Abstract
Aquaporin-4 (AQP4) is a water-selective transporter expressed in astrocytes throughout the central nervous system, as well as in kidney, lung, stomach and skeletal muscle. The two AQP4 isoforms produced by alternative spicing, M1 and M23 AQP4, form heterotetramers that assemble in cell plasma membranes in supramolecular structures called orthogonal arrays of particles (OAPs). Phenotype analysis of AQP4-null mice indicates the involvement of AQP4 in brain and spinal cord water balance, astrocyte migration, neural signal transduction and neuroinflammation. AQP4-null mice manifest reduced brain swelling in cytotoxic cerebral edema, but increased brain swelling in vasogenic edema and hydrocephalus. AQP4 deficiency also increases seizure duration, impairs glial scarring, and reduces the severity of autoimmune neuroinflammation. Each of these phenotypes is likely explicable on the basis of reduced astrocyte water permeability in AQP4 deficiency. AQP4 is also involved in the neuroinflammatory demyelinating disease neuromyelitis optica (NMO), where autoantibodies (NMO-IgG) targeting AQP4 produce astrocyte damage and inflammation. Mice administered NMO-IgG and human complement by intracerebral injection develop characteristic NMO lesions with neuroinflammation, demyelination, perivascular complement deposition and loss of glial fibrillary acidic protein and AQP4 immunoreactivity. Our findings suggest the potential utility of AQP4-based therapeutics, including small-molecule modulators of AQP4 water transport function for therapy of brain swelling, injury and epilepsy, as well as small-molecule or monoclonal antibody blockers of NMO-IgG binding to AQP4 for therapy of NMO.
Keywords: AQP4, water transport, transgenic mice, brain edema, astrocyte migration, neuroexcitation, neuroinflammation, epilepsy, neuromyelitis optica
Aquaporin-4 identification, distribution, structure and function
Aquaporin-4 (AQP4) was originally cloned by our lab in 1994 from rat lung1 and subsequently from different species and tissues2. AQP4 is most strongly expressed in the central nervous system (CNS), but is found as well in kidney collecting duct, gastric parietal cells, skeletal muscle, airway epithelium and various glandular epithelia3, 4. In the CNS, AQP4 is expressed in astrocytes, and is particularly concentrated at pial and ependymal surfaces in contact with the cerebrospinal fluid (CSF) in the subarachnoid space and the ventricles5. At the cell level, AQP4 expression is polarized in astrocytic foot processes in contact with blood vessels.
AQP4 is present in two major isoforms produced by alternative splicing: a relatively long (M1) isoform with translation initiation at Met-1, and a shorter (M23) isoform with translation initiation at Met-23 (Figure 1A)2, 6, 7. In rat, but not human or mouse, a longer isoform (Mz) is also found, but at very low levels8, 9. The M1 and M23 isoforms of AQP4 associate in membranes as heterotetramers10, 11. AQP4 functions as a water-selective transporter with a relatively high single channel water permeability compared to other aquaporins12, 13. A high-resolution X-ray crystal structure along with molecular dynamics simulations suggest a structural basis of AQP4 water selectivity involving steric and electrostatic factors14. Like other aquaporins15, 16, AQP4 monomers, each of about 30 kdalton molecular size, contain 6 membrane-spanning helical domains and two short helical segments surrounding cytoplasmic and extracellular vestibules connected by a narrow aqueous pore.
Figure 1.
