Abstract
Receptor-induced Ca2+ oscillations provide ‘digitized’ signals that confer precise activation of downstream targets. New studies reveal that STIM proteins — sensors of endoplasmic reticulum Ca2+ levels — cyclically translocate during oscillations, transiently coupling to activate cell-surface Ca2+ entry channels, resulting in a spatially unique signal that selectively triggers immediate-early gene expression.
Calcium signals, crucial to the control of a plethora of cellular functions, involve extraordinary spatial and temporal precision within cells [1]. In most cells, physiological receptor activation induces repetitive oscillations of cytosolic Ca2+ mediated by cyclic release of Ca2+ from endoplasmic reticulum (ER) stores [2,3]. These ‘digital’ Ca2+ signals confer unique specificity, sensitivity and accuracy in the activation of downstream target functions [3]. As ER Ca2+ stores empty, Ca2+ enters through highly specific store-operated channels (SOCs) in the plasma membrane that are controlled by STIM proteins, sensors of ER luminal Ca2+ levels [4]. Rather than merely replenishing depleted stores, it has recently been revealed that Ca2+ entry through SOCs contributes crucially to the spatial signature of Ca2+ oscillations [5]. Indeed, in a study by Bird et al. [6] in this issue of Current Biology, the STIM1 protein is now shown to translocate cyclically in and out of ER–plasma membrane junctions during each Ca2+ oscillatory spike. This STIM-mediated Ca2+ entry component of the digitized Ca2+ signals appears to be crucial for the Ca2+-mediated control of gene expression [5,6].
In all cells, Ca2+ release from ER stores and Ca2+ entry through SOCs in the plasma membrane are highly coordinated events [2,4]. Indeed, the dynamic ER Ca2+-sensing STIM proteins (STIM1 and STIM2) are remarkable integrators of the two processes [4,7,8]. STIM proteins detect subtle changes in ER luminal Ca2+ levels and undergo profound migration within the ER membrane to enter ER–plasma membrane junctions, where they trap and activate members of the Orai family of highly Ca2+-selective SOCs [4,7–10]. The activation and function of STIM1 and STIM2 proteins is significantly different [11,12]. STIM1 requires quite substantial Ca2+ release from the ER to undergo translocation, but activates opening of Orai channels very efficiently [4]. In contrast, STIM2 appears sensitive to small changes in ER luminal Ca2+ and rapidly translocates into ER–plasma membrane junctions with minimal store depletion [6,12]; however, STIM2 is poor at activating Orai channels [6,13] and, when overexpressed, has a dominant-inhibitory effect on channel activation [11].
The function of STIM proteins and their translocation and coupling to Orai channels has mainly been studied in response to substantial emptying of ER Ca2+ stores activated, for example, by high levels of agonists for phospholipase C (PLC)-coupled receptors that produce large global increases in inositol (1,4,5) trisphosphate (InsP3) levels, or by applying the powerful ER Ca2+ pump blocker thapsigargin [4]. However, these are non-physiological conditions. Instead, cells in vivo are exposed to much lower levels of receptor agonist, resulting in lower InsP3 production, which may remain local to the cell periphery and penetrate less deeply into the cell interior. The new study of Bird et al. [6] and the recent work by Di Capite et al. [5] examined the function of SOCs under physiological agonist activation conditions in which continuous oscillations of Ca2+ are observed, driven by fluctuating InsP3-induced Ca2+ release through InsP3 receptors (InsP3Rs) in the ER membrane. The repetitive Ca2+ spikes are regenerative ER Ca2+ release events thought to involve complex negative and positive feedback of Ca2+ on the InsP3R [2,3].
Clearly, external Ca2+ entry is required for the oscillations to continue: without such entry, stores cannot refill and the oscillations run down and cease. Although the Ca2+ entry route had been debated, it is now clear that SOCs mediate the entry to maintain oscillations [6,14,15]. The elimination of STIM1 or Orai1 channels suppresses the oscillations [6,15]. Since each spike induced by low levels of agonist represents a rather small total release of ER Ca2+, it might have been expected that the more ‘sensitive’ Ca2+ sensor, STIM2, would be the major instigator of the replenishing Ca2+ entry process. Surprisingly, only knockdown of STIM1, and not STIM2, inhibited Ca2+ oscillations [6]. An interesting inference can be drawn from this observation. Given that STIM1 needs a more substantial discharge of luminal Ca2+ to be activated [6,12], the rather small release events detected by STIM1 during Ca2+ spikes likely represent large decreases of Ca2+ in just a relatively few discrete Ca2+ stores (Figure 1), as opposed to a more global ER Ca2+ release event involving a relatively small overall change in luminal Ca2+. The results could also suggest that these local stores are predominantly localized at the cell periphery, likely in close proximity to pre-existing ER–plasma membrane junctions into which STIM1 molecules could easily and rapidly move to activate Orai channels. Indeed, these might also be the stores closest to the source of InsP3 production, as depicted in Figure 1.
