Summary
Background
Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are potentially fatal blistering diseases caused by autoantibodies targeting desmoglein (Dsg) adhesion proteins. Previous studies have shown an IgG4 > IgG1 predominance of anti-Dsg antibodies in pemphigus; however, no studies have examined total serum IgG4 levels in pemphigus. IgG4 is induced by chronic antigen stimulation, which could occur with persistent skin blistering and potentially elevate the total serum IgG4 relative to other IgG subclasses in patients with pemphigus.
Objectives
The primary aim of the study was to quantitate total and Dsg-specific IgG subclasses in patients with pemphigus.
Methods
IgG subclasses and Dsg-specific IgG1 and IgG4 were quantitated in patients with PV and PF, and in sera from age-matched controls using a subclass enzyme-linked immunosorbent assay. The effectiveness of IgG4 depletion in blocking IgG pathogenicity in PV was determined using a keratinocyte dissociation assay.
Results
Dsg-specific antibodies comprised a median of 7·1% and 4·2% of total IgG4 in patients with PV and PF, respectively, with eightfold and fourfold enrichment in IgG4 vs. IgG1. Total serum IgG4, but not other IgG subclasses, was enriched in patients with PV and PF compared with age-matched controls (P = 0·004 and P = 0·005, respectively). IgG4 depletion of PV sera reduced pathogenicity in a keratinocyte dissociation assay and showed that affinity-purified IgG4 is more pathogenic than other serum IgG fractions.
Conclusions
Dsg-specific autoantibodies are significantly enriched in IgG4, which may explain the enrichment of total serum IgG4 in some patients with pemphigus. By preferentially targeting autoimmune rather than beneficial immune antibodies, IgG4-targeted therapies may offer safer treatment options for pemphigus.
Pemphigus is a potentially fatal antibody-mediated, tissue-specific autoimmune disease caused by autoantibodies against desmoglein (Dsg) cell adhesion proteins.1 In pemphigus foliaceus (PF), autoantibodies against Dsg1 cause superficial blisters in the skin. In pemphigus vulgaris (PV), Dsg3 autoantibodies cause deeper suprabasal blisters in the mucous membrane epithelia. Some patients with PV develop Dsg1 in addition to Dsg3 autoantibodies, which correlate with the presence of suprabasal blisters in both mucosa and skin. The clinical and histological site of blister formation in patients with PF and PV can be explained by the expression patterns of the different Dsg isoforms in mucosa and skin.2 The pathogenicity of Dsg3- and Dsg1-specific PV and PF IgG has been experimentally validated, indicating that anti-Dsg IgG is both necessary and sufficient for blister formation, and that serum autoantibody enzyme-linked immunosorbent assay (ELISA) titres correlate with disease activity.3–7
Although the Fc region of pemphigus autoantibodies is not required for blister formation in experimental pemphigus models,8–11 anti-Dsg antibodies have been shown to preferentially associate with the IgG4 subclass. In both PV and PF, patients with active disease demonstrate Dsg-reactive IgG4 and IgG1, while patients in remission and some healthy relatives of patients with pemphigus can demonstrate only anti-Dsg IgG1.12–15 IgG2 and IgG3 anti-Dsg autoantibodies have not been associated with disease.16,17 Longitudinal studies of patients with an endemic form of PF indicate that a preferential rise in the ratio of IgG4 to IgG1 Dsg-reactive antibodies accompanies the onset of disease activity. Additionally, an IgG4-specific Dsg ELISA was shown to have greater sensitivity and specificity than a total IgG Dsg ELISA in detecting active disease in endemic PF, suggesting a more significant clinical association of pathogenic antibodies with IgG4 rather than with other IgG subclasses in this patient population.18 Collectively, these studies indicate that the acquisition of an anti-Dsg IgG4 response is a characteristic serological finding in patients with active pemphigus.
Although Dsg-specific IgG4 has been described in patients with pemphigus, particularly those with endemic PF, to our knowledge no studies have investigated levels of total serum IgG4 in pemphigus. A hyper-IgG4 state is uncommon in humans, having been described only in people receiving repetitive cutaneous immunization with monoclonal or oligoclonal antigens, such as beekeepers and individuals undergoing allergic desensitization therapy.19 We postulated that skin blisters could act as a form of chronic autovaccination to Dsg antigens in pemphigus, leading to an anti-Dsg IgG4 response that could potentially elevate the total serum IgG4 relative to other IgG subclasses. We therefore examined whether total serum IgG4 is enriched in patients with pemphigus. To determine what percentage of total IgG is Dsg-specific, we quantitated Dsg-specific IgG1 and IgG4 in patients with pemphigus. Finally, we evaluated whether IgG4 depletion abrogates the pathogenicity of PV sera.
Materials and methods
Patient characteristics, sera and antibodies
All studies were performed under research protocols approved by the Institutional Review Board. PV, PF and age-matched control sera were obtained from banked samples at the University of Pennsylvania clinical and research laboratories (median ages 48, 46 and 55 years, respectively). For some samples, sera were derived from citrated plasma by incubation with 15 mmol L−1 CaCl2 for 30 min at 37 °C followed by overnight incubation at 4 °C and centrifugation/filtration, or from heparinized plasma by incubation with 120 mmol L−1 CaCl2 and thrombin followed by centrifugation/filtration. Unaffected individuals were blood donors at the Hospital of the University of Pennsylvania. The diagnosis of pemphigus was confirmed by clinical presentation, histology and at least one positive serological test (direct immunofluorescence, indirect immunofluorescence or Dsg ELISA). Disease activity measurements, patient demographics and clinical treatment information were not available for all banked serum samples, although all patients had active disease at the time of serum collection. Patients treated with rituximab were excluded from analysis.
