Abstract
A new manzamine-related alkaloid with unprecedented δ-lactone and ε-lactam rings called acantholactone (2), was isolated from the Indonesian sponge Acanthostrongylophora sp. The relative configuration of the two new ring systems was established through detailed analysis of NOESY correlations combined with molecular modeling studies. The absolute configuration of 2 was determined as 12S, 24R, 25R, 26R by comparing the computed electronic circular dichroism (ECD) spectra with experimental values.
Keywords: Manzamine alkaloids
The manzamine alkaloids have proven to be a source of promising biological activities as well as fascinating and novel chemical structures.1 Since the discovery of manzamine A (1),2 over 80 related structures have been reported3 including the novel structures: dimer kauluamine,4 dimer neo-kauluamine,5 manadomanzamines A and B,6 and zamamidines A and B.7
As a part of our lead optimization of 1 against malaria and other targets, a large scale purification method that enables the isolation of pure 1 and related alkaloids in gram quantities1a from the Indonesian sponge Acanthostrongylophora sp. was developed. We investigated the polar fractions and isolated the new manzamine acantholactone. Herein, we report the isolation and structure elucidation of 2.
The sponge, Acanthostrongylophora sp., was collected from Indonesia and successively extracted with acetone. The crude acetone extract was subjected first to an acid–base1a process to obtain the total alkaloids content. Silica gel fractionation and purification of the more polar fractions by successive RP-HPLC resulted in the isolation of the new manzamine acantholactone (2) as well as the known manzamines manzamine A (1),2 8-hydroxymanzamine A,8 manzamine F,7 12,34-oxamanzamine E,9 and 31-keto-12,34-oxa-32,33-dihydroircinal A.8
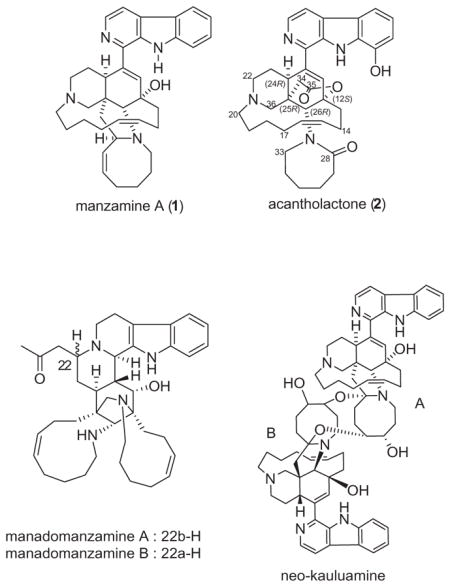
