Abstract
Aquaporins (AQPs) are membrane water channels that are involved in a diverse set of functions in mammalian physiology including epithelial fluid transport, brain water balance, cell migration, cell proliferation, neuroexcitation, fat metabolism, epidermal hydration, and others. Phenotype analysis of knockout mice has demonstrated an important role for AQPs in transepithelial fluid transport in kidney tubules, salivary and airway submucosal glands, choroid plexus and ciliary epithelium. The physiological functions of these epithelia, such as absorption of glomerular filtrate by proximal tubule and secretion of saliva by salivary gland, involve rapid transcellular water transport across epithelial cell barriers. Studies in knockout mice have also provided evidence that AQPs are not physiologically important in some epithelia where they are expressed, including lacrimal gland, sweat gland, gallbladder, alveoli and airways. Rates of transepithelial fluid transport per unit membrane surface area in these epithelia are substantially lower than transepithelial fluid transport rates in proximal tubule and salivary gland. Pharmacological inhibition of AQP water permeability in epithelia, with consequent reduced fluid transport, offers potential therapy for human diseases involving water imbalance such as congestive heart failure, hypertension and glaucoma.
Keywords: water channel, water transport, kidney, salivary gland, epithelium
INTRODUCTION
The aquaporins (AQPs) are a family of water transporting proteins, some of which (the ‘aquaglyceroporins’) also transport glycerol. AQPs are expressed at plasma membranes in epithelial, endothelial and other cell types. Studies from knockout mice have identified several unanticipated roles of AQPs in mammalian physiology (1). An expected role of AQPs is in transepithelial fluid transport, which is the focus of this review. AQP-facilitated water transport is also involved in water movement into and out of brain, in cell migration, in neuroexcitation, and in sensory signal transduction (2, 3). Aquaglyceroporin-facilitated glycerol transport is involved in cell proliferation, epidermal hydration and adipocyte metabolism (4–6). The involvement of AQPs in cell migration and proliferation is of particular interest in cancer biology, as the strong expression of AQPs in some high-grade tumors may be related to enhanced invasiveness, metastatic potential and growth (7). Clinically, though the best described human disease of AQP mutation is the rare genetic disorder nephrogenic diabetes insipidus (8, 9), there is increasing interest in the role of AQP polymorphisms in human disease, in AQP-based diagnostics, and in AQP-targeted therapeutics (10).
AQPs AND TRANSEPITHELIAL FLUID TRANSPORT IN KIDNEY TUBULES
AQP-facilitated transepithelial water transport in kidney tubules is necessary for the formation of a concentrated urine. AQP1 is expressed in apical and basolateral plasma membranes in epithelial cells in proximal tubule and thin descending limb of Henle (TDLH), and in the microvascular endothelial cells in outer medullary descending vasa recta (OMDVR) (Fig. 1A). AQP2 in collecting duct principal cells undergoes vasopressin-regulated trafficking between an intracellular vesicular compartment and the cell apical plasma membrane. AQP3 and AQP4 are co-expressed at the basolateral membrane of collecting duct epithelial cells, with AQP3 prominently in proximal segments of collecting duct and AQP4 in inner medullary collecting duct. AQP1 and AQP3 null mice are remarkably polyuric, consuming 2–3 (AQP1) to 7–10 (AQP3) times more fluid than wildtype mice, whereas the AQP4 null mice are not polyuric (11–13) (Fig. 1B). Urinary osmolality is reduced in AQP1 and AQP3 null mice (Fig. 1C).
Fig. 1.
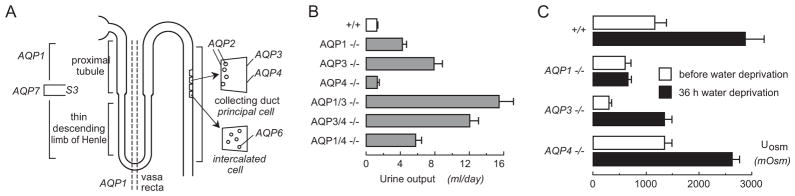
AQPs facilitate transepithelial fluid transport in kidney tubules. A. Location of AQPs in kidney tubules. B. Daily urine output of mice of indicated genotypes given free access to food and water. C. Urine osmolality before and after a 36-hour water deprivation in mice of indicated genotypes. Data from refs. 11–13.