Recombinant anti-Dsg IgG1 and IgG4 expressing the same variable region were produced by subcloning variable regions from human PV monoclonal antibodies (mAbs) (D31)12b/c and (D31)2/2910 into the PIGG vector (for production of IgG1) as previously described.20 The constant region of the IgG4 heavy chain was amplified by polymerase chain reaction from whole blood genomic DNA (purified using the Qiagen QIAamp DNA Blood Mini Kit; Qiagen, Venlo, the Netherlands) and subcloned by restriction digest into the ApaI-NheI sites of PIGG to create the hPIGG4 vector (for production of human IgG4). All inserts were sequence verified, allowing for discrepancies in intronic sequences that would not be predicted to interfere with RNA splicing. IgG mAbs were produced by transient transfection of 293T cells using jetPEI transfection reagent (Polyplus; Polyplus Transfection Inc., New York, NY, U.S.A.) and purification by protein A chromatography as described.20 Antibodies were quantitated by A280 and immunoglobulin subclass ELISA (Invitrogen, Grand Island, NY, U.S.A.).
Total and antigen-specific immunoglobulin subclass enzyme-linked immunosorbent assay
Total IgG1, IgG2, IgG3 and IgG4 concentrations were quantitated in control and pemphigus sera using an immunoglobulin subclass ELISA (Invitrogen) according to the manufacturer’s instructions.
For Dsg-specific subclass ELISA, titrations of anti-Dsg3 and anti-Dsg1 IgG1 and IgG4 mAbs, incubated on Dsg3- or Dsg1-coated wells (MBL International, Woburn, MA, U.S.A.) and developed with specific horseradish peroxidase (HRP)-conjugated anti-IgG1 (diluted 1 : 500) and anti-IgG4 (diluted 1 : 10 000) secondary antibodies (Alpha Diagnostic International, San Antonio, TX, U.S.A.), were used to generate a quantitative standard curve for ELISA. At the same time, patient sera, appropriately diluted to be within the linear range of the standard curves, were incubated on Dsg-coated wells and detected with the same subclass-specific secondary antibodies. The resulting OD450 value was used to calculate the concentration of anti-Dsg3 subclass-specific antibody in each patient sample, based on the equation from the standard curve regression analysis. Standard curves and equations were derived using Microsoft Excel and XLSTAT software (Addinsoft, Andernach, Germany).
Statistical analysis
Statistical analysis was performed with XLSTAT software, using the Wilcoxon rank sum or Wilcoxon signed rank tests. P-values < 0·05 were considered significant.
Affinity adsorption and purification of serum IgG
Anti-Dsg3 serum IgG was adsorbed using recombinant Dsg3 affinity chromatography. Baculoviral vector pET Dsg3E, encoding the extracellular domains of Dsg3 expressing a carboxyterminal E and 6x histidine tag,21 was transfected into Sf9 cells using the BaculoGold expression system (BD Biosciences, San Jose, CA, U.S.A.). Recombinant baculovirus was amplified for four passages in Sf9 cells, followed by infection of Hi5 cells for recombinant protein expression. Expression of proteins was confirmed by immunoblot with HRP-labelled anti-E tag antibody (Abcam, Cambridge, U.K.) and chemiluminescence detection (GE Healthcare, Waukesha, WI, U.S.A.). Hi5 and Sf9 insect cells (both from Invitrogen) were cultured at 27 °C in Express Five SFM or Sf-900 medium (Gibco, from Invitrogen), respectively, supplemented with Antibiotic-Antimycotic (Gibco) and L-glutamine. Recombinant Dsg3 protein was purified by Talon cobalt affinity chromatography (Clontech Laboratories, Inc., Mountain View, CA, U.S.A.).
Serum IgG4 was adsorbed and purified using anti-IgG4 affinity chromatography. Purified mouse anti-human IgG4 (Invitrogen) was coupled to Affigel 10 matrix (BioRad, Hemel Hempstead, U.K.) as previously described.20 The specificity of the IgG4-depleted and IgG4-purified serum IgG fractions was measured by IgG subclass ELISA (Invitrogen).
Pathogenicity assays
In vitro human keratinocyte dissociation assays with pemphigus IgG fractions were performed as previously described.20,22 The number of cell sheet fragments was quantitated by ImageJ (National Institutes of Health, Bethesda, MD, U.S.A.). The number of cell sheet fragments in the negative control phosphate- buffered saline well was subtracted from all other wells for calculations.
