Abstract
Introduction:
Article 8 of the Framework Convention on Tobacco Control mandates all signatory countries to “protect citizens from exposure to tobacco smoke in workplaces, public transport and indoor public places.” Even though there has been great progress in the implementation of Article 8, still most of the world population remains exposed to secondhand smoke (SHS). In this article, we sought to summarize the research that supports Article 8, where do we stand, and current research gaps and future directions.
Discussion:
Secondhand smoke is an established cause of heart disease and several types of cancer. Additional research is needed to reach final conclusions for diseases where evidence is only suggestive of causality. The only solution to SHS exposure in public places is banning smoking indoors. Research on the gaming industry and nightclubs, particularly in developing countries, needs to be disseminated to support their inclusion in smoke-free laws. Aside from indoor bans, additional research is needed for outdoor and multiunit housing bans and in support of measures that protect children and other vulnerable populations. The impact of smoke-free laws on other health outcomes, besides heart disease and respiratory outcomes, is another area where further research is needed. Thirdhand smoke assessment and health effects are also likely to be a topic of further research. As new tobacco products emerge, evaluating SHS exposure and effects will be vital.
Conclusions:
Furthering research in support of Article 8 can contribute to reach the final goal of protecting everyone from SHS exposure.
Secondhand smoke (SHS), the tobacco smoke generated by active smokers, remains a widespread health hazard worldwide (U.S. Department of Health and Human Services, 2006). Stimulated by the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC), countries and subnational entities are implementing smoke-free legislations to protect populations from the health effects of SHS exposure. In this article, we sought to summarize research efforts that have contributed to the advancement of smoke-free environments and those that are needed to ensure complete prevention and control of SHS exposure worldwide (World Health Organization, 2011).
PROTECTION FROM EXPOSURE TO TOBACCO SMOKE: FCTC ARTICLE 8
The WHO FCTC, the world’s first public health international treaty, aims to halt the tobacco epidemic. Among its key provisions, Article 8 mandates Parties to the treaty to “protect citizens from exposure to tobacco smoke in workplaces, public transport and indoor public places” (WHO Framework Convention on Tobacco Control, 2007). Recognizing that there is no safe level of SHS exposure, Article 8 implementation guidelines state that effective measures require total elimination of smoking and tobacco smoke in all indoor public places and workplaces as well as in other public places such as outdoor or quasi-outdoor places (WHO Framework Convention on Tobacco Control, 2007). Moreover, the guidelines indicate that legislation is needed, that all people must be protected, and that the implementation and enforcement of the legislation should be adequately monitored and evaluated.
The guidelines are thus straightforward in establishing that the only solution to SHS exposure is enforcing 100% smoke-free environments by law. The guidelines could have been stronger by qualifying SHS exposure in the workplace as a serious hazard that governments have to address in their occupational safety and health laws. Despite this limitation, the guidelines provide a powerful legislative framework for policy makers. Tobacco control advocates play an essential role in the process, ensuring the effective dissemination and implementation of the guidelines into evidence-based smoke-free policy including occupational safety and health policy. The International Agency for Research on Cancer (IARC) has also developed a model to evaluate smoke-free policy development and the factors to be considered when implementing and evaluating the resulting intermediate and distal effects (IARC, 2009). Moreover, resources to adequately monitor legislation development, implementation, and enforcement are critical to guarantee legislation success.
As of April 2012, 176 parties/countries had signed and ratified the FCTC. Partly stimulated by it, 66 countries have implemented nationwide legislation, although only 46 include bars and restaurants (American Nonsmokers’ Rights Foundation, 2012). Worldwide in 2011, there were an additional 739 million people protected from SHS exposure, an increase of more than 385 million since 2008 (World Health Organization, 2011). Most of the population, however, remains still unprotected, especially children and women. Additional efforts are needed to ensure widespread protection from SHS exposure in public places and workplaces.
THE IMPORTANCE OF SMOKE-FREE ENVIRONMENTS
Major Health Consequences of SHS
Scientific evidence has unequivocally established that SHS causes premature death and disease (Table 1) (Barnes & Bero, 1996; National Research Council Committee on Passive Smoking, 1986; U.S. Department of Health and Human Services, 1986). Most of the SHS exposure disease burden results from cardiovascular disease, lung cancer, and respiratory disease and developmental effects in children (California Environmental Protection Agency, 2005; Oberg, Jaakkola, Woodward, Peruga, & Prüss-Ustün, 2011; U.S. Department of Health and Human Services, 2006). The WHO estimates that 603,000 deaths were attributable to SHS exposure in 2004, corresponding to 1.0% of the worldwide mortality (Oberg et al., 2011). While increasing evidence suggests that SHS also affects smokers (Lai et al., 2011; Lam et al., 2005), the WHO burden of disease analyses considered only non-smokers, likely underestimating the burden of disease from SHS exposure.
Table 1.
Major Reports on SHS and Disease
| Number of diseases | ||||
|---|---|---|---|---|
| SHS associated with | SHS causally linked to | Diseases first established to be caused by SHS | ||
| U.S. SGRa | 1986 | 4 | 1 | Lung cancer |
| U.S. EPAb | 1992 | 3 | 3 | Middle ear diseaseIncrease severity and asthma symptoms |
| California EPAc | 1997 | 1 | 8 | Coronary heart diseaseNew asthmaSudden infant death syndrome |
| United Kingdomd | 1998 | 2 | 4 | — |
| WHOe | 1999 | 0 | 6 | — |
| IARCf | 2004 | 0 | 1 | — |
| Cal EPAg | 2005 | 1 | 10 | Breast cancer |
| U.S. SGRh | 2006 | 20i | 10 | — |
Note. Adapted from International Agency for Research on Cancer (IARC, 2009). EPA = Environmental Protection Agency; SGR = Surgeon General report; WHO = World Health Organization.
f IARC (2004).
iRefers to diseases where the evidence is suggestive but not sufficient.
In addition to the diseases with sufficient evidence to conclude that the association is causal, there is a long list of diseases for which there is suggestive but not sufficient evidence for causality (Table 2). Breast cancer, the most common cancer in women, has been related to SHS exposure (California Environmental Protection Agency, 2005; Miller et al., 2007; U.S. Department of Health and Human Services, 2006), although the relationship is still subject to debate (Pirie et al., 2008). Recent cohort studies have supported the notion that breast cancer is indeed caused by SHS and should be added to the list of diseases SHS cause (Boffetta & Autier, 2011; Johnson et al., 2011; Luo et al., 2011). Evidence also supports the association of SHS exposure with chronic obstructive pulmonary disease (COPD) (Yin et al., 2007) and tuberculosis (Basu, Stuckler, Bitton, & Glantz, 2011; Leung et al., 2010). Additional research is needed to complete the spectrum of diseases caused by SHS. This information will be important to understand the total burden of disease and economic impact of SHS exposure.
Table 2.
Diseases for Which Evidence Is Suggestive but Not Sufficient to Infer a Causal Relationship With SHS
| Reproductive and developmental effects |
| Preterm delivery |
| Childhood cancer |
| Leukemia |
| Lymphoma |
| Brain tumors |
| Respiratory effects on children |
| Middle ear effusion |
| Onset of childhood asthma |
| Cancer |
| Breast cancer |
| Nasal sinus cancer |
| Cardiovascular diseases |
| Stroke |
| Atherosclerosis |
| Respiratory effects |
| Nasal allergies |
| Acute respiratory symptoms |
| Chronic respiratory symptoms |
| Decline in lung function in asthmatics |
| Decrement in lung function in healthy individuals |
| Adult-onset and worsening of asthma |
| Chronic obstructive pulmonary disease |
| Tuberculosis |
Note. Adapted from U.S. Surgeon General Report (U.S. Department of Health and Human Services, 2006). Tuberculosis data are from California Environmental Protection Agency (2005), Leung et al. (2010), and U.S. Department of Health and Human Services (2006).
Economic burden of SHS exposure
Secondhand smoke exposure results in a considerable economic burden to individuals, businesses, and society. Australia, Canada, Hong Kong, Ireland, the United Kingdom, and the United States have found significant costs derived from SHS exposure. In the United States, in 2005, the annual costs of excess medical care, mortality, and morbidity exceeded $10 billion and an additional $5 billion from indirect costs (e.g., disability, lost wages) (Behan, Eriksen, & Lin, 2005). Even though most expenses are derived from treating SHS-caused diseases (Waters, Foldes, Alesci, & Samet, 2009), there is additional economic burden from SHS exposure to businesses, such as higher renovation, cleaning costs, and insurance premiums. Little is known about these economic costs in developing countries, in part due to the fact that reliable local data of SHS exposure are often lacking.
SMOKE-FREE ENVIRONMENTS: HISTORY, INEFFECTIVE ALTERNATIVES, AND INDUSTRY CHALLENGES
History of Smoke-Free Environments
The smoke-free environment movement began in the early 1970s (IARC, 2009), moving slowly from restrictions (e.g., the state of Arizona restricted smoking in public places in 1973) to comprehensive smoking bans (e.g., Vermont established a comprehensive smoking ban that excluded only establishments holding a cabaret license in 1995; Institute of Medicine, 2010). Worldwide, when FCTC discussions began in 1996, an incipient smoke-free movement was taking root in developed countries. By that time, research documenting the harmful effects of SHS and proving ventilation systems to be ineffective had accumulated.
Employees and nonsmokers’ rights organizations have played a key role in fighting for smoke-free legislation, particularly in the United States. In the mid-1970s, driven by the annoyance and potential health hazards from SHS exposure, nonsmokers began to organize educational campaigns and eventually pursued legislation. Then, in 1976, American for Nonsmokers’ Rights was founded. Initially United States—focused, it is now active worldwide (American Nonsmokers’ Rights Foundation, 2012). Flight attendants, exposed to extraordinarily high SHS levels in airplanes (Neilsen & Glantz, 2004; Repace, 2004a, 2004b), were instrumental in spearheading the push for legislation. Their efforts led to the elimination of smoking on U.S. flights. In 2000, the Flight Attendant Medical Research Institute, the largest foundation that supports SHS research was created through a court settlement. Two years later, in 2002, the International Commission on Occupational Health in their position document stated that “[e]mployees at their workplace must not breathe air that is contaminated by tobacco smoke” and concluded that the only way to achieve smoke-free workplaces is through legislation implementation and enforcement (International Commission on Occupational Health, 2002).
In the early 2000s, the enactment of highly influential smoke-free legislations in New York City (2002), Ireland and Norway (2004), and Uruguay (2006) contributed to the spreading of legislations worldwide. The successful implementation of these initiatives represents a turning point in the history of smoking, showing that implementing smoke-free legislation is relatively easy to do, has many health, societal, and economical benefits, and is largely supported by most populations. Moreover, evidence shows that support for smoke-free legislation increases markedly following legislation implementation (Borland et al., 2006; Heloma & Jaakkola, 2003).
