Abstract
Background
Event Related Potential (ERP) studies have highlighted some measures, notably P3 amplitude, that are associated with both state and trait deficits in alcoholism, while studies examining N400 amplitude in alcoholism are few. The present study aims to examine changes in the N400 component, an electrophysiological correlate of semantic priming, in event-related potentials from a lexical decision task in 87 alcohol dependent subjects and 57 community controls.
Method
Each subject was presented with 300 stimuli sequentially in a quasi-randomized design, where 150 stimuli were words and 150 were non-words. The subjects made a lexical decision indicating the word/nonword status with a button press. Among the words, 50 words (primed) were always preceded by their antonyms (prime, n=50), whereas the remaining 50 words were unrelated. N400 amplitude and latency measures were compiled from ERPs to the primed and unprimed words. Corresponding reaction time and response characteristics were also analyzed.
Results
Control subjects revealed a significant attenuation of the N400 response to the primed word when compared to the unprimed word. Significantly less attenuation was observed in alcohol dependent subjects. No significant group differences were seen for latency and behavioral measures. All subjects had slower RT for unprimed words compared to primed words; however significantly less reaction time savings between the unprimed and primed condition was noted for alcoholics.
Conclusion
These results suggest a reduced flexibility in the cognitive networks and a lack of resource optimization in alcoholics. The reduced attenuation of N400 during the primed condition in the alcohol dependent subjects may reflect an inability to engage similar neuronal substrates associated with semantic relatedness as seen in the controls. As diminished N400 attenuation during priming is observed in both alcoholics and high risk subjects, it may be a marker of risk and a good endophenotype for alcoholism.
Keywords: N400, Semantic Priming, Lexical Decision, Event-Related-Potentials, Alcoholism
1. INTRODUCTION
Cognitive deficits in alcohol dependent subjects have been described extensively, and can vary from severe memory problems as observed in Korsakoff’s syndrome to subtle deficits observed in the processing of a stimulus, as seen in decreased P3 amplitude (Ceballos et al., 2009; Oscar-Berman and Zola-Morgan, 1980; Oscar-Berman et al., 1982; Porjesz and Begleiter, 2003; Porjesz et al., 2005; Tarter and Ryan, 1983). Electrophysiological studies of adult alcoholics and high risk children of alcoholics have found alterations generally in N1, N2, P2 and P3, where only the reduced P3 amplitude was a robust finding across laboratories and across experimental paradigms (Cohen et al., 1997; Fein and Chang, 2006; Kamarajan et al., 2005a; Miyazato and Ogura, 1993; Oscar-Berman, 1987; Pfefferbaum et al., 1991; Porjesz and Begleiter, 1985; Porjesz and Begleiter, 1987; Prabhu et al., 2001; Realmuto et al., 1993; Rodriguez Holguin et al., 1998; Rodriguez Holguin et al., 1999a; Rodriguez Holguin et al., 1999b; Zhang et al., 2001). In addition, alcohol dependence has been shown to adversely affect several cognitive functions, including stimulus discrimination (Porjesz et al., 1987), response inhibition (Cohen et al., 1997; Kamarajan et al., 2005a; Kamarajan et al., 2005b), and semantic processing (Ji et al., 1999; Maylor et al., 1987; Williams and Rundell, 1984). These abnormalities observed in ERP components reveal cognitive impairment in alcoholics [For a detailed review see (Porjesz and Begleiter, 2003)].
In contrast, not many studies have examined semantic processing substrates in the context of alcohol dependence. A negative peak in the ERP (designated as N400) occurring predominantly over the centroparietal scalp region and approximately 300 to 650 ms after the presentation of a word that is incongruent with its semantic context, has been the cornerstone of semantic processing studies (Bentin, 1989; Bentin et al., 1993; Gunter and Friederici, 1999; Hamberger et al., 1995; Kutas and Hillyard, 1980; Nixon et al., 2002). Classically it was understood to be elicited only to semantic violations (Kutas and Hillyard, 1980; Kutas and Van Petten, 1988; Nixon et al., 2002), but recent studies have shown that the N400 varies systematically with the processing of potentially meaningful stimuli, where the amplitude is reduced by a number of factors (Kutas and Federmeier, 2000); some of these factors are semantic congruity, antonyms, repetitions and stimuli that occur with high frequency (Brown and Hagoort, 1993; Fischler et al., 1983; Kutas and Federmeier, 2000; Nobre and McCarthy, 1995; Penke et al., 1997).
N400 can be elicited from different experimental paradigms. One such paradigm is the lexical decision task where letter-strings are presented in sequence and the subject’s task is to decide whether the stimulus presented is a word or a non-word. Within this framework the task involves a semantic priming paradigm, where some of the words were antonym-pairs. Semantic priming task has been one of the most extensively used ERP paradigms to study the effect of priming on N400 (Bentin, 1989; Ganis et al., 1996). The semantic priming effect refers to the faster reaction time to the related targets than to the unrelated targets in a lexical decision task (Meyer and Schvaneveldt, 1971). This effect can also be observed in ERP tasks. Further, there is parallel between the N400 amplitude observed for words in a lexical decision task and the N400 amplitude observed to words in sentences (Kutas and Federmeier, 2000; Kutas and Hillyard, 1984; Taylor, 1953). In the semantic priming paradigm, a word preceded by an unrelated word (unprimed condition) produces a larger N400 in comparison to a word preceded by a related word (primed condition) (McCarthy and Nobre, 1993).
There are several proposed theories regarding the N400 in a priming paradigm and the most favored one is from Neely and Keefe (1989), who propose that these mechanisms are automatic spreading activation (Collins and Loftus, 1975), expectancy and semantic matching (den Heyer et al., 1983; Neely and Keefe, 1989; Silva-Pereyra et al., 1999). Expectancy and semantic matching mechanisms are referred to as controlled priming mechanisms and are generally believed to act more effectively at relatively long stimulus onset asynchrony (SOA) of greater than 500 ms, whereas automatic spreading activation mechanism is said to have influence on the priming effect at short SOAs (De Groot et al., 1986; Neely, 1991). However, studies have shown that semantic matching strategies (controlled priming mechanisms) can be active at SOAs as short as 150 ms (Koivisto, 1998) and automatic spreading activation can influence priming as long as 2000 ms (Deacon et al., 1999). In general, research evidence suggests that the N400 in priming paradigms reflects different mechanisms, such as automatic spreading activation (Deacon et al., 2004; Deacon et al., 2000; Kiefer, 2002; Kiefer and Spitzer, 2000; Kutas and Hillyard, 1989), expectancy (Kutas et al., 1984; Silva-Pereyra et al., 1999) and semantic matching (Chwilla et al., 1998; Holcomb, 1993), depending on the paradigm used. Similarly, the topography of N400 depends on the modality, type of stimulus and the paradigm of the study. For example, auditory N400 seems to be more evenly distributed over the scalp, whereas the visual N400 shows clear centroparietal predominance (Domalski et al., 1991).