Results
Dsg-specific antibodies comprise a significantly higher proportion of total serum IgG4 than total serum IgG1 in patients with pemphigus vulgaris and pemphigus foliaceus
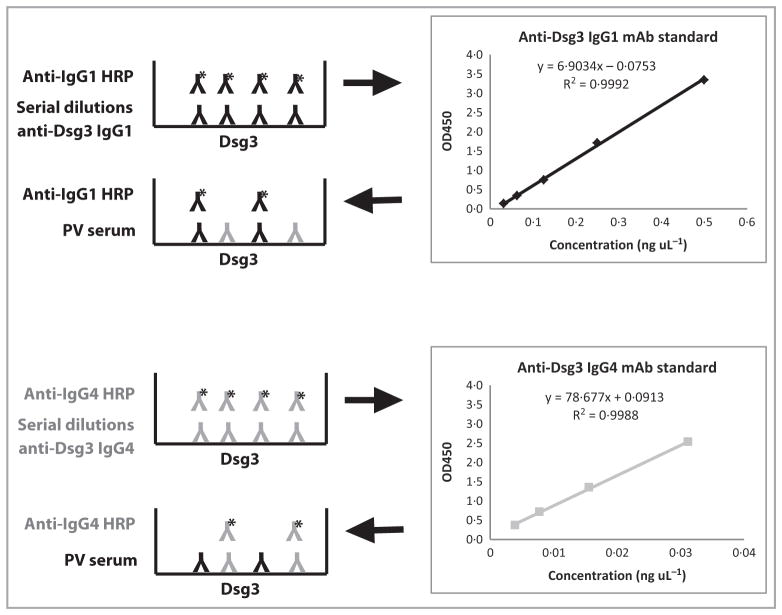
To determine the percentage of total IgG that is antigen specific, we developed a novel Dsg-specific subclass ELISA, using IgG1 and IgG4 recombinant mAbs expressing the same anti-Dsg variable region as quantitative standards for the assay (diagrammed in Fig. 1). Briefly, serial dilutions of anti-Dsg IgG1 or IgG4 mAb standards were incubated on Dsg-coated wells, followed by detection with IgG1- or IgG4-specific secondary antibodies. Anti-Dsg mAb standards were used at concentrations in the linear range of antibody binding and detection. Anti-IgG1 secondary antibodies showed no detectable binding to anti-Dsg IgG4 bound to Dsg-coated wells, and anti-IgG4 secondary antibodies showed no detectable binding to anti-Dsg IgG1 bound to Dsg-coated wells (data not shown). At the same time, pemphigus sera, appropriately diluted to be within the linear range of the assay, were incubated on Dsg-coated wells and developed with either IgG1- or IgG4-specific secondary antibodies. The resulting OD450 value was used to calculate the concentration of anti-Dsg subclass-specific antibody in each sample based on the equation from the standard curve regression analysis.
Fig 1.
Diagram of desmoglein (Dsg)-specific subclass enzyme-linked immunosorbent assay (ELISA). Serial dilutions of IgG1 or IgG4 recombinant human monoclonal antibodies (mAbs) expressing the same anti-Dsg3 variable region were incubated on Dsg3-coated wells and detected with subclass-specific horseradish peroxidase (HRP)-conjugated secondary antibodies to generate a quantitative standard curve. At the same time, pemphigus vulgaris (PV) serum was incubated on Dsg3-coated wells and developed with either IgG1 or IgG4-specific secondary antibodies. The resulting OD450 value was used to calculate the concentration of anti-Dsg3 subclass-specific antibody in each patient sample based on the equation from the standard curve regression analysis. A similar design was used for Dsg1-specific subclass ELISA, except that anti-Dsg1 mAb standards and pemphigus foliaceus sera were incubated on Dsg1-coated wells.
In initial experiments to validate the accuracy of the ELISA to quantitate Dsg-specific IgG4 in patient samples, we compared results for one sample from a patient with PV using two different methods. In the first method, we used Dsg3-specific subclass ELISA to measure the concentration of Dsg3-specific IgG4, and divided this by the total serum IgG4 concentration to determine the percentage of total IgG4 that is Dsg3 specific. This assay yielded values of 15·5% and 16·7% in two independent experiments. In the second method, purified PV IgG was adsorbed with recombinant Dsg3 bound to agarose beads, using naked agarose beads as a control. Dsg3 ELISA of samples before and after adsorption showed that 97% of Dsg3-reactive antibodies were depleted by the Dsg3 column, whereas < 1% of Dsg3-reactive antibodies were adsorbed by naked agarose beads. The total serum IgG4 measurement before and after adsorption of Dsg-specific antibodies indicated a decrease of 18·5% in total IgG4. Therefore, although we cannot be certain that these mAb standards are reflective of the composite affinity of polyclonal IgG1 vs. IgG4 in all patients, these data suggest that the Dsg3-specific subclass ELISA is both accurate and reproducible.
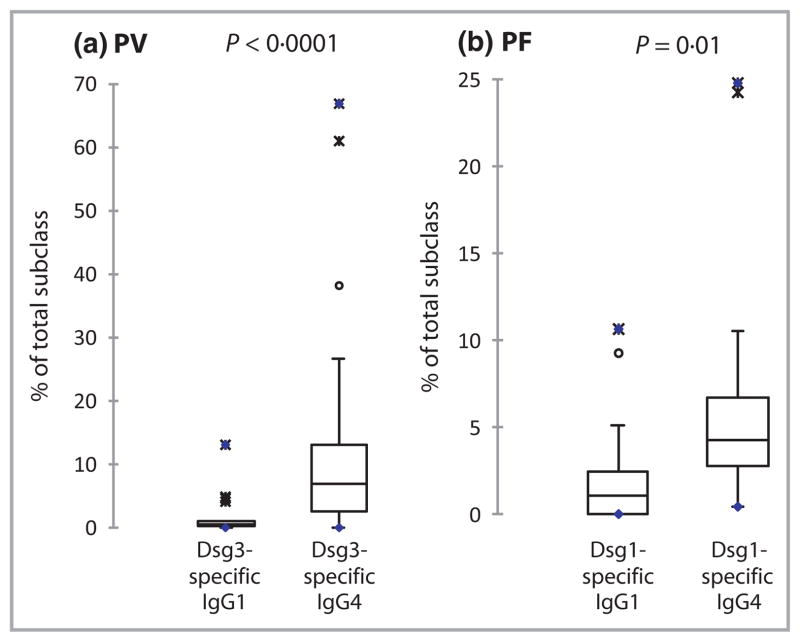
The quantitation of Dsg3-specific IgG1 and IgG4 in 27 patients with PV appears in Table S1 (see Supporting Information). The percentage of Dsg3-specific antibodies in the total IgG4 fraction ranged from 0·1% to 67·0%. The percentage of IgG4 that was Dsg3 specific was significantly higher than the percentage of IgG1 that was Dsg3 specific, with medians of 7·1% and 0·5%, respectively (P < 0·0001 by Wilcoxon signed rank test, Fig. 2a). In 12 patients with PV (44%), the serum concentration of Dsg3-specific IgG4 exceeded the serum concentration of Dsg3-specific IgG1. Overall, the median ratio of Dsg3-specific IgG4 to Dsg3-specific IgG1 in patients with PV was 0·88, compared with a 0·11 median ratio of total IgG4 to total IgG1 in the same patients (P < 0·0001 by Wilcoxon signed rank test), and a 0·05 median ratio of total IgG4 to total IgG1 in unaffected individuals. These data indicate an approximately eightfold enrichment of anti-Dsg antibodies in the serum IgG4 vs. the serum IgG1 fraction in patients with PV.