Acantholactone (2)10 was obtained as a pale yellow powder from DCM, and it was revealed to have a molecular formula of C36H43N4O4 by HRESIMS (m/z 595.3416 (M+H)+) combined with 1H and 13C NMR data (Table 1). The 1H NMR resonances at δ 8.28 (1H, d, J = 5.2), 8.04 (1H, d, J = 5.2 Hz), 7.73 (1H, d, J = 7.5 Hz), 7.17 (1H, t, J = 8.0 Hz), and 7.09 (1H, d, J = 7.2 Hz) combined with 13C NMR resonances at 136.9, 121.7, 116.5, 113.7, and 113.4 confirmed the presence of an 1,8-disubstituted β-carboline moiety with a hydroxy group at C-8 (144.4). HMBC correlation of H-11 (δH 6.09, δC 121.4) to C-12 (83.6), C-26 (70.9), C-10 (140.2), and C-24 (44.0) confirmed that the β-carboline moiety is coupled with the typical cyclohexene ring found in many manzamine alkaloids. The presence of the piperidine ring was confirmed by the HMBC correlations of H-26 (δH 3.86, δC 70.9) to C-36 (70.7), C-25 (46.8), and C-24 (44.0). The 13C NMR resonance at 56.6 (δH 4.69, 3.97) was assigned to C-20 which showed HMBC correlations with the other two α-carbons of N-21 (C-22, 61.0; C-36, 70.7) in the piperidine ring. The presence of the 13-membered ring in the molecule was confirmed by the 1H–1H-COSY and HMBC correlations of the two olefinic 1H NMR resonances, H-15 (δH 5.47, δC 131.4) and H-16 (δH 5.50, δC 131.8). The unprecedented δ-lactone ring was established based on the HMBC correlations of H-34 (δC 36.4) methylene resonances at δ 3.04 and 2.90 to C-24 (δH 4.07, 44.0), C-25 (46.8), and C-35 (ester carbonyl) (173.9). The downfield chemical shifts of C-12 (83.6), C-25 (46.8), C-36 (70.7), and C-26 (70.9) with the HMBC correlations confirmed the δ-lactone ring to be connected between C-12 and C-26. The 13C NMR spectra showed an additional carbonyl group which was shown to be the amidic carbonyl at C-28 of the ε-lactam ring based on the HMBC correlations of H-26 (δH 3.86, δC 70.9), H-33 (δH 3.80, 3.58; δC 55.8), and H-29 (δH 1.92, 1.67; δC 29.3) to the C-28 carbonyl (179.6). The relative configuration of acantholactone (2) was deduced from the detailed analysis of the NOESY spectrum (Table 1, 2Fig. 1). The chair-boat conformation of the piperidine and the cyclohexene rings was assigned based on the NOESY correlations of H-24, H-26, and H-22. The new δ-lactone ring was assigned as α-orientation relative to the cyclohexene ring based on the NOESY correlations of H-24/H-36. The NOESY correlation between H-20 and H-33 suggested the α-conformation of the ε-lactam ring as seen in the 3D structure of the most stable conformer of (Fig. 2).