Studies have shown that the distribution of N400 is different for different types of auditory stimuli. For example, the N400 elicited by concrete words tends to have a more anterior distribution compared to those elicited by abstract words (Holcomb et al., 1999; Kounios and Holcomb, 1994). On the other hand, both hemispheres are said be involved in different types of semantic processing (Federmeier and Kutas, 1999; Federmeier and Kutas, 2002). These differences point out that the effects observed with different types of stimuli are from non-identical neural generators, which in turn implies that semantic information is not stored in a modality-independent manner (Kutas and Federmeier, 2000). Intracranial recording studies suggest that the scalp-recorded N400 is associated with waves of activity across multiple brain areas, such as ventrolateral prefrontal cortex, inferotemporal cortex, superior temporal sulcus, medial temporal lobe and hippocampus (Halgren et al., 1994a; Halgren et al., 1994b; McCarthy et al., 1995; Nobre and McCarthy, 1995). Therefore, the N400 recorded at the scalp is an outcome of coordinated activity in multiple brain regions.
There is a dearth of studies exploring N400 in alcoholism and few studies have examined priming and the N400 component in adult alcoholics. Nixon et al., (2002) examined processing efficiency using a sentence paradigm in which the responses to the terminal word were compared between alcohol dependent subjects and community controls. The authors reported reduced amplitudes of the difference waveform only in the temporal regions in alcohol dependent subjects. Using a sentences paradigm, Ceballos et al. (2003) showed increased N400 latencies in alcohol dependent subjects who also had a diagnosis of antisocial personality (ASP). In a later study examining single substance and dual substance dependence, Ceballos et al., (2005) reported that reduced N400 amplitude was associated with alcohol dependence irrespective of cocaine co-dependence when compared to non-dependent controls. Of the studies mentioned above, two showed a reduction in N400 amplitude and one study reported increased latency in adult alcoholics. These studies have not clarified if the N400 amplitude reductions were specific to differences in priming related activations. Hence, the current study was conducted to examine the semantic priming effects on N400 in male adult alcoholics. A recent study from our laboratory (Roopesh et al., 2009) showed lack of attenuation of N400 amplitude for primed stimulus in high risk offspring of alcoholics.
2. METHODS AND MATERIALS
2.1. Subjects
Eighty-seven male alcohol dependent subjects with an age range of 19 - 47 years (Mean = 30.12, SD = ± 4.88) and 57 male control subjects between 20 - 47 years (Mean = 29.47, SD = ± 5.36) constituted the present study. Alcoholic subjects were recruited from inpatient and outpatient treatment facilities. Controls were recruited from health maintenance organizations, driver’s license records and dental clinics. The diagnosis of alcohol dependence was made based on the DSM IV criteria for alcohol dependence. The alcohol dependent group consisted of subjects who completed the de-addiction program in the treatment centers, and were abstinent from alcohol intake for at least 28 days before the EEG recording session. Further, subjects who tested positive in the urine screen (for their recent drug use) or breathalyzer test were excluded from the study. Subjects with hepatic encephalopathy/cirrhosis of the liver, multiple sclerosis, stroke, Huntington’s disease, a history of psychosis, head injury, seizures or neurosurgical procedures were excluded. Subjects who tested positive for HIV, had uncorrected sensory deficits, or had used any psychoactive substances in the past 5 days were also excluded.
All the subjects were right handed and had at least a minimum of ten years of education. In the alcohol dependent group, some subjects also had a history of other substance abuse. This comorbid poly-substance use/abuse is commonly observed in alcohol dependent subjects, especially in the US population (Bierut et al., 1998; Compton et al., 2007; Hasin et al., 2007). Assessments, recordings and analyses were conducted at the State University of New York – Downstate Medical Center and the study was approved by the Institutional Review Board (IRB).
2.2. Data recording
Subjects were seated on a comfortable chair in a sound-attenuated, temperature-regulated, and dimly lit booth (Industrial Acoustics Company; Bronx, NY). An Electro-Cap (Electro-Cap International Inc.; Eaton, OH) with 32 leads based on the International 10-20 system (Jasper, 1958) was placed on the scalp of each subject. A forehead electrode served as the ground and the nose electrode served as the common reference. Electrode impedance was maintained below 5 kΩ. Vertical and horizontal electro-ocular (EOG) activities were recorded with the electrodes placed supraorbitally and at the outer canthus of the left eye. Amplifier gain was set at 10,000 times on Sensorium EPA-2 Electrophysiology amplifiers (Charlotte, VT), with a high pass filter of 0.02 Hz and low pass filter of 50 Hz and digitized on a Concurrent 5550 computer (Concurrent Computer Corp. Atlanta, GA). The sampling rate was 256 Hz with sampling beginning 187 ms prior to and continuing for 1413 ms after the stimulus onset. Artifact rejection thresholds were set at 73.3 μV. Waveforms were computed using epochs filtered with 32 Hz low pass digital filter. The epochs were 1000 milliseconds long and included 200 milliseconds prior to the stimulus and 800 ms post-stimulus. Only subjects with good, artifact-free visually inspected waveforms were included. The trials in which the response time exceeded 1000 ms were coded as missed responses and excluded from all analyses. Further, only the correct response trials and with 15 minimum numbers of good trials in each conditions were included for the statistical analysis.