Fig 2.
Desmoglein (Dsg)-specific antibodies are enriched in the serum IgG4 subclass. (a) In patients with pemphigus vulgaris (PV, n = 27) the median percentage of Dsg3-specific IgG4 was 7.1% (IQR 2.6– 13.2%) compared with 0.5% (IQR 0.2–1.1%) for percentage Dsg3- specific IgG1 (P < 0.0001 by Wilcoxon signed rank test). (b) In patients with pemphigus foliaceus (PF, n = 16) the median percentage of Dsg1-specific IgG4 was 4.0% (IQR 2.1–6.6%) compared with 1.2% (IQR 0–3.3%) for percentage Dsg1-specific IgG1 (P = 0.04 by Wilcoxon signed rank test). IQR, interquartile range.
The quantitation of Dsg1-specific IgG1 and IgG4 in 16 patients with PF appears in Table S2 (see Supporting Information). The percentage of Dsg1-specific antibodies in the total IgG4 fraction ranged from 0% to 24·8%. One patient (PF1) did not have any detectable serum IgG4 or Dsg1-specific IgG4 and therefore the percentage of Dsg1-specific IgG4 could not be calculated. In the remaining 15 patients with PF, the percentage of IgG4 that was Dsg1 specific was significantly higher than the percentage of IgG1 that was Dsg1 specific, with medians of 4·2% and 1·0%, respectively (P = 0·01 by Wilcoxon signed rank test, Fig. 2b). In seven patients with PF (44%), the serum concentration of Dsg1-specific IgG4 exceeded the serum concentration of Dsg1-specific IgG1. The median ratio of Dsg-specific IgG4 to Dsg1-specific IgG1 in patients with PF was 0·54, compared with a 0·15 median ratio of total IgG4 to total IgG1 in the same patients (P = 0·01 by Wilcoxon signed rank test), indicating an approximately fourfold enrichment of anti-Dsg1 antibodies in the serum IgG4 fraction.
Enrichment of total serum IgG4, but not other IgG subclasses, in patients with pemphigus vulgaris and pemphigus foliaceus compared with unaffected individuals
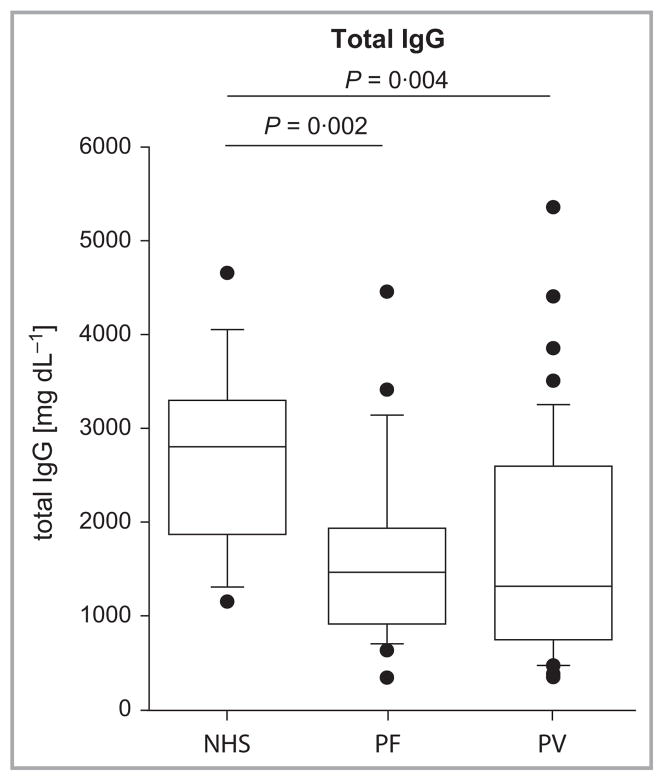
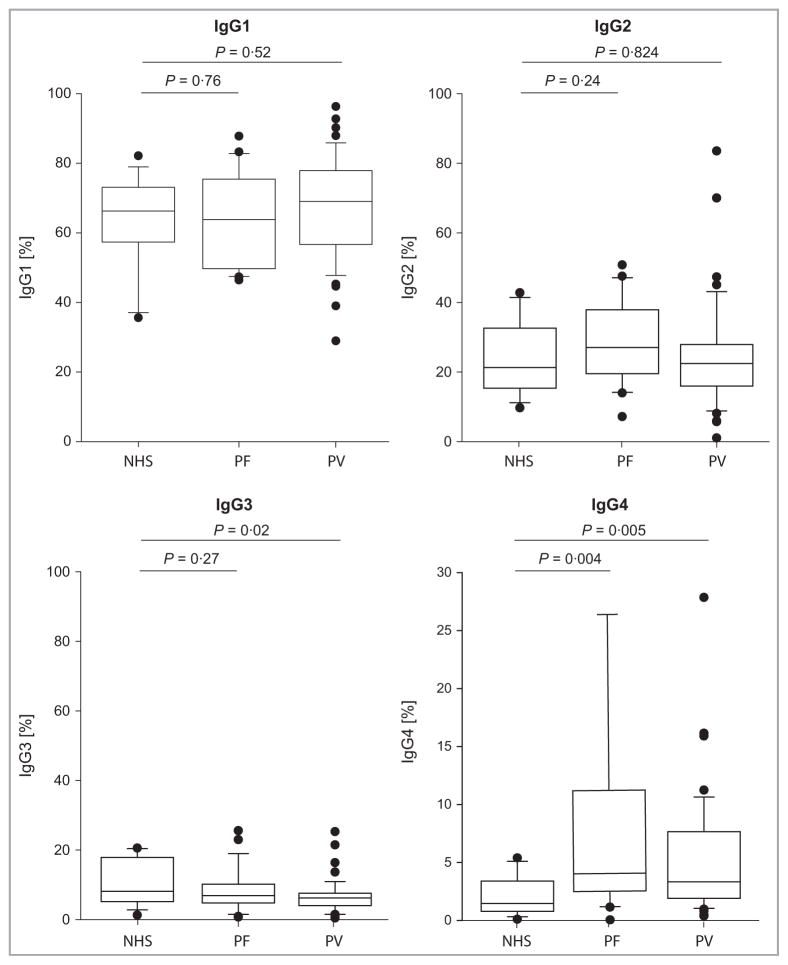