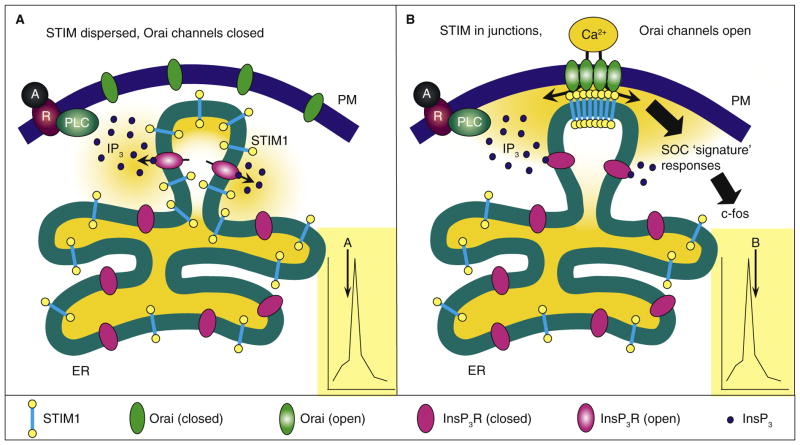
Figure 1. Coordination of spatially distinct Ca2+ signals during oscillations.
The figure shows a hypothetical model depicting the coordinated opening of InsP3R Ca2+ release channels in the ER and store-operated Orai Ca2+ entry channels in the plasma membrane (PM) during different phases of oscillations induced by submaximal stimulation of PLC-coupled receptors. (A) During the initial rising phase of spikes (see inset), activation of PLC-coupled receptors (denoted by R) by agonist (denoted by A) results in locally increased InsP3 levels opening InsP3Rs predominantly in peripheral ER. The rise in cytoplasmic Ca2+ represents rapid Ca2+ release and depletion of stores near the plasma membrane, while the bulk of the ER remains Ca2+ replete without compromising crucial ER functions. (B) During the falling phase, Ca2+ has been exhausted and/or InsP3Rs are deactivated. The substantial luminal Ca2+ decrease surpasses the Ca2+-sensing threshold required for STIM1 activation, causing STIM1 molecules to aggregate and translocate into ER–plasma membrane junctions. There, the STIM1 carboxyl termini lie within 10–20 nm of the plasma membrane where they bind, trap and activate Orai channels within the plasma membrane. Ca2+ enters through the Orai channels where it provides a spatially unique local Ca2+ signaling ‘signature’ required for triggering a number of crucial processes, including immediate early gene expression. The entering Ca2+ also bathes the region of the depleted stores and is pumped back to effect their refilling. The STIM1 Ca2+ sensor is saturated causing a rapid reversal of Orai activation, disaggregation of STIM1 and diffusion of STIM1 away from junctions. The cyclical movement of STIM1 is hence an important part of controlling the generation of Ca2+ oscillations. The specific spatial ‘signature’ of SOC-mediated Ca2+ entry provides a unique signal controlling gene expression.
The studies of Bird et al. [6] provide remarkable evidence for the movement of STIM proteins during Ca2+ oscillations and also reveal the proximity of discrete plasma-membrane-associated subsets of ER. The authors used total internal reflection microscopy to view STIM1 (tagged with enhanced yellow fluorescent protein, EYFP), in the ER undergoing translocation within close proximity (<100 nm) of the plasma membrane, while simultaneously recording low-agonist-induced Ca2+ oscillations in the cytosol. A small amount of the EYFP–STIM1 was clearly observed to translocate into ER–plasma membrane junctions then move away again during each oscillation. This is consistent with studies indicating that STIM1 molecules act locally, moving on average less than 2 μm to reach ER–plasma membrane junctions [16]. The oscillation-driven STIM1 translocation was found to be even more pronounced if Ca2+ were excluded from the cytosol [6]. In this case, STIM1 appeared to continue to accumulate to even higher levels during the course of Ca2+ oscillations. Obviously, with no Ca2+ outside, the movement of STIM1 molecules to activate Orai channels results in no entry of Ca2+ and hence no replenishment of stores. Presumably, in this case, the flight of STIM1 molecules into junctional ER is essentially irreversible and they continue to accumulate. The lack of Ca2+ entry prevents the stores from refilling and the oscillations wind down.