The urinary concentrating defect in AQP1 null mice represents a combination of defective proximal tubule fluid absorption and defective counter-current multiplication, producing a relatively hypoosmolar medullary interstitium. Transepithelial osmotic water permeability (Pf) in microperfused proximal tubule is ~5-fold lower in AQP1 knockout mice than wildtype mice (14), indicating that the major pathway for osmotically-driven water transport in proximal tubule is transcellular and mediated by AQP1. Free-flow renal micropuncture showed an approximately 2-fold reduction in isosmolar fluid absorption (14), supporting a model in which mild luminal hypotonicity drives osmotic water movement through highly water permeable cell membranes. Supporting this interpretation is the marked decrease in luminal fluid osmolality in end proximal tubule fluid in AQP1 knockout mice (15), indicating active pumping of salt out of the tubule lumen without adequate water movement to dissipate the transepithelial osmotic gradient.
Defective countercurrent multiplication also contributes to the defective urinary concentrating ability in AQP1 null mice. The inability of vasopressin to increase urine osmolality (11) in water-deprived AQP1 null mice indicates a relatively hypoosmolar medullary interstitium. The TDLH and OMDVR are required to generate a hypertonic medullary interstitium. AQP1 deletion results in an ~10-fold decreased transepithelial Pf in microperfused segments of TDLH (16) and a large reduction in Pf in microperfused OMDVR (17). Collecting duct epithelial cells express AQP2, AQP3 and AQP4. The urinary concentrating defect produced by deletion of these AQPs is related to impaired osmotic equilibration of luminal fluid in collecting duct because of reduced transepithelial osmotic water permeability. AQP-facilitated transepithelial fluid transport in kidney tubules is thus crucial to the urinary concentrating mechanism.
AQPs IN TRANSEPITHELIAL FLUID SECRETION
We have investigated the involvement of AQPs in fluid secretion by glands (salivary, submucosal, sweat, lacrimal, choroid plexus and ciliary epithelium using knockout mice. The general conclusion is that AQPs facilitate active fluid secretion when sufficiently rapid, in which case AQP deletion in general reduces the volume and increases the ion/solute content of secreted fluid. AQPs appear not to be needed when fluid secretion rate (per unit epithelial surface area) is low, as AQP-independent water permeability is high enough to support slow fluid secretion (or absorption). Studies of slow transepithelial fluid transport in lung alveoli and airways support this conclusion.
AQP5 deletion reduces fluid secretion by salivary glands
In salivary gland the interstitial-to-luminal transport of sodium and chloride across the acinar epithelium is the driving force for osmotic water flow. The salivary duct epithelium, where sodium and chloride are absorbed and potassium and bicarbonate are secreted, is relatively water-impermeable so as to produce a hypotonic saliva. Upon stimulation the salivary gland can secrete saliva at high rates (up to 50 ml/min/100 g tissue in humans), which relies on rapid water movement from serosa-to-mucosa across the capillary endothelium and acinar cells. AQP1 is expressed in microvascular endothelial cells of salivary gland, AQP5 in apical membrane of acinar cells, and possibly AQP8 in the basolateral membrane. We found that pilocarpine-stimulated saliva production was reduced by more than 60% in AQP5 null mice, producing a viscous secretion (18) (Fig. 2A). Compared with the saliva from wildtype mice, the saliva from AQP5 null mice was hypertonic and hypernatremic (Fig. 2B). Amylase and protein secretion, functions of salivary mucous cells, were not affected by AQP5 deletion. Saliva secretion was not impaired in AQP1, AQP4 of AQP8 knockout mice (18, 19). Similar data were subsequently reported for AQP5 null mice, with the expected redued osmotic water permeability in acinar cells (20).
Fig. 2.
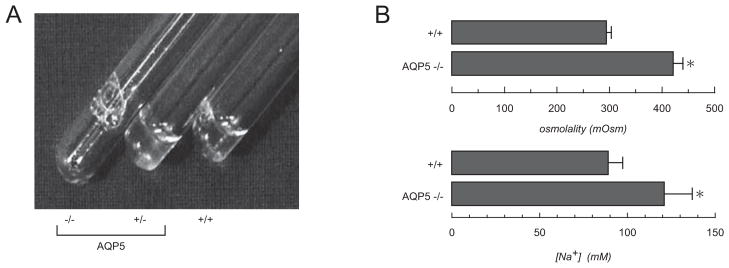
Impaired secretion of saliva in AQP5 knocout mice. A. Saliva collected over 5 min from mice of indicated genotype in response to pilocarpine simulation. B. Mean (±SE) osmolality and sodium concentration of saliva collected from wildtype and AQP5 null mice. From ref. 18.