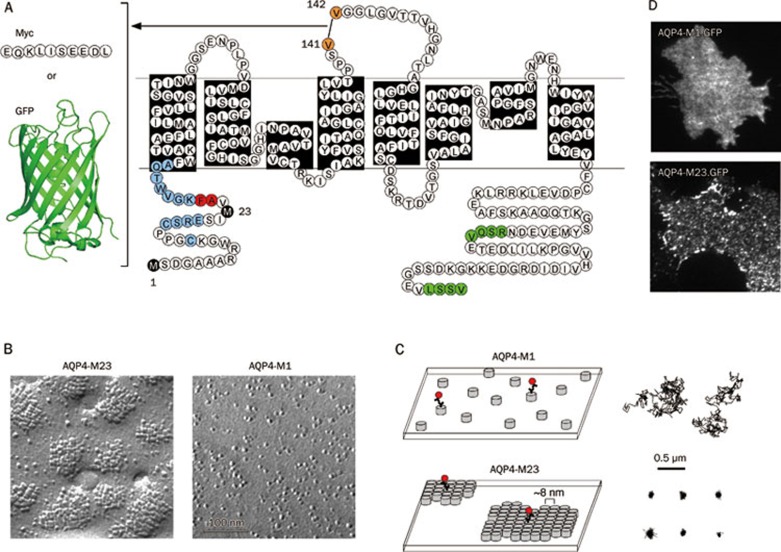
Visualization of AQP4 in OAPs in live cells. (A) AQP4 sequence and topology showing site of Myc or GFP insertion in the second extracellular loop for fluorescence labeling. Black: Met1 and Met23 translation initiation sites; blue: residues where single mutations do not affect OAP formation or disruption; red: residues where single mutations strongly disrupt OAPs; green: C-terminal PDZ-binding domains. (B) Freeze-fracture electron micrographs of COS-7 cells expressing Myc-tagged AQP4-M23 (left) and AQP4-M1 (right). (C) Schematic showing the organization of AQP4 tetramers (left) and representative single particle trajectories (right) of quantum dot-labeled AQP4 molecules in cells expressing AQP4-M1 (top) or AQP4-M23 (bottom). Each grey cylinder represents one AQP4 tetramer. A subset of AQP4 molecules is labeled with quantum dots (red) for single particle tracking. (D) Visualization of AQP4 OAPs by total internal reflection fluorescence microscopy, showing GFP tagged M1 (top) and M23 (bottom) AQP4.
AQP4 assembly in orthogonal arrays of particles
AQP4 is a structural component of orthogonal arrays of particles (OAPs), which are square arrays of intramembrane particles seen in cell membranes by freeze-fracture electron microscopy (FFEM)17, 18. Based on the finding that AQP4 is expressed in the same cells in which OAPs were identified, we originally proposed that AQP4 was the OAP protein. Experimental support for this hypothesis came from FFEM on AQP4-transfected CHO cells showing characteristic OAPs19, and from the absence of OAPs in brain and other tissues from AQP4 null mice20. Immunogold labeling of AQP4 in OAPs in tissues confirmed the conclusion that AQP4 was the key structural component of OAPs21. The biological significance of OAP formation by AQP4 remains unknown, though it has been proposed that OAPs might facilitate AQP4 water transport, polarization to astrocyte foot processes, and cell-cell adhesion12, 22, 23. As discussed further below, AQP4 OAPs have also been proposed to be the target of neuromyelitis optica (NMO) autoantibodies (NMO-IgG)24.
FFEM in cells transfected with the M1 and M23 isoforms of AQP4 show that M23 assembles into large OAPs, whereas M1 tetramers are largely dispersed (Figure 1B)25. In primary astrocytes, and in cells co-transfected with M1 and M23, OAPs are considerably smaller on average than OAPs in cells expressing only M2323, 25, suggesting that M1 interacts with the array-forming M23 in the plasma membrane, limiting the size to which OAPs assemble in vivo. Prior to 2008, the only method for identifying OAPs was FFEM. However, various technical challenges limit the usefulness of FFEM when examining questions regarding the mechanisms involved in OAP formation and regulation. Limitations include fixation artifacts, difficulty in obtaining statistically rigorous information about numbers of AQP4 tetramers in OAPs, and difficulty in identifying OAPs in cells expressing low levels of AQP4.
We developed a series of optical methods to study AQP4 OAP assembly in live cells. One method used in our studies has been single particle tracking (SPT), a technique that is technically and conceptually simple. A c-myc epitope is engineered into the second extracellular loop of the AQP4 molecule (Figure 1A), cells are transiently transfected, and a subset of AQP4 molecules at the plasma membrane in live cells are labeled, via antibody coupling, to fluorescent quantum dots (Figure 1C, left). The movement of individual quantum dots, due to AQP4 diffusion, is followed using a fluorescence microscope, and trajectories of individual quantum dots are reconstructed and analyzed. We initially applied SPT to study AQP1 diffusion, finding long-range free diffusion over a wide variety of conditions, indicating that AQP1 exists in the plasma membrane largely free of specific interactions26. In applying SPT to AQP4, we reasoned that individual AQP4 tetramers should be mobile, whereas AQP4 in large OAPs should be relatively immobile. As expected, in a variety of cell lines and primary astrocytes, we found remarkable immobility of AQP4-M23 and rapid diffusion of AQP4-M1 (Figure 1C, right)27.
