Abstract
STIM proteins are ubiquitous endoplasmic reticulum Ca2+ sensors that rapidly translocate to couple with ‘store-operated’ Orai Ca2+ channels when luminal Ca2+ levels are low. STIM1 also senses heat changes, which trigger a similar translocation and prime STIM1 to activate Orai, suggesting that STIM1 functions as a sensor of multiple stress signals.
The generation of Ca2+ signals is crucial to cells. Such signals control not only rapid functions such as secretion and contraction but also longer-term responses including transcription, cell division and apoptosis1. Ca2+ stored in the endoplasmic reticulum (ER) of cells is released to generate rapid and robust Ca2+ signals, but the ER Ca2+ stores must be replenished to protect the protein-folding environment within the ER. Depleted ER Ca2+ levels are sensed by STIM proteins, which are remarkably dynamic ER membrane proteins functioning as sensors of small changes in Ca2+ stored within the ER2,3. STIMs physically couple to Orai channels in the plasma membrane to activate store-operated Ca2+ entry. This Ca2+ entry not only replenishes stores but provides important spatially restricted and longer-term cytoplasmic Ca2+ signals that control crucial cellular responses such as gene expression and growth2,4,5. A new study6 now reveals that STIM1, in addition to sensing Ca2+, also senses small physiologically encountered increases in temperature. These changes in temperature trigger the same translocation of STIM1 toward plasma membrane junctions as Ca2+ store depletion, indicating that STIM proteins have multifunctional sensing capabilities.
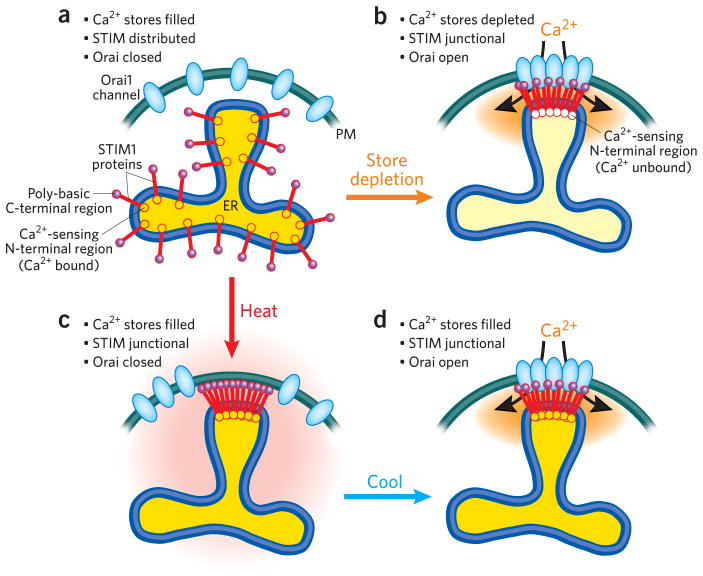
As stores release Ca2+ to generate cytoplasmic signals, Ca2+ dissociates from the luminal N-terminal Ca2+-sensing domain on STIM1, triggering STIM1 to aggregate and rapidly translocate within the ER membrane into discrete junctional regions closely apposed with the plasma membrane (Fig. 1a, b)2,3. In these junctions, the C-terminal cytoplasmic STIM1 domain makes contact with the plasma membrane, where it activates the highly selective Orai Ca2+ entry channels, which mediate longer-term Ca2+ signals crucial to turning on gene expression in many cell types2,3. The large cytoplasmic STIM1 domain unfolds and extends across the ~15-nm ER–plasma membrane junctional space to make contact with the plasma membrane. Two important STIM1 cytoplasmic domains mediate coupling to the plasma membrane: a poly-basic, lysine-rich region at the far C terminus attaches to the plasma membrane surface, perhaps to negatively charged lipids7,8, and a discrete segment of approximately 100 amino acids, the Ca2+-activating domain (CAD), interacts with and tethers Orai channels in the plasma membrane, activating their Ca2+ entry channel function7.
Figure 1.