If Dsg-specific IgG4 is significantly elevated, this could lead to elevation of total serum IgG4 in patients with pemphigus. We first examined total serum IgG levels in a larger cohort of 48 patients with PV and 24 with PF, compared with 15 age-matched controls. Total serum IgG was significantly elevated in unaffected individuals compared with patients with PV and PF (P = 0·004 and P = 0·002, respectively, Fig. 3), presumably due to immunosuppressive therapy in patients with pemphigus. To normalize for differences in total IgG and to determine which IgG subclasses differ between pemphigus and unaffected individuals, we examined the IgG subclasses as a percentage of total IgG. Total serum IgG4, but not IgG1, IgG2 or IgG3, was enriched in patients with PV and PF compared with unaffected individuals (Fig. 4). Patients with PV and PF demonstrated a median percentage IgG4 of 3·3% [interquartile range (IQR) 1·9–7·5%] and 4·1% (IQR 2·6–10·9%), compared with 1·5% (IQR 0·8–3·4%) in age-matched unaffected individuals (P = 0·005 and P = 0·004, respectively). IgG3 was significantly lower in patients with PV (median 5·8%), but not in patients with PF (median 6·5%), compared with age-matched controls (median 8·2%, P = 0·02).
Fig 3.
Total serum IgG is decreased in patients with pemphigus. Normal human sera (NHS) showed a median total IgG level of 2800 mg dL−1 (IQR 1890–3260 mg dL−1), compared with 1320 mg dL−1 (IQR 750–2550 mg dL−1) and 1460 mg dL−1 (IQR 930–1910 mg dL−1) in patients with pemphigus vulgaris (PV) and pemphigus foliaceus (PF), respectively. IQR, interquartile range.
Fig 4.
Enrichment of total serum IgG4, but not other IgG subclasses, in patients with pemphigus. Serum concentrations of IgG1, IgG2, IgG3 and IgG4 as a percentage of total IgG were measured by quantitative subclass enzyme-linked immunosorbent assay in 48 patients with pemphigus vulgaris (PV) and 24 with pemphigus foliaceus (PF), relative to 15 age-matched normal human serum (NHS) controls. A significant enrichment of total serum IgG4 was observed in patients with PV and PF (P = 0.005 and P = 0.004 by Wilcoxon rank sum test, respectively).