We exploited AQP4 diffusion as a 'read-out' of OAP assembly in live cells to investigate a series of questions regarding the biophysics and determinants of OAP formation. From measurements on AQP4 mutants and chimeras, we concluded that OAP formation by M23 involved hydrophobic intermolecular interactions of N-terminal AQP4 residues just downstream of Met-23, and that lack of OAP formation by M1 results from non-specific blocking of N-terminal interactions by residues just upstream of Met-2328. We also demonstrated rapid and reversible temperature-dependent assembly into OAPs of certain weakly associating AQP4 mutants29, and found that the M1 and M23 isoforms of AQP4 co-mingled in OAPs10. OAPs in live cells were visualized directly by total internal reflection microscopy of GFP-AQP4 chimeras (Figure 1D)30. Single-molecule step-photobleaching and intensity analysis of GFP-labeled M1-AQP4 in the presence of excess unlabeled AQP4 isoforms/mutants indicated heterotetrameric AQP4 association. Time-lapse total internal reflection fluorescence imaging of AQP4-M23 in live cells indicated that OAPs diffused slowly and rearranged over tens of minutes. Together, our measurements in live cells revealed extensive AQP4 monomer-monomer and tetramer-tetramer interactions as well as regulated AQP4 assembly in OAPs. Current work is focused on mathematical modeling of AQP4 assembly in OAPs and super-resolution imaging of individual OAPs.
Functions of AQP4 in the CNS
We have systematically investigated the roles of AQP4 in the CNS utilizing AQP4 knockout mice created in 1997 by targeted gene disruption31. As described below, we confirmed the anticipated involvement of AQP4 in brain water balance, and discovered unexpected roles of AQP4 in astrocyte migration, neuroexcitatory phenomena and neuroinflammation. For each of these phenotypes there is a direct or at least plausible link between AQP4 molecular function as a water channel with the CNS phenotype. AQP4 knockout mice have normal growth and survival, as well as CNS anatomy and histology, vascularity, baseline intracranial pressure and blood-brain barrier integrity32, 33, 34. More recently, Hu and coworkers independently generated AQP4 knockout mice, though they reported significant baseline abnormalities in their mice including impaired blood-brain barrier integrity35. The mice of Hu et al manifest a variety of neurochemical and other abnormalities36, 37, 38, which are difficult to interpret because of their baseline abnormalities and the difficulty in reconciling the various brain phenotypes with the water transporting role of AQP4. There are also confusing data in the literature from Frigeri and coworkers who reported marked abnormalities in cell structure and proliferation in astrocyte cell cultures after AQP4 knockdown39. The original and follow-on data by that group appear to be incorrect, as AQP4 knockdown or knockout in astrocyte cultures does not affect cell growth or morphology40, 41, 42.
AQP4 and brain edema
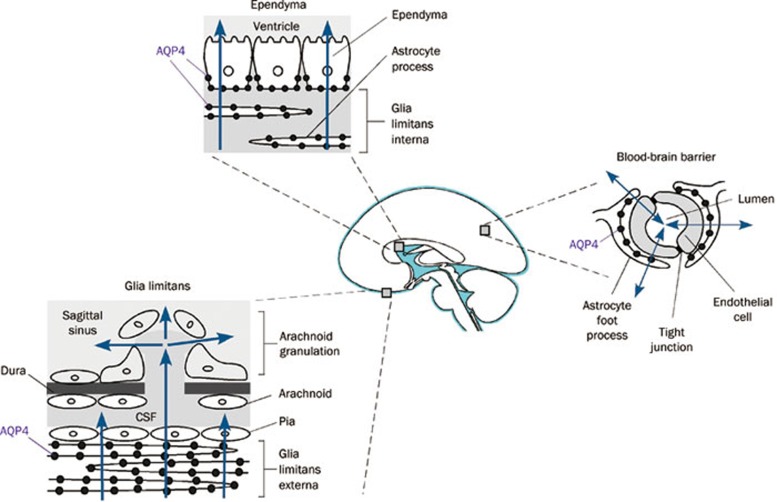
The pattern of AQP4 expression in the brain (at interfaces between brain parenchyma and major fluid compartments) as well as regulation studies (correlating AQP4 expression and brain edema) provide indirect evidence for involvement of AQP4 in brain water balance. We thus postulated the involvement of AQP4 in water movement into and out of brain. There are several types of brain edema that can occur independently or together. In cytotoxic (cellular) brain edema, water moves into the brain through an intact blood-brain barrier in response to osmotic driving forces (Figure 2). The archetypal example of cytotoxic edema is water intoxication in which acute serum hyponatremia causes brain swelling by a simple osmotic mechanism. Mice lacking AQP4 show improved clinical outcome and reduced brain water accumulation compared to wildtype mice in water intoxication as well as in other models of primarily cytotoxic brain edema, including ischemic stroke and bacterial meningitis32, 43. Increased AQP4 protein expression in a transgenic AQP4-overexpressing mouse worsens brain swelling in water intoxication44. Recently, an additional mechanism of AQP4-dependent brain swelling has been proposed involving altered cell volume regulation and loss of AQP4-TRPV4 interaction in AQP4 deficiency45, though further work is needed to prove the relevance of this mechanism in vivo.
