; and an iron–sulfur protein (ISP, Rip1) with a Rieske-type 2Fe–2S cluster (Fig. 3). Bacterial bc1 complexes usually contain only these core subunits, and at most, a single supernumerary (containing no redox cofactors) subunit, whereas the mitochondrial enzymes contain up to eight supernumerary subunits per monomer. The supernumerary subunits are not required for either electron flow or proton translocation by the bc1 complexes. In accordance with the endosymbiotic theory of mitochondrial origin, the catalytic core of each bc1 monomer in eukaryotes is comprised of the same essential redox cofactor-containing subunits with a high degree of conservation between kingdoms.
Fig. 3.
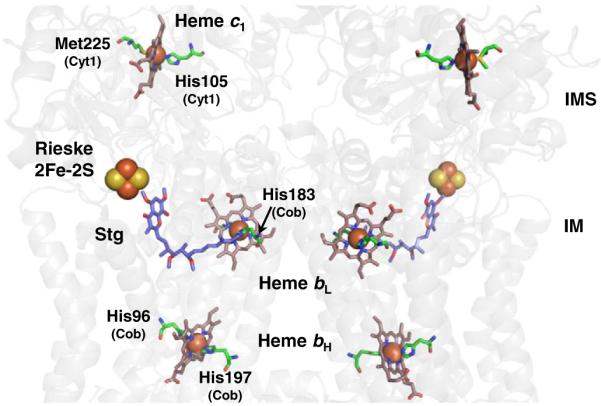
Arrangement of the hemes and the Rieske iron–sulfur clusters in the bc1 dimeric complex. For clarity, the protein backbone is in transparent grey and only the cofactors corresponding to a monomeric unit are labeled. The axial ligands to the heme iron are highlighted along with respective residue numbers and subunits (PDB ID: 3CX5 numbering). The axial ligands to heme bL are His183 and His82 (not shown) of Cob. The quinone analogue inhibitor stigmatellin (Stg) is also shown and denotes the p-side quinone-binding site that is located between the low potential b heme (Heme bL) and the Rieske iron–sulfur cluster. The n-side quinone-binding site (not shown) lies close to the high potential b heme (Heme bH).
The redox protein Cob is the only bc1 subunit encoded by the yeast mitochondrial genome. It contains eight TM helices containing two heme b moieties and serves as the foundational protein in bc1 biogenesis. The other subunits are encoded by the nuclear genome and must be imported into the mitochondria. Both the Cyt1 and ISP catalytic subunits are anchored in the IM by a single TM domain and contain a soluble globular domain containing its heme c1 and 2Fe–2S and prosthetic groups, respectively, in the IMS. The ISP protein is intertwined between adjacent monomers of the complex, with its globular domain in one unit and its TM helix in the adjacent unit. Thus, electron transfer can only occur when the complex is assembled as a dimer. Furthermore, movement of the globular catalytic domain in ISP is essential to the electron transfer to cytochrome c1 in the catalytic mechanism. The dimeric bc1 complex forms higher-order supercomplexes with CcO (Section 4) in yeast and with both CcO and the NADH dehydrogenase (complex I) in higher eukaryotes.
3.2. Organization and function of bc1 complex redox sites
3.2.1. Structure of bc1 complex heme sites
High-resolution crystal structures of the bovine, chicken and yeast bc1 complexes identify the redox-active sites within the enzyme [39–42]. Each bc1 monomer has three 6-coordinate, low-spin heme centers. The midpoint reduction potentials for heme bH, heme bL, and heme c1 are~+120 mV, ~−30 mV, and ~+240 mV, respectively [43], although there is slight variation between species and strains (Figs. 1 and 3).
The di-heme cytochrome b polypeptide forms the two quinone-binding sites. According to the crystal structures, the two planar b-type hemes are rotated approximately 45° to one another, but are positioned perpendicular to the IM within a four-helix bundle of the cytochrome b subunit. Earlier spectroscopic and mutagenesis [44] studies determined that two His residues in each of the second (helix B) and fourth (helix D) TM helices bind the hemes. These His residues are invariant in over 800 sequences of b-type cytochromes, including cytochrome b6 of the b6f complex [45]. The distance between the heme iron atoms and the ligating His varies from 2.05 to 2.08 Å [39–42]. The distance between the iron centers of the b hemes in the same monomer is approximately 21 Å [40–42]. Interestingly, this same distance is also observed for the bL hemes in opposite monomers, implying that electron transfer may be possible between them [46]. The low-potential heme bL is located close to the IMS side of the IM, but protected from solvent by the cytochrome c1 subunit. Conversely, the high-potential heme bH is located near the surface of Cob in a solvent-filled cavity accessible from the matrix. In this location, heme bH can receive electrons from bL to drive the Q cycle.
The IMS-localized domain of the cytochrome c1 subunit (Cyt1) contains a single heme c1 moiety that is covalently attached to the polypeptide by two thioether bonds to Cyt1 cysteine residues [40] (a rare one thioether bond Cyt1 exists [47]). Cyt1 is larger and differs in structural elements from other c-type cytochromes in addition to having a TM segment [48]. Like most apo-cytochromes c, a CxxCH motif provides the sulfhydryls for the two thioether bonds and the imidazole nitrogen of histidine serves as the fifth axial ligand to the heme iron. Methionine is typically the sixth axial iron ligand.
3.2.2. Catalytic mechanism of the bc1 complex
Details of the bc1 catalytic mechanism can be found in many excellent reviews [49–51]. Here, we provide a general overview while highlighting hemes’ roles. A central function of the two hemes, bH and bL, is to provide a bifurcation pathway to the two electrons abstracted from the oxidation of quinol. The third heme of bc1, heme c1, accepts electrons from the Rieske 2Fe–2S cluster and mediates them to the soluble cytochrome c. Specifics are outlined below.
The bc1 complex contains two quinone reaction centers located on opposite sides within the IM [52] (Fig. 3). Ubiquinol binds at the Qo, or the P site of the complex. After deprotonation, one electron is released to reduce sequentially the Rieske 2Fe–2S cluster, heme c1 and then heme c of the soluble cytochrome c. The second electron is passed to the low-potential heme bL and then to the high-potential heme bH which is then used to reduce the quinone at the Qi, or N site, forming a semiquinone. Another cycle of quinol oxidation at the Qo site provides the second electron necessary for the reduction of the semiquinone at the Qi site from the first reaction sequence. The resulting quinol re-enters the Q cycle at the Qo site. Thus, the net product of the Q cycle is the translocation of four protons to the IMS, the uptake of two protons from the mitochondrial matrix, and the reduction of two molecules of cytochrome c.
While the overall reaction mechanism remains largely undisputed, the bifurcated oxidation of ubiquinol is proposed to occur by two different mechanisms. In the sequential mechanism, the electron is first passed to the high-potential Rieske protein. This reaction results in the remaining electron, now present as an unstable semiquinone (Q•), to be passed to bL and then to bH. However, this historic mechanism lacks the experimental evidence to support the existence of a semiquinone intermediate at the Qo center. Trumpower and colleagues proposed a refined model in which the oxidation of ubiquinol occurs by a concerted mechanism in which electrons are simultaneously donated to the Rieske protein and heme bL [53]. Cytochrome bL immediately reduces cytochrome bH, which then reduces ubiquinone to the semiquinone at the Qi site. The electron transfer from bL to bH also results in a protein conformational change that facilitates movement of the Rieske Fe/S protein between cytochromes b and c1 [54].
3.3. Formation and maturation of bc1 redox sites
The individual maturation of cofactor-containing subunits requires several deliberate steps, including cofactor delivery to the appropriate cellular compartment, manipulation and/or preservation of the apoprotein in a conformation amenable to cofactor insertion, and ligation of the cofactor to the specific functional groups of the subunit polypeptide. An additional complication is that all known c-type holocytochromes are located in a different cellular compartment than the site of either apo-polypeptide or heme synthesis [55]. It is also unclear which side of the plasma membrane/inner membrane hemylation takes place to form cytochromes b. Additionally, little is known about the mechanism of b-type cytochrome maturation because assembly defects have severe cellular phenotypes [56].
3.3.1. Di-heme cytochrome b maturation
In prokaryotes, mutagenesis of the ligating histidine residues in cytochrome b prevents cofactor insertion and results in a nonfunctional enzyme that impairs bc1 assembly [56]. The non-covalent chemical nature and lack of known specific assembly factors has led to the suggestion that heme is spontaneously inserted into the apoprotein. This assumption can be supported by in vitro experiments where heme is able to bind synthetic peptides mimicking cytochrome b helices [57]. Thus, the model for bacterial bc1 complex assembly assumes that the b hemes are inserted either very soon, after, or concurrent, with the folding of the cytochrome b polypeptide into the membrane.