IgG4 depletion blocks pemphigus vulgaris IgG pathogenicity
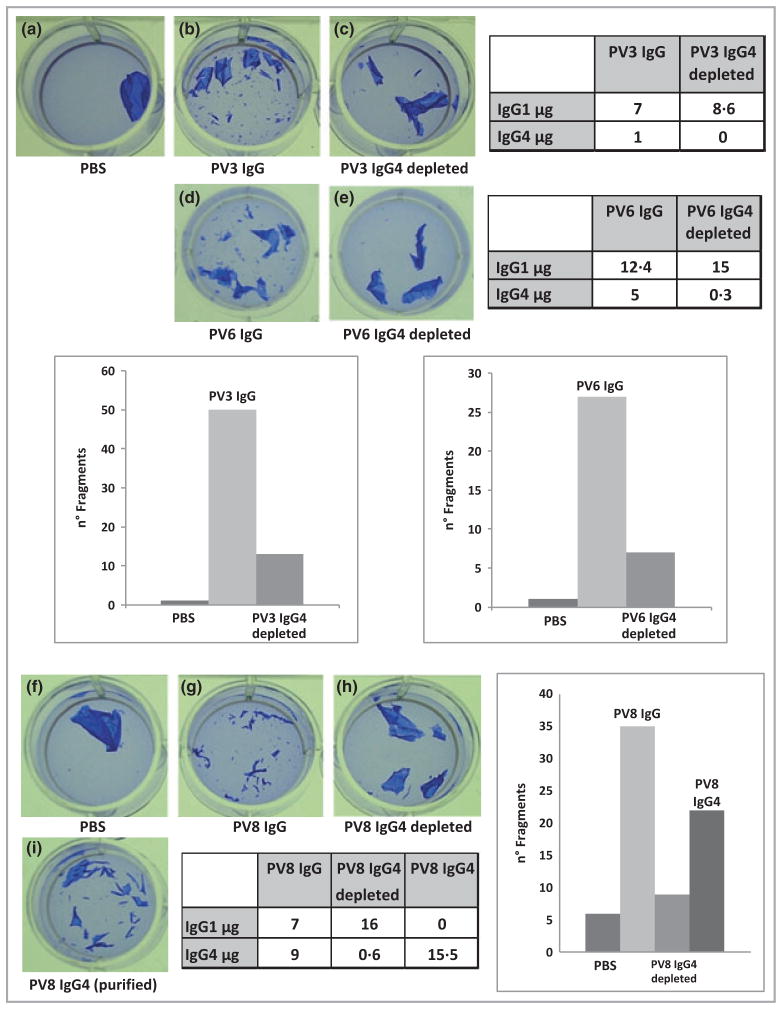
If the IgG4 subclass comprises most of the pathogenic autoantibodies, then IgG4 depletion of pemphigus sera should deplete pathogenic activity. Pathogenic but not nonpathogenic anti-Dsg3 PV autoantibodies have been shown to cause dissociation of skin keratinocyte cell sheets.10,22 For testing, we selected three samples from patients with PV who had new-onset mild disease (PV3), early moderate disease (PV6) and chronic severe disease (PV8). Adsorption of serum IgG4 from all three patient samples resulted in a mean 81% drop in cell sheet fragments (Fig. 5, panels b–c, d–e, g–h). IgG subclass measurement in the original and IgG4-depleted samples confirmed that IgG1 levels were not affected by IgG4 depletion.
Fig 5.
IgG4 depletion blocks pathogenic activity of pemphigus IgG. Specific adsorption of serum IgG4 abrogated pathogenicity of three IgG samples from patients with pemphigus vulgaris (PV) without affecting serum IgG1 levels (panels b–c, d–e, g–h). Bar graphs indicate the number of cell sheet fragments. Incubation of skin keratinocyte cell sheets with PV IgG4 resulted in a fivefold higher fragment number compared with IgG4-depleted serum fractions (panels h–i). Results for PV8 are representative of three independent experiments.
To compare the relative pathogenic activities of the IgG1 and IgG4 subclasses, we incubated keratinocyte cell sheets with equal μg amounts of IgG1 and IgG4. A fivefold increase in cell sheet fragments was observed with purified IgG4 compared with IgG4-depleted fractions (Fig. 5, panels h–i), indicating that pathogenic antibodies are enriched in the serum IgG4 subclass compared with other IgG subclasses.
Discussion
Previously, it has been shown that the Dsg-specific autoantibodies in patients with pemphigus with active disease tend to be IgG4,12–15 although to our knowledge no previous studies have reported elevation of total serum IgG4 in patients with pemphigus. In the current study, we have shown that serum IgG4 is enriched in patients with pemphigus compared with unaffected individuals (Fig. 4). Dsg-specific autoantibodies comprise a median of 7·1% and 4·2% of total serum IgG4 in patients with PV and PF, respectively (Fig. 2). Overall, an eightfold enrichment of anti-Dsg3 antibodies and a fourfold enrichment of anti-Dsg1 antibodies were observed in the serum IgG4 vs. the serum IgG1 fractions in patients with PV and PF.
In some patients, antigen-specific antibodies comprised the majority of serum IgG4, which could account for the elevation of total serum IgG4. However, in other patients with elevated serum IgG4, Dsg-specific antibodies did not comprise the majority. It is possible that in these patients antibodies against other keratinocyte autoantigens (such as Dsg1 in mucocutaneous PV, or other keratinocyte cell surface antigens) may contribute to elevation of serum IgG4. Alternatively, chronic cutaneous antigen stimulation may generally skew the immune response towards a hyper-IgG4 state (discussed further below).
Why enrichment of autoantibodies in the IgG4 fraction occurs in pemphigus has not been well established. The variable region of an antibody is formed in developing B cells, before exposure to antigen, through a process of germline DNA rearrangement known as VDJ recombination. After the variable regions have recombined, the constant region of the antibody is joined to the variable region through further germline DNA rearrangement. Initially all B cells produce IgM, but subsequently they may undergo class switch recombination to a new isotype (IgG1, IgG2, IgG3, IgG4, IgA1, IgA2 or IgE). Due to the order of constant region genes in chromosomal DNA, if a variable region recombines initially with a proximal constant region, such as IgG1, it can later recombine again to a distal constant region, such as IgG4. However, because DNA recombination involves irreversible excision of intervening DNA, once a variable region recombines with a more distal constant region, such as IgG4, that variable region can never switch back to a more proximal isotype such as IgG1. IgG1 is the most abundant serum IgG subclass and is associated with an interleukin (IL)-4/T-helper2 (Th2) immune response.23 Repeated antigenic exposure can encourage subsequent isotype switching. IL-4 and IL-13 promote isotype switching first to IgG4 and subsequently to IgE.24 IL-10 may potentiate IL-4-induced switching to IgG4 over IgE.25 Upregulation of the Th2 cytokines IL-4, IL-10 and IL-13 has been described in patients with pemphigus.26,27 These cytokines would promote an IgG4 > IgG1 serum autoantibody profile, coinciding with the observed autoantibody IgG subclasses in patients with active disease.