Ineffective Alternatives to SHS Exposure Hazards
Research has been instrumental in determining the most efficient and complete way to solve the SHS exposure problem. The American Society of Heating, Refrigeration, and Air Conditioning Engineers (ASHRAE, 2011), in its 2008 position on SHS, concluded that a smoking ban was the only means of effectively eliminating indoor exposure to SHS and “no other engineering approaches, including current and advanced dilution ventilation or air cleaning technologies, have been demonstrated or should be relied upon to control health risk from ETS exposure…” (ASHRAE, 2008). Separation of smokers from nonsmokers, ventilation systems, air cleaning, and filtration are all ineffective strategies to eliminate SHS exposure and its harmful effects. The tobacco industry has been supporting each of these ineffective strategies, especially the accommodation strategy, at different levels worldwide (Aguinaga Bialous, Pressman, Gigliotti, Muggli, & Hurt, 2010; Bialous & Glantz, 2002; Campbell & Balbach, 2011; Dearlove, Bialous, & Glantz, 2002; Drope, Bialous, & Glantz, 2004; Sebrie & Glantz, 2007). Increasing evidence also indicates that SHS can infiltrate from separated smoking into nonsmoking areas in multifamily dwellings (Bohac, Hewett, Hammond, & Grimsrud, 2011; King, Travers, Cummings, Mahoney, & Hyland, 2010; Kraev, Adamkiewicz, Hammond, & Spengler, 2009). Furthermore, air monitoring results (using particulate matter [PM] and airborne nicotine levels as SHS markers) have consistently proven the industry’s “accommodation program” and partial smoking restrictions to be ineffective in bars, restaurants, and casinos (Agbenyikey et al., 2011; Akbar-Khanzadeh, Milz, Ames, Spino, & Tex, 2004; Barnoya, Mendoza-Montano, & Navas-Acien, 2007; Erazo et al., 2010; Jiang et al., 2011; Kim, Sohn, & Lee, 2010; Lambert, Samet, & Spengler, 1993; Milz et al., 2007; Repace, 2009; Repace et al., 2011).
Since the main reason for smoke-free environments is the health consequences of SHS exposure, research was instrumental in documenting the ineffectiveness of ventilation systems. The ventilation rate required to reduce SHS to “acceptable” levels of cancer risk would have to be increased 22,500 times compared with current ventilation standards (Repace, 2005; Repace & Johnson, 2006).
The Tobacco Industry and Smoke-Free Environments
Previously secret industry documents became available for free as a result of litigation in the 1990s in the United States (http://legacy.library.ucsf.edu/about/about_collections.jsp). These expose the industry’s strategies to prevent and obstruct the spread of smoke-free laws, investing in multimillion-dollar campaigns to confuse the public and slow down the rate of decline in cigarette consumption and social acceptability of smoking (Glantz, Barnes, Bero, Hanauer, & Slade, 1995; Muggli, Hurt, & Blanke, 2003). Worldwide, the industry has secretly hired consultants, sponsored symposia, financed research, and engaged in lobbying in order to fuel the controversy on the relationship between SHS and disease (Barnoya & Glantz, 2002; Hammond & Assunta, 2003; Muggli et al., 2003; Repace, 2004a). In recent years, especially in the United States, the industry has shifted the focus to economic claims that smoke-free environments are disastrous for the hospitality industry, investing millions of dollars in restaurant associations (Dearlove et al., 2002) and in the gaming industry (Mandel & Glantz, 2004). These claims continue to be pressed vigorously to oppose legislation, despite the fact that all high-quality independently funded and peer-reviewed research regarding the effects of smoke-free policies on the hospitality industry has consistently shown no effect or a positive effect on revenue (Hahn, 2010; Scollo, Lal, Hyland, & Glantz, 2003). It remains a challenge to educate restaurateurs and others in the hospitality industry, who often become (unknowingly) the foot soldiers for the tobacco industry in its effort to halt or delay the smoke-free movement.
RESEARCH TO SUPPORT THE IMPLEMENTATION AND ENFORCEMENT OF SMOKE-FREE ENVIRONMENTS
Many countries and subnational entities have assessed the extent of SHS exposure in their efforts to advance and evaluate the development, implementation, and enforcement of smoke-free legislation (Breysse & Navas-Acien, 2010). SHS exposure can be measured using questionnaires, environmental markers (personal or area monitoring), and biomarkers (Samet, 1999). Objective measures of SHS (in the environment or in the human body) are excellent tools to quantify exposure and its health effects, educate policy makers and the public about the importance of smoke-free legislation, and evaluate the impact of legislation after implementation.
Environmental Measures of SHS
The most widely used methods for determining SHS exposure in indoor public places and workplaces are airborne nicotine and PM <2.5 µm (PM2.5) (Barnoya et al., 2007; Hyland, Travers, Dresler, Higbee, & Cummings, 2008; Liu et al., 2010; Lopez et al., 2008; Navas-Acien et al., 2004; Nebot et al., 2005). Airborne SHS studies generally measure nicotine for several days, reflecting time-weighted average concentrations over the period of assessment. PM2.5 studies generally measure air PM during short periods of time (minutes or hours), reflecting concentrations during actual occupancy. The main advantage of nicotine over PM2.5 is that it is tobacco specific. Measuring PM2.5 concentrations has the advantage of providing immediate information on SHS levels and allowing comparisons with safety standards. Additionally, no permission is required to measure PM2.5 as it can be done discretely using a portable machine. On the other hand, air nicotine measurements require the establishments’ permission to place the monitor. In addition to direct environmental SHS measurements, mathematical models can be used to estimate exposure according to different patterns of cigarette smoking as well as to compare different control measures. Based on a mass balance model, standard techniques require information on room volume, generation rate (cigarettes smoked), and removal rate (e.g., air exchange and deposition rates) (Repace, 2007; U.S. Department of Health and Human Services, 2006).
Multinational research has contributed to evaluate SHS exposure in public places and workplaces (Agbenyikey et al., 2011; Barnoya et al., 2007; Hyland et al., 2008; Jones et al., 2012; Liu et al., 2010; Lopez et al., 2008; Navas-Acien et al., 2004; Nebot et al., 2005; Schoj et al., 2010; Stillman et al., 2007). This research has shown the usefulness of measuring air nicotine and PM2.5 for SHS surveillance, support for policy initiatives, and implementation evaluation. For instance, Guatemala used airborne nicotine levels to work with Congress to have bars and restaurants included in the 2009 smoking ban (Barnoya et al., 2007). Furthermore, implementing common methods and similar protocols has allowed comparing SHS concentrations across countries. Before legislation implementation, research in the Americas, Europe, and Asia found that nicotine was found in most locations surveyed (including hospitals and schools; Barnoya et al., 2007; Nebot et al., 2005; Stillman et al., 2007) and that the highest concentrations were in bars and restaurants (Barnoya et al., 2007; Liu et al., 2010; Nebot et al., 2005; Stillman et al., 2007), raising major concerns for employees’ health.
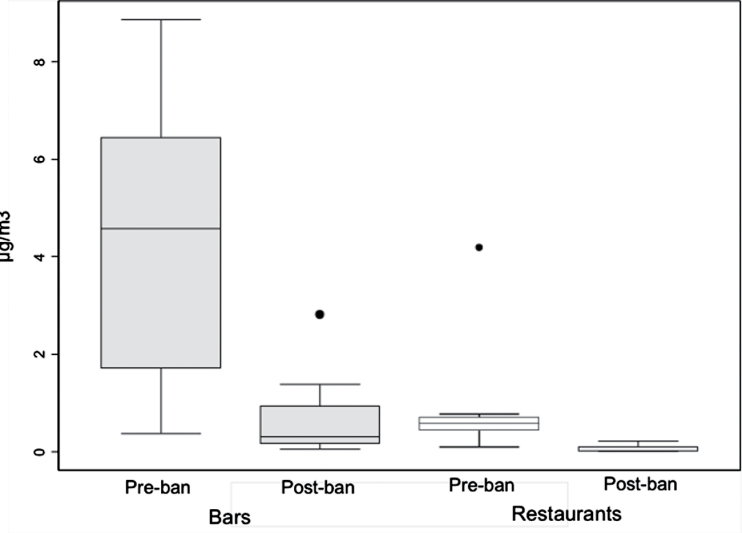
Evaluation of the successful implementation of and compliance with smoke-free legislations is another use of environmental measures. Indeed, major reductions in SHS exposure (>75%) have been documented after the implementation of comprehensive bans in Ireland (Mulcahy, Evans, Hammond, Repace, & Byrne, 2005), Norway (Ellingsen et al., 2006), Scotland (Semple et al., 2007), Uruguay (Blanco-Marquizo et al., 2010), and Guatemala (Figure 2) (Barnoya et al., 2011). Conversely, in countries without or with a partial smoking ban, no change has been documented (Erazo et al., 2010; Gleich, Mons, & Potschke-Langer, 2011; Gorini et al., 2008; Lopez et al., 2008). These evaluations identify opportunities for improvement. For example, in Uruguay, SHS concentrations in bars and restaurants decreased only 81% compared with 97% in schools, indicating that there is an additional need for enforcement in the former (Blanco-Marquizo et al., 2010).
Figure 2.
Airborne nicotine concentrations in Guatemala before (2006) and 6 months after (2009) smoke-free legislation was implemented. Nicotine levels decreased 87% in bars and 95% in restaurants. Horizontal lines within boxes indicated the medians. Boxes indicate the the interquartile range. Bars indicate values within 1.5 times the interquartile range. Solid circles indicate outlying points. Reproduced from Barnoya et al. (2011). Secondhand smoke exposure in bars and restaurants in Guatemala City: Before and after smoking ban evaluation. Cancer Causes & Control, 22(1), 151–156, Figure 1. With kind permission from Springer Science+Business Media.
Secondhand Smoke Biomarkers
Biomarkers are critical for quantifying personal SHS exposure among nonsmokers. They integrate SHS exposure at home, work, leisure, and transportation but cannot distinguish from different SHS sources. Also, they cannot distinguish between SHS exposure and occasional or light smoking. Nicotine and cotinine (a nicotine metabolite), the most commonly used biomarkers to assess personal exposure, are tobacco specific and can be measured in serum, saliva, urine, hair, or toenails (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009). Hair and toenail nicotine have longer half-lives and are easier to sample, store, and transport compared with urine, saliva, or serum (Nafstad, Jaakkola, Hagen, Zahlsen, & Magnus, 1997). In addition to nicotine and its metabolites, tobacco carcinogens can also be measured. The most commonly used is NNAL ([4-methylnitrosamino]-1-[3-pyridyl]-1-butanol), a metabolite of NNK (nicotine-derived nitrosamine ketone), a potent tobacco-specific carcinogen that can be measured in urine (Centers for Disease Control and Prevention, 2012). Other markers reflecting cardiotoxic and carcinogenic compounds in SHS, but not tobacco specific, include heavy metals (e.g., lead, cadmium), acrolein, benzene (Institute of Medicine, 2010), and polycyclic aromatic hydrocarbons (Bolte et al., 2008; Suwan-ampai, Navas-Acien, Strickland, & Agnew, 2009). Non-specific biomarkers of early effect can also be measured, including inflammatory and oxidative stress markers and DNA-adducts. There is room for further research to identify inexpensive, easy, noninvasive, and specific biomarkers of SHS exposure.