Interestingly, EYFP-tagged STIM2 molecules expressed in cells also accumulated into junctions close to the plasma membrane during oscillations [6]. The STIM2 translocation initiated a little faster than that of STIM1 during the onset of oscillations and was not as enhanced as that of STIM1 following Ca2+ removal. This would be consistent with the more constitutive ability of STIM2 to enter junctions, with greater store depletion having little further effect. However, as stated above, STIM2 does not effectively couple with endogenous Orai channels to mediate Ca2+ entry. Whereas Bird et al. [6] suggest that this is because the STIM2 molecule is intrinsically less able to couple with Orai channels, our own recent studies militate against a simple coupling difference between STIM1 and STIM2 [17]: the cytoplasmic carboxy-terminal Orai-interacting domains of STIM1 and STIM2 can each bind to and activate Orai1 channels [17]. The functional differences between STIM1 and STIM2 lie more within their amino-terminal ER luminal regions. Although reportedly more sensitive to ER Ca2+ changes [12], the difference in affinity between the Ca2+-binding EF-hands of STIM1 and STIM2 is not substantial [18]. Instead, a small amino-terminal domain that differs between STIM1 and STIM2 may confer substantial differences in the ability of STIM1 and STIM2 to aggregate and conformationally couple to Orai channels [13,19]. Hence, STIM2 can have a dominant-inhibitory effect by virtue of it being a poor partial agonist in the activation of Orai channels. Certainly, STIM2 is more constitutively ‘available’ at ER–plasma membrane junctions, but this may be a result of poor clearance from junctions rather than increased sensitization to enter junctions. Regardless, it is likely important that STIM2’s constitutive presence in junctions is attenuated by its poor ability to activate channels, having a distinct role in controlling Ca2+ homeostasis [12] and/or functioning as a negative regulator of STIM1-mediated Ca2+ entry [13].
Perhaps the most significant conclusion from the new work linking SOCs with the Ca2+ oscillatory response is the functional role of Ca2+ entering through SOCs during Ca2+ oscillations. Particularly in the work of Di Capite et al. [5] we learn that the SOC-mediated Ca2+ entry provides a ‘spatial signature’ that specifically controls Ca2+-dependent gene expression. Thus, the prevailing theory on the significance of store-operated Ca2+ entry during oscillations is that it is necessary to prevent run-down and to allow replenishment of Ca2+ stores to facilitate the continuation of oscillations. While this function is clearly important, Di Capite et al. [5] used a trick which allowed Ca2+ oscillations to continue normally without any Ca2+ entry component. Thus, they applied high levels of La3+ to block movement of Ca2+ across the plasma membrane — both entry through SOCs and exit via plasma membrane Ca2+ pumps — essentially isolating the cytoplasm from the cell exterior, allowing InsP3-mediated oscillations in response to low-agonist-induced activation to continue normally without any net Ca2+ entry. In mast cells, the proinflammatory leukotriene LTC4 induces expression of the immediate early gene c-fos through PLC-driven InsP3-mediated oscillatory Ca2+ signals. Using the La3+ block to prevent SOC-mediated Ca2+ entry, submaximal LTC4 levels led to activation of Ca2+ oscillations identical to those observed without the block, but there was no c-fos expression. Thus, it is concluded that an important function of Ca2+ oscillations is to activate SOCs, which mediate spatially defined entry of Ca2+ that is essential for the activation of gene expression. Indeed, both recent papers [5,6] refer to a number of other examples in which the SOC-mediated Ca2+ entry component is important for coupling to Ca2+-dependent downstream effectors. Such a specific role for Ca2+ entry is highly consistent with studies in neuronal cells which revealed that the signature of Ca2+ entry signals through L-type channels or NMDA receptors is crucial for defining gene induction responses [20].
The function of a specific subset of ER Ca2+ stores tightly coupled to the activation of SOCs makes much sense. Thus, the maintenance of Ca2+ within the bulk ER is essential in order to preserve protein synthesis and trafficking and to prevent protein misfolding and stress responses. Considering that STIM1 is the major mediator of coupling to activate SOCs and requires substantial luminal Ca2+ decreases to become activated, it is logical that such release be restricted to a small fraction of stores. As shown in Figure 1, it is also logical that these restricted stores are near the plasma membrane, where they are not only exposed to the highest levels of InsP3, but also proximal to ER–plasma membrane junctions to optimize STIM1-mediated coupling to activate Orai channels.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW, Bird GS. Cytoplasmic calcium oscillations and store-operated calcium influx. J Physiol. 2008;586:3055–3059. doi: 10.1113/jphysiol.2008.153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 4.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai - dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 6.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RB, Putney JW. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 8.Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 10.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 12.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, Soboloff J. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–19168. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird GS, Putney JW., Jr Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedel B, Boyles RR, Putney JW, Jr, Bird GS. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Deng X, Zhou Y, Hendron E, Ritchie MF, Tang XD, Kurosaki T, Mori Y, Soboloff J, Gill DL. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci USA. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM)1 and STIM2 EF-SAM regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 20.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]