Maturation of cytochrome b is the nucleating factor in eukaryotic bc1 complex biogenesis (Fig. 4). COB pre-mRNA is processed by the matrix protein Cbp2 and the mitochondrial proteins Cbs1 and Cbs2, which activate translation via interaction with the 5′ untranslated region of COB mRNA and the mitochondrial ribosome [58]. Cbp6 was also identified as a translational activator of COB mRNA, but was more recently found to form a complex with the matrix assembly protein Cbp3 at the ribosomal exit tunnel, where the two proteins interact with the newly synthesized Cob. The Cob–Cbp6–Cbp3 complex recruits the additional IMS assembly factor Cbp4 after dissociation with the ribosome [59]. An early assembly intermediate containing Cob was identified by native gel electrophoresis in yeast lacking important bc1 subunits [60,61]. This intermediate also contained two small bc1 subunits, Qcr7 and Qcr8. Although it remains unclear whether the newly synthesized Cob associated with Cbp3, Cbp6 and Cbp4 is a distinct complex from the Cob/Qcr7/Qcr8 assembly intermediate, it is likely that the initial assembly intermediate contains each of these assembly factors. This putative Cob-containing complex with Cbp3, Cbp4, Cbp6, Qcr7 and Qcr8 may exist to stabilize Cob for population of the two heme centers.
Fig. 4.
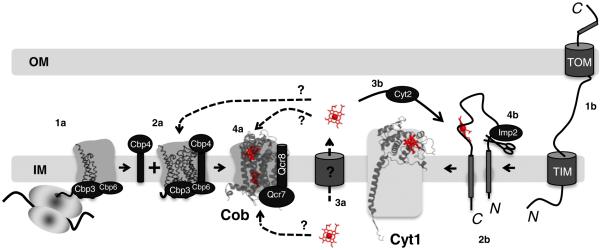
Hemylation of cytochrome b and cytochrome c1 polypeptides in eukaryotic bc1 complex. Translation of COB mRNA initiates bc1 complex biogenesis (1a). Cob forms a ternary complex with the Cbp3/Cbp4/Cbp6 proteins independent of the mitochondrial ribosome (2a). Following transport of newly synthesized heme across the IM (3a), this is a possible point at which heme is inserted into the apo-cytochrome b from the IMS side of the inner membrane. This insertion could occur prior to, in conjunction with, or post Qcr7 and Qcr8 additions (4a). Transport across the IM would not be required if the product heme from ferrochelatase is deposited into the IM (see Fig. 6a). Hemylation of Cob could also occur directly from the matrix side. Apo-cytochrome c1 is synthesized in the cytosol and must be imported into the mitochondria through the inner and outer membrane translocases (1b). A bipartite signaling sequence directs insertion into the IM (2b). Following transport of newly synthesized heme across the IM, the heme lyase, Cyt2, participates in the covalent attachment of heme c1 to apocytochrome c1 (3b). The final step in holocytochrome c1 maturation is cleavage by Imp2 to remove the N-terminal membrane anchor, resulting in an Nout–Cin topology (4b). PDB accession number: 3CX5 was used to generate the figure.
The mechanism of Cob hemylation is unknown. It remains unclear whether the reaction occurs from the matrix or the IMS side of the IM. Heme synthesis occurs on the matrix side of the IM by ferrochelatase. The two heme moieties associated with Cob lie within a four-helix bundle. If Cob hemylation occurs within the assembly intermediate containing Qcr7, Qcr8, Cbp3, Cbp4 and Cbp6, the Qcr7 subunit and the N-terminus of Qcr8 are packed against the matrix-facing loops of Cob, blocking access to the four-helix bundle interior. In addition, Cbp3 and Cbp6 are matrix-facing subunits that may also block access to Cob. In contrast, the IMS side of the Cob intermediate would be devoid of steric constraints by Qcr8. Cbp4 is projected to have a globular domain facing the IMS, but Cbp4 is not conserved through eukaryotes. Thus, hemylation may occur through the IMS surface of Cob. No information exists on how heme is translocated across the IM, yet that is an essential step, as heme is used in other cellular compartments.
Hemylation of Cob is likely required for subsequent maturation of the Cob/Qcr7/Qcr8 complex through the addition of subunits Cor1, Cor2, Cyt1 and Qcr9 [61,62]. The late maturation step includes the addition of 2Fe–2S-containing Rip1 and Qcr10. Since Rip1 contains the essential 2Fe–2S center that mediates electron transfer to the cytochrome c1 subunit Cyt1, the late core intermediate is devoid of function. The addition of Rip1 and Qcr10 enables association with CcO, forming the supercomplex in yeast [62,63].
3.3.2. Cytochrome c1 heme maturation
In prokaryotes, the Sec translocon delivers cytochrome c1 to the periplasm where the covalent attachment of heme c to apo-cytochrome by the system I machinery occurs early in bacterial bc1 complex biogenesis [64]. Deletion, mutation, or removal of the transmembrane anchor of the cytochrome c1 gene or mutation of the heme binding site of cytochrome c1 protein prevents the addition of either cytochrome b or Rieske protein subunits [65–67] to the complex. Furthermore, removal of the C-terminal membrane anchor in R. sphaeroides causes retention of detectable amounts of the soluble holo-protein in the periplasm [67], suggesting that heme c1 insertion occurs independently of Cyt1 membrane insertion and that a functional holo-Cyt1 is required for assembly and association of the remaining bc1 complex subunits.
Cyt1 is the only bc1 TM subunit oriented with its C-terminus directed towards the matrix. Therefore, proper localization and topogenesis requires a bipartite targeting signal sequence in Cyt1 to direct its intra-mitochondrial sorting. Following inner membrane insertion of the C-terminal tail, the heme c1 cofactor is inserted into the new IMS-localized N-terminal domain of Cyt1 [68,69]. The final step in Cyt1 maturation is cleavage by the Imp2 signal peptidase to remove the matrix and N-terminal TM portions of Cyt1, resulting in mature holo-Cyt1 (Fig. 4). Positive heme staining on SDS-PAGE of the intermediate form of Cyt1 is evidence that heme attachment occurs prior to Imp2 processing. Furthermore, in the absence of Imp2, the intermediate holo-cytochrome c1 persists [70].
In yeast, the covalent attachment of heme to apo-Cyt1 is mediated by either of the two heme lyases in the IMS, CCHL and CC1HL [70]. In mammals, the activities of both CCHL and CC1HL are possessed by the HCCS heme synthetase, which functions to assemble both cytochrome c1 and the mobile IMS protein cytochrome c. Both CCHL and HCCS can functionally substitute for CC1HL in yeast, although the converse is not true [70]. Therefore, it remains possible that CCHL and CC1HL are actually part of a larger complex that is required for covalent bond formation in cytochrome c-type proteins, and that the so-called lyases are not the catalytic component of this complex.
The fungal CC1HL, designated Cyt2, is a small protein of 216 residues in S. cerevisiae. The N-terminal segments of CC1HL orthologs exhibit considerable length variation, but contain dual CP motifs that may transiently bind hemes [71]. It is unclear whether Cyt2 or any CCHL protein catalyzes the thioether bond formation directly. An alternative scenario is that Cyt2 is a chaperone that stabilizes a conformation of Cyt1, allowing spontaneous thioether bond formation.
The IMS protein Cyc2 has been identified both in a screen for mutants deficient in hemylated cytochrome c and also isolated as a multicopy suppressor in cells lacking CC1HL, suggesting a role in the maturation of mitochondrial c-type cytochromes [72]. Cyc2 has a non-covalently bound FAD cofactor with a proposed role in the redox modulation of the heme lyase reaction. Cyc2 is essential for hemylation of Cyt1 by CCHL, especially when the CxxCH (CAPCH) heme-binding motif of Cyt1 is mutated to affect the redox chemistry of the cysteinyl thiols [73]. However, Cyc2 is not required for the hemylation of Cyt1 by its cognate CC1HL lyase. This observation suggests that a different candidate reductase than Cyc2 may be necessary for Cyt1 maturation.