AQP5 deletion reduces fluid secretion by airway submucosal glands
Submucosal glands in mammalian airways secrete a mixture of water, ions and macromolecules onto the airway surface. Glandular secretions are important in establishing airway surface liquid (ASL) composition and volume, and in antimicrobial defense mechanisms. Submucosal glands contain serous tubules, where active salt secretion into the gland lumen creates a small osmotic gradient driving water transport across a water permeable epithelium, as well as mucous cells and tubules, where viscous glycoproteins are secreted. AQP5 is expressed at the luminal membrane of serous epithelial cells. We found that pilocarpine-stimulated fluid secretion by submucosal glands was reduced by >2-fold in AQP5 null mice compared to wildtype mice (21). Analysis of secreted fluid showed a >2-fold increase in total protein in AQP5 null mice, suggesting intact protein secretion across a relatively water impermeable epithelial barrier. Submucosal gland morphology and density did not differ significantly in wild type vs. AQP5 null mice. Therefore, as in salivary gland, the luminal membrane of submucosal gland epithelial cells is the rate-limiting barrier to water movement.
AQP1-dependent cerebrospinal fluid (CSF) secretion by choroid plexus and aqueous fluid secretion by ciliary epithelium
AQP1 is expressed strongly at the ventricular-facing surface of choroid plexus epithelium. To investigate its role in CSF secretion water permeability was measured in isolated choroid plexus of wildtype vs. AQP1 null mice, as well as intracranial pressure (ICP) and the kinetics CSF secretion and drainage (22). Osmotically induced water transport was rapid in choroid plexus from wild-type mice and reduced by 5-fold by AQP1 deletion, confirming AQP1 is the major water pathway in choroid plexus. AQP1 deletion did not affect choroid plexus size or structure. By stereotaxic puncture of the lateral ventricle with a microneedle, ICP was 9.5 cmH2O in wildtype mice and 4.2 cmH2O in AQP1 null mice. CSF production, as measured by a dye dilution method involving fluid collections using a second microneedle introduced into the cisterna magna, was 0.37 μL/min in wildtype mice, and reduced ~25% in AQP1 null mice. However, pressure-dependent CSF outflow, measured from steady-state ICP at different ventricular infusion rates, was not affected by AQP1 deletion. The potential clinical relevance of the reduced ICP in AQP1 null mice was demonstrated in a model of focal brain injury, where AQP1 null mice had remarkably reduced ICP and improved survival compared with wildtype mice. AQP1 thus plays a role in CSF secretion and ICP regulation.
In the eye, the principal determinants of intraocular pressure (IOP) include the rate of aqueous fluid secretion by the ciliary epithelium and the rate of fluid drainage (outflow) in the canal of Schlemm. Aqueous fluid secretion involves near-isosmolar fluid secretion driven by active salt transport across the ciliary epithelium. Aqueous fluid drainage involves pressure-driven bulk fluid flow in the canal of Schlemm as well as fluid movement through the sclera by seepage across the ciliary muscle and supraciliary space. AQP1 and AQP4 are expressed in non-pigmented ciliary epithelium, and AQP1 in trabecular meshwork endothelium. We found reduced IOP was found in mice lacking AQP1 and/or AQP4 compared to wildtype mice in both outbred (CD1) inbred (C57/bl6) and mouse genetic backgrounds (23, 24). Whether larger differences in IOP will be found with AQP deficiency in models of glaucoma remains to be determined. We also developed methods to measure aqueous fluid secretion and drainage rate in mice in order to resolve whether the reduced steady-state IOP in AQP deficiency is due to reduced aqueous fluid production and/or reduced outflow. Aqueous fluid outflow in wildtype and AQP1 deficient mice was ~0.35 μl/hr/mmHg. The lack of effect of AQP1 deletion on aqueous fluid outflow is consistent with a bulk fluid-flow mechanism that is predicted not to involve AQPs. Aqueous fluid secretion was measured from the disappearance, as measured by confocal microscopy, of fluorescein from the aqueous fluid space following pulsed iontophoretic introduction. The aqueous fluid production rate was 3.6 μl/hr and slowed significantly in AQP1 deficiency. Thus, the decreased IOP in AQP1 null mice probably results from reduced aqueous fluid production, which is probably a consequence of impaired isosmolar fluid secretion across the ciliary epithelium.