Biomarkers contribute to several important areas in SHS research. First, they improve exposure assessment and the magnitude of the association with related health endpoints. Second, they contribute to estimate the burden from SHS exposure in different population groups. In children, biomarkers yield that individual internal dose is higher compared with adults, even in the presence of similar exposure (Benowitz et al., 2009; Kim et al., 2010). Third, biomarkers ultimately evaluate the implementation of tobacco control programs (Jones et al., 2012). Currently the United States (serum), Germany, and Canada (urine) maintain national cotinine databases (Centers for Disease Control and Prevention, 2012; Health Canada, 2010; Heinrich et al., 2005). In the United States, serum cotinine levels have declined 70% from 1988–1991 to 2001–2002 (Centers for Disease Control and Prevention, 2012; Pirkle, Bernert, Caudill, Sosnoff, & Pechacek, 2006). Likewise, in the United Kingdom, serum cotinine levels declined 86% from 1978–1980 to 1998–2000 (Jefferis et al., 2009). These data provide evidence of the overall positive impact of tobacco control measures implemented during the 1990s.
BENEFITS OF SMOKE-FREE LAWS
Research on Outcomes Measures
Since the implementation of smoke-free laws in indoor places and workplaces, evidence has accumulated on the health benefits accruing shortly after. The main reason to implement these laws is to protect employees (exposed for several hours) and customers from the harmful effects of SHS exposure. Therefore, it is fundamental to document the impact legislation has on SHS-caused disease. These data will help to evaluate the legislation implementation and enforcement and raise public awareness and support.
Heart Disease
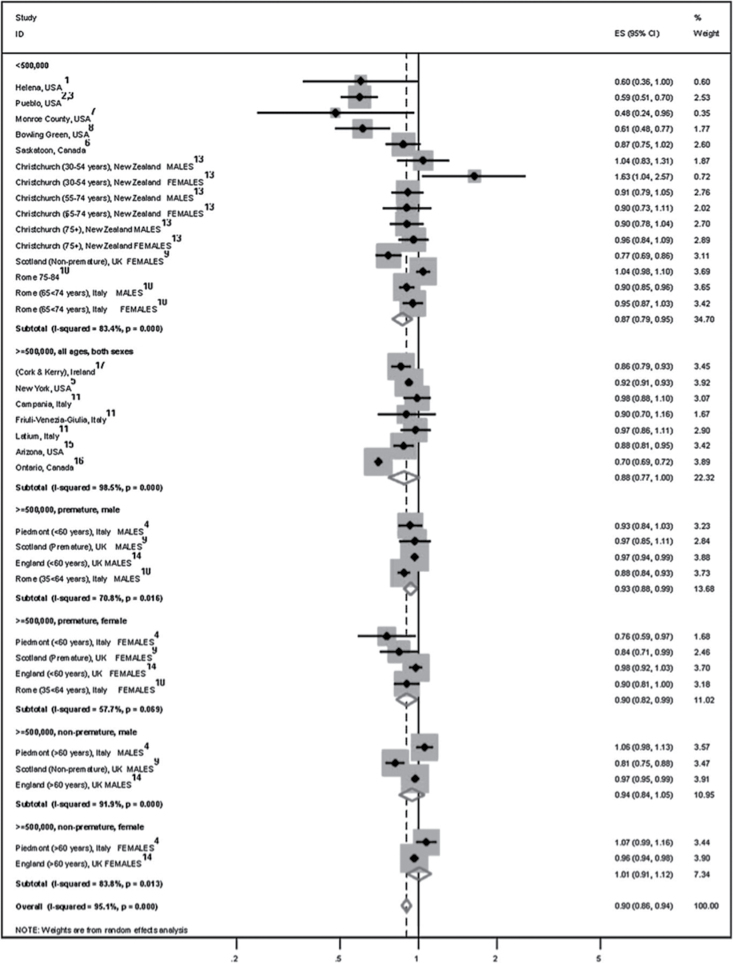
Smoke-free laws produce an immediate and substantial drop in hospital admissions for acute myocardial infarction (AMI), and the effect grows with time. One year after law implementation, AMI incidence drops by approximately 10% (95% confidence interval = 6%–14%), an effect that increases to about 30% after 3 years (Lightwood & Glantz, 2009; Mackay, Irfan, Haw, & Pell, 2010; Meyers, Neuberger, & He, 2009) (Figure 3). While there is substantial heterogeneity in results across different studies, the variable months of follow-up after legislation is the main predictor of the magnitude of the reduction in a model that is adjusted for other study characteristics including age and study location (Barnoya & Colditz, 2011; Mackay et al., 2010). In 2010, the U.S. Institute of Medicine report concluded that “there is a causal relationship between smoking bans and decreases in acute coronary events” (Institute of Medicine, 2010).
Figure 3.
Forest plot of stratified random effects meta-analysis of studies evaluating the effects of smoke-free environments on acute coronary events. Combined results yield a 30% decrease in acute coronary events with the introduction of smoke-free environments. CI = confidence interval. Reproduced from Mackay et al. (2010) with permission from BMJ Publishing Group Ltd.
Lung Cancer
As expected, the beneficial impact of smoke-free laws on lung cancer incidence takes longer than the impact on heart disease. This, in part, reflects the lower frequency and longer latency of cancer compared with heart disease. Cancer incidence, however, has been found to decrease at a faster rate in U.S. states with strong tobacco control programs that include smoking bans (Barnoya & Glantz, 2004; Jemal, Cokkinides, Shafey, & Thun, 2003; Kabir, Connolly, Clancy, Jemal, & Koh, 2007; Pierce, Messer, White, Kealey, & Cowling, 2010; Polednak, 2008). While additional research is needed to document the impact in lung cancer incidence, it can be concluded that there is a decline associated with a smoke-free law, probably from protecting nonsmokers and from helping smokers to quit.
Respiratory and Sensory Symptoms
Multiple studies have documented the positive short-term impact of smoke-free legislation in the improvement of self-reported sensory and respiratory symptoms as well as of lung function measures (Allwright et al., 2005; Ayres et al., 2009; Eagan, Hetland, & Aaro, 2006; Eisner, Smith, & Blanc, 1998; Farrelly et al., 2005; Lai et al., 2011; Larsson, Boethius, Axelsson, & Montgomery, 2008; Menzies et al., 2006; Pearson, Windsor, El-Mohandes, & Perry, 2009; Schoj et al., 2010; Skogstad et al., 2006; Wakefield, Cameron, Inglis, Letcher, & Durkin, 2005). On the contrary, incomplete bans do not improve workers’ respiratory health (Fernandez et al., 2009). Additional research in the general population should contribute to evaluate the benefits of legislation on symptoms.
Tobacco Consumption and Cessation
Smoking prevalence and tobacco consumption decrease and cessation increases after the implementation of smoke-free laws (IARC, 2009). Meta-analyses yield a 3.5% absolute decrease in smoking prevalence associated with smoke-free laws (Fichtenberg & Glantz, 2002; Hopkins et al., 2010). This decrease is twice as large as that observed with partial smoking bans (Fichtenberg & Glantz, 2002). Even though most of these data are from developed nations, developing nations’ data are starting to emerge. In China, workplaces with a smoking ban have smoking prevalence 18% lower (55.5% vs. 73.3%) than workplaces that only restrict smoking (Gao, Zheng, Gao, Chapman, & Fu, 2011). Other benefits include the decrease in cigarette consumption (Hargreaves et al., 2010; Hopkins et al., 2010) and a reduction in the percentage of heavy smokers (≥25 cigarettes/day) (Hopkins et al., 2010). Furthermore, voluntary bans at home have also been found to decrease cigarette consumption among smokers (Mills, Messer, Gilpin, & Pierce, 2009).
Cessation and quit attempts also increase after the implementation of a smoking ban in workplaces (Bauer, Hyland, Li, Steger, & Cummings, 2005) and homes (Hyland et al., 2009). Cessation is an integral part of tobacco control, and provision of medications for cessation is included as part of the FCTC (World Health Organization, 2003). While medications help smokers quit, most smokers quit unaided (Chapman & MacKenzie, 2010). Smoke-free initiatives represent, in this regard, a powerful indirect measure to help smokers quit and remain abstinent (Figure 1).
Figure 1.

Direct and indirect health benefits of smoke-free places.
Econometric Studies
The economic impact of smoke-free legislations has been one of the major arguments used by the tobacco industry to influence the hospitality industry and oppose legislation. Not surprisingly, a 2003 review concluded that all studies reporting a negative economic impact of smoke-free legislation were funded by the tobacco industry (Scollo et al., 2003). We discuss here some examples of evaluations published in the peer-review literature. In the United States and Canada, studies in counties, states, or provinces yield no evidence of change in hospitality industry revenues (Alamar & Glantz, 2007; Alpert, Carpenter, Travers, & Connolly, 2007; Hyland & Cummings, 1999; IARC, 2009; Luk, Ferrence, & Gmel, 2006; Pyles & Hahn, 2011; Young, Szychowski, Karp, Liu, & Diedrich, 2010). Similar results have been shown in European countries, Australia (Wakefield et al., 2002), and New Zealand (Edwards et al., 2008; Melberg & Lund, 2012). Although there is no reason to suspect that the findings will be different in Asia, Latin America, or Africa, few studies are available from those regions. In Mexico City, the 2008 legislation showed a non-statistically significant increase in restaurants’ revenue, total wages, and employment rates (Guerrero Lopez, Jimenez Ruiz, Reynales Shigematsu, & Waters, 2011). Furthermore, in some cases, legislation can have a positive economic impact in the hospitality industry (Alamar & Glantz, 2007; Collins, Shi, Forster, Erickson, & Toomey, 2010; Hyland & Tuk, 2001).
RESEARCH GAPS AND PRIORITIES
Even though much progress has occurred regarding research to support the implementation, enforcement, and evaluation of smoke-free laws, several important areas remain to be explored and are needed to ensure the complete implementation of Article 8. Table 3 summarizes what we consider to be the research priorities to further implement Article 8.
Table 3.
Article 8 Research Priorities
| Secondhand smoke exposure in children and vulnerable populations (using novel technology) |
| Smoke-free bars, nightclubs, casinos, and gambling industry venues |
| Method development and evaluation of exposure to secondhand smoke in spaces that are commonly not regulated, such as multiunit housing, motor vehicles, and outdoor areas |
| Health benefits of smoke-free policies besides cardiovascular disease |
| Thirdhand smoke exposure and health effects |
Evaluation and Enforcement
Evaluation and enforcement of smoke-free environments has been centered on SHS exposure markers. However, as smoke-free environments spread to multiunit housing and private enclosed spaces, new technologies that should allow monitoring enforcement without invading subjects’ privacy will emerge. Exposure in multiunit housing, in particular those of low socioeconomic status, has been shown to be a problem that needs to be urgently addressed (King et al., 2010; Kraev et al., 2009; Wilson, Klein, Blumkin, Gottlieb, & Winickoff, 2011). Even though it has been shown that most parents agree having their children tested for SHS exposure at home (Winickoff et al., 2011), the best assessment method is yet to be determined. In this regard, nanotechnology might be a promising tool in developing new SHS exposure assessment technology. These technologies will need to be cost-effective as most likely resource-limited countries will be the ones requiring enforcement monitoring.