STIM1 activation results from either Ca2+ sensing or temperature sensing. The diagram represents the dynamic interactions between STIM proteins in the ER and Orai channels in the plasma membrane in response to Ca2+ store depletion or transient increased temperature. (a) In the resting state, ER Ca2+ stores are replete, and STIM is distributed throughout the ER; Orai channels are inactive and distributed across the plasma membrane. (b) When ER Ca2+ is depleted after activation of Ca2+ release channels, Ca2+ dissociates from the luminal Ca2+-sensing EF hand domain of STIM1, and STIM1 aggregates and translocates into ER–plasma membrane junctions assisted by interaction of the poly-basic region (purple) with the plasma membrane. The CAD region of STIM1 (not shown) attaches to and activates Orai channels. (c) An increase in temperature to above 40 °C causes STIM1 to aggregate and translocate into ER–plasma membrane junctions. The poly-basic C terminus of STIM1 is essential for this to occur, as the ΔK-STIM1 truncated version of STIM1 does not undergo heat-induced translocation. The increased temperature prevents STIM-Orai interactions, hence Orai channels are not tethered or activated at the higher temperature. (d) After heat-primed cells are returned to a lowered temperature, the junctional STIM can now bind and activate Orai channels, even though the Ca2+ stores within the ER have not been depleted.
The notion that activation of STIM1 leading to Orai coupling is triggered exclusively by ER luminal Ca2+ depletion has already been challenged; recent evidence reveals that a cysteine residue close to the luminal N-terminal Ca2+-sensing domain of STIM1 can become glutathionylated under conditions of oxidative stress9. In this case, STIM1 may function as a redox sensor and trigger coupling to activate Ca2+ entry through Orai channels without the necessity for store depletion9.
In this issue, Xiao et al.6 reveal that temperature profoundly alters STIM1 function, with modest temperature increases to above 40 °C causing STIM1 to constitutively aggregate and translocate into near–plasma membrane junctions without Ca2+ store depletion (Fig. 1c). However, the effect of temperature on the functional coupling between STIM1 and Orai1 is more complex. The STIM-induced coupling and activation of Orai channels mediated by the CAD domain is actually inhibited at elevated temperatures (above 40 °C). Interestingly, short-term (1–2 min) exposure of cells expressing STIM1 and Orai1 to the higher temperatures seems to prime the coupling process such that a return to lower temperatures results in profound activation of Orai channels (Fig. 1d), this ‘heat-off’ response again occurring independently of Ca2+ store depletion. The lysine-rich, polybasic STIM1 C terminus is essential for the heat-induced translocation of STIM1 into junctions (Fig. 1c), as shown by C-terminal truncation of STIM1 (ΔK-STIM1), which prevents this movement into junctions. However, ΔK-STIM1 can still translocate into junctions and activate Orai channels in response to Ca2+ store depletion, as the CAD-Orai1 interaction is sufficiently strong at normal temperature. Thus, activation of STIM1 by Ca2+ store emptying is not equivalent to heat-mediated activation of STIM1, the latter requiring the poly-basic region of STIM1 for attachment to the plasma membrane. However, the CAD domain in STIM1 is essential for both the heat-off– and Ca2+ store depletion– induced functional coupling to activate Orai channels.
These recent studies reveal STIM1 in a new light—as a remarkable sensor of multiple stress signals. The depletion of Ca2+ from ER stores, the levels of reactive oxygen species and the effects of increased temperature are each stress conditions that result in misfolding and damage to proteins with severe consequences on cellular function and growth. STIM proteins seem designed to sense each of these three stress conditions, triggering the same dynamic translocation and plasma membrane–coupling pathway. Moreover, STIM has targets other than just the stringently Ca2+-selective Orai channels—it couples to modify function of the widely expressed transient receptor potential cation channel family and also voltage-operated Ca2+ channels10. Elimination of STIM proteins has profound effects on the function and development of many cell types, especially hematopoietic cells; other cells that seem primarily influenced by STIM knockdown include keratinocytes, endothelial cells and skeletal and smooth muscle cells2–4. Until recently, the focus of understanding STIM proteins has been their role in Ca2+ signaling and Ca2+ homeostasis. However, the ability of STIM1 to transduce temperature and redox changes into calcium and downstream signals may have special significance in each one of these tissues. Indeed, the role of STIM proteins in modulating Ca2+ signals in response to stress conditions may be profoundly important in the function, development and pathophysiology of many distinct cell types.
Footnotes
Competing financial interests: The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturechemicalbiology/.
References
- 1.Clapham DE. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins SR, Meyer T. Trends Cell Biol. 2011;21:202–211. doi: 10.1016/j.tcb.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan PG, Lewis RS, Rao A. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancarella S, Wang Y, Gill DL. Curr Biol. 2009;19:R950–R952. doi: 10.1016/j.cub.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao B, Coste B, Mathur J, Patapoutian A. Nat Chem Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CY, et al. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Deng X, Gill DL. Sci Signal. 2010;3:pe42. doi: 10.1126/scisignal.3148pe42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins BJ, et al. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]