Table 1.
1H, 13C NMR, and 2D data of 2 (δ in ppm, J in Hz)a
| Position | Acantholactone (2)b
|
||||
|---|---|---|---|---|---|
| 13C NMR | 1H NMR | HMBC | COSY | NOESY | |
| 1 | 147.5 s | – | – | – | |
| 3 | 136.9 d | 8.28 d, 5.2 | 3, 4a, 10 | 4 | 4, 6, 7, 11, 15, 16 |
| 4 | 116.5 d | 8.04 d, 5.2 | 3, 4a | 3 | 3, 6, 7, 11 |
| 4a | 131.2 s | – | – | – | |
| 4b | 131.0 s | – | – | – | |
| 5 | 113.4 d | 7.73 d, 7.5 | 4b, 6, 7 | 6 | 6, 7, 11 |
| 6 | 121.7 d | 7.17 t, 8.0 | 8a, 8b | 5 | 5, 11, 15, 16 |
| 7 | 113.7 d | 7.09 d, 7.2 | 5, 8, 9 | 6 | 5, 11, 15, 16 |
| 8 | 144.4 s | – | – | – | |
| 8a | 121.9 s | – | – | – | |
| 9b | 132.2 s | – | – | – | |
| 10 | 140.2s | – | – | – | |
| 11 | 121.4 d | 6.09 s | 10, 12, 24, 26 | – | 3, 4, 5, 6, 7, 24, 26 |
| 12 | 83.6 s | – | – | ||
| 13 | 39.1 t | 2.94 m, 2.57 m | 26 | 14 | |
| 14 | 28.7 t | 1.91 m, 1.81 m | 13, 15 | ||
| 15 | 131.4 d | 5.47 dd, 9.5. 4.8 | 14, 16 | 14, 16 | 26, 24, 17, 16 |
| 16 | 131.8 d | 5.50 m | 15, 17 | 26, 24, 17, 15 | |
| 17 | 25.5 t | 1.77 m, 1.65 m | 16 | 16, 17a | 16, 15 |
| 18 | 27.5 t | 2.47 m, 2.26 m | |||
| 19 | 23.0 t | 1.91 m, 1.74 m | |||
| 20 | 56.6 t | 4.69 d, 14, 3.97 m | 19, 18 | 20a, 19 | |
| 21 | - | – | – | – | |
| 22 | 61.0 t | 4.55 m, 3.57 m | 36, 23, 20 | 23 | |
| 23 | 30.5 t | 2.17 m, 1.51 m | 24 | 22, 24 | |
| 24 | 44.0 d | 4.07 d, 4.1 | 12, 22, 25, 26 | 23 | 34, 16, 15, 11 |
| 25 | 46.8 s | – | – | – | |
| 26 | 70.9 d | 3.86 s | 12, 13, 24, 33, 36 | – | 22 |
| 28 | 179.6 s | – | – | – | |
| 29 | 29.3 t | 1.92 m, 1.67 m | 28 | ||
| 30 | 24.0 t | 1.87 m, 1.64 m | |||
| 31 | 26.1 t | 1.76 m, 1.62 m | |||
| 32 | 23.6 t | 1.91 m, 1.74 m | |||
| 33 | 55.8 t | 3.80 d,12, 3.58 m | 26, 28, 32 | 32 | |
| 34 | 36.4 t | 3.04 d, 18, 2.90 d, 18 | 24, 26, 35 | – | 26, 24 |
| 35 | 173.9 s | – | – | – | |
| 36 | 70.7 t | 3.77 m, 3.49 m | 12, 22, 25, 26,34 | 36 | |
400 MHz for 1H and 100 MHz for 13C NMR. Carbon multiplicities were determined by DEPT135 experiments. s = C, d = CH, t = CH2.