Displacement of SHS exposure from the workplace to the household has been an argument frequently made by the tobacco industry, in particular, that this would result in higher childhood exposure. Even though there is some evidence suggesting that children’s exposure at home has increased (using questionnaire data; Ho et al., 2010), most results (including those using biomarkers) have proven otherwise (Akhtar, Currie, Currie, & Haw, 2007; Holliday, Moore, & Moore, 2009; Hyland et al., 2009). For those countries pending smoke-free legislation implementation, collecting data on household exposure to compare before and after implementation would be useful to garner additional support for smoke-free workplaces and households. Furthermore, these data would allow comparing the effects on household exposure in countries with different smoking prevalence.
Short-Term and Long-Term Health Benefits of Smoke-Free Environments
As described above, the short- and long-term health benefits of smoke-free environments are well documented. Heart disease and lung cancer have been the central research topic. This, in part driven by the rapid and large increased risk observed with SHS exposure, has allowed ecological analysis to find a beneficial effect over these diseases. However, as time since implementation grows, research will be able to evaluate the impact of smoke-free legislations on SHS-caused diseases that are less common and are with lower associated risk (e.g., bladder cancer, spontaneous abortion) compared with heart disease and lung cancer. For example, in California, as with lung cancer, bladder cancer also experienced a nearly significant drop (Barnoya & Glantz, 2004). Furthermore, research on nonfatal but equally important diseases (e.g., otitis media) is now emerging. In the United States, as the percentage of smoke-free households has increased, the percentages of ambulatory visits and hospital discharges of otitis media have also decreased (Alpert, Behm, Connolly, & Kabir, 2011). In addition, as the smoke-free movement moves from the workplace to households, this type of analysis and disease burden research might become more common and needed to support enforcement.
Secondhand Smoke in Outdoor Spaces
Countries and subnational entities with comprehensive legislation are now considering smoking bans in open spaces including parks, beaches, and areas near building entrances. Although some evidence has been recently published on SHS exposure in outdoor areas (Brennan et al., 2010; Kaufman, Kharrazi, Delorenze, Eskenazi, & Bernert, 2002; Kaufman, Zhang, Bondy, Klepeis, & Ferrence, 2011; Klepeis, Ott, & Switzer, 2007; Mage et al., 2010; Repace, 2008; Stafford, Daube, & Franklin, 2010), research to understand exposure levels in these areas and the contribution to internal dose is urgently needed. This research can have a major impact in supporting smoking bans in outdoor areas.
Thirdhand Smoke
Thirdhand smoke, the residual tobacco smoke pollutants that remain on surfaces and in dust after tobacco has been smoked (Matt et al., 2011), is now a subject of growing research interest. Even though most research has focused on the aging of tobacco smoke and on possible markers of exposure and its constituents, it is likely that improved exposure assessment will play a major role in determining internal dose and its health consequences (Hovell & Hughes, 2009; Matt et al., 2011; Thomas et al., 2011).
Focus on Developing Countries
Developing countries have moved forward in the smoke-free movement but most of them still lack comprehensive legislation. Therefore, straightforward data (e.g., airborne nicotine or PM2.5 levels) that has proven useful elsewhere to support legislation approval should aid in the development, implementation, and enforcement of legislation. Publication of results in high-quality and locally relevant academic journals and press conferences with the media should be part of a dissemination plan. Furthermore, researchers should communicate with and involve policy makers early in the research design process. Even though influencing policy is more a process than a product and requires the interaction of current activities and relationships, researchers should make an effort to approach policy makers and public health advocates in order to translate research into policy (Carden, 2009).
Basic SHS exposure indicators should also be collected on a continuous basis. Exposure at the workplace and household and costs associated with exposure are examples of data that, even though not publishable as novel research, are key to other types of analyses (e.g., economic and health burden analyses) that are publishable at international and local level and would aid in getting additional legislation support.
Resource-limited countries should choose the most cost-effective method to monitor SHS exposure. Airborne nicotine levels and PM2.5 are examples that have been effectively used in developing countries (e.g., Guatemala, Uruguay) to support legislation (Barnoya et al., 2011; Blanco-Marquizo et al., 2010). In addition, these are relatively easy to implement following readily available protocols (Avila-Tang, Travers, & Navas-Acien, 2010). In some instances, these measurements might not be feasible for economic or logistical reasons. Observational tools, modeling exposure, and interviews and questionnaires on SHS exposure and smoking ban support are additional monitoring tools that could be used in resource-limited settings (Hyland et al., 2009; Repace, 2007; Wilson et al., 2010). Ideally the government or agency in-charge of policy implementation and enforcement should also be involved in funding data collection, publication, and dissemination. However, academic and nongovernmental institutions can play important roles especially when governments are slow in action.
Research on the health benefits from smoke-free environments should also be conducted in developing countries. As described above, heart disease and lung cancer have been the main focus. Regarding heart disease, reliable data on trends of hospital admissions and adjusted incidence and mortality rates are needed. Furthermore, given that many other confounders need to be controlled for (e.g., access to intensive and emergency care, seasonal trends), researchers and governments need to collect data even before legislation is implemented. Even though the same is true for lung cancer, it might be easier to have trend data, although documenting SHS exposure status is still needed. Research on short- and long-term changes in respiratory symptoms and disease, such as changes in asthma attacks, active tuberculosis, and COPD, will also be important. Regardless of the hurdles to conduct health outcomes research in developing countries, researchers and funders need to address these topics because ultimately we aim to decrease the burden of disease from SHS exposure.
Vulnerable Populations
Secondhand smoke and thirdhand smoke might be particularly harmful to vulnerable populations. Individuals with asthma (SHS is associated with disease exacerbation; U.S. Department of Health and Human Services, 2006) might be more susceptible to thirdhand smoke exposure. The fetus, infants, and young children are also vulnerable, given their early stage of development. Therefore, SHS and thirdhand smoke might pose an additional threat. For example, assessing the influence of SHS and thirdhand smoke on breast tissue at early ages might yield additional data to support the increased risk of breast cancer with SHS exposure. In addition, the low and middle socioeconomic strata of society are the more likely to be exposed and the less likely to be protected. Likewise, those with low educational level need to be included in the research agenda. Research documenting SHS exposure among vulnerable populations, for instance, using biomarkers, can be done and should be used for smoke-free law advocacy.
Data Needs for Countries/ Subnational Entities That Are Still Not Smoke-Free
Innovative research remains critical to help countries that are still not smoke-free to enact legislation and ensure complete protection of all people as mandated by the FCTC. The following areas are important.
Exposure Assessment
High-quality, standardized methods that objectively measure SHS remain a powerful tool to unquestionably document that the nonsmoking population is exposed. This research can use air nicotine, PM2.5, nicotine-related biomarkers, or SHS exposure modeling (Repace, 2007; U.S. Department of Health and Human Services, 2006). Less frequently used methods include measuring NNK in the environment or NNAL in biospecimens. These two have the advantage of reflecting exposure to a tobacco-specific carcinogen that could help make the argument regarding tobacco as an occupational health hazard that must and can be eliminated through a smoking ban. However, costs and feasibility should be taken into account when selecting the method to assess SHS exposure.
Improvements in exposure assessment can also contribute to better estimate the risk for those health outcomes where the debate continues such as in breast cancer (California Environmental Protection Agency, 2005; Johnson et al., 2011; U.S. Department of Health and Human Services, 2006) and chronic rhinosinusitis (Reh et al., 2009; Reh & Navas-Acien, 2010; U.S. Department of Health and Human Services, 2006). Also, while current SHS exposure methods can be used outdoors, better methods can be developed or adjusted.
Ventilation, Separated Areas, and Other Measures
Despite the overwhelming amount of evidence proving that the only effective way to solve the SHS exposure problem is to establish comprehensive smoke-free environments, the industry continues to push ventilation as an alternative solution. Engineering approaches such as complete separation and isolation can reduce exposure and the corresponding risks but not as comprehensively and completely as smoke-free environments. Furthermore, adverse health effects of the occupants of the smoking rooms cannot be controlled by ventilation (ASHRAE, 2010). Therefore, research on ventilation systems and standards will remain much needed, in particular for developing countries. Epidemiologists and ventilation/environmental experts in developed and developing countries should work together to maximize resources and generalizability. Economic analysis of ventilation systems in developing countries should yield additional data to support legislation. The high cost in setting up and maintaining well-ventilated smoking rooms, in particular in resource-limited countries, is another reason why smoke-free environments are the only solution to SHS exposure. In this regard, economists should also be part of a research team to support legislation.
Social and Political Evaluations
Smoke-free environments, as an example of a population-based strategy of preventive medicine, are a social process. Therefore, social and political forces interact within each other, leading to social change. Sociological and anthropological research on the process should be part of the smoke-free movement evaluations. For example, how does the Uruguay experience where smoke-free environments were mandated by a Presidential decree compare with the U.S. experience where going from local municipalities to state laws has been the most effective strategy? The experience in China also deserves additional attention. In this country, smoke-free environments have turned out to be a breakthrough (in particular compared with the slow progress of other FCTC Articles) as local cities have implemented legislation that is now spreading to the national level (China, May 2011). Despite enforcement issues, this experience should provide useful lessons to countries that have trouble enacting legislation. These social evaluations should include continuous support monitoring. Evidence has shown that support for smoke-free legislation increases after implementation (Barnoya et al., 2011; Crosbie, Sebrie, & Glantz, 2011; Etter, 2009; Hyland et al., 2009; IARC, 2009). Also, as legislation spreads, worldwide support is increasing, and these should have a positive impact on other tobacco control measures.
Other forces influencing support for smoke-free legislation before, during, and after implementation should also be a matter of research. For example, media coverage can often increase support for legislation and awareness of the harmful effects of SHS (Durrant, Wakefield, McLeod, Clegg-Smith, & Chapman, 2003; Magzamen, Charlesworth, & Glantz, 2001; Nagelhout et al., 2012). Furthermore, smoke-free environments are also an equity issue, and as they become the norm we need to document that everyone, regardless of socioeconomic or educational status, is equally protected from the harmful effects of SHS. For example, in Bangladesh, data from the International Tobacco Control project have yielded that those with low educational level are less likely to be concerned about SHS or to have a smoke-free home (Abdullah et al., 2011). These data should also be adequately disseminated among advocates and policy makers to further increase legislation support and implementation.
Tobacco Industry Tactics
Even though several industry tactics have been adequately described using the internal industry documents, Article 5.2 of the FCTC that aims to monitor industry interference should prove useful in helping with the enactment and implementation of smoke-free environments. Research on industry-sponsored hospitality groups, smokers’ rights organizations, ineffective ventilation systems, and lobbying is still needed during implementation and enforcement of smoke-free environments.
Health Effects From SHS Exposure
As exposure methods improve, the health effects (acute and chronic) that are still subject to debate will become easier to determine. Research on the SHS components that lead to the acute effects will be fundamental to support the expansion of smoke-free laws to include other combustion products (e.g., water pipe, electronic cigarette). Therefore, the list of heavy metals in SHS in the IOM Report (Institute of Medicine, 2010) is likely to grow over time.