2.3. Lexical Decision Task
Semantic priming in the current study was investigated using a lexical decision task that required subjects to indicate when a stimulus was a word or a non-word with a button press using a different hand for each category. The hand used for the button press to indicate word and non-word were counter-balanced across subjects. Subjects were told to respond as quickly and as accurately as possible. The subjects were sequentially presented with a partially randomized list of 150 words and 150 non-words with a uniform inter-stimulus interval of 1600 ms. The exposure time for each stimulus was 150 ms. Among the 150 words, 100 words were part of 50 antonym pairs. For example, antonyms like TOP, BOTTOM, were always presented consecutively. Here the first word of the antonym pair was considered as the priming word or ‘Prime’, and the second word of the antonym pair as ‘Primed’. Thus, in total there were 50 Prime and 50 Primed words. These antonym pairs were always preceded and followed by non-words. The remaining 50 words were not part of antonym pairs and were considered as unrelated words (Unprimed); these words were generally interspersed among the non-words, but sometimes two unrelated words followed each other. (Please refer to figure 1).
Figure 1.
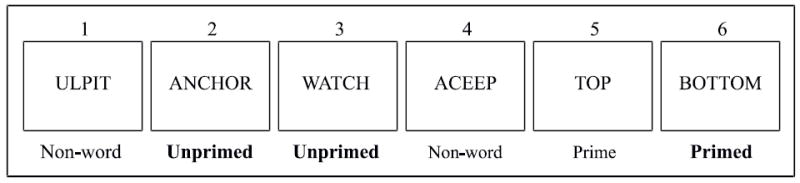
Sample of words used in the task, its corresponding priming condition and the order of presentation.
Word length was the same for primed and unprimed conditions and averaged 4.5 letters. Non-word length also averaged 4.5 letters and consisted of pronounceable combinations of letters. Both word and non-word stimuli were of 2.5 cm in height and were of white color presented over a black background. Word familiarity, using the scale of Toglia et al. (1978), for both the primed and unprimed words, averaged 6.3 on a scale of 1 (unfamiliar) to 7 (very familiar). A standardized spelling list (Forbes, 1968) placed the average grade level of the primed and unprimed words in the 3rd grade and ranged from 2nd to 7th grade. Imaginability of the words ranged from 215 to 641 on the scale of 100 to 700 with an average of 484.31. The average concreteness of the words was 447.36, with the range from 242 to 624. Both imaginability and concreteness scores were obtained online from the MRC Psycholinguistic Database (Refer http://www.psy.uwa.edu.au/mrcdatabase/mrc2.html). Written frequency, according to Kucera-Francis frequency count, averaged 456.80 and ranged from one to 21,341 (Kucera and Francis, 1967). The parts of speech of the words included noun, verb, adjective, adverb and preposition; some words fell into more than one category.
Averaged waveforms for the primed (words) and unprimed stimuli (words) for each subject were used to identify and measure the N400 component. The N400 component was selected as the largest negative peak between 300 and 500 ms and occurring before the large positive component after the stimulus onset in mean waveforms. Peak amplitude and latency values were extracted for each subject using a semi-automatic peak-picking program. The analysis for the current study was limited to words.
2.4. Statistical analysis
Data were analyzed using SPSS version 12.0. Of the 32 channels, data from fifteen electrodes were selected and grouped into three regions, viz. frontal (F7, F3, Fz, F4, F8), temporo-central (T7, C3, Cz, C4, T8), and parietal (P7, P3, Pz, P4, P8). Subject and task related variables such as age, education, reaction time and details of drinking were analyzed for group differences using t-tests. As a primary analysis, repeated measures ANOVAs were used separately for amplitude and latency, with group as the between-subjects variable. Semantic condition (primed and unprimed) and scalp region (frontal, central, and parietal) were entered as the within-subjects factors. Secondary analysis included examining group differences using separate repeated measures ANOVAs for amplitude and latency measures for both primed and unprimed conditions. Scalp region and electrode position were entered as within-subjects factors. Greenhouse-Geisser correction was employed when required. Unless otherwise mentioned, the results were non-significant.
3. RESULTS
3.1. Demographic, clinical and Performance variables
Both groups showed no significant difference with respect to age of the subjects (t = -0.755, p = .452). Average alcohol intake per month for the last six months were significantly different between the two groups (t = -6.88, p < .000), but alcohol intake did not show significant correlations with ERP measures. With respect to the performance measures, no significant differences across semantic conditions were noted for the following - correct response, wrong response, missed response and reaction time for conditions. However, both groups of subjects showed differences in performance between primed and unprimed words: in the number of wrong responses, missed responses and reaction time (RT), where subjects in both groups had significantly more wrong and missed responses as well as slower RT to unprimed words compared to primed words (Table 1). Finally, reaction time difference between the unprimed and primed condition was computed for each individual and the RT difference value was found to be significantly larger for control subjects in comparison to the alcoholics (t = 2.47, p = 0.015).
Table 1.
Comparison of performance measures between semantic conditions in alcoholic and control groups
| Primed | Unprimed | t | Sig. | ||
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | ||||
| Alcoholics | Correct Response | 29.02 (0.92) | 29.84 (0.89) | -1.346 | .182 |
| Wrong Response | 1.53 (0.26) | 2.95 (0.26) | -5.355 | .000*** | |
| Missed Response | 0.91 (0.14) | 1.35 (0.19) | -2.587 | .011* | |
| Reaction Time | 0.565 (0.009) | 0.598 (0.008) | -7.470 | .000*** | |
| Controls | Correct Response | 28.30 (1.15) | 29.21 (0.99) | -1.083 | .284 |
| Wrong Response | 0.96 (0.20) | 2.98 (0.34) | -5.809 | .000*** | |
| Missed Response | 0.63 (0.15) | 1.02 (0.19) | -2.415 | .019* | |
| Reaction Time | 0.557 (0.14) | 0.609 (0.01) | -7.541 | .000*** | |
3.2. Amplitude Measure
The mean N400 amplitudes at frontal, temporo-central and parietal regions in primed and unprimed conditions for control and alcohol-dependent groups are shown in table 2. The main effect of Group (F = 3.25; p = 0.074) showed only a trend towards differences in N400 amplitudes between groups. A significant main effect was observed for Condition (F = 34.07; p = 0.000) confirming a larger negative amplitude for unprimed compared to primed words. The results of the repeated measures ANOVA revealed a significant Group x Condition (F = 5.25; p = 0.023) interaction effect, indicating that alcohol dependent subjects had significantly larger negative amplitudes for the primed word condition when compared to control subjects. The Group × Region (F = 2.86; p = 0.06) interaction effect showed a trend towards significance because the highest differences between controls and alcoholics were centroparietal and the smallest were at the frontal regions.