AQP-independent fluid secretion
As in salivary and airway submucosal glands, AQP5 is expressed at the apical membrane in acinar epithelial cells in lacrimal and sweat glands. However, unlike salivary gland, the secretion of tear fluid (25) and sweat (26) are not impaired AQP5 knockout mice. The substantially lower rates of transepithelial fluid secretion in lacrimal and sweat glands compared to salivary gland probably accounts for the lack of physiological importance of AQP5. The fluid residence time in the acinar lumen is another determinant of whether maximal fluid secretion requires high, AQP-facilitated water permeability. In lacrimal and sweat glands the basal (AQP-independent) water permeability of the glandular acinar epithelia are sufficiently high and the fluid residence time sufficiently long to support the relatively slow rates of fluid secretion. Studies in lung (27, 28), airways (29) and gallbladder (30) indicate that AQPs are present in these tissues and facilitate osmotically driven water transport ; however, AQP deletion does not affect a series of tested physiological function including alveolar fluid absorption, airway fluid absorption and surface liquid properties, and bile composition. We conclude that AQPs are not required for active fluid absorption/secretion when fluid transport rates are low.
CONCLUDING STATEMENT
Phenotype studies in AQP knockout mice have confirmed the anticipated role of AQPs in transepithelial fluid transport in kidney tubules and certain exocrine glands. A perhaps less anticipated finding is that many epithelia express AQPs and show AQP-dependent osmosis, but without apparent physiological importance. Why AQPs are expressed in various epithelia without apparent physiological importance remains unclear. In terms of translational aspects of the basic research, we suggest that pharmacological inhibition of AQP water permeability in epithelia may offer potential therapy for human diseases involving water imbalance such as congestive heart failure, hypertension and glaucoma.
References
- 1.Verkman AS. Mammalian aquaporins : diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10 :e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456 :693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulos MC, Verkman AS. Potential utility of aquaporin modulators for therapy of brain disorders. Prog Brain Res. 2008;170 :589–601. doi: 10.1016/S0079-6123(08)00446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojek A, Praetorius J, Frøkiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70 :307–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 5.Maeda N, Hibuse T, Funahashi T. Role of aquaporin-7 and aquaporin-9 in glycerol metabolism ; involvement in obesity. Handb Exp Pharmacol. 2009;190 :233–249. doi: 10.1007/978-3-540-79885-9_12. [DOI] [PubMed] [Google Scholar]
- 6.Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in epidermis. J Invest Dermatol. 2008;128 :2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 7.Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins-new players in cancer biology. J Mol Med. 2008;86 :523–529. doi: 10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264 :92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 9.King LS, Choi M, Fernandez PC, Cartron JP, Agre P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N Engl J Med. 2001;345 :175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- 10.Verkman AS. Aquaporins : translating bench research to human disease. J Exper Biol. 2009;212 :1707–1715. doi: 10.1242/jeb.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273 :4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 12.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA. 2000;97 :4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100 :957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA. 1998;95 :9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallon V, Verkman AS, Schnermann J. Luminal hypotonicity in proximal tubules of aquaporin-1-knockout mice. Am J Physiol Renal Physiol. 2000;278 :F1030–1033. doi: 10.1152/ajprenal.2000.278.6.F1030. [DOI] [PubMed] [Google Scholar]
- 16.Chou CL, Knepper MA, Hoek AN, Brown D, Yang B, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J Clin Invest. 1999;103 :491–496. doi: 10.1172/JCI5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallone TL, Edwards A, Ma T, Silldorff EP, Verkman AS. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J Clin Invest. 2000;105 :215–222. doi: 10.1172/JCI8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274 :20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 deficient mice. Am J Physiol. 2005;288 :C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 20.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001b;276 :23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276 :41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- 22.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. Faseb J. 2005;19 :76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Vetrivel L, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002;119 :561–569. doi: 10.1085/jgp.20028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verkman AS, Ruiz-Ederra J, Levin M. Functions of aquaporins in the eye. Prog Ret Eye Res. 2008;27 :420–433. doi: 10.1016/j.preteyeres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore M, Ma T, Yang B, Verkman AS. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp Eye Res. 2000;70 :557–562. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Sonawane N, Verkman AS. Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J Physiol. 2002;541 :561–568. doi: 10.1113/jphysiol.2001.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest. 1999;103 :555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105 :93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J Gen Physiol. 2001;117 :573–582. doi: 10.1085/jgp.117.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Zhang H, Verkman AS. Very high aquaporin-1 facilitated water permeability in mouse gallbladder. Am J Physiol. 2009;296 :G816–822. doi: 10.1152/ajpgi.90680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