Sensory symptoms (irritation and related symptoms) and even simple annoyance of SHS that also occurs after briefs periods of exposure have in general been underresearched. Annoyance can be sufficient for legislative control, such as noise level in residential districts. These data can motivate nonsmokers to become more intolerant to SHS and support smoke-free legislation (Junker, Danuser, Monn, & Koller, 2001). Given that this research is based on questionnaires and follow-up is required, the human and economic resources needed are less than those needed to assess health effects. Therefore, this approach might be particularly relevant for resource-limited settings. At the same time, since more SHS awareness and sensitivity can be expected after smoke-free legislation, potential increases in reporting SHS exposures and symptoms must be carefully evaluated.
Research Dissemination
Several venues have been used for research dissemination, and all have a specific target audience and policy reach. Research that yields new/original knowledge at the global level is likely to reach the peer-reviewed literature. Public health, policy, anthropology, and medical journals are some examples of peer-reviewed venues where these results can be found. Results that may not represent a particular novel finding from a global perspective but may be relevant at the local level to support public policy are often found in mass media, government reports, or nongovernmental organization policy briefs. Finally, there are results that can reach local medical or public health journals that might not be indexed and therefore not as visible as the usual peer-reviewed literature. Regardless of, from a researcher’s perspective, dissemination efforts and policy implications of research results should be taken into consideration early in the research process.
CONCLUSIONS
It has been almost 30 years since the first U.S. Surgeon General Report (U.S. Department of Health and Human Services, 1986) referred to SHS as a cause of serious diseases. Since then, research measuring SHS exposure and proving the health and economical benefits of smoke-free environments has had a major policy impact. Smoke-free environments provide an example of how research can be translated into policy. Implementation and enforcement result from a combination of research, politics, and culture. In this article, we have focused on research needs and current gaps from exposure assessment and epidemiological perspectives. In addition, the political and cultural determinants should be a matter of further research. Experts in political and behavioral sciences and international law can play a crucial role in further understanding the critical aspects that contribute to differences in the successful implementation of FCTC Article 8 (Hovell & Hughes, 2009). Clinical, epidemiological, social, experimental, and exposure sciences, coupled with research on tobacco industry documents, have all contributed to the movement and will likely be part of the spread of smoke-free environments worldwide (including private enclosed spaces and outdoor or quasi-outdoor environments; Hovell & Hughes, 2009). However, considering all the evidence, progress is still slow, in particular in developing countries. The tobacco industry is powerful and has strong alliances. Governments and public entities need to allocate more resources, preferably from tobacco tax revenue, to support SHS research and related control measures. More collaborative and creative efforts by researchers, public health advocates, and policy makers are needed to mobilize nonsmokers, to change social norms that help smokers not to smoke around others and to make them quit, and to prevent young people from starting smoking.
FUNDING
This publication was made possible by contract number HHSN261201100185P from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, National Institutes of Health. JB receives additional support from the American Cancer Society. AN-A receives additional support from the Flight Attendant Medical Research Institute, the Bloomberg Initiative to Reduce Tobacco Use, and from the U.S. National Cancer Institute (R03CA153959).
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Abdullah A. S., Hitchman S. C., Driezen P., Nargis N., Quah A. C., Fong G. T. (2011). Socioeconomic differences in exposure to tobacco smoke pollution (TSP) in Bangladeshi households with children: Findings from the International Tobacco Control (ITC) Bangladesh Survey. International Journal of Environmental Research and Public Health, 8, 842–860 doi:10.3390/ijerph8030842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbenyikey W., Wellington E., Gyapong J., Travers M. J., Breysse P. N., McCarty K. M., Navas-Acien A. (2011). Secondhand tobacco smoke exposure in selected public places (PM2.5 and air nicotine) and non-smoking employees (hair nicotine) in Ghana. Tobacco Control, 20, 107–111 doi:10.1136/tc.2010.036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguinaga Bialous S., Pressman S., Gigliotti A., Muggli M., Hurt R. (2010). A resposta da industria do tabaco a criacao de espacos livres de fumo no Brasil. Revista Panamericana de Salud Publica, 27(4), 283–290 [DOI] [PubMed] [Google Scholar]
- Akbar-Khanzadeh F., Milz S., Ames A., Spino S., Tex C. (2004). Effectiveness of clean indoor air ordinances in controlling environmental tobacco smoke in restaurants. Archives of Environmental Health, 59, 677–685 doi:10.1080/00039890409602953 [DOI] [PubMed] [Google Scholar]
- Akhtar P. C., Currie D. B., Currie C. E., Haw S. J. (2007). Changes in child exposure to environmental tobacco smoke (CHETS) study after implementation of smoke-free legislation in Scotland: National cross sectional survey. BMJ, 335, 545 doi:10.1136/bmj.39311.550197.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamar B., Glantz S. A. (2007). Effect of smoke-free laws on bar value and profits. American Journal of Public Health, 97, 1400–1402 doi:10.2105/AJPH.2006.095315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwright S., Paul G., Greiner B., Mullally B. J., Pursell L., Kelly A., Perry I. J. (2005). Legislation for smoke-free workplaces and health of bar workers in Ireland: Before and after study. BMJ, 331, 1117 doi:10.1136/bmj.38636.499225.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert H. R., Carpenter C. M., Travers M. J., Connolly G. N. (2007). Environmental and economic evaluation of the Massachusetts Smoke-Free Workplace Law. Journal of Community Health, 32, 269–281 [DOI] [PubMed] [Google Scholar]
- Alpert H. R., Behm I., Connolly G. N., Kabir Z. (2011). Smoke-free households with children and decreasing rates of paediatric clinical encounters for otitis media in the United States. Tobacco Control, 20, 207–211 doi:10.1136/tc.2010.038711 [DOI] [PubMed] [Google Scholar]
- American Nonsmokers’ Rights Foundation (2012). Smokefree status of workplaces and hospitality venues around the world (July 11, 2012), 1–9. Retrieved from http://www.no-smoke.org/pdf/internationalbarsandrestaurants.pdf [Google Scholar]
- American Society of Heating, Refrigeration, and Air Conditioning Engineers (2008). ASHRAE position document on environmental tobacco smoke. Atlanta, GA: ASHRAE; [Google Scholar]
- American Society of Heating, Refrigeration, and Air Conditioning Engineers (2010). ASHRAE position document on environmental tobacco smoke. Atlanta, GA: ASHRAE; [Google Scholar]
- American Society of Heating, Refrigeration, and Air Condi tioning Engineers (2011). ASHRAE mission. Atlanta, GA: ASHRAE; (May 23). [Google Scholar]
- Avila-Tang E., Travers M. J., Navas-Acien A. (2010). Promoting smoke-free environments in Latin America: A comparison of methods to assess secondhand smoke exposure. Salud Pública de México, 52(Suppl. 2), S138–S148 doi:S0036-36342010000800009 [pii] [DOI] [PubMed] [Google Scholar]
- Ayres J. G., Semple S., MacCalman L., Dempsey S., Hilton S., Hurley J. F., Petticrew M. (2009). Bar workers’ health and environmental tobacco smoke exposure (BHETSE): Symptomatic improvement in bar staff following smoke-free legislation in Scotland. Occupational and Environmental Medicine, 66, 339–346 doi:10.1136/oem.2008.040311 [DOI] [PubMed] [Google Scholar]
- Barnes D. E., Bero L. A. (1996). Industry-funded research and conflict of interest: An analysis of research sponsored by the tobacco industry through the Center for Indoor Air Research. Journal of Health Politics, Policy and Law, 21, 515–542 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Arvizu M., Jones M. R., Hernandez J. C., Breysse P. N., Navas-Acien A. (2011). Secondhand smoke exposure in bars and restaurants in Guatemala City: Before and after smoking ban evaluation. Cancer Causes & Control, 22, 151–156 doi:10.1007/s10552-010-9673-8 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Colditz G. A. (2011). Meta-analysis of before and after studies shows a 10% reduction in acute coronary events after introduction of comprehensive smoke-free legislation. Evidence-Based Nursing, 14, 46–47 doi:10.1136/ebn.14.2.46 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Glantz S. (2002). Tobacco industry success in preventing regulation of secondhand smoke in Latin America: The “Latin Project.”. Tobacco Control, 11, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnoya J., Glantz S. (2004). Association of the California tobacco control program with declines in lung cancer incidence. Cancer Causes & Control, 15, 689–695 doi:10.1023/B:CACO.0000036187.13805.30 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Mendoza-Montano C., Navas-Acien A. (2007). Secondhand smoke exposure in public places in Guatemala: Comparison with other Latin American countries. Cancer Epidemiology, Biomarkers and Prevention, 16, 2730–2735 [DOI] [PubMed] [Google Scholar]
- Basu S., Stuckler D., Bitton A., Glantz S. A. (2011). Projected effects of tobacco smoking on worldwide tuberculosis control: Mathematical modelling analysis. BMJ, 343, d5506 doi:10.1136/bmj.d5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. E., Hyland A., Li Q., Steger C., Cummings K. M. (2005). A longitudinal assessment of the impact of smoke-free worksite policies on tobacco use. American Journal of Public Health, 95(6), 1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan D., Eriksen M., Lin Y. (2005). Economic effects of environmental tobacco smoke. Atlanta, GA: Georgia State University; Retrieved from http://www.soa.org/research/research-projects/life-insurance/research-economic-effect.aspx [Google Scholar]
- Benowitz N. L., Bernert J. T., Caraballo R. S., Holiday D. B., Wang J. (2009). Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology, 169, 236–248 [DOI] [PubMed] [Google Scholar]
- Bialous S. A., Glantz S. A. (2002). ASHRAE Standard 62: Tobacco industry’s influence over national ventilation standards. Tobacco Control, 11, 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Marquizo A., Goja B., Peruga A., Jones M. R., Yuan J., Samet J. M., Navas-Acien A. (2010). Reduction of secondhand tobacco smoke in public places following national smoke-free legislation in Uruguay. Tobacco Control, 19, 231–234 doi:10.1136/tc.2009.034769 [DOI] [PubMed] [Google Scholar]
- Boffetta P., Autier P. (2011). Is breast cancer associated with tobacco smoking? BMJ, 342, d1093 doi:10.1136/bmj.d1093 [DOI] [PubMed] [Google Scholar]
- Bohac D. L., Hewett M. J., Hammond S. K., Grimsrud D. T. (2011). Secondhand smoke transfer and reductions by air sealing and ventilation in multiunit buildings: PFT and nicotine verification. Indoor Air, 21, 36–44 doi:10.1111/j.1600-0668.2010.00680.x [DOI] [PubMed] [Google Scholar]