Figure 2.
Routes of AQP4-facilitated water entry and exit from the brain, showing blood-brain barrier, ependyma and pial surface.
In vasogenic (leaky-vessel) brain edema, water moves into the brain by a bulk fluid flow mechanism through a leaky blood-brain barrier, and exits the brain through the AQP4-rich glia limitans lining brain ventricles and the brain surface (Figure 2). When these water exit routes are impaired in obstructive hydrocephalus, water movement out of the brain through microvessels at the blood-brain barrier becomes more significant. The archetypal example of vasogenic edema is brain tumor-associated edema. AQP4 knockout mice manifest worse clinical outcome and greater brain water accumulation in brain tumor edema as well as in other models of vasogenic edema including intraparenchymal fluid infusion, cortical-freeze injury, brain abscess and subarachnoid hemorrhage33, 46, 47. AQP4 null mice also manifest an accelerated course of brain swelling in obstructive hydrocephalus48, which is generally classified as a cause of interstitial edema. As a bidirectional water channel, AQP4 thus facilitates brain water accumulation in cytotoxic edema and clearance of excess brain water in vasogenic and interstitial edema. AQP4 appears to play a similar role in spinal cord, with reduced swelling and improved clinical outcome in AQP4 deficiency in spinal cord compression injury49, which is primarily associated with cytotoxic edema, while worse swelling and clinical outcome in spinal cord contusion injury50, which is primarily vasogenic in nature.
AQP4 and astrocyte migration
Work done by our lab in tumor angiogenesis led to the discovery of AQP4 involvement in astrocyte migration. Motivated by the strong expression of AQP1 in tumor microvessels, we found impaired angiogenesis and tumor growth in AQP1 null mice after subcutaneous or intracranial tumor cell implantation51. Studies in primary aortic endothelial cell cultures from wildtype and AQP1 null mice revealed similar adhesion and proliferation, though impaired cell migration. Supporting a general role of aquaporins in cell migration were the findings that transfection of non-endothelial cells with various AQPs accelerated their migration, and that migrating AQP1-expressing cells had prominent membrane ruffles at their leading edge with polarization of AQP1 protein to lamellipodia. Similar observations were made in brain, where astrocyte migration is important in glial scar formation. Glial scar formation can both be beneficial, by sequestering an acute lesion such as in brain injury, and deleterious, by inhibiting neuronal regeneration and axonal sprouting52. We found that astrocyte cultures from brains of wildtype and AQP4 knockout mice had similar morphology, proliferation and adhesiveness, but showed markedly impaired migration in the AQP4-deficient cultures in wound healing and transwell Boyden chamber assays41. AQP4 was polarized to the leading edge of migrating cells, with more lamellipodia formed in wildtype vs AQP4 null astrocyte cultures. Further, glial scarring was impaired in AQP4 null mice following a stab injury. In a follow-on study, we showed remarkably impaired migration of AQP4-null astrocytes in intact brain in a model of stab injury involving injection of fluorescently labeled astrocytes53. Of relevance to brain, the tumor grade of astrocytomas has been correlated in a number of studies with AQP4 expression54. We found that aquaporin expression in tumor cells increased their extravasation from blood vessels and local invasiveness55, providing a potential explanation for the expression of aquaporins in many high-grade tumors.