Table 2.
N400 amplitudes at the three Antero-posterior Regions defined in the analysis
| Region | Primed Word | Unprimed Word | |||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Controls [n = 57] | Frontal | -.749 | .415 | -2.679 | .349 |
| Temporo-Central | -.873 | .441 | -3.058 | .390 | |
| Parietal | -1.657 | .547 | -3.609 | .500 | |
| Alcoholics [n = 87] | Frontal | -1.515 | .336 | -2.298 | .282 |
| Temporo-Central | -2.252 | .357 | -3.359 | .316 | |
| Parietal | -3.649 | .443 | -4.404 | .404 | |
To examine the Group × Condition interaction effect more closely, the groups were compared for primed and unprimed word conditions separately. The groups were significantly different only in the primed word condition (F = 6.391; p = .013), but not in the unprimed word condition (F = 0.248; p = .619) (Figure 2). In figure 3, attenuation of N400 amplitude (the area within the circle at the right) in the primed condition is striking in controls and not observed as strongly in alcoholics. The current source density (CSD) map of N400 response (Figure 4) reveals a strong frontal sink and a posterior source in the controls. However, while the alcoholics have a comparable response to controls for the unprimed word condition, the sink and source are severely diminished in alcoholics in the primed condition.
Figure 2.
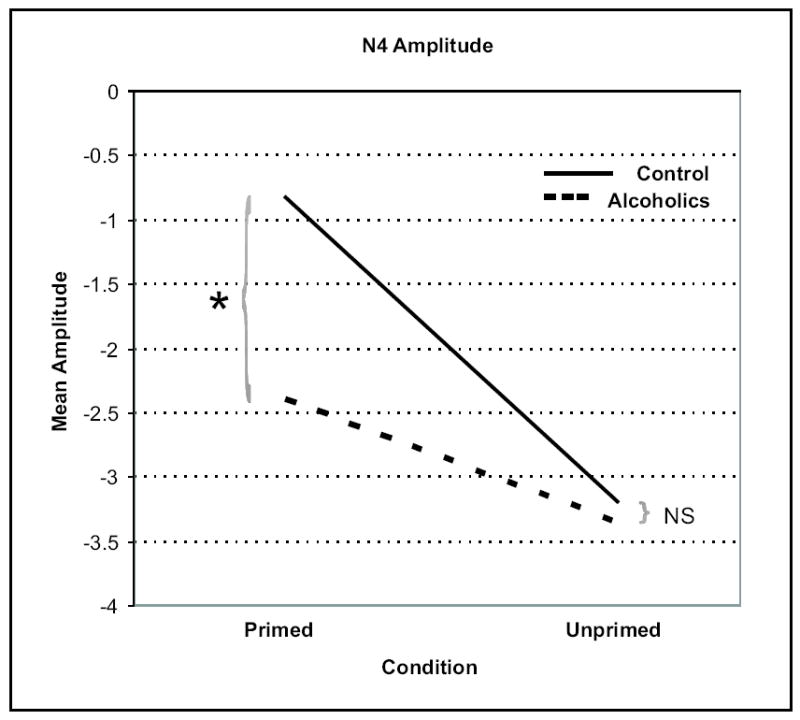
Grand average waveforms for alcoholics and controls for the primed and unprimed condition.
Figure 3.
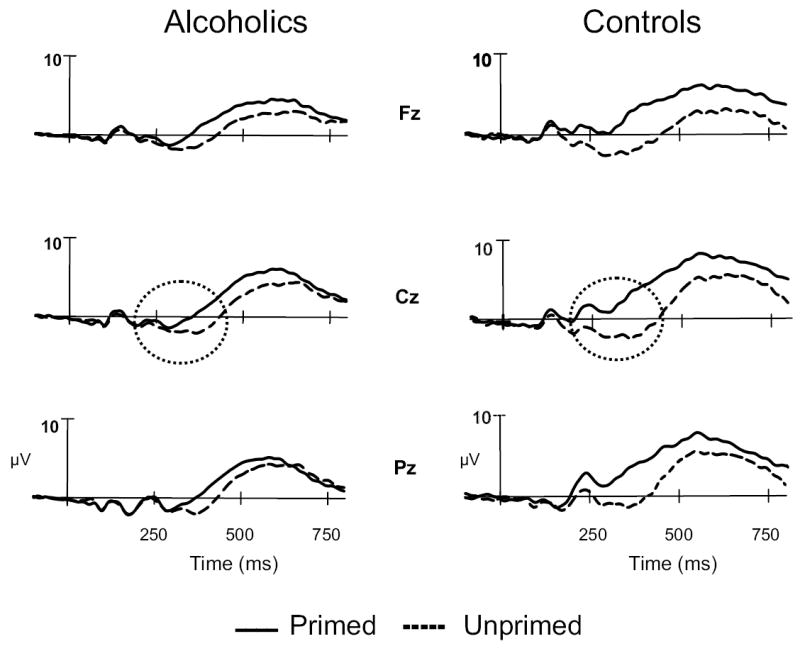
ERP waveform in primed and unprimed condition illustrating the N400 component for alcoholics and controls
Figure 4.