- Bolte G., Heitmann D., Kiranoglu M., Schierl R., Diemer J., Koerner W., Fromme H. (2008). Exposure to environmental tobacco smoke in German restaurants, pubs and discotheques. Journal of Exposure Science and Environmental Epidemiology, 18, 262–271 doi:10.1038/sj.jes.7500590 [DOI] [PubMed] [Google Scholar]
- Borland R., Yong H. H., Siahpush M., Hyland A., Campbell S., Hastings G., Fong G. T. (2006). Support for and reported compliance with smoke-free restaurants and bars by smokers in four countries: Findings from the International Tobacco Control (ITC) Four Country Survey. Tobacco Control, 15(Suppl. 3), iii34–iii–41 doi:10.1136/tc.2004.008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan E., Cameron M., Warne C., Durkin S., Borland R., Travers M. J., Wakefield M. A. (2010). Secondhand smoke drift: Examining the influence of indoor smoking bans on indoor and outdoor air quality at pubs and bars. Nicotine and Tobacco Research, 12, 271–277 doi:10.1093/ntr/ntp204 [DOI] [PubMed] [Google Scholar]
- Breysse P. N., Navas-Acien A. (2010). The smoking gun: Working to eliminate tobacco smoke exposure. Journal of Exposure Science and Environmental Epidemiology, 20, 397–398 doi:10.1038/jes.2010.34 [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency (2005). Proposed identification of environmental tobacco smoke as a toxic air contaminant, part B: Health effects. Sacramento, CA: State of California Office of Environmental Health Hazard Assessment; [Google Scholar]
- Campbell R. B., Balbach E. D. (2011). Manufacturing credibility: The National Energy Management Institute and the Tobacco Institute’s strategy for indoor air quality. American Journal of Public Health, 101, 497–503 doi:10.2105/AJPH.2010.199695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden F. (2009). Knowledge to policy. Making the most of development research. Ottawa, Canada: Sage Publication; [Google Scholar]
- Centers for Disease Control and Prevention (2012). Fourth national report on human exposure to environmental chemicals, updated tables. Atlanta, GA: Center for Disease Control and Prevention; [Google Scholar]
- Chapman S., MacKenzie R. (2010). The global research neglect of unassisted smoking cessation: Causes and consequences. PLoS Medicine, 7, e1000216 doi:10.1371/journal.pmed.1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N. M., Shi Q., Forster J. L., Erickson D. J., Toomey T. L. (2010). Effects of clean indoor air laws on bar and restaurant revenue in Minnesota cities. American Journal of Preventive Medicine, 39(6, Suppl. 1), S10–S15 doi:10.1016/j.amepre.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Crosbie E., Sebrie E. M., Glantz S. A. (2011). Strong advocacy led to successful implementation of smokefree Mexico City. Tobacco Control, 20, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearlove J. V., Bialous S. A., Glantz S. A. (2002). Tobacco industry manipulation of the hospitality industry to maintain smoking in public places. Tobacco Control, 11, 94–104 doi:10.1136/tc.2010.037010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J., Bialous S. A., Glantz S. A. (2004). Tobacco industry efforts to present ventilation as an alternative to smoke-free environments in North America. Tobacco Control, 13(Suppl. 1), i41–i47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant R., Wakefield M., McLeod K., Clegg-Smith K., Chapman S. (2003). Tobacco in the news: An analysis of newspaper coverage of tobacco issues in Australia, 2001. Tobacco Control, 12(Suppl. 2), ii75–ii81 doi:10.1136/tc.12.suppl_2.ii75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagan T. M., Hetland J., Aaro L. E. (2006). Decline in respiratory symptoms in service workers five months after a public smoking ban. Tobacco Control, 15, 242–246 doi:10.1136/tc.2005.015479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R., Thomson G., Wilson N., Waa A., Bullen C., O’Dea D., Woodward A. (2008). After the smoke has cleared: Evaluation of the impact of a new national smoke-free law in New Zealand. Tobacco Control, 17, e2 doi:10.1136/tc.2007.020347 [DOI] [PubMed] [Google Scholar]
- Eisner M. D., Smith A. K., Blanc P. D. (1998). Bartenders’ respiratory health after establishment of smoke-free bars and taverns. JAMA, 280, 1909–1914 [DOI] [PubMed] [Google Scholar]
- Ellingsen D. G., Fladseth G., Daae H. L., Gjølstad M., Kjaerheim K., Skogstad M., Molander P. (2006). Airborne exposure and biological monitoring of bar and restaurant workers before and after the introduction of a smoking ban. Journal of Environmental Monitoring: JEM, 8, 362–368 doi:10.1039/b600050a [DOI] [PubMed] [Google Scholar]
- Erazo M., Iglesias V., Droppelmann A., Acuña M., Peruga A., Breysse P. N., Navas-Acien A. (2010). Secondhand tobacco smoke in bars and restaurants in Santiago, Chile: Evaluation of partial smoking ban legislation in public places. Tobacco Control, 19, 469–474 doi:tc.2009.035402 [pii]10.1136/tc.2009.035402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J. F. (2009). Secondhand smoke in Geneva, 1996–2006: Changes in exposure, opinions, and workplace smoking bans in the absence of national legislation. International Journal of Occupational and Environmental Health, 15, 159–165 [DOI] [PubMed] [Google Scholar]
- Farrelly M. C., Nonnemaker J. M., Chou R., Hyland A., Peterson K. K., Bauer U. E. (2005). Changes in hospitality workers’ exposure to secondhand smoke following the implementation of New York’s smoke-free law. Tobacco Control, 14, 236–241 doi:10.1136/tc.2004.008839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E., Fu M., Pascual J. A., López M. J., Pérez-Ríos M., Schiaffino A., Nebot M. and the Spanish Smoking Law Evaluation Group (2009). Impact of the Spanish smoking law on exposure to second-hand smoke and respiratory health in hospitality workers: A cohort study. PloS One, 4, e4244 doi:10.1371/journal.pone.0004244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenberg C. M., Glantz S. A. (2002). Effect of smoke-free workplaces on smoking behaviour: Systematic review. BMJ, 325, 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Zheng P., Gao J., Chapman S., Fu H. (2011). Workplace smoking policies and their association with male employees’ smoking behaviours: A cross-sectional survey in one company in China. Tobacco Control, 20, 131–136 doi:10.1371/journal.pone.0004244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz S. A., Barnes D. E., Bero L., Hanauer P., Slade J. (1995). Looking through a keyhole at the tobacco industry. The Brown and Williamson documents. JAMA, 274, 219–224 [PubMed] [Google Scholar]
- Gleich F., Mons U., Potschke-Langer M. (2011). Air contamination due to smoking in German restaurants, bars, and other venues–before and after the implementation of a partial smoking ban. Nicotine and Tobacco Research, 13, 1155–1160 doi:10.1093/ntr/ntr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G., Moshammer H., Sbrogiò L., Gasparrini A., Nebot M., Neuberger M., Serrahima E. Italy & Austria Before and After Study Working Group (2008). Italy and Austria before and after study: Second-hand smoke exposure in hospitality premises before and after 2 years from the introduction of the Italian smoking ban. Indoor Air, 18, 328–334 doi:INA534 [pii] 10.1111/j.1600-0668.2008.00534.x [DOI] [PubMed] [Google Scholar]
- Guerrero Lopez C. M., Jimenez Ruiz J. A., Reynales Shigematsu L. M., Waters H. R. (2011). The economic impact of Mexico City’s smoke-free law. Tobacco Control, 20, 273–278 doi:tc.2010.036467 [pii]10.1136/tc.2010.036467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. J. (2010). Smokefree legislation: A review of health and economic outcomes research. American Journal of Preventive Medicine, 39(6, Suppl. 1), S66––S76 doi:10.1016/j.amepre.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Hammond R., Assunta M. (2003). The Framework Convention on Tobacco Control: Promising start, uncertain future. Tobacco Control, 12, 241–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K., Amos A., Highet G., Martin C., Platt S., Ritchie D., White M. (2010). The social context of change in tobacco consumption following the introduction of ‘smokefree’ England legislation: A qualitative, longitudinal study. Social Science and Medicine, 71, 459–466 doi:10.1016/j.socscimed.2010.04.025 [DOI] [PubMed] [Google Scholar]
- Health Canada (2010). Environmental and workplace health, report on human biomonitoring of environmental chemicals in Canada (Vol. 8.11). Ottawa, Canada: Health Canada; [Google Scholar]
- Heinrich J., Hölscher B., Seiwert M., Carty C. L., Merkel G., Schulz C. (2005). Nicotine and cotinine in adults’ urine: The German Environmental Survey 1998. Journal of Exposure Analysis and Environmental Epidemiology, 15, 74–80 doi:10.1038/sj.jea.7500373 [DOI] [PubMed] [Google Scholar]
- Heloma A., Jaakkola M. S. (2003). Four-year follow-up of smoke exposure, attitudes and smoking behaviour following enactment of Finland’s national smoke-free work-place law. Addiction, 98, 1111–1117 [DOI] [PubMed] [Google Scholar]
- Ho S. Y., Wang M. P., Lai H. K., Hedley A. J., Lam T. H. (2010). “Carcinogens in a puff”: Smoking in Hong Kong movies. Tobacco Control, 19, 518–519 doi:10.1136/tc.2009.032003 [DOI] [PubMed] [Google Scholar]
- Holliday J. C., Moore G. F., Moore L. A. (2009). Changes in child exposure to secondhand smoke after implementation of smoke-free legislation in Wales: A repeated cross-sectional study. BMC Public Health, 9, 430 doi:10.1186/1471-2458-9-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. P., Razi S., Leeks K. D., Priya Kalra G., Chattopadhyay S. K., Soler R. E. (2010). Smokefree policies to reduce tobacco use: A systematic review. American Journal of Preventive Medicine, 38(2, Suppl. 1), S275––S289 doi:10.1016/j.amepre.2009.10.029 [DOI] [PubMed] [Google Scholar]
- Hovell M. F., Hughes S. C. (2009). The behavioral ecology of secondhand smoke exposure: A pathway to complete tobacco control. Nicotine and Tobacco Research, 11, 1254–1264 doi:10.1093/ntr/ntp133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Cummings K. M. (1999). Restaurateur reports of the economic impact of the New York City Smoke-Free Air Act. Journal of Public Health Management and Practice, 5, 37–42 [DOI] [PubMed] [Google Scholar]
- Hyland A., Hassan L. M., Higbee C., Boudreau C., Fong G. T., Borland R., Hastings G. (2009). The impact of smokefree legislation in Scotland: Results from the Scottish ITC: Scotland/UK longitudinal surveys. European Journal of Public Health, 19, 198–205 doi:10.1093/eurpub/ckn141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Travers M. J., Dresler C., Higbee C., Cummings K. M. (2008). A 32-country comparison of tobacco smoke derived particle levels in indoor public places. Tobacco Control, 17, 159–165 doi:10.1136/tc.2007.020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Tuk J. (2001). Restaurant employment boom in New York City. Tobacco Control, 10, 199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Secondhand smoke exposure and cardiovascular effects: Making sense of the evidence. Washington, DC: Institute of Medicine. (2010) [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2004). IARC monographs on the evaluation of carcinogenic risks to humans (Vol. 83). Lyon, France: International Agency for Research on Cancer; [Google Scholar]
- International Agency for Research on Cancer. (2009). IARC handbooks on tobacco control, volume 13: Evaluating the effectiveness of smoke-free policies. Lyon, France: International Agency for Research on Cancer.