NMR obtained in d6-acetone.
Figure 1.
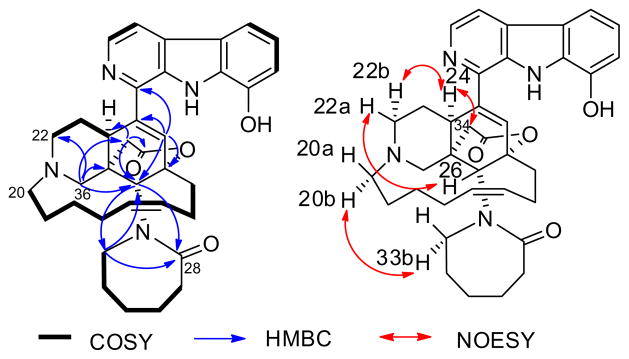
Selected 2D correlations of acantholactone (2).
Figure 2.
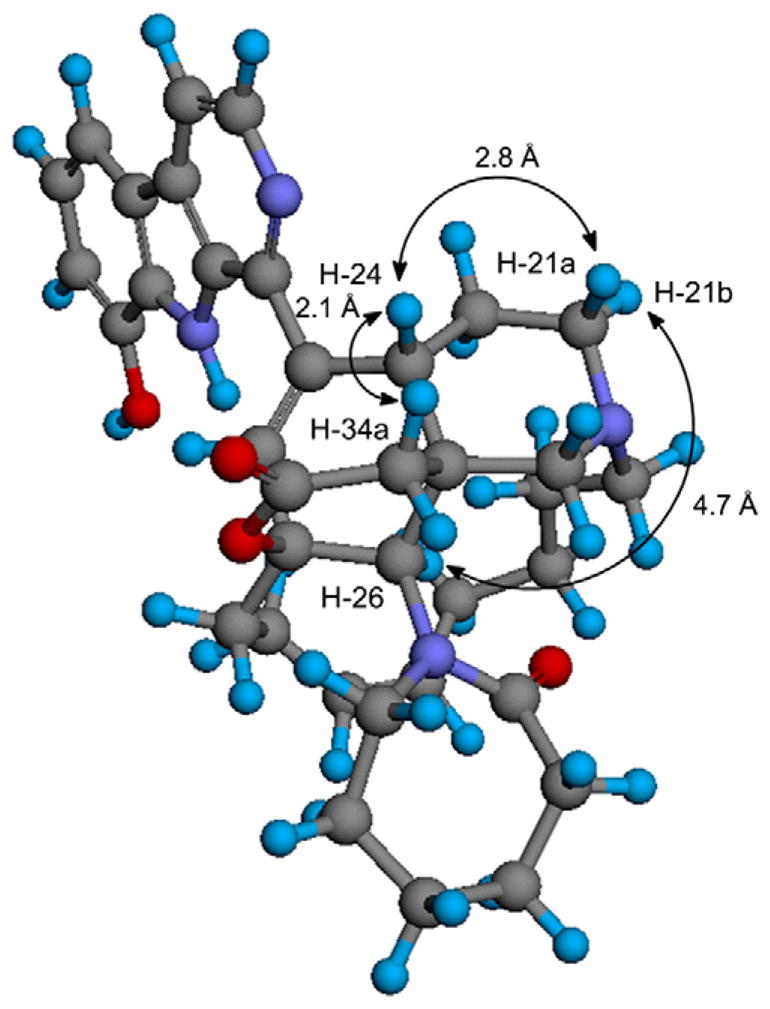
3D structure of the most stable conformer of acantholactone (2) in acetonitrile with the key NOESY correlations and the distance between the hydrogen atoms.
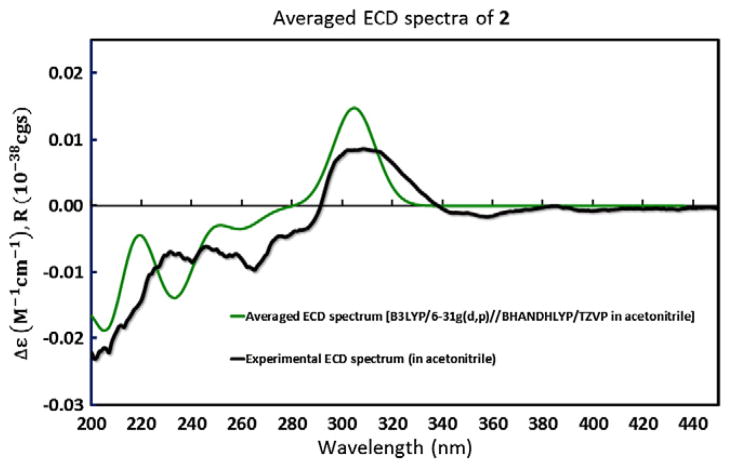
The absolute configuration of acantholactone (2) was established by comparing the computed electronic circular dichroism (ECD) spectra with experiment (Fig. 3). As a result of conformational analysis, six low-energy conformations were generated using the Merck Molecular Force Field (MMFF) calculations followed by the PM6 semi-empirical optimizations. The optimized structures were then used for the hybrid density-functional theory (DFT) calculation at the B3LYP/6-31G(d,p) level for the gas phase and for acetonitrile (using the Polarizable Continuum Model (PCM)).11 The DFT optimized structures were subsequently used for the TDDFT excited-state calculations at the BHANDHLYP/TZVP level for the gas phase and for acetonitrile (PCM). The obtained rotational strengths were Boltzmann averaged and fitted to Gaussian functions with the calculated excitation energies to simulate ECD curves of 2 which were then overlaid with the experimental ECD spectrum for comparison. Based on the relative configuration elucidated by the NMR experiments, the absolute configuration of acantholactone (2) was thus assigned as 12S, 24R, 25R, 26R.
Figure 3.
Computed ECD curve (in acetonitrile) overlaid with the experimental ECD spectrum of 2.
Acantholactone (2) is the first manzamine-related alkaloid with a unique ε-lactam as well as δ-lactone rings. The fact that the manzamine family showed a diverse range of biological activities makes acantholactone a good candidate for evaluation against malaria, neuroprotection, and TB. However, the low isolated yield of 2 from the sponge extract limited its biological evaluation. This will encourage the synthetic community to develop efficient synthetic routes that will enable the biological evaluation of 2.