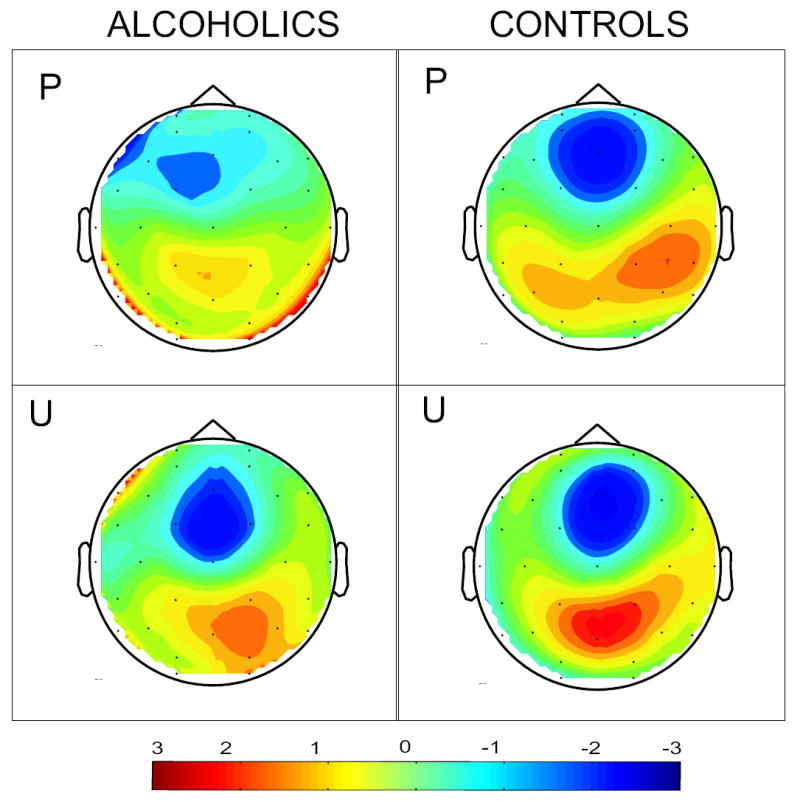
CSD (current source density) map in alcoholics and controls describing the source (red) and sink (blue) for primed (P) and unprimed (U) conditions from a fixed latency slice at 325 ms near the peak N400 response.
3.3. Latency Measures
Analysis of the latency values showed a significant main effect for the Condition (F = 32.679, p < .000); that is, as a whole, both groups had significantly more delayed latency for the unprimed word compared to the primed word condition. There were no other significant effects observed.
4. DISCUSSION
The results of the current study showed that the primed word was processed much faster than the unprimed word in all subjects, and the control subjects had significantly more time savings, as measured by RT difference between the two conditions, than the alcoholics; the controls also showed significant attenuation of N400 amplitude to the primed word, a correlate of the priming effect, which was not observed in alcoholics. While N400 amplitude was not significantly different between groups for the unprimed condition, the alcoholic subjects were significantly different from control subjects in producing consistently larger N400 amplitudes for primed words (table 2). These findings suggest that alcoholics are perhaps unaffected by priming cues when compared to controls in a semantic priming paradigm using a lexical decision task (Figure 2, 3 and 4). Similar lack of N400 attenuation for primed words in non-alcohol dependent (high risk) children of alcoholics, when compared to a normal control low-risk group, was recently reported from our laboratory (Roopesh et al., 2009).
The groups did not differ significantly for other measures of performance in both primed and unprimed conditions (table 1). In addition, no significant main or interaction effects for group and condition differences for the ERP latency measures were observed in this study. Similar results for latency were reported by Nixon et al. (2002) and Ceballos et al. (2005). On the other hand, another study by Ceballos et al. (2003) obtained a significant difference in latency between alcoholics and community controls. However, the paradigm used in that study was a classical N400 sentence paradigm, which is different from the one used in the present study, and the alcoholic subjects had co-morbid diagnosis of antisocial personality disorder. The lack of significant group differences in most performance and latency measures, as well as the lack of significant difference between groups in N400 amplitude for the unprimed word condition indicates that the observed deficit cannot be attributed to slowing of cognitive process, intelligence and/or general cognitive impairment in alcoholics (Grillon et al., 1991; Nixon et al., 2002), but rather a specific deficit in semantic priming processes.
In the lexical decision paradigm, the stimuli are presented sequentially and the subjects are instructed to respond by depressing a key, indicating that each stimulus is either a ‘word’ or a ‘non-word’. This paradigm is primarily said to measure automatic spreading activation, in other words the association strength (Hutchison, 2003), and to reduce post-lexical strategies on the semantic priming effect (Silva-Pereyra et al., 1999; Timothy et al., 1988). However, the current study had a long SOA of 1750 ms. The stimuli studied included antonym-pairs, which by their very nature had a high degree of relatedness, the proportion of related-pairs was thirty-three percent, and there were high rates of non-words (fifty-percent). This suggests that the N400 in the current study possibly reflects relatively more controlled mechanisms/post-lexical strategies and less automatic spreading activation. Within the controlled mechanism, the more active process would be ‘semantic expectancy’, where upon presentation of the first word of the antonym pair, subjects tend to generate the expectation for the second word of the pair. Therefore, the lack of N400 attenuation observed in alcoholics in the current study indicates that they fail to efficiently process the inherent semantic relatedness present in the antonym-pairs that is supported by a significantly lower reaction time reduction between unprimed and primed conditions in alcoholics when compared to controls. This shows the presence of deficits in semantic expectancy and post-lexical processes in adult alcoholics. Apart from semantic congruity and repetitions, N400 amplitude also varies inversely with factors such as contextual integration with the information currently held in working memory, as well as association strength of the semantic memory and the ease of accessing information from memory (Brown and Hagoort, 1993; Fischler et al., 1983; Hutchison, 2003; Kutas and Federmeier, 2000; Nobre and McCarthy, 1995; Penke et al., 1997). As a result, it can be hypothesized that alcoholics have deficits in contextual integration, and assessing information from working memory. In addition, these might also be due to poor association strength of the lexical network or semantic memory networks. This is substantiated by earlier work in our laboratory by Ji et al. (1999), which suggested that alcoholics are less efficient in the semantic mnemonic match/nonmatch processes.
Research findings from monkey studies have shown less firing in masses of nerve cells with response to repeated or primed stimuli, suggesting selective inhibition (Miller et al., 1991; Miller et al., 1993). This selective inhibition can be hypothesized to facilitate better processing for familiar stimuli, such as repeated and/or primed stimuli. If this selective inhibition is not observed, each incoming stimulus can be said to be processed as a new stimulus (Porjesz and Begleiter, 1995), thus implying deficits in cognitive inhibition. In the current study in contrast with controls, the N400 amplitude is not decreased for the primed stimulus in alcoholics, where each word can be assumed to be processed anew, which suggests that alcoholics show deficits in inhibition. Parallels can be drawn between semantic priming deficits and deficits in cognitive inhibition, as several studies have theorized that alcoholism falls into a spectrum of disinhibitory disorders (Cohen et al., 1997; Hada et al., 2000; Kamarajan et al., 2006; Kamarajan et al., 2005a; Pfefferbaum et al., 1991; Porjesz et al., 2005). It should be emphasized that most of the higher cognitive functions, including semantic memory or semantic working memory are dependent on basic cognitive functions, at least partially and thus we can also attribute the semantic priming deficits observed in this study to deficits in cognitive inhibition.