- International Commission on Occupational Health (2002). ICOH network on tobacco-free workplaces. Retrieved from http://www.icohweb.org/site_new/ico_news_detail.asp?id=34 [Google Scholar]
- Jefferis B. J., Thomson A. G., Lennon L. T., Feyerabend C., Doig M., McMeekin L., Whincup P. H. (2009). Changes in environmental tobacco smoke (ETS) exposure over a 20-year period: Cross-sectional and longitudinal analyses. Addiction, 104, 496–503 doi:10.1111/j.1360-0443.2008.02473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Cokkinides V. E., Shafey O., Thun M. J. (2003). Lung cancer trends in young adults: An early indicator of progress in tobacco control (United States). Cancer Causes and Control, 14, 579–585 [DOI] [PubMed] [Google Scholar]
- Jiang R. T., Cheng K. C., Acevedo-Bolton V., Klepeis N. E., Repace J. L., Ott W. R., Hildemann L. M. (2011). Measurement of fine particles and smoking activity in a statewide survey of 36 California Indian casinos. Journal of Exposure Science and Environmental Epidemiology, 21, 31–41 doi:10.1038/jes.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. C., Miller A. B., Collishaw N. E., Palmer J. R., Hammond S. K., Salmon A. G., Turcotte F. (2011). Active smoking and secondhand smoke increase breast cancer risk: The report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tobacco Control, 20, e2 doi:10.1136/tc.2010.035931 [DOI] [PubMed] [Google Scholar]
- Jones M. R., Wipfli H., Shahrir S., Avila-Tang E., Samet J. M., Breysse P. N., Navas-Acien A. FAMRI Bar Study Investigators (2012). Secondhand tobacco smoke: An occupational hazard for smoking and non-smoking bar and nightclub employees. Tobacco Control. Advance online publication. doi:10.1136/tobaccocontrol-2011–050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker M. H., Danuser B., Monn C., Koller T. (2001). Acute sensory responses of nonsmokers at very low environmental tobacco smoke concentrations in controlled laboratory settings. Environmental Health Perspectives, 109, 1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir Z., Connolly G. N., Clancy L., Jemal A., Koh H. K. (2007). Reduced lung cancer deaths attributable to decreased tobacco use in Massachusetts. Cancer Causes and Control, 18, 833–838 doi:10.1007/s10552-007-9027-3 [DOI] [PubMed] [Google Scholar]
- Kaufman F. L., Kharrazi M., Delorenze G. N., Eskenazi B., Bernert J. T. (2002). Estimation of environmental tobacco smoke exposure during pregnancy using a single question on household smokers versus serum cotinine. Journal of Exposure Analysis and Environmental Epidemiology, 12, 286–295 doi:10.1038/sj.jea.7500224 [DOI] [PubMed] [Google Scholar]
- Kaufman P., Zhang B., Bondy S. J., Klepeis N., Ferrence R. (2011). Not just “a few wisps”: Real-time measurement of tobacco smoke at entrances to office buildings. Tobacco Control, 20, 212–218 doi:10.1136/tc.2010.041277 [DOI] [PubMed] [Google Scholar]
- Kim S., Sohn J., Lee K. (2010). Exposure to particulate matters (PM2.5) and airborne nicotine in computer game rooms after implementation of smoke-free legislation in South Korea. Nicotine and Tobacco Research, 12, 1246–1253 doi:10.1093/ntr/ntq189 [DOI] [PubMed] [Google Scholar]
- King B. A., Travers M. J., Cummings K. M., Mahoney M. C., Hyland A. J. (2010). Secondhand smoke transfer in multiunit housing. Nicotine and Tobacco Research, 12, 1133–1141 doi:10.1093/ntr/ntq162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepeis N. E., Ott W. R., Switzer P. (2007). Real-time measurement of outdoor tobacco smoke particles. Journal of the Air and Waste Management Association, 57, 522–534 [DOI] [PubMed] [Google Scholar]
- Kraev T. A., Adamkiewicz G., Hammond S. K., Spengler J. D. (2009). Indoor concentrations of nicotine in low-income, multi-unit housing: Associations with smoking behaviours and housing characteristics. Tobacco Control, 18, 438–444 doi:10.1136/tc.2009.029728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. K., Hedley A. J., Repace J., So C., Lu Q. Y., McGhee S. M., Wong C. M. (2011). Lung function and exposure to workplace second-hand smoke during exemptions from smoking ban legislation: An exposure-response relationship based on indoor PM2.5 and urinary cotinine levels. Thorax, 66, 615–623 doi:10.1136/thx.2011.160291 [DOI] [PubMed] [Google Scholar]
- Lam T. H., Ho L. M., Hedley A. J., Adab P., Fielding R., McGhee S. M., et al. (2005). Secondhand smoke and respiratory ill health in current smokers. Tobacco Control, 14, 307–314 doi:10.1136/tc.2005.011775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert W. E., Samet J. M., Spengler J. D. (1993). Environmental tobacco smoke concentrations in no-smoking and smoking sections of restaurants. American Journal of Public Health, 83, 1339–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M., Boethius G., Axelsson S., Montgomery S. M. (2008). Exposure to environmental tobacco smoke and health effects among hospitality workers in Sweden—Before and after the implementation of a smoke-free law. Scandinavian Journal of Work, Environment and Health, 34, 267–277 [DOI] [PubMed] [Google Scholar]
- Leung C. C., Lam T. H., Ho K. S., Yew W. W., Tam C. M., Chan W. M., Au K. F. (2010). Passive smoking and tuberculosis. Archives of Internal Medicine, 170, 287–292 doi:10.1001/archinternmed.2009.506 [DOI] [PubMed] [Google Scholar]
- Lightwood J. M., Glantz S. A. (2009). Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation, 120, 1373–1379 doi:10.1161/circulationaha.109.870691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. L., Yang Y., Travers M. J., Fong G. T., O’Connor R. J., Hyland A., Jiang Y. (2010). A cross-sectional study on levels of second-hand smoke in restaurants and bars in five cities in China. Tobacco Control, 19(Suppl. 2), i24–i29 doi:10.1136/tc.2009.029959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. J., Nebot M., Albertini M., Birkui P., Centrich F., Chudzikova M., Fernandez E. (2008). Secondhand smoke exposure in hospitality venues in Europe. Environmental Health Perspectives, 116, 1469–1472 doi:10.1289/ehp.11374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk R., Ferrence R., Gmel G. (2006). The economic impact of a smoke-free bylaw on restaurant and bar sales in Ottawa, Canada. Addiction, 101, 738–745 doi:10.1111/j.1360-0443.2006.01434.x [DOI] [PubMed] [Google Scholar]
- Luo J., Margolis K. L., Wactawski-Wende J., Horn K., Messina C., Stefanick M. L., Rohan T. E. (2011). Association of active and passive smoking with risk of breast cancer among postmenopausal women: A prospective cohort study. BMJ, 342, d1016 doi:10.1136/bmj.d1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. F., Irfan M. O., Haw S., Pell J. P. (2010). Meta-analysis of the effect of comprehensive smoke-free legislation on acute coronary events. Heart, 96, 1525–1530 doi:10.1136/hrt.2010.199026 [DOI] [PubMed] [Google Scholar]
- Mage C., Goldstein A. O., Colgan S., Skinner B., Kramer K. D., Steiner J., Staples A. H. (2010). Secondhand smoke policies at state and county fairs. North Carolina Medical Journal, 71, 409–412 [PubMed] [Google Scholar]
- Magzamen S., Charlesworth A., Glantz S. A. (2001). Print media coverage of California’s smokefree bar law. Tobacco Control, 10, 154–160 doi:10.1136/tc.10.2.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. L., Glantz S. A. (2004). Hedging their bets: Tobacco and gambling industries work against smoke-free policies. Tobacco Control, 13, 268–276 doi:10.1136/tc.2004.007484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt G. E., Quintana P. J., Destaillats H., Gundel L. A., Sleiman M., Singer B. C., Hovell M. F. (2011). Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environmental Health Perspectives, 119, 1218–1226 doi:10.1289/ehp.1103500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melberg H. O., Lund K. E. (2012). Do smoke-free laws affect revenues in pubs and restaurants? European Journal of Health Economics, 13, 93–99 doi:10.1007/s10198-010-0287-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies D., Nair A., Williamson P. A., Schembri S., Al-Khairalla M. Z., Barnes M., Lipworth B. J. (2006). Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA, 296, 1742–1748 doi:10.1001/jama.296.14.1742 [DOI] [PubMed] [Google Scholar]
- Meyers D. G., Neuberger J. S., He J. (2009). Cardiovascular effect of bans on smoking in public places: A systematic review and meta-analysis. Journal of the American College of Cardiology, 54, 1249–1255 doi:10.1016/j.jacc.2009.07.022 [DOI] [PubMed] [Google Scholar]
- Miller M. D., Marty M. A., Broadwin R., Johnson K. C., Salmon A. G., Winder B., Steinmaus C. ; California Environmental Protection Agency (2007). The association between exposure to environmental tobacco smoke and breast cancer: A review by the California Environmental Protection Agency. Preventive Medicine, 44, 93–106 doi:10.1016/j.ypmed.2006.08.015 [DOI] [PubMed] [Google Scholar]
- Mills A. L., Messer K., Gilpin E. A., Pierce J. P. (2009). The effect of smoke-free homes on adult smoking behavior: A review. Nicotine and Tobacco Research, 11, 1131–1141 doi:10.1093/ntr/ntp122 [DOI] [PubMed] [Google Scholar]
- Milz S., Akbar-Khanzadeh F., Ames A., Spino S., Tex C., Lanza K. (2007). Indoor air quality in restaurants with and without designated smoking rooms. Journal of Occupational and Environmental Hygiene, 4, 246–252 doi:10.1080/15459620701204801 [DOI] [PubMed] [Google Scholar]
- Muggli M. E., Hurt R. D., Blanke D. D. (2003). Science for hire: A tobacco industry strategy to influence public opinion on secondhand smoke. Nicotine and Tobacco Research, 5, 303–314 [DOI] [PubMed] [Google Scholar]
- Mulcahy M., Evans D. S., Hammond S. K., Repace J. L., Byrne M. (2005). Secondhand smoke exposure and risk following the Irish smoking ban: An assessment of salivary cotinine concentrations in hotel workers and air nicotine levels in bars. Tobacco Control, 14, 384–388 doi:14/6/384 [pii]10.1136/tc.2005.011635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafstad P., Jaakkola J. J., Hagen J. A., Zahlsen K., Magnus P. (1997). Hair nicotine concentrations in mothers and children in relation to parental smoking. Journal of Exposure Analysis and Environmental Epidemiology, 7, 235–239 [PubMed] [Google Scholar]
- Nagelhout G. E., van den Putte B., de Vries H., Crone M., Fong G. T., Willemsen M. C. (2012). The influence of newspaper coverage and a media campaign on smokers’ support for smoke-free bars and restaurants and on secondhand smoke harm awareness: Findings from the International Tobacco Control (ITC) Netherlands Survey. Tobacco Control, 21, 24–29 doi:10.1136/tc.2010.040477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee on Passive Smoking (1986). Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington, DC: National Academy Press; [PubMed] [Google Scholar]