IgG4 autoantibodies are predominant in other chronic autoimmune conditions, such as bullous pemphigoid, cicatricial pemphigoid, epidermolysis bullosa acquisita, thrombotic thrombocytopenic purpura, autoimmune sclerosing pancreatitis and Mikulicz’s disease.28–33 Elevated serum IgG4 is also observed in immune conditions characterized by repetitive antigen exposure, such as in beekeepers and individuals undergoing allergic desensitization therapy.19 These observations support the hypothesis that IgG4 is potentially a specific marker of antibodies resulting from chronic immune reaction against antigen, or autoantigen in the case of autoantibody-mediated diseases.
The enrichment of anti-Dsg antibodies in the IgG4 fraction raises the question of whether IgG4 could serve as a therapeutic target in pemphigus, for example through subclass-specific immunoadsorption or by selective depletion of surface-IgG4 positive memory B cells. Currently there are no therapies approved by the Food and Drug Administration for pemphigus, which is life-threatening if left untreated.34 Most therapies for pemphigus rely on general immune suppression, which is associated with significant side-effects including fatal infection and secondary cancers. Thus, a major frontier for autoimmunity research is to identify and target only the disease- causing antibodies. IgG4 is a potentially attractive therapeutic target, as it is the least abundant IgG subclass, and unlike other IgG subclasses it does not activate complement and therefore is thought to be relatively unimportant for fighting infection. Although selective IgG4 deficiency has been associated with recurrent upper respiratory tract infections in some patients, other studies have estimated that up to 50% of children have no detectable serum IgG4 and are otherwise healthy.35,36 Our finding that patients with PV and PF have lower overall total IgG levels compared with unaffected individuals (Fig. 3) suggests that current immunosuppressive therapy generally targets all subclasses, but does not correct the IgG4 enrichment in pemphigus. Therefore, selective depletion of IgG4 or the cells that produce it could represent a novel and safer strategy for the treatment of pemphigus.
Demonstrating the feasibility of this approach, we have found that IgG4 depletion reduces the pathogenic activity of pemphigus sera. IgG4 depletion reduces keratinocyte dissociation by PV IgG by a mean of 81%, indicating that pathogenic antibodies are preferentially enriched in the serum IgG4 fraction (Fig. 5). One remaining question is whether remaining Dsg-specific IgG1 could perpetuate active disease in patients who are depleted of IgG4. Our studies suggest that IgG4 is the major pathogenic IgG subclass in patients with PV relative to the other subclasses, as PV IgG depleted of IgG4 (which would contain the IgG1, IgG2 and IgG3 subclasses) demonstrated pathogenicity similar to a negative control (Fig. 5). Prior studies have shown that patients in clinical remission, and even unaffected relatives of patients with pemphigus, can express Dsg-specific IgG1 without evidence of clinical disease. 12–15 Additionally, if IgG1 autoantibodies subsequently switch to IgG4 with chronic active disease, IgG4 depletion strategies will ultimately capture these pathogenic autoantibody populations. We are currently pursuing studies to investigate the genetic relationships of pathogenic and nonpathogenic antibodies among the different IgG subclasses in patients with pemphigus at the mAbs level. Even if some pathogenic antibodies reside in the IgG1 subclass, sparing of the IgG1 population could be considered a prudent compromise in designing treatment strategies for chronic autoimmune diseases that are currently without a cure. If IgG4 depletion is safe, then chronic IgG4 depletion may be preferable to pan-B-cell depletion therapies that risk fatal infection and often require repeated infusions for maintenance of disease control.
Supplementary Material
What’s already known about this topic?
Previous studies have shown that the anti-desmoglein (Dsg) autoantibodies in patients with pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are predominantly of the IgG4 subclass during active disease, but how much of total IgG4 they comprise, and whether this increases the total IgG4 concentration relative to the other IgG subclasses, is unknown.
What does this study add?
Using a novel Dsg-specific subclass enzyme-linked immunosorbent assay, we quantitated Dsg-specific IgG1 and IgG4 in pemphigus sera.
Dsg-specific IgG4 comprises a median of 7·1% and 4·2% of total serum IgG4 in patients with PV and PF, respectively, with four- to eightfold enrichment of autoantibodies in IgG4 vs. IgG1.
Total serum IgG4 is enriched in some patients with pemphigus. These data suggest that IgG4 may be a preferable target for therapy.
Acknowledgments
We thank Eun Jung Choi for technical assistance and John R. Stanley for provision of pemphigus sera and helpful discussions.
Funding sources
This study was supported by the Department of Dermatology at the University of Milan (L.L.), the National Institute of Arthritis and Musculoskeletal and Skin Diseases AR053505 and AR057001 (A.S.P.), Deutsche Forschungsgemeinschaft Research Foundation (C.T.E.), T32-AR007465 (A.R.N.), and the Penn Skin Disease Research Center (P30-AR057217).
Footnotes
Conflicts of interest
None declared.