The scalp distributions of N400 have shown regional and hemispheric differences based on the stimuli, modality and paradigm used for the studies (Curran et al., 1993). It is generally found that the auditory N400 is distributed over the scalp more evenly compared to the visual N400, which shows more of a centroparietal and right hemisphere predominance (Domalski et al., 1991; Franklin et al., 2007; Hill et al., 2002; Kutas and Hillyard, 1982; Kutas and Van Petten, 1988). The word stimuli used in the current study are in the visual modality, and our CSD map (figure 5) reveals a centroparietal and right hemisphere predominant source for the control group only. In contrast, for the primed condition the posterior source is largely central and the sink shifts to the left in the alcoholic group, while the controls have a strong source that is predominantly towards the right hemisphere. The deficits perhaps involve hemispherically asymmetric processes.
Several electrophysiological studies have suggested that the deficits observed in alcoholics can be due to trait factors rather than alcohol related state factors (Kamarajan et al., 2005a; Kamarajan et al., 2005b; Porjesz and Begleiter, 1994; Porjesz and Begleiter, 1998). Furthermore, studies have reported that certain ERP components are highly heritable (Begleiter et al., 1998; Hesselbrock et al., 2001; Porjesz et al., 2002a; Porjesz et al., 2002b; van Beijsterveldt and van Baal, 2002) and may serve as endophenotypes for a predisposition to develop alcoholism (Frederick and Iacono, 2006; Kamarajan et al., 2005b; Porjesz et al., 2005). A large scale genome wide linkage study using the same semantic priming – lexical decision paradigm as in the current study showed significant heritability for N400 amplitude in response to primed and unprimed words, and the N400 component showed significant genetic correlations, indicating shared genetic effects (Almasy et al., 1999; Almasy et al., 2001). In addition, using the same paradigm, Roopesh et al. (2009) found that high risk offspring of alcoholics compared to low risk children showed a similar lack of N400 attenuation for primed words observed in the alcoholics in this study. Similarly, in another semantic priming study, alcoholic subjects with positive parental history compared to alcoholic subjects with negative parental history showed impairment for associated targets (Sayette et al., 2001). Among the several features required to meet criteria for an endophenotype (Gottesman and Shields, 1972, 1973; Gottesman and Gould, 2003) affected individuals (newly diagnosed, abstinent/chronic alcoholics) should manifest the trait, and the trait must be present in unaffected relatives of affected individuals with levels significantly higher than in random controls. Given our results of the lack of N400 attenuation for primed words and the deficits in discriminating between primed and unprimed words in both adult alcoholics and the offspring of alcoholics, it is suggested that N400 may have great utility as an electrophysiological endophenotype that characterizes genetic vulnerability to alcohol dependence. Future work examining the correlations between P3 amplitude changes and N400 attenuation would help to determine if similar or independent processes underlie the deficits observed in these two cognitive tasks.
CONCLUSION
The results of the study highlight a significant lack of N400 attenuation for primed words in alcohol dependent subjects compared to controls, although both groups respond similarly in the unprimed condition. This lack of attenuation in N400 amplitude in alcoholics possibly reflects an inability to engage similar neuronal substrates as those associated with semantic relatedness in the control subjects. While these differences in the brain responses to semantic relatedness between groups are reflected in one aspect of behavior (reaction time difference between conditions) in this simple antonym pair task, it is likely that in more demanding tasks the brain responses would be associated with stronger behavioral effects. These altered mechanisms in alcoholics lead to less efficient information processing as, in contrast to controls, they are unable to benefit from available information (e.g. prime stimulus) to facilitate and optimize information processing. This is supported by the significantly less reaction time reduction between the unprimed and primed condition, observed in alcoholics when compared to controls. Similar findings of reduced N400 attenuation during priming have also been reported in young non-alcoholic offspring of alcoholics (Roopesh et al., 2009), and hence they may represent an electrophysiological endophenotype that characterizes a genetic vulnerability to develop alcoholism and related disorders. Studies are underway as part of the Collaborative Study on the Genetics of Alcoholism (COGA) to determine possible underlying genes involved in these electrophysiological features.
Acknowledgments
We wish to acknowledge with great admiration the founder of the laboratory and our mentor Dr. Henri Begleiter, whose seminal work and scientific spirit inspires us towards excellence. This study was supported by the National Institutes of Health (NIH) Grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) R01AA02686 and R37AA005524. We are grateful for the valuable technical assistance of Arthur Stimus, Evgenia Rosenberg, Carlene Haynes, Joyce Alonzia, Edward Babington, Aquanette Sass, Tracy Crippen and Dr. Mohini Ranganathan for work in defining the clinical sample for the present study.