- Navas-Acien A., Peruga A., Breysse P., Zavaleta A., Blanco-Marquizo A., Pitarque R., Samet J. (2004). Secondhand tobacco smoke in public places in Latin America, 2002-2003. JAMA, 291, 2741–2745 doi:10.1001/jama.291.22.2741 [DOI] [PubMed] [Google Scholar]
- Nebot M., López M. J., Gorini G., Neuberger M., Axelsson S., Pilali M., Hammond S. K. (2005). Environmental tobacco smoke exposure in public places of European cities. Tobacco Control, 14, 60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilsen K., Glantz S. A. (2004). A tobacco industry study of airline cabin air quality: Dropping inconvenient findings. Tobacco Control, 13(Suppl. 1), i20–i29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg M., Jaakkola M. S., Woodward A., Peruga A., Prüss-Ustün A. (2011). Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet, 377, 139–146 doi:10.1016/S0140-6736(10)61388-8 [DOI] [PubMed] [Google Scholar]
- Pearson J., Windsor R., El-Mohandes A., Perry D. C. (2009). Evaluation of the immediate impact of the Washington, D.C., smoke-free indoor air policy on bar employee environmental tobacco smoke exposure. Public Health Reports, 124(Suppl. 1), 134–142 [PMC free article] [PubMed] [Google Scholar]
- Pierce J. P., Messer K., White M. M., Kealey S., Cowling D. W. (2010). Forty years of faster decline in cigarette smoking in California explains current lower lung cancer rates. Cancer Epidemiology, Biomarkers and Prevention, 19, 2801–2810 doi:10.1158/1055–9965.EPI-10–0563 [DOI] [PubMed] [Google Scholar]
- Pirie K., Beral V., Peto R., Roddam A., Reeves G., Green J. Million Women Study Collaborators (2008). Passive smoking and breast cancer in never smokers: Prospective study and meta-analysis. International Journal of Epidemiology, 37, 1069–1079 doi:10.1093/ije/dyn110 [DOI] [PubMed] [Google Scholar]
- Pirkle J. L., Bernert J. T., Caudill S. P., Sosnoff C. S., Pechacek T. F. (2006). Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environmental Health Perspectives, 114, 853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polednak A. P. (2008). Tobacco control indicators and lung cancer rates in young adults by state in the United States. Tobacco Control, 17, 66–69 doi:10.1136/tc.2007.020925 [DOI] [PubMed] [Google Scholar]
- Pyles M. K., Hahn E. J. (2011). Economic effects of Ohio’s smoke-free law on Kentucky and Ohio border counties. Tobacco Control, 20, 73–76 doi:10.1136/tc.2009.035493 [DOI] [PubMed] [Google Scholar]
- Reh D. D., Lin S. Y., Clipp S. L., Irani L., Alberg A. J., Navas-Acien A. (2009). Secondhand tobacco smoke exposure and chronic rhinosinusitis: A population-based case-control study. American Journal of Rhinology and Allergy, 23, 562–567 doi:10.2500/ajra.2009.23.3377 [DOI] [PubMed] [Google Scholar]
- Reh D. D., Navas-Acien A. (2010). Relationship between second-hand tobacco smoke exposure and chronic rhinosinusitis: Evidence for causality. Expert Review of Respiratory Medicine, 4, 445–449 doi:10.1586/ers.10.44 [DOI] [PubMed] [Google Scholar]
- Repace J. (2004a). Flying the smoky skies: Secondhand smoke exposure of flight attendants. Tobacco Control, 13(Suppl. 1), i8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repace J. (2004b). Respirable particles and carcinogens in the air of delaware hospitality venues before and after a smoking ban. Journal of Occupational and Environmental Medicine, 46, 887–905 doi:00043764-200409000-00001 [pii] [DOI] [PubMed] [Google Scholar]
- Repace J. (2005). Controlling tobacco smoke pollution. ASHRAE IAQ Applications, 6 (3), 11–15 [Google Scholar]
- Repace J. (2007). Exposure to secondhand smoke. In Ott W. R., Steinemann A. C., Wallace L. A. (Eds.), Exposure analysis (pp. 201–231). Boca Raton, FL: Taylor & Francis; [Google Scholar]
- Repace J. (2008). Benefits of smoke-free regulations on outdoor settings: Beaches, golf courses, patios, and in motor vehicles. Willam Mitchell Law Review, 34 (4), 1621–1638 [Google Scholar]
- Repace J., Johnson K. C. (2006). Can displacement ventilation control secondhand ETS? Technical feature. ASHRAE IAQ Applications, 7, 1–6 [Google Scholar]
- Repace J. L. (2009). Secondhand smoke in Pennsylvania casinos: A study of nonsmokers’ exposure, dose, and risk. American Journal of Public Health, 99, 1478–1485 doi:10.2105/AJPH.2008.146241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repace J. L., Jiang R. T., Acevedo-Bolton V., Cheng K. C., Klepeis N. E., Ott W. R., Hildemann L. M. (2011). Fine particle air pollution and secondhand smoke exposures and risks inside 66 US casinos. Environmental Research, 111, 473–484 doi:10.1016/j.envres.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Samet J. M. (1999). Workshop summary: Assessing exposure to environmental tobacco smoke in the workplace. Environmental Health Perspectives, 107(Suppl. 2), 309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoj V., Alderete M., Ruiz E., Hasdeu S., Linetzky B., Ferrante D. (2010). The impact of a 100% smoke-free law on the health of hospitality workers from the city of Neuquen, Argentina. Tobacco Control, 19 (2), 134–137 doi:10.1136/tc.2009.032862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollo M., Lal A., Hyland A., Glantz S. (2003). Review of the quality of studies on the economic effects of smoke-free policies on the hospitality industry. Tobacco Control, 12, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebrie E. M., Glantz S. A. (2007). “Accommodating” smoke-free policies: Tobacco industry’s Courtesy of Choice programme in Latin America. Tobacco Control, 16, e6 doi:10.1136/tc.2006.018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S., Maccalman L., Naji A. A., Dempsey S., Hilton S., Miller B. G., Ayres J. G. (2007). Bar workers’ exposure to second-hand smoke: The effect of Scottish smoke-free legislation on occupational exposure. The Annals of Occupational Hygiene, 51, 571–580 doi:10.1093/annhyg/mem044 [DOI] [PubMed] [Google Scholar]
- Skogstad M., Kjaerheim K., Fladseth G., Gjølstad M., Daae H. L., Olsen R., Ellingsen D. G. (2006). Cross shift changes in lung function among bar and restaurant workers before and after implementation of a smoking ban. Occupational and Environmental Medicine, 63, 482–487 doi:10.1136/oem.2005.024638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford J., Daube M., Franklin P. (2010). Second hand smoke in alfresco areas. Health Promotion Journal of Australia, 21, 99–105 [DOI] [PubMed] [Google Scholar]
- Stillman F., Navas-Acien A., Ma J., Ma S., Avila-Tang E., Breysse P., Samet J. (2007). Second-hand tobacco smoke in public places in urban and rural China. Tobacco Control, 16, 229–234 doi:10.1136/tc.2006.018333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwan-ampai P., Navas-Acien A., Strickland P. T., Agnew J. (2009). Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiology, Biomarkers and Prevention, 18, 884–893 doi:10.1158/1055–9965.EPI-08-0939 [DOI] [PubMed] [Google Scholar]
- Thomas J. L., Guo H., Carmella S. G., Balbo S., Han S., Davis A., Hecht S. S. (2011). Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiology, Biomarkers and Prevention, 20, 1213–1221 doi:1055–9965.EPI-10–1027 [pii]10.1158/1055–9965.EPI-10–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Department of Health (1998). Report of the Scientific Committee on Tobacco and Health: Department of Health. United Kingdom: Department of Health and Social Services, Northern Ireland, The Scottish Office Department of Health, Welsh Office; [Google Scholar]
- U.S. Department of Health and Human Services (1986). The health consequences of involuntary smoking. A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control; [Google Scholar]
- U.S. Department of Health and Human Services (2006). The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general.. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control. [Google Scholar]
- U.S. Environmental Protection Agency (1992). Respiratory health effects of passive smoking: Lung cancer and other disorders. Washington, DC: US Environmental Protection Agency; [Google Scholar]
- Wakefield M., Cameron M., Inglis G., Letcher T., Durkin S. (2005). Secondhand smoke exposure and respiratory symptoms among casino, club, and office workers in Victoria, Australia. Journal of Occupational and Environmental Medicine, 47, 698–703 [DOI] [PubMed] [Google Scholar]
- Wakefield M., Siahpush M., Scollo M., Lal A., Hyland A., McCaul K., Miller C. (2002). The effect of a smoke-free law on restaurant business in South Australia. Australian and New Zealand Journal of Public Health, 26, 375–382 [DOI] [PubMed] [Google Scholar]
- Waters H. R., Foldes S. S., Alesci N. L., Samet J. (2009). The economic impact of exposure to secondhand smoke in Minnesota. American Journal of Public Health, 99, 754–759 doi:10.2105/AJPH.2008.137430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Framework Convention on Tobacco Control (2007). Guidelines on protection from exposure to tobacco smoke. Article 8 of the FCTC. Geneva, Switzerland: WHO Framework Convention on Tobacco Control. [Google Scholar]
- Wilson K. M., Klein J. D., Blumkin A. K., Gottlieb M., Winickoff J. P. (2011). Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics, 127, 85–92 doi:10.1542/peds.2010–2046 [DOI] [PubMed] [Google Scholar]
- Wilson N., Weerasekera D., Blakely T., Edwards R., Thomson G., Gifford H. (2010). What is behind smoker support for new smokefree areas? National survey data. BMC Public Health, 10, 498 doi:10.1186/1471-2458-10-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winickoff J. P., Tanski S. E., McMillen R. C., Ross K. M., Lipstein E. A., Hipple B. J., Klein J. D. (2011). Acceptability of testing children for tobacco-smoke exposure: A national parent survey. Pediatrics, 127, 628–634 doi:10.1542/peds.2010–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1999). International consultation on environmental tobacco smoke (ETS) and child health. Geneva, Switzerland: World Health Organization; [Google Scholar]
- World Health Organization (2003). WHO Framework Convention on Tobacco Control. Geneva, Switzerland: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011). WHO report on the global tobacco epidemic, 2011: The MPOWER package. Warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization; [Google Scholar]
- Yin P., Jiang C. Q., Cheng K. K., Lam T. H., Lam K. H., Miller M. R., Adab P. (2007). Passive smoking exposure and risk of COPD among adults in China: The Guangzhou Biobank Cohort Study. Lancet, 370, 751–757 doi:10.1016/S0140-6736(07)61378-6 [DOI] [PubMed] [Google Scholar]
- Young W. F., Szychowski J., Karp S., Liu L., Diedrich R. T. (2010). Economic impacts of the Pueblo Smoke-Free Air Act. American Journal of Preventive Medicine, 38, 340–343 doi:10.1016/j.amepre.2009.11.008 [DOI] [PubMed] [Google Scholar]