Cyt1 along with Cor1, Cor2 and Qcr9 are added to the early core assembly intermediate (Cob, Qcr7, Qcr8) to form the late core assembly intermediate lacking only Rip1 and Qcr10. Hemylation of Cyt1 likely occurs prior to this assembly step, since it appears to precede Imp2 proteolytic processing (Fig. 4). No information is available regarding whether hemylation of Cob and Cyt1 is coupled. Further research is needed to define the steps and protein components necessary for these hemylation reactions.
4. Heme a of cytochrome c oxidase
4.1. Overview
Eukaryotic CcO is the terminal enzyme of the mitochondrial electron transport chain. A member of the heme-copper oxidase superfamily, this enzyme catalyzes the reduction of molecular oxygen to water concurrently with the oxidation of ferrocytochrome c. These redox reactions, like that in complex III, are coupled to the translocation of protons across the inner mitochondrial membrane contributing to the formation of the electrochemical proton gradient utilized to generate ATP.
In contrast to its 3–4 subunit bacterial counterparts, eukaryotic CcOs consist of 11 (yeast) to 13 (mammals) subunits; nevertheless, the catalytic core of the enzyme formed by the three largest proteins, Cox1, Cox2 and Cox3, shows a significant degree of conservation throughout evolution [74]. Three core subunits are encoded by mitochondrial DNA, while the additional subunits are products of the nuclear genome [75,76].
Structures of CcO enzymes have been elucidated from bovine heart mitochondria as well as from bacterial counterparts Paracoccus denitrificans and Thermus thermophilus [9,77–80]. Cox1 is the largest and most conserved of the core subunits. It is a highly hydrophobic protein with 12 TM helices, and it contributes to both proton pumping and oxygen reduction reactions. The second catalytic subunit Cox2 has only 2 TM helices. Studies both in bacteria and eukaryotes suggest that Cox3 is likely important for stability of the catalytic core and modulates the proton pumping pathways in Cox1 [81]. The additional nuclear-encoded subunits are important for both assembly and function of the enzyme [75,76].
4.2. Organization and function of CcO redox sites
4.2.1. Structure of CcO redox sites
Redox sites of CcO are represented by the copper ions and the a-type heme cofactors that together form very unique cofactor sites found only in terminal oxidases (Fig. 5). The Cox2 subunit contains the binuclear CuA center located within the IMS-exposed C-terminal domain of Cox2. The other redox cofactors are present in Cox1. First, the low-spin six-coordinate heme a is ligated by two axial histidyl ligands. The fully extended farnesyl group of this heme is inside an α-helical bundle formed by helices 1, 11, and 12 of Cox1 [77,82]. The second heme molecule exists within a heterobimetallic center consisting of copper (designated CuB) and heme a. This heme, designated a3, is five-coordinate high-spin, with a single axial ligand.
Fig. 5.
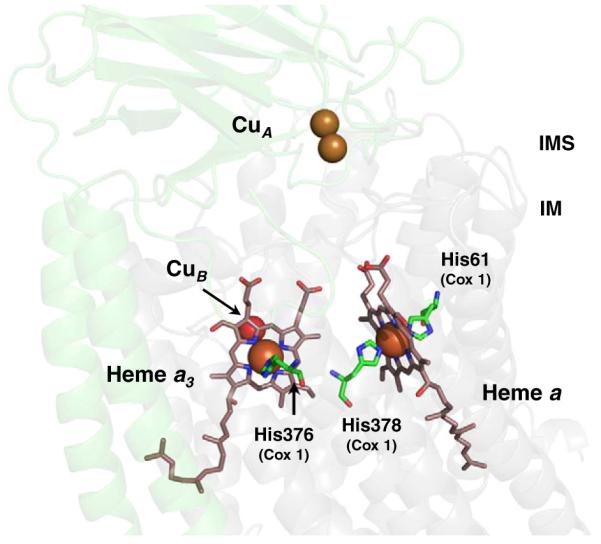
Arrangement of the hemes a and a3:CuB and CuA in CcO. The binuclear CuA center is located in Cox2 subunit (transparent green) and is the entrance site for electrons from reduced cytochrome c. Electrons are subsequently passed to the low-spin, bis-His heme a and then to the heterobimetallic heme a3:CuB center in Cox1 (transparent grey) where O2 reduction occurs. The axial ligands to the heme iron are highlighted along with respective residue numbers and subunits (PDB ID: 2OCC numbering).
The CuB site resides 13 Å below the IM surface, and the heme a3 is localized in a cavity with its farnesyl tail located at the interface of the Cox1 and Cox2 subunits. Consistently, the association of the Cox1 and Cox2 subunits has been reported to be an important factor for proper coordination of heme a3 in mammalian CcO [83]. The cavity is partially solvent-accessible from the IMS side, and Cox2 docking in the course of assembly appears to occlude the cavity [9,77]. Both hemes have their farnesyl and vinyl groups extending toward the mitochondrial matrix side, while hydrophilic propionate moieties are facing the IMS side.
4.2.2. Function of CcO redox sites
The CcO redox sites in combination with hydrogen bonds and the peptide backbone form an intricate molecular wire that allows shuttling of the electrons through the enzyme [84]. The electrons from the reduced cytochrome c enter CcO through the CuA center located near the docking site of Cox2 and Cyc1. The CuA site is located 19 Å away from the heme a prosthetic group. Electron transfer between the CuA and heme a does not involve proton movement [85]. Electrons in heme a are subsequently passed to the heterobimetallic heme a3:CuB center where oxygen reduction occurs. For more detailed reviews on CcO catalysis see [85,86].
4.3. Formation and maturation of CcO redox sites
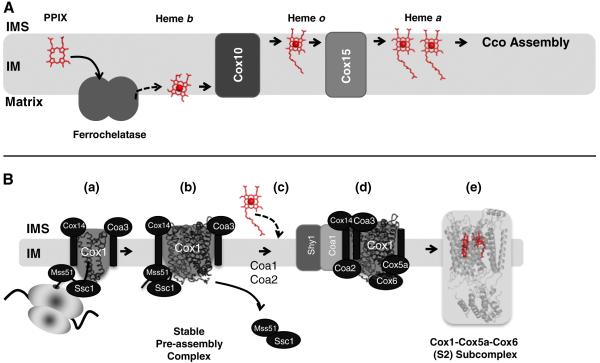
As mentioned in Section 1, heme a is generated by the sequential modification of the protoheme, the product of ferrochelatase (Figs. 6 and 7a). In the first step, a 17-hydroxyethylfarnesyl group is added to the C2 vinyl side chain of heme b catalyzed by a farnesyltransferase-type enzyme, generally referred to as heme o synthase (HOS). In many bacterial oxidases, this intermediate heme o serves as a terminal prosthetic group. Unlike in bacteria, only trace amounts of heme o are found in normal mitochondria, but detectable levels are observed in CcO assembly mutants [87,88].
Fig. 6.
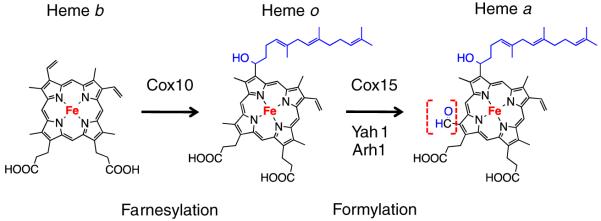
Chemical modifications of heme b to heme a via heme o catalyzed by the enzymes Cox10 (Heme o synthase, HOS) and Cox15 (Heme a synthase, HAS). In mitochondria, heme o is considered to be an intermediate and does not serve as a cofactor; where as in bacteria, heme o can functionally replace heme a in its terminal oxidase (e.g., cytbo3). Ferredoxin Yah1 and adrenodoxin Arh1 supply electrons for Cox15-mediated heme o to heme a conversion.
Fig. 7.
(A) Synthesis of heme a from protoporphyrin IX (PPIX) for hemylation of CcO in the mitochondria. PPIX (product of PPIX oxidase, not shown) likely enters ferrochelatase (shown as a dimer) from the IM. The product heme b exits either directly to the IM, or to the matrix, where it is utilized for hemylation of complex II and complex III. A sub population of heme b is converted to heme a via Cox10 and Cox15 within the IM. The exit path of heme a from Cox15 is unknown. As drawn, the heme exits on the matrix side of the IM to be used for CcO assembly. (B) Possible route of hemylation of Cox1 subunit in yeast CcO. Translation of COX1 mRNA commences CcO biogenesis (a). Newly synthesized Cox1 forms a stable complex with Mss51, Cox14, Coa3 and mitochondrial Hsp70 chaperone, Ssc1, independent of the mitochondrial ribosome (b). Subsequently, Coa1 appears to mediate progression of Cox1 to a downstream complex for cofactor insertion. Mss51 is released for further rounds of Cox1 translation/assembly (c). With the help of Shy1 and Coa2, heme a is incorporated into maturing Cox1 (d) to form the stable Cox1–Cox5a–Cox6 S2 complex (e). PDB accession number: 2OCC was used to generate the figure.