Supplementary Material
Acknowledgments
This work was funded by NIH grants NCRR P20 RR021929 (Center of Research Excellence in Natural Products Neuroscience); NIAID 5RO1AI1036596; and an NIH research facilities improvement grant C06 RR-14503-01. A.E.W. gratefully thanks The Ministry of Higher Education of Egypt for a predoctoral fellowship. We thank the Mississippi Center for Supercomputing Research for the computation time.
Footnotes
Supplementary data (copies of the 1D and 2D spectra of acantholactone and experimental details of the isolation and the computational part)associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2012.08.140.
References and notes
- 1.For reviews on manzamine alkaloids: Peng J, Rao KV, Choo YM, Hamann MT. Manzamine Alkaloids. In: Fattorusso E, Taglialatela-Scafati O, editors. Modern Alkaloids. Wiley-VCH, GmbH, KgaA; Weinheim, Germany: 2007. pp. 189–232.Hamann MT. Curr Pharm Des. 2007;13:653–660. doi: 10.2174/138161207780162818.
- 2.Sakai R, Higa T, Jefford CW, Bernardinelli G. J Am Chem Soc. 1986;108:6404–6405. [Google Scholar]
- 3.Hu J, Hamann MT, Hill RT, Kelly M. Alkaloids. Vol. 6. Elseiver Science; San Diego, USA: 2003. pp. 207–285. [Google Scholar]
- 4.Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, Scheuer PJ. J Am Chem Soc. 1995;117:10743–10744. [Google Scholar]
- 5.El-Sayed KA, Kelly M, Kara UAK, Ang KKH, Katsuyama I, Dunbar DC, Khan AA, Hamann MT. J Am Chem Soc. 2001;123:1804–1808. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- 6.Peng J, Hu J, Kazi AB, Li Z, Avery M, Peraud O, Hill RT, Franzblau SG, Zhang F, Schinazi RF, Wirtz SS, Tharnish P, Kelly M, Wahyuono S, Hamann MT. J Am Chem Soc. 2003;125:13382–13386. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi Y, Kubota T, Fromont J, Kobayashi J. Org Lett. 2009;11:21–24. doi: 10.1021/ol802251q. [DOI] [PubMed] [Google Scholar]
- 8.Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. J Nat Prod. 2004;67:1314–1318. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao KV, Donia MS, Peng J, Garcia-Palomero E, Alonso D, Martinez A, Medina M, Franzblau SG, Tekwani BL, Khan SI, Wahyuono S, Willett KL, Hamann MT. J Nat Prod. 2006;69:1034–1040. doi: 10.1021/np0601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acantholactone (2) 4 mg; yellow powder (DCM); 31.6 (c 0.045, MeOH); UV (MeOH) λmax 352, 292, 255, 234 nm; CD (CH3CN) λmax 306 nm (Δε+0.0084), 274 nm (Δε−0.0047), 244 nm (Δε−0.0065), 230 nm (Δε−0.0075); NMR data, see Table 1; HRESIMS m/z 595.3416 (calcd for C36H43N4O4, (M+H)+, 595.3498).
- 11.(a) Becke AD. J Chem Phys. 1993;98:1372–1377. [Google Scholar]; (b) Schaefer A, Horn H, Ahlrichs R. J Chem Phys. 1992;97:2571–2577. [Google Scholar]; (c) Schaefer A, Huber C, Ahlrichs R. J Chem Phys. 1994;100:5829–5835. [Google Scholar]; (d) Wang J, Zhai WZ, Zou Y, Zhu JJ, Xiong J, Zhao Y, Yang GX, Fan H, Hamann MT, Xia G, Hu JF. Tetrahedron Lett. 2012;53:2654. doi: 10.1016/j.tetlet.2012.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.