References
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O’Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Goate A, Edenberg HJ, Chorlian DB, Kuperman S, O’Connor SJ, Rohrbaugh J, Bauer LO, Foroud T, Rice JP, Reich T, Begleiter H. Genetics of event-related brain potentials in response to a semantic priming paradigm in families with a history of alcoholism. Am J Hum Genet. 2001;68:128–135. doi: 10.1086/316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, Van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li TK, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J, Jr, Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Bentin S. Electrophysiological studies of visual word perception, lexical organization, and semantic processing: a tutorial review. Lang Speech. 1989;32:205–220. doi: 10.1177/002383098903200302. [DOI] [PubMed] [Google Scholar]
- Bentin S, Kutas M, Hillyard SA. Electrophysiological evidence for task effects on semantic priming in auditory word processing. Psychophysiology. 1993;30:161–169. doi: 10.1111/j.1469-8986.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Brown C, Hagoort P. The processing nature of the N400: evidence from masked priming. J Cogn Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Bauer LO, Houston RJ. Recent EEG and ERP findings in substance abusers. Clin EEG Neurosci. 2009;40:122–128. doi: 10.1177/155005940904000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos NA, Houston RJ, Smith ND, Bauer LO, Taylor RE. N400 as an index of semantic expectancies: differential effects of alcohol and cocaine dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:936–943. doi: 10.1016/j.pnpbp.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Nixon SJ, Phillips JA, Tivis R. Semantic processing in alcoholics with and without antisocial symptomatology. J Stud Alcohol. 2003;64:286–291. doi: 10.15288/jsa.2003.64.286. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Hagoort P, Brown CM. The mechanism underlying backward priming in a lexical decision task: Spreading activation versus semantic matching. The Quarterly Journal of Experimental Psychology. 1998;51A:531–560. [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997;21:1398–1406. [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–428. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Curran T, Tucker DM, Kutas M, Posner MI. Topography of the N400: brain electrical activity reflecting semantic expectancy. Electroencephalogr Clin Neurophysiol. 1993;88:188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- De Groot AMB, Thomassen AJWM, Hudson PTW. Primed lexical decision: The effect of varying the stimulus-onset asynchrony of prime and target. Acta Psychologica. 1986;61:17–36. [Google Scholar]
- Deacon D, Grose-Fifer J, Yang CM, Stanick V, Hewitt S, Dynowska A. Evidence for a new conceptualization of semantic representation in the left and right cerebral hemispheres. Cortex. 2004;40:467–478. doi: 10.1016/s0010-9452(08)70140-0. [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang C, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: evidence that the N400 does not reflect a post-lexical process. Brain Res Cogn Brain Res. 2000;9:137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Deacon D, Uhm TJ, Ritter W, Hewitt S, Dynowska A. The lifetime of automatic semantic priming effects may exceed two seconds. Brain Res Cogn Brain Res. 1999;7:465–472. doi: 10.1016/s0926-6410(98)00034-2. [DOI] [PubMed] [Google Scholar]
- den Heyer K, Briand K, Dannenbring GL. Strategic factors in a lexical-decision task: evidence for automatic and attention-driven processes. Mem Cognit. 1983;11:374–381. doi: 10.3758/bf03202452. [DOI] [PubMed] [Google Scholar]
- Domalski P, Smith ME, Halgren E. Cross-modal repetition effects on the N4. Psychol Sci. 1991;2:173–178. [Google Scholar]
- Federmeier KD, Kutas M. Right words and left words: electrophysiological evidence for hemispheric differences in meaning processing. Brain Res Cogn Brain Res. 1999;8:373–392. doi: 10.1016/s0926-6410(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M. Picture the difference: electrophysiological investigations of picture processing in the two cerebral hemispheres. Neuropsychologia. 2002;40:730–747. doi: 10.1016/s0028-3932(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler I, Bloom PA, Childers DG, Roucos SE, Perry NW., Jr Brain potentials related to stages of sentence verification. Psychophysiology. 1983;20:400–409. doi: 10.1111/j.1469-8986.1983.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Forbes CT. Graded and Classified Spelling Lists for Teachers Grades 2-8. Educators Publishing Service; Cambridge, MA: 1968. [Google Scholar]
- Franklin MS, Dien J, Neely JH, Huber E, Waterson LD. Semantic priming modulates the N400, N300, and N400RP. Clin Neurophysiol. 2007;118:1053–1068. doi: 10.1016/j.clinph.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Frederick JA, Iacono WG. Beyond the DSM: defining endophenotypes for genetic studies of substance abuse. Curr Psychiatry Rep. 2006;8:144–150. doi: 10.1007/s11920-006-0014-2. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kutas M, Sereno MI. The search for “common sense”: an electrophysiological study of the comprehension of words and pictures in reading. J Cogn Neurosci. 1996;8:89–106. doi: 10.1162/jocn.1996.8.2.89. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and Genetics: A Twin Study Vantage Point. Academic Press; New York: 1972. [Google Scholar]
- Grillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biol Psychiatry. 1991;29:467–480. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Friederici AD. Concerning the automaticity of syntactic processing. Psychophysiology. 1999;36:126–137. doi: 10.1017/s004857729997155x. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. J Physiol Paris. 1994a;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M. Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes [corrected] J Physiol Paris. 1994b;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Friedman D, Ritter W, Rosen J. Event-related potential and behavioral correlates of semantic processing in Alzheimer’s patients and normal controls. Brain Lang. 1995;48:33–68. doi: 10.1006/brln.1995.1002. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism--evidence from the collaborative study on the genetics of alcoholism. J Biomed Sci. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hill H, Strube M, Roesch-Ely D, Weisbrod M. Automatic vs. controlled processes in semantic priming--differentiation by event-related potentials. Int J Psychophysiol. 2002;44:197–218. doi: 10.1016/s0167-8760(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ. Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology. 1993;30:47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Kounios J, Anderson JE, West WC. Dual-coding, context-availability, and concreteness effects in sentence comprehension: an electrophysiological investigation. J Exp Psychol Learn Mem Cogn. 1999;25:721–742. doi: 10.1037//0278-7393.25.3.721. [DOI] [PubMed] [Google Scholar]
- Hutchison KA. Is semantic priming due to association strength or feature overlap? A microanalytic review. Psychon Bull Rev. 2003;10:785–813. doi: 10.3758/bf03196544. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. EEG Journal. 1958;10:371–375. [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H. Event-related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol Psychiatry. 1999;45:494–507. doi: 10.1016/s0006-3223(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005a;69:353–73. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005b;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. The N400 is modulated by unconsciously perceived masked words: further evidence for an automatic spreading activation account of N400 priming effects. Cognitive Brain Research. 2002;13:27–39. doi: 10.1016/s0926-6410(01)00085-4. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Spitzer M. Time course of conscious and unconscious semantic brain activation. Neuroreport. 2000;11:2401–2407. doi: 10.1097/00001756-200008030-00013. [DOI] [PubMed] [Google Scholar]