In the subsequent step, the C8 methyl group of heme o is converted to a formyl group. Based on studies in bacteria, it has been postulated that conversion occurs by sequential oxidation of the methyl group through a short-lived C8-alcohol intermediate, sometimes referred to as heme i or heme ox [89,90]. This reaction is performed by heme a synthase (HAS). Oxidation of the C8 methyl group has been proposed to proceed via two mono-oxygenase reactions [89]. However, this mechanism has been challenged by O2 isotope-labeling studies demonstrating no significant incorporation of the label into either heme a nor heme i [91]. The same study suggested that water might be a donor of the oxygen atom in this reaction.
4.3.1. Heme o synthase
The heme o synthase (HOS) is designated Cox10 [92]. It is a large membrane protein that in yeast assembles into a multimeric complex of ~300 kDa believed to be a homo-oligomer [93]. The stability of the Cox10 oligomer appears to depend on the presence of newly synthesized Cox1 and its early assembly intermediates. This connection of heme a biosynthesis and CcO biogenesis may serve regulatory purposes to minimize excess and toxic levels of highly reactive heme a. Cox10-catalyzed conversion of protoheme appears to be a rate-limiting step in heme a formation, given its ~8-fold dearth over Cox15 in yeast mitochondria [94]. It is therefore likely that regulation of Cox10 abundance/activity is the way to modulate the heme a biosynthetic pathway.
An N196K gain-of-function mutation in Cox10 was identified as a dominant suppressor in cells lacking the CcO assembly factor Coa2. These studies established that Coa2 functions in concert with Cox10 in the hemylation of Cox1 (see Section 4.4.2). While Cox10 exists only as the monomer in coa2Δ cells and, apparently, is largely inactive, the N196K mutant Cox10 forms a stable oligomer, and the enzyme is active as the cells respire normally [93]. Cox10 oligomerization appears to be a key feature of HOS enzymatic activity.
4.3.2. Heme a synthase
Heme a synthase was first described in B. subtilis [95–97]. Further studies identified eukaryotic orthologs commonly designated Cox15 [98,99]. Purified CtaA has been shown to contain sub-stoichiometric amounts of both hemes b and a. This led to a conclusion that HAS is a heme b-containing enzyme utilizing the cofactor at the active site, akin to other oxygenases. Bound heme a was interpreted as an unreleased reaction product [97]. A bioinformatic approach helped to identify proteins acting in concert with Cox15. In Schizosaccharomyces pombe, HAS exists as a part of a natural fusion protein, consisting of Cox15 and a Yah1-like ferredoxin extension [100,101]. Further studies confirmed the involvement of Yah1 as well as ferredoxin reductase Arh1 in heme a biosynthesis [88].
The catalytic domain of Cox15 is projected to exist on the IMS side of the IM. Since the catalytic domain of Cox10 resides on the matrix side of the IM, an unresolved question concerns how heme o is translocated to the IMS side of the IM for Cox15-mediated oxidation. Electrons for this step are likely passed through the heme b in Cox15 from ferredoxin in the matrix.
Similar to HOS, heme a synthases are polytopic membrane-embedded proteins with up to eight predicted TM segments [90,98]. Studies in yeast demonstrated that Cox15 forms a high molecular weight complex [93,98], but its composition is unknown. Brown et al. overexpressed B. subtilis CtaB and CtaA, as well as R. sphaeroides Cox10 and Cox15 in E. coli and showed interaction of HOS and HAS in both cases [91]. This observation led to the hypothesis that heme o is channeled directly from HOS to HAS. Studies in yeast, however, failed to demonstrate interaction of Cox10 and Cox15. Moreover, the deletion of COX15 does not affect the stability of the Cox10 complex and vice versa [93] (and our unpublished results). Unlike Cox10, the Cox15 high-mass complex is not responsive to perturbations of Cox1 synthesis or maturation.
Yeast lacking Cox15 contain no heme a, but show low levels of heme o, suggesting that the two enzymes are not intricately linked [87]. Likewise, fibroblasts isolated from patients with fatal infantile hypertrophic cardiomyopathy associated with mutations in Cox15 are characterized by reduced heme a levels, but heme o content is elevated [102]. It should be noted, however, that overexpression of COX10 in Cox15-deficient yeast cells does not result in the increase of heme o levels. Based on this observation, Barros and Tzagoloff suggested that there is positive regulation of HOS function by either Cox15 or the product of the Cox15-catalyzed reaction.
4.4. Maturation of heme a-containing reactive sites within CcO
Hemylation of Cox1 is one of the key events in CcO biogenesis. Heme a is required for stability and folding of the Cox1 subunit [92,103,104]. This is consistent with structural data indicating that heme a is not only a reactive site but also a “structural unit” linking together distant helical bundles of Cox1. In some bacteria, such as R. sphaeroides, CcO assembly proceeds normally even in the absence of heme a [105]. Based on these observations, it has been assumed that hemylation of Cox1 occurs either co-translationally or upon its incorporation into the lipid bilayer [106,107].
A more recent study indicated that Cox1 hemylation is a post-translational event [108]. In yeast, CcO biogenesis initiates with Cox1 synthesis on mitochondrial ribosomes tethered to the IM by IM-associated translational activators Pet309 and Mss51 (Fig. 7b). Mss51 has a second function in translational elongation of Cox1, which is performed within a high-molecular-weight complex consisting of Mss51, chaperone-like factors Cox14 and Coa3/Cox25, and mHsp70 chaperone Ssc1, which represents, along with newly synthesized Cox1, an early CcO assembly intermediate [75,76]. Cox1 maturation proceeds to downstream assembly complexes containing assembly factors designated Coa1, Coa2 and Shy1 (SURF1 in humans) [108–111]. Neither the Mss51- nor Coa1-containing Cox1 intermediates have the redox sites populated; removal of either Cox10 or Cox15 (or other molecules involved in cofactor synthesis or incorporation) has no effect on the stability of either complex. Likewise, these intermediates are not affected in cox1 strains with mutated heme a axial ligands [108].
The formation of both the heme a and the heme a3-CuB sites appears to occur within or adjacent to the Shy1/SURF1-containing Cox1 assembly intermediate. Studies with fibroblasts from patients with mutations in Cox10 and Cox15 reveal the absence of the partially assembled Cox1 intermediate [102,112,113]. Similarly, the lack of each protein in yeast affects the Shy1 associated Cox1 assembly intermediate [108]. These observations are supported by the genetic interactions in yeast between Cox10, Coa1, Shy1 and Coa2 [110,111]. CcO assembly blocked at either Cox2 maturation or addition of the CuB moiety results in accumulation of a transient Cox1 pro-oxidant intermediate. The nature of such a pro-oxidant is not fully understood, but it appears to correlate with the presence of a reactive five-coordinate heme a3 [114]. The pro-oxidant heme a3:Cox1 intermediate is absent in cells lacking Shy1 or Coa1 [111], another observation supporting the postulate about the heme a maturation point.
There are a few lines of evidence that suggest the heme a site may be formed earlier than the heme a3 center. First, CcO isolated from R. sphaeroides or P. denitrificans cells lacking SURF1 orthologs is deficient in heme a3 but not heme a [115,116]. Second, a stalled Cox1 assembly intermediate containing a subset of nuclear-encoded subunits is observed in the fibroblasts from SURF1, but not Cox10- or Cox15-deficient patients [102,112,113]. It is plausible that heme a insertion may be necessary for formation/stabilization of the intermediate. Likewise, studies on the assembly of E. coli bo3 oxidase, where heme b is analogous to the heme a of CcO, demonstrated that formation of the heme b site is critical for further maturation of the enzyme [117].
4.4.1. Shy1/Surf1 proteins
Heme a can spontaneously self-insert into maquettes, chemically engineered four-helix bundles mimicking the ones of Cox1 [118]. However, questions exist regarding the speed and efficacy of such insertion, which become critical aspects in vivo, where unfolded hydrophobic proteins are quickly shielded or removed by quality control systems to prevent aggregation and/or deleterious side effects [119]. It is therefore plausible that dedicated factors exist to facilitate heme a insertion.