References
- 1.Payne AS, Stanley JR. Pemphigus. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Dermatology in General Medicine. 8. New York: McGraw Hill; 2012. [Google Scholar]
- 2.Mahoney MG, Wang Z, Rothenberger K, et al. Explanation for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461–8. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amagai M, Karpati S, Prussick R, et al. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J Clin Invest. 1992;90:919–26. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amagai M, Hashimoto T, Shimizu N, et al. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest. 1994;94:59–67. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amagai M, Hashimoto T, Green KJ, et al. Antigen-specific immunoabsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895–901. doi: 10.1111/1523-1747.ep12606168. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Diaz LA, Fairley JA, et al. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–43. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SW, Kobayashi M, Tanikawa A, et al. Monitoring disease activity in pemphigus with enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3. Br J Dermatol. 2002;147:261–5. doi: 10.1046/j.1365-2133.2002.04838.x. [DOI] [PubMed] [Google Scholar]
- 8.Rock B, Labib RS, Diaz LA. Monovalent Fab′ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990;85:296–9. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascaro JM, Jr, España A, Liu Z, et al. Mechanisms of acantholysis in pemphigus vulgaris: role of IgG valence. Clin Immunol Immunopathol. 1997;85:90–6. doi: 10.1006/clin.1997.4408. [DOI] [PubMed] [Google Scholar]
- 10.Payne AS, Ishii K, Kacir S, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii K, Lin CY, Siegel DL, et al. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–48. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhol K, Natarajan K, Nagarwalla N, et al. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–43. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock B, Martins CR, Theofilopoulos AN, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 14.Kricheli D, David M, Frusic-Zlotkin M, et al. The distribution of pemphigus vulgaris-IgG subclasses and their reactivity with desfmoglein 3 and 1 in pemphigus patients and their first-degree relatives. Br J Dermatol. 2000;143:337–42. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- 15.Warren SJ, Arteaga LA, Rivitti EA, et al. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol. 2003;120:104–8. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 16.Futei Y, Amagai M, Ishii K, et al. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 17.Torzecka JD, Wozniak K, Kowalewski C, et al. Circulating pemphigus autoantibodies in healthy relatives of pemphigus patients: coincidental phenomenon with a risk of disease development? Arch Dermatol Res. 2007;299:239–43. doi: 10.1007/s00403-007-0760-y. [DOI] [PubMed] [Google Scholar]
- 18.Qaqish BF, Prisayanh P, Qian Y, et al. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2008;129:110–18. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. 1. Prolonged immunization results in an IgG4- restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- 20.Payne AS, Siegel DL, Stanley JR. Targeting pemphigus autoantibodies by their heavy chain variable region genes. J Invest Dermatol. 2007;127:1681–91. doi: 10.1038/sj.jid.5700790. [DOI] [PubMed] [Google Scholar]
- 21.Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–17. [PubMed] [Google Scholar]
- 22.Ishii K, Harada R, Matsuo I, et al. In vitro keratinocyte dissociation assay for evaluation of the pathogenicity of anti-desmoglein 3 IgG autoantibodies in pemphigus vulgaris. J Invest Dermatol. 2005;124:939–46. doi: 10.1111/j.0022-202X.2005.23714.x. [DOI] [PubMed] [Google Scholar]
- 23.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, Mills FC, Saxon A. Switch circles from Il-4-directed epsilon- class switching from human B-lymphocytes – evidence for direct, sequential, and multiple step sequential switch from mu to epsilon Ig heavy-chain gene. J Immunol. 1994;152:3427–35. [PubMed] [Google Scholar]
- 25.Jeannin P, Lecoanet S, Delneste Y, et al. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–61. [PubMed] [Google Scholar]
- 26.Hertl M, Eming R, Veldman C. T cell control in autoimmune bullous skin disorders. J Clin Invest. 2006;116:1159–66. doi: 10.1172/JCI28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Amagai M, Tanikawa A, et al. T helper type 2-biased natural killer cell phenotype in patients with pemphigus vulgaris. J Invest Dermatol. 2006;127:324–30. doi: 10.1038/sj.jid.5700527. [DOI] [PubMed] [Google Scholar]
- 28.Dopp R, Schmidt E, Chimanovitch I, et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42:577–83. [PubMed] [Google Scholar]
- 29.Laffitte E, Skaria M, Jaunin F, et al. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br J Dermatol. 2001;144:760–8. doi: 10.1046/j.1365-2133.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 30.Bernard P, Prost C, Aucouturier P, et al. The subclass distribution of IgG autoantibodies in cicatricial pemphigoid and epidermolysis bullosa acquisita. J Invest Dermatol. 1991;97:259–63. doi: 10.1111/1523-1747.ep12480369. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari SE, Mudde GC, Rieger M, et al. IgG subclass distribution of antibodies against ADAMTS13 in patients with thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7:1703–10. doi: 10.1111/j.1538-7836.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Ohara M, Suzuki C, et al. Elevated IgG4 concentrations in serum of patients with Mikulicz’s disease. Scand J Rheumatol. 2004;33:432–3. doi: 10.1080/03009740410006439. [DOI] [PubMed] [Google Scholar]
- 34.Mao X, Payne AS. Seeking approval: present and future therapies for pemphigus vulgaris. Curr Opin Invest Drugs. 2008;9:497–504. [PMC free article] [PubMed] [Google Scholar]
- 35.Plebani A, Ugazio AG, Avanzini MA, et al. Serum IgG subclass concentrations in healthy subjects at different age: age normal percentile charts. Eur J Pediatr. 1989;149:164–7. doi: 10.1007/BF01958271. [DOI] [PubMed] [Google Scholar]
- 36.Moss RB, Carmack MA, Esrig S. Deficiency of IgG4 in children: association of isolated IgG4 deficiency with recurrent respiratory tract infection. J Pediatr. 1992;120:16–21. doi: 10.1016/s0022-3476(05)80590-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.