- Koivisto M. Categorical priming in the cerebral hemispheres: automatic in the left hemisphere, postlexical in the right hemisphere? Neuropsychologia. 1998;36:661–668. doi: 10.1016/s0028-3932(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. J Exp Psychol Learn Mem Cogn. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kucera, Francis WN. Computational Analysis of Present-Day American English. Brown University Press; Providence: 1967. [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. The lateral distribution of event-related potentials during sentence processing. Neuropsychologia. 1982;20:579–590. doi: 10.1016/0028-3932(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. An electrophysiological probe of incidental semantic association. Journal of Cognitive Neuroscience. 1989;1:38–49. doi: 10.1162/jocn.1989.1.1.38. [DOI] [PubMed] [Google Scholar]
- Kutas M, Lindamood T, Hillyard SA. Word expectancy and event-related brain potentials during sentence processing. In: Kornblum S, Requin J, editors. Preparatory states and processes. Lawrence Erlbaum; Hillsdale, NJ: 1984. pp. 217–238. [Google Scholar]
- Kutas M, Van Petten C. Event-related potential studies of language. In: Ackles PK, Jennings JR, Coles GH, editors. Advances in psychophysiology. JAI Press; Greenwich, CT: 1988. pp. 138–187. [Google Scholar]
- Maylor EA, Rabbitt PM, Kingstone A. Effects of alcohol on word categorization and recognition memory. Br J Psychol. 1987;78:233–239. doi: 10.1111/j.2044-8295.1987.tb02242.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC. Modulation of semantic processing by spatial selective attention. Electroencephalogr Clin Neurophysiol. 1993;88:210–219. doi: 10.1016/0168-5597(93)90005-a. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. J Neurosci. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J Exp Psychol. 1971;90:227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato Y, Ogura C. Abnormalities in event-related potentials: N100, N200 and P300 topography in alcoholics. Jpn J Psychiatry Neurol. 1993;47:853–862. doi: 10.1111/j.1440-1819.1993.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. Erlbaum; Hillsdale, NJ: 1991. [Google Scholar]
- Neely JH, Keefe DE. Semantic context effects on visual word processing: A hybrid prospective-retrospective processing theory. In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 24. Academic Press; New York: 1989. pp. 207–248. [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. J Neurosci. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. Alcohol-related ERP changes in cognition. Alcohol. 1987;4:289–292. doi: 10.1016/0741-8329(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Zola-Morgan SM. Comparative neuropsychology and Korsakoff’s syndrome. I.--Spatial and visual reversal learning. Neuropsychologia. 1980;18:499–512. doi: 10.1016/0028-3932(80)90152-9. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Zola-Morgan SM, Oberg RG, Bonner RT. Comparative neuropsychology and Korsakoff’s syndrome. III--Delayed response, delayed alternation and DRL performance. Neuropsychologia. 1982;20:187–202. doi: 10.1016/0028-3932(82)90009-4. [DOI] [PubMed] [Google Scholar]
- Penke M, Weyerts H, Gross M, Zander E, Munte TF, Clahsen H. How the brain processes complex words: an event-related potential study of German verb inflections. Brain Res Cogn Brain Res. 1997;6:37–52. doi: 10.1016/s0926-6410(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002a;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RD, Van Thiel D, editors. Alcohol and the Brain. Plenum Press; New York: 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butters N, Nathan P, editors. Neuropsychology of alcoholism Implications for diagnosis and treatment. Plenum Press; New York: 1987. pp. 45–63. [Google Scholar]
- Porjesz B, Begleiter H. Annual meeting of the Research Society on Alcoholism. Maui, Hawaii: 1994. Neurophysiology in individuals at high risk for alcoholism. [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials and cognitive function in alcoholism. Alcohol Health Res World. 1995;19:108–112. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. Vol. 2. Oxford University Press; 1996. [Google Scholar]
- Porjesz B, Begleiter H. Genetic basis of event-related potentials and their relationship to alcoholism and alcohol use. J Clin Neurophysiol. 1998;15:44–57. doi: 10.1097/00004691-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27:153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. The N2 component of the event-related brain potential in abstinent alcoholics. Electroencephalogr Clin Neurophysiol. 1987;66:121–131. doi: 10.1016/0013-4694(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O’Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002b;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcohol Clin Exp Res. 2001;25:531–539. [PubMed] [Google Scholar]
- Realmuto G, Begleiter H, Odencrantz J, Porjesz B. Event-related potential evidence of dysfunction in automatic processing in abstinent alcoholics. Biol Psychiatry. 1993;33:594–601. doi: 10.1016/0006-3223(93)90097-w. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Corral M, Cadaveira F. Visual and auditory event-related potentials in young children of alcoholics from high- and low-density families. Alcohol Clin Exp Res. 1998;22:87–96. doi: 10.1111/j.1530-0277.1998.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcohol Clin Exp Res. 1999a;23:582–591. [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biol Psychiatry. 1999b;46:281–291. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Stimus A, Bauer LO, Rohrbaugh J, O’Connor SJ, Kuperman S, Schuckit M, Porjesz B. Priming Deficiency in Male Subjects at Risk for Alcoholism: The N4 During a Lexical Decision Task. Alcohol Clin Exp Res. 2009;33:2027–2036. doi: 10.1111/j.1530-0277.2009.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Perrott MA, Wertz JM. Parental alcoholism and the effects of alcohol on mediated semantic priming. Exp Clin Psychopharmacol. 2001;9:409–417. [PubMed] [Google Scholar]
- Silva-Pereyra J, Harmony T, Villanueva G, Fernandez T, Rodriguez M, Galan L, Diaz-Comas L, Bernal J, Fernandez-Bouzas A, Marosi E, Reyes A. N400 and lexical decisions: automatic or controlled processing? Clin Neurophysiol. 1999;110:813–824. doi: 10.1016/s1388-2457(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Ryan CM. Neuropsychology of alcoholism. Etiology, phenomenology, process, and outcome. Recent Dev Alcohol. 1983;1:449–469. [PubMed] [Google Scholar]
- Taylor WL. ‘Cloze procedure’: a new tool for measuring readability. Journalism Quarterly. 1953;30:415–433. [Google Scholar]
- Timothy P, McNamara T, Altarriba J. Depth of spreading activation revisited: Semantic mediated priming occurs in lexical decisions. Journal of Memory and Language. 1988;27:545–559. [Google Scholar]
- Toglia MP, Battig WF, Barrow K, Cartwright DS, Posnansky CJ, Pellegrino JW, Moore TJ, Camilli GA. Handbook of Semantic Word Norms. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH. Effect of alcohol on recall and recognition as functions of processing levels. J Stud Alcohol. 1984;45:10–15. doi: 10.15288/jsa.1984.45.10. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Cohen HL, Porjesz B, Begleiter H. Mismatch negativity in subjects at high risk for alcoholism. Alcohol Clin Exp Res. 2001;25:330–337. doi: 10.1097/00000374-200103000-00003. [DOI] [PubMed] [Google Scholar]