SURF1/Shy1 proteins have been implicated in heme a site formation. Although the exact role of the protein remains nebulous, P. denitrificans SURF1 isoforms were shown to bind heme a in a 1:1 stoichiometry when co-expressed along with heme a biosynthetic enzymes in E. coli [120]. The same study identified a conserved histidine residue important for heme binding and implicated SURF1 as a heme a insertase. In Thermacea bacteria, the ba3-type CcO biogenesis is independent of a SURF1 protein, but an unrelated CbaX factor is required for heme a3 site formation [121]. The CbaX factor lacks any histidine residues, and no heme binding studies were conducted. The heme-insertase scenario may not hold true for eukaryotic Shy1/SURF1; mutation of the conserved histidine residues in yeast Shy1 has only minor or no effect on respiration [122]. Consistently, yeast cells lacking Shy1, as well as SURF1-deficient patient cell lines retain substantial respiratory activity. Therefore, it remains unclear whether Shy1/SURF1 simply acts as a chaperone stabilizing the maturing Cox1 protein or whether it has a more direct role in heme a3 insertion.
4.4.2. Coa2
Genetic studies in yeast identified another molecule, designated Coa2, critical for Cox1 hemylation. Coa2 has been identified as a highcopy suppressor of the respiratory defect in coa1Δ and shy1Δ deletion strains and has been shown to transiently interact with Shy1 [111]. Subsequent genetic studies found that Coa2 functions downstream of Mss51 and Coa1 [93]. Cells lacking Coa2 exhibit a facile degradation of Cox1 that appears to arise from impaired hemylation and subsequent misfolding of the molecule [93,123]. The respiratory defect of coa2Δ cells is suppressed by the dominant N196K gain-of-function mutation in Cox10 [93]. The Cox10 high-mass complex is severely attenuated in coa2Δ cells. The suppressive effect of the mutation is restricted to Coa2-deficient cells and appears to depend on restoration of the Cox10 oligomeric state and the abundance of the oligomer. In addition, attenuation of Oma1 protease substantially restores respiration in coa2Δ cells [93,123]. The absence of the Oma1 protease appears to increase the time for the inefficient Cox1 hemylation process in coa2Δ cells.
Similar to Shy1/SURF1, Coa2 does not appear to be a dedicated heme chaperone; substitutions or removal of the residues that potentially could constitute a heme-binding motif have no effect on respiratory function (our unpublished results). Coa2 is important in coupling Cox1 synthesis to Cox10 oligomerization and in addition appears to have a role in the formation of the low spin heme a site in Cox1. It remains to be determined whether formation of the heme a site occurs in a similar fashion in human mitochondria. No robust mammalian homolog of Coa2 has been identified so far.
5. Concluding remarks and perspective
Hemes are integral components of the respiratory complexes II, III, and IV that perform multi-electron transport and catalysis. The functional significance of the heme in complex II remains unclear, as does the relevance of its second quinone-binding site. An intriguing observation that the SDH subunit Sdh3 exists in a second complex with Tim18 creates the possibility that heme has a role in the TIM22 translocase for mitochondrial import of carrier proteins. Future studies will need to resolve whether the hemes in SDH and the Sdh3/Tim18 complex have only a structural role or participate in an electron transfer reaction.
Important biological questions persist regarding the maturation of heme centers in complex III and complex IV. Hemylation of the subunit Cob of complex III, for example, is likely required for subsequent nucleation biogenesis of additional subunits. It will be important to resolve at which stage of Cob maturation the heme is inserted, and whether additional proteins function in the hemylation reaction. Covalent association of heme with cytochrome c and c1 occurs in the IMS, yet information is lacking on how heme is translocated from the site of its formation on the matrix-facing surface of the IM back to the IMS. The translocation step is likely coupled to heme presentation to the proteins involved in covalent heme addition. Complex IV biogenesis is closely linked to heme site maturation. Significant questions persist on the mechanism of heme a insertion into Cox1 from the IMS side of the IM as well as the translocation of heme o from the matrix side of the IM to Cox15. The heme b in Cox15 is likely a key cofactor for the electron transfer from ferredoxin in the matrix to the Cox15 reaction center. A final key question remains on whether bioavailable heme b pools exist within the mitochondria or IMS for hemylation reactions. Although much information is known for heme reaction mechanisms, the biological movement of heme for use in hemylation reactions remains uncharacterized.
Acknowledgments
We acknowledge the grant support from the National Institutes of Health, GM083292 and ES03817 to D.R.W. H.J.K. and P.M.S. were supported by training grant T32 DK007115. O.K. was supported by American Heart Association grant 10POST4300044.
References
- [1].Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Elsevier; Amsterdamn and New York: 1971. [Google Scholar]
- [2].Rodgers KR. Heme-based sensors in biological systems. Curr. Opin. Chem. Biol. 1999;3:158–167. doi: 10.1016/S1367-5931(99)80028-3. [DOI] [PubMed] [Google Scholar]
- [3].Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem. Rev. 1996;96:2841–2888. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- [4].Mense SM, Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell. Res. 2006;16:681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- [5].Hu R-G, Wang H, Xia Z, Varshavsky A. The N-end rule pathway is a sensor of heme. Proc. Nat. Acad. Sci. U. S. A. 2008;105:76–81. doi: 10.1073/pnas.0710568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gray HB, Winkler JR. Electron transfer in proteins. Annu. Rev. Biochem. 1996;65:537–561. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- [7].Reedy CJ, Gibney BR. Heme protein assemblies. Chem. Rev. 2004;104:617–649. doi: 10.1021/cr0206115. [DOI] [PubMed] [Google Scholar]
- [8].Zhuang J, Reddi AR, Wang Z, Khodaverdian B, Hegg EL, Gibney BR. Evaluating the roles of the heme a side chains in cytochrome c oxidase using designed heme proteins. Biochemistry. 2006;45:12530–12538. doi: 10.1021/bi060565t. [DOI] [PubMed] [Google Scholar]
- [9].Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- [10].Tezcan FA, Winkler JR, Gray HB. Effects of ligation and folding on reduction potentials of heme proteins. J. Am. Chem. Soc. 1998;120:13383–13388. [Google Scholar]
- [11].Barker PD, Ferguson SJ. Still a puzzle: why is haem covalently attached in c-type cytochromes? Structure. 1999;7:281–290. doi: 10.1016/s0969-2126(00)88334-3. [DOI] [PubMed] [Google Scholar]
- [12].Kranz RG, Richard-Fogal C, Taylor J-S, Frawley ER. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009;73:510–528. doi: 10.1128/MMBR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lemire BD, Oyedotun KS. The Saccharomyces cerevisiae mitochondrial succinate:ubiquinone oxidoreductase. Biochim. Biophys. Acta. 2002;1553:102–116. doi: 10.1016/s0005-2728(01)00229-8. [DOI] [PubMed] [Google Scholar]
- [14].Lancaster CRD. Succinate:quinone oxidoreductases: an overview. Biochim. Biophys. Acta. 2002;1553:1–6. doi: 10.1016/s0005-2728(01)00240-7. [DOI] [PubMed] [Google Scholar]
- [15].Lemos RS, Fernandes AS, Pereira MM, Gomes CM, Teixeira M. Quinol:fumarate oxidoreductases and succinate:quinone oxidoreductases: phylogenetic relationships, metal centres and membrane attachment. Biochim. Biophys. Acta. 2002;1553:158–170. doi: 10.1016/s0005-2728(01)00239-0. [DOI] [PubMed] [Google Scholar]
- [16].Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase—assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iverson TM, Luna-Chavez C, Schröder I, Cecchini G, Rees DC. Analyzing your complexes: structure of the quinol-fumarate reductase respiratory complex. Curr. Opin. Struct. Biol. 2000;10:448–455. doi: 10.1016/s0959-440x(00)00113-5. [DOI] [PubMed] [Google Scholar]
- [18].Yankovskaya V, Horsefield R, Törnroth S, Luna-Chavez C, Miyoshi H, Léger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- [19].Dym O, Eisenberg D. Sequence–structure analysis of FAD-containing proteins. Protein Sci. 2001;10:1712–1728. doi: 10.1110/ps.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- [21].Hägerhäll C. Succinate: quinone oxidoreductases variations on a conserved theme. Biochim. Biophys. Acta. 1997;1320:107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- [22].Oyedotun KS, Sit CS, Lemire BD. The Saccharomyces cerevisiae succinate dehydrogenase does not require heme for ubiquinone reduction. Biochim. Biophys. Acta. 2007;1767:1436–1445. doi: 10.1016/j.bbabio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- [23].Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH. Escherichia coli succinate dehydrogenase variant lacking the heme b. Proc. Nat. Acad. Sci. U. S. A. 2007;104:18007–18012. doi: 10.1073/pnas.0707732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maklashina E, Rajagukguk S, McIntire WS, Cecchini G. Mutation of the heme axial ligand of Escherichia coli succinate-quinone reductase: implications for heme ligation in mitochondrial complex II from yeast. Biochim. Biophys. Acta. 2010;1797:747–754. doi: 10.1016/j.bbabio.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Page CC, Moser CC, Chen X, Dutton PL. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature. 1999;402:47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- [26].Blatt Y, Pecht I. Allosteric cooperative interactions among redox sites of Pseudomonas cytochrome oxidase. Biochemistry. 1979;18:2917–2922. doi: 10.1021/bi00580a037. [DOI] [PubMed] [Google Scholar]
- [27].Moore GR, Pettigrew GW. Cytochromes c. Springer-Verlag; 1987. [Google Scholar]
- [28].Iverson TM, Luna-Chavez C, Cecchini G, Rees DC. Structure of the Escherichia coli fumarate reductase respiratory complex. Science. 1999;284:1961–1966. doi: 10.1126/science.284.5422.1961. [DOI] [PubMed] [Google Scholar]
- [29].Holmgren E, Hederstedt L, Rutberg L. Role of heme in synthesis and membrane binding of succinic dehydrogenase in Bacillus subtilis. J. Bacteriol. 1979;138:377–382. doi: 10.1128/jb.138.2.377-382.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakamura K, Yamaki M, Sarada M, Nakayama S, Vibat CR, Gennis RB, Nakayashiki T, Inokuchi H, Kojima S, Kita K. Two hydrophobic subunits are essential for the heme b ligation and functional assembly of complex II (succinate-ubiquinone oxidoreductase) from Escherichia coli. J. Biol. Chem. 1996;271:521–527. doi: 10.1074/jbc.271.1.521. [DOI] [PubMed] [Google Scholar]
- [31].Nihei C, Nakayashiki T, Nakamura K, Inokuchi H, Gennis RB, Kojima S, Kita K. Abortive assembly of succinate-ubiquinone reductase (complex II) in a ferrochelatase-deficient mutant of Escherichia coli. Mol. Genet. Genomics. 2001;265:394–404. doi: 10.1007/s004380100444. [DOI] [PubMed] [Google Scholar]
- [32].Vibat CR, Cecchini G, Nakamura K, Kita K, Gennis RB. Localization of histidine residues responsible for heme axial ligation in cytochrome b556 of complex II (succinate:ubiquinone oxidoreductase) in Escherichia coli. Biochemistry. 1998;37:4148–4159. doi: 10.1021/bi9716635. [DOI] [PubMed] [Google Scholar]
- [33].Maklashina E, Rothery RA, Weiner JH, Cecchini G. Retention of heme in axial ligand mutants of succinate-ubiquinone oxidoreductase (complex II) from Escherichia coli. J. Biol. Chem. 2001;276:18968–18976. doi: 10.1074/jbc.M011270200. [DOI] [PubMed] [Google Scholar]
- [34].Oyedotun KS, Yau PF, Lemire BD. Identification of the heme axial ligands in the cytochrome b562 of the Saccharomyces cerevisiae succinate dehydrogenase. J. Biol. Chem. 2004;279:9432–9439. doi: 10.1074/jbc.M311877200. [DOI] [PubMed] [Google Scholar]
- [35].Lemarie A, Grimm S. Mutations in the heme b-binding residue of SDHC inhibit assembly of respiratory chain complex II in mammalian cells. Mitochondrion. 2009;9:254–260. doi: 10.1016/j.mito.2009.03.004. [DOI] [PubMed] [Google Scholar]
- [36].Gebert N, Gebert M, Oeljeklaus S, von der Malsburg K, Stroud DA, Kulawiak B, Wirth C, Zahedi RP, Dolezal P, Wiese S, Simon O, Schulze-Specking A, Truscott KN, Sickmann A, Rehling P, Guiard B, Hunte C, Warscheid B, van der Laan M, Pfanner N, et al. Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Mol. Cell. 2011;44:811–818. doi: 10.1016/j.molcel.2011.09.025. [DOI] [PubMed] [Google Scholar]
- [37].Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- [38].Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- [41].Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- [42].Lange C, Hunte C. Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. Proc. Nat. Acad. Sci. U. S. A. 2002;99:2800–2805. doi: 10.1073/pnas.052704699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sun J, Trumpower BL. Superoxide anion generation by the cytochrome bc1 complex. Arch. Biochem. Biophys. 2003;419:198–206. doi: 10.1016/j.abb.2003.08.028. [DOI] [PubMed] [Google Scholar]
- [44].Yun CH, Crofts AR, Gennis RB. Assignment of the histidine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry. 1991;30:6747–6754. doi: 10.1021/bi00241a017. [DOI] [PubMed] [Google Scholar]
- [45].Esposti MD, De Vries S, Crimi M, Ghelli A, Patarnello T, Meyer A. Mitochondrial cytochrome b: evolution and structure of the protein. Biochim. Biophys. Acta. 1993;1143:243–271. doi: 10.1016/0005-2728(93)90197-n. [DOI] [PubMed] [Google Scholar]
- [46].Swierczek M, Cieluch E, Sarewicz M, Borek A, Moser CC, Dutton PL, Osyczka A. An electronic bus bar lies in the core of cytochrome bc1. Science. 2010;329:451–454. doi: 10.1126/science.1190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fülöp V, Sam KA, Ferguson SJ, Ginger ML, Allen JWA. Structure of a trypanosomatid mitochondrial cytochrome c with heme attached via only one thioether bond and implications for the substrate recognition requirements of heme lyase. FEBS J. 2009;276:2822–2832. doi: 10.1111/j.1742-4658.2009.07005.x. [DOI] [PubMed] [Google Scholar]
- [48].Ambler RP. Sequence variability in bacterial cytochromes c. Biochim. Biophys. Acta. 1991;1058:42–47. doi: 10.1016/s0005-2728(05)80266-x. [DOI] [PubMed] [Google Scholar]
- [49].Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- [50].Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu. Rev. Physiol. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- [51].Osyczka A, Moser CC, Dutton PL. Fixing the Q cycle. Trends Biochem. Sci. 2005;30:176–182. doi: 10.1016/j.tibs.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [52].Zhu QS, Berden JA, De Vries S, Folkers K, Porter T, Slater EC. Identification of two different Q-binding sites in QH2-cytochrome c oxidoreductase, using the Q analogue n-heptadecylmercapto-6-hydroxy-5,8-quinolinequinone. Biochim. Biophys. Acta. 1982;682:160–167. doi: 10.1016/0005-2728(82)90130-x. [DOI] [PubMed] [Google Scholar]
- [53].Trumpower B. A concerted, alternating sites mechanism of ubiquinol oxidation by the dimeric cytochrome bc1 complex. Biochim. Biophys. Acta. 2002;1555:166–173. doi: 10.1016/s0005-2728(02)00273-6. [DOI] [PubMed] [Google Scholar]
- [54].Covian R, Trumpower BL. Regulatory interactions in the dimeric cytochrome bc(1) complex: the advantages of being a twin. Biochim. Biophys. Acta. 2008;1777:1079–1091. doi: 10.1016/j.bbabio.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hamel P, Corvest V, Giegé P, Bonnard G. Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim. Biophys. Acta. 2009;1793:125–138. doi: 10.1016/j.bbamcr.2008.06.017. [DOI] [PubMed] [Google Scholar]
- [56].Brasseur G, Saribaş AS, Daldal F. A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim. Biophys. Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- [57].Robertson DE, Farid RS, Moser CC, Urbauer JL, Mulholland SE, Pidikiti R, Lear JD, Wand AJ, DeGrado WF, Dutton PL. Design and synthesis of multi-haem proteins. Nature. 1994;368:425–432. doi: 10.1038/368425a0. [DOI] [PubMed] [Google Scholar]
- [58].Rödel G. Two yeast nuclear genes, CBS1 and CBS2, are required for translation of mitochondrial transcripts bearing the 5′-untranslated COB leader. Curr. Genet. 1986;11:41–45. doi: 10.1007/BF00389424. [DOI] [PubMed] [Google Scholar]
- [59].Gruschke S, Kehrein K, Römpler K, Gröne K, Israel L, Imhof A, Herrmann JM, Ott M. Cbp3–Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J. Cell Biol. 2011;193:1101–1114. doi: 10.1083/jcb.201103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zara V, Conte L, Trumpower BL. Identification and characterization of cytochrome bc(1) subcomplexes in mitochondria from yeast with single and double deletions of genes encoding cytochrome bc(1) subunits. FEBS J. 2007;274:4526–4539. doi: 10.1111/j.1742-4658.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- [61].Crivellone MD, Wu MA, Tzagoloff A. Assembly of the mitochondrial membrane system analysis of structural mutants of the yeast coenzyme QH2–cytochrome c reductase complex. J. Biol. Chem. 1988;263:14323–14333. [PubMed] [Google Scholar]
- [62].Zara V, Conte L, Trumpower BL. Evidence that the assembly of the yeast cytochrome bc1 complex involves the formation of a large core structure in the inner mitochondrial membrane. FEBS J. 2009;276:1900–1914. doi: 10.1111/j.1742-4658.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cruciat CM, Brunner S, Baumann F, Neupert W, Stuart RA. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 2000;275:18093–18098. doi: 10.1074/jbc.M001901200. [DOI] [PubMed] [Google Scholar]
- [64].Thony-Meyer L, Künzler P. Translocation to the periplasm and signal sequence cleavage of preapocytochrome c depend on sec and lep, but not on the ccm gene products. Eur. J. Biochem. 1997;246:794–799. doi: 10.1111/j.1432-1033.1997.t01-1-00794.x. [DOI] [PubMed] [Google Scholar]
- [65].Gerhus E, Steinrücke P, Ludwig B. Paracoccus denitrificans cytochrome c1 gene replacement mutants. J. Bacteriol. 1990;172:2392–2400. doi: 10.1128/jb.172.5.2392-2400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thony-Meyer L, James P, Hennecke H. From one gene to two proteins: the biogenesis of cytochromes b and c1 in Bradyrhizobium japonicum. Proc. Nat. Acad. Sci. U. S. A. 1991;88:5001–5005. doi: 10.1073/pnas.88.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Konishi K, Van Doren SR, Kramer DM, Crofts AR, Gennis RB. Preparation and characterization of the water-soluble heme-binding domain of cytochrome c1 from the Rhodobacter sphaeroides bc1 complex. J. Biol. Chem. 1991;266:14270–14276. [PubMed] [Google Scholar]
- [68].Hartl FU, Ostermann J, Guiard B, Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987;51:1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- [69].Arnold I, Fölsch H, Neupert W, Stuart RA. Two distinct and independent mitochondrial targeting signals function in the sorting of an inner membrane protein, cytochrome c1. J. Biol. Chem. 1998;273:1469–1476. doi: 10.1074/jbc.273.3.1469. [DOI] [PubMed] [Google Scholar]
- [70].Bernard DG, Gabilly ST, Dujardin G, Merchant S, Hamel PP. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 2003;278:49732–49742. doi: 10.1074/jbc.M308881200. [DOI] [PubMed] [Google Scholar]
- [71].Steiner H, Kispal G, Zollner A, Haid A, Neupert W, Lill R. Heme binding to a conserved Cys–Pro–Val motif is crucial for the catalytic function of mitochondrial heme lyases. J. Biol. Chem. 1996;271:32605–32611. doi: 10.1074/jbc.271.51.32605. [DOI] [PubMed] [Google Scholar]
- [72].Bernard DG, Quevillon-Cheruel S, Merchant S, Guiard B, Hamel PP. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J. Biol. Chem. 2005;280:39852–39859. doi: 10.1074/jbc.M508574200. [DOI] [PubMed] [Google Scholar]
- [73].Corvest V, Murrey DA, Bernard DG, Knaff DB, Guiard B, Hamel PP. c-type cytochrome assembly in Saccharomyces cerevisiae: a key residue for apocytochrome c1/lyase interaction. Genetics. 2010;186:561–571. doi: 10.1534/genetics.110.120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Khalimonchuk O, Rödel G. Biogenesis of cytochrome c oxidase. Mitochondrion. 2005;5:363–388. doi: 10.1016/j.mito.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [75].Mick DU, Fox TD, Rehling P. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 2011;12:14–20. doi: 10.1038/nrm3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Soto IC, Fontanesi F, Liu J, Barrientos A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core, Biochim, Biophys. Acta (BBA) - Bioenergetics. 2012;1817:883–897. doi: 10.1016/j.bbabio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yoshikawa S. Beef heart cytochrome c oxidase. Curr. Opin. Struct. Biol. 1997;7:574–579. doi: 10.1016/s0959-440x(97)80124-8. [DOI] [PubMed] [Google Scholar]
- [78].Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- [79].Ostermeier C, Harrenga A, Ermler U, Michel H. Structure at 27 A resolution of the Paracoccus denitrificans two-subunit cytochrome c oxidase complexed with an antibody FV fragment. Proc. Nat. Acad. Sci. U. S. A. 1997;94:10547–10553. doi: 10.1073/pnas.94.20.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Williams PA, Blackburn NJ, Sanders D, Bellamy H, Stura EA, Fee JA, McRee DE. The CuA domain of Thermus thermophilus ba3-type cytochrome c oxidase at 1.6 A resolution. Nat. Struct. Biol. 1999;6:509–516. doi: 10.1038/9274. [DOI] [PubMed] [Google Scholar]
- [81].Varanasi L, Hosler J. Alternative initial proton acceptors for the D pathway of Rhodobacter sphaeroides cytochrome c oxidase. Biochemistry. 2011;50:2820–2828. doi: 10.1021/bi102002v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- [83].Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, Meunier B, Hanna MG, García JJ, Capaldi RA, Lake BD, Leonard JV, Schapira AH. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am. J. Hum. Genet. 1999;65:1030–1039. doi: 10.1086/302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yoshikawa S, Muramoto K, Shinzawa-Itoh K, Aoyama H, Tsukihara T, Ogura T, Shimokata K, Katayama Y, Shimada H. Reaction mechanism of bovine heart cytochrome c oxidase. Biochim. Biophys. Acta. 2006;1757:395–400. doi: 10.1016/j.bbabio.2006.04.028. [DOI] [PubMed] [Google Scholar]
- [85].Kaila VRI, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem. Rev. 2010;110:7062–7081. doi: 10.1021/cr1002003. [DOI] [PubMed] [Google Scholar]
- [86].Kim YC, Hummer G. Proton-pumping mechanism of cytochrome c oxidase: A kinetic master-equation approach. Biochim. Biophys. Acta. 2012;1817:526–536. doi: 10.1016/j.bbabio.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Barros M, Tzagoloff A. Regulation of the heme A biosynthetic pathway in Saccharomyces cerevisiae. FEBS Lett. 2002;516:119–123. doi: 10.1016/s0014-5793(02)02514-0. [DOI] [PubMed] [Google Scholar]
- [88].Barros MH, Nobrega FG, Tzagoloff A. Mitochondrial ferredoxin is required for heme A synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:9997–10002. doi: 10.1074/jbc.M112025200. [DOI] [PubMed] [Google Scholar]
- [89].Brown KR, Allan BM, Do P, Hegg EL. Identification of novel hemes generated by heme A synthase: evidence for two successive monooxygenase reactions. Biochemistry. 2002;41:10906–10913. doi: 10.1021/bi0203536. [DOI] [PubMed] [Google Scholar]
- [90].Hederstedt L, Lewin A, Throne-Holst M. Heme A synthase enzyme functions dissected by mutagenesis of Bacillus subtilis CtaA. J. Bacteriol. 2005;187:8361–8369. doi: 10.1128/JB.187.24.8361-8369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Brown K, Brown B, Hoagland E, Mayne C. Heme A synthase does not incorporate molecular oxygen into the formyl group of heme A. Biochemistry. 2004;43:8616–8624. doi: 10.1021/bi049056m. [DOI] [PubMed] [Google Scholar]
- [92].Nobrega MP, Nobrega FG, Tzagoloff A. COX10 codes for a protein homologous to the ORF1 product of Paracoccus denitrificans and is required for the synthesis of yeast cytochrome oxidase. J. Biol. Chem. 1990;265:14220–14226. [PubMed] [Google Scholar]
- [93].Bestwick M, Khalimonchuk O, Pierrel F, Winge DR. The role of Coa2 in hemylation of yeast Cox1 revealed by its genetic interaction with Cox10. Mol. Cell. Biol. 2010;30:172–175. doi: 10.1128/MCB.00869-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Z, Wang Y, Hegg EL. Regulation of the heme A biosynthetic pathway: differential regulation of heme A synthase and heme O synthase in Saccharomyces cerevisiae. J. Biol. Chem. 2009;284:839–847. doi: 10.1074/jbc.M804167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Svensson B, Lübben M, Hederstedt L. Bacillus subtilis CtaA and CtaB function in haem A biosynthesis. Mol. Microbiol. 1993;10:193–201. doi: 10.1111/j.1365-2958.1993.tb00915.x. [DOI] [PubMed] [Google Scholar]
- [96].Svensson B, Hederstedt L. Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J. Bacteriol. 1994;176:6663–6671. doi: 10.1128/jb.176.21.6663-6671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Svensson B, Andersson KK, Hederstedt L. Low-spin heme A in the heme A biosynthetic protein CtaA from Bacillus subtilis. Eur. J. Biochem. 1996;238:287–295. doi: 10.1111/j.1432-1033.1996.0287q.x. [DOI] [PubMed] [Google Scholar]
- [98].Glerum DM, Muroff I, Jin C, Tzagoloff A. COX15 codes for a mitochondrial protein essential for the assembly of yeast cytochrome oxidase. J. Biol. Chem. 1997;272:19088–19094. doi: 10.1074/jbc.272.30.19088. [DOI] [PubMed] [Google Scholar]
- [99].Petruzzella V, Tiranti V, Fernandez P, Ianna P, Carrozzo R, Zeviani M. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics. 1998;54:494–504. doi: 10.1006/geno.1998.5580. [DOI] [PubMed] [Google Scholar]
- [100].Barros MH, Carlson CG, Glerum DM, Tzagoloff A. Involvement of mitochondrial ferredoxin and Cox15p in hydroxylation of heme O. FEBS Lett. 2001;492:133–138. doi: 10.1016/s0014-5793(01)02249-9. [DOI] [PubMed] [Google Scholar]
- [101].Bureik M, Schiffler B, Hiraoka Y, Vogel F, Bernhardt R. Functional expression of human mitochondrial CYP11B2 in fission yeast and identification of a new internal electron transfer protein, etp1. Biochemistry. 2002;41:2311–2321. doi: 10.1021/bi0157870. [DOI] [PubMed] [Google Scholar]
- [102].Antonicka H, Leary SC, Guercin G-H, Agar JN, Horvath R, Kennaway NG, Harding CO, Jaksch M, Shoubridge EA. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003;12:2693–2702. doi: 10.1093/hmg/ddg284. [DOI] [PubMed] [Google Scholar]
- [103].Steinrücke P, Ludwig B. Genetics of Paracoccus denitrificans. FEMS Microbiol. Rev. 1993;10:83–117. doi: 10.1016/0378-1097(93)90505-v. [DOI] [PubMed] [Google Scholar]
- [104].Williams S, Valnot I, Rustin P, Taanman JW. Cytochrome c oxidase subassemblies in fibroblast cultures from patients carrying mutations in COX10, SCO1, or SURF1. J. Biol. Chem. 2004;279:7462–7469. doi: 10.1074/jbc.M309232200. [DOI] [PubMed] [Google Scholar]
- [105].Hiser L, Hosler JP. Heme a is not essential for assembly of the subunits of cytochrome c oxidase of Rhodobacter sphaeroides. J. Biol. Chem. 2001;276:45403–45407. doi: 10.1074/jbc.M107016200. [DOI] [PubMed] [Google Scholar]
- [106].Carr HS, Winge DR. Assembly of cytochrome c oxidase within the mitochondrion. Acc. Chem. Res. 2003;36:309–316. doi: 10.1021/ar0200807. [DOI] [PubMed] [Google Scholar]
- [107].Zee JM, Glerum DM. Defects in cytochrome oxidase assembly in humans: lessons from yeast. Biochem. Cell Biol. 2006;84:859–869. doi: 10.1139/o06-201. [DOI] [PubMed] [Google Scholar]
- [108].Khalimonchuk O, Bestwick M, Meunier B, Watts TC, Winge DR. Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol. Cell. Biol. 2010;30:1004–1017. doi: 10.1128/MCB.00640-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mashkevich G, Repetto B, Glerum DM, Jin C, Tzagoloff A. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 1997;272:14356–14364. doi: 10.1074/jbc.272.22.14356. [DOI] [PubMed] [Google Scholar]
- [110].Pierrel F, Bestwick ML, Cobine PA, Khalimonchuk O, Cricco JA, Winge DR. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 2007;26:4335–4346. doi: 10.1038/sj.emboj.7601861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pierrel F, Khalimonchuk O, Cobine PA, Bestwick M, Winge DR. Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis that facilitates the maturation of Cox1. Mol. Cell. Biol. 2008;28:4927–4939. doi: 10.1128/MCB.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003;72:101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stiburek L, Vesela K, Hansikova H, Pecina P, Tesarova M, Cerna L, Houstek J, Zeman J. Tissue-specific cytochrome c oxidase assembly defects due to mutations in SCO2 and SURF1. Biochem. J. 2005;392:625–632. doi: 10.1042/BJ20050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Khalimonchuk O, Bird A, Winge DR. Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J. Biol. Chem. 2007;282:17442–17449. doi: 10.1074/jbc.M702379200. [DOI] [PubMed] [Google Scholar]
- [115].Smith D, Gray J, Mitchell L, Antholine WE, Hosler JP. Assembly of cytochrome-c oxidase in the absence of assembly protein Surf1p leads to loss of the active site heme. J. Biol. Chem. 2005;280:17652–17656. doi: 10.1074/jbc.C500061200. [DOI] [PubMed] [Google Scholar]
- [116].Bundschuh FA, Hoffmeier K, Ludwig B. Two variants of the assembly factor Surf1 target specific terminal oxidases in Paracoccus denitrificans. Biochim. Biophys. Acta. 2008;1777:1336–1343. doi: 10.1016/j.bbabio.2008.05.448. [DOI] [PubMed] [Google Scholar]
- [117].Stenberg F, von Heijne G, Daley DO. Assembly of the cytochrome bo3 complex. J. Mol. Biol. 2007;371:765–773. doi: 10.1016/j.jmb.2007.05.045. [DOI] [PubMed] [Google Scholar]
- [118].Gibney BR, Isogai Y, Rabanal F, Reddy KS, Grosset AM, Moser CC, Dutton PL. Self-assembly of heme A and heme B in a designed four-helix bundle: implications for a cytochrome c oxidase maquette. Biochemistry. 2000;39:11041–11049. doi: 10.1021/bi000925r. [DOI] [PubMed] [Google Scholar]
- [119].Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- [120].Bundschuh FA, Hannappel A, Anderka O, Ludwig B. Surf1, associated with Leigh syndrome in humans, is a heme-binding protein in bacterial oxidase biogenesis. J. Biol. Chem. 2009;284:25735–25741. doi: 10.1074/jbc.M109.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Werner C, Richter O-MH, Ludwig B. A novel heme a insertion factor gene cotranscribes with the Thermus thermophilus cytochrome ba3 oxidase locus. J. Bacteriol. 2010;192:4712–4719. doi: 10.1128/JB.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bestwick M, Jeong M-Y, Khalimonchuk O, Kim H, Winge DR. Analysis of Leigh syndrome mutations in the yeast SURF1 homolog reveals a new member of the cytochrome oxidase assembly factor family. Mol. Cell. Biol. 2010;30:4480–4491. doi: 10.1128/MCB.00228-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Khalimonchuk O, Jeong M-Y, Watts T, Ferris E, Winge DR. Selective Oma1-mediated proteolysis of the Cox1 subunit of cytochrome oxidase in assembly mutants. J. Biol. Chem. 2012 doi: 10.1074/jbc.M111.313148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Harmon PA, Hendler RW, Levin IW. Resonance Raman and optical spectroscopic monitoring of heme a redox states in cytochrome c oxidase during potentiometric titrations. Biochemistry. 1994;33:699–707. doi: 10.1021/bi00169a011. [DOI] [PubMed] [Google Scholar]
- [125].Dutton P, Wilson D, Lee C-P. Oxidation–reduction potentials of cytochromes in mitochondria. Biochemistry. 1970;9:5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]