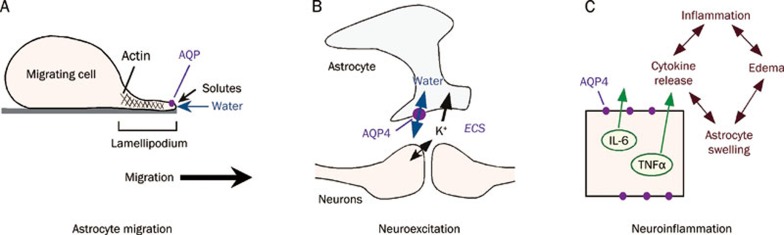
We propose that aquaporin-facilitated cell migration involves enhanced water movement at the plasma membrane in lamellipodial protrusions56. The importance of water fluxes across the plasma membrane in causing localized swelling of lamellipodia has been considered in the early literature on cell migration57. As diagrammed in Figure 3A, we propose that actin de-polymerization and ion influx at the leading edge of a migrating cell increase cytoplasmic osmolality locally, driving water influx across the cell plasma membrane. Supporting the idea of water flow into and out of migrating cells is evidence that migration can be modulated by changes in extracellular osmolality and transcellular osmotic gradients41. The resultant water transport and expansion of the adjacent plasma membrane caused by increased hydrostatic pressure is followed by actin re-polymerization to stabilize the cell membrane protrusion. In support of this idea is the observation that regional hydrostatic pressure changes within cells do not equilibrate throughout the cytoplasm on scales of ten microns and ten seconds58, and could thus contribute to the formation of localized cell membrane protrusions. Further studies are required to validate our ideas relating aquaporin water permeability to cell migration.
Figure 3.
Roles of AQP4 in brain. (A) Proposed mechanism of aquaporin-facilitated cell migration. Aquaporin-facilitated water influx across lamellipodia at the leading edge of a migrating cell promotes membrane protrusion. (B) Proposed pseudo-solvent drag mechanism of AQP4-facilitated neuroexcitation. K+ released into the extracellular space (ECS) following neuroexcitation is mainly taken up by astrocytes. K+ reuptake results in osmotic water influx into astrocytes and consequent ECS shrinkage, maintaining the electrochemical driving force for K+ reuptake. (C) Proposed mechanism for AQP4 involvement in neuroinflammation. AQP4 facilitates cytokine secretion, as well as astrocyte swelling and local cytotoxic edema.
AQP4 and neuroexcitation
The brain extracellular space (ECS) comprises ∼20% of brain tissue volume, consisting of a jelly-like matrix in which neurons, glial cells and blood vessels are embedded. The ECS contains ions, neurotransmitters, metabolites, peptides, and extracellular matrix molecules, forming the microenvironment for neurons, astrocytes and other brain cells. During neuronal activity, depolarization of neurons and adjacent glial cells increases extracellular glutamate and K+. Excess K+ in the ECS is taken up and 'siphoned' largely by astrocytes. AQP4 is expressed in electrically excitable tissues in supportive cells adjacent to excitable cells, including glia but not neurons in brain, Müller but not bipolar cells in retina, supportive but not hair cells in the inner ear, and supportive cells but not olfactory receptor neurons in olfactory epithelium. Postulating from its expression pattern the involvement of AQP4 in neuroexcitatory phenomena, we characterized various neurosensory and neuroexcitatory phenotypes in the AQP4 knockout mice. Electrophysiological measurements showed impaired vision59, hearing60 and olfaction61 in AQP4 null mice, as demonstrated by increased auditory brainstem response thresholds, and reduced electroretinogram and electroolfactogram potentials. Also, seizure threshold is reduced and seizure duration is prolonged in AQP4 knockout mice62. Possible mechanisms for these phenomena supported by experimental data include delayed K+ reuptake by astrocytes in AQP4 deficiency following neuroexcitation62, 63, and mild ECS expansion64, 65, 66, 67. Delayed K+ reuptake was also found in α-syntrophin null mice in which astrocyte AQP4 is mislocalized68. Slowed K+ reuptake in brain would prolong seizure duration, as found experimentally.
The precise link between K+ reuptake by astrocytes and AQP4 water permeability remains speculative. It had been postulated that interaction between AQP4 and the inwardly rectifying K+ channel, Kir4.1, was responsible69; however, patch-clamp analysis indicated that AQP4 deficiency did not affect Kir4.1 K+ channel function in retinal Müller cells or brain astrocytes70, 71. As diagrammed in Figure 3B, we propose a simple mechanism in which AQP4-dependent water permeability enhances K+ transport by pseudo-solvent drag. Excess K+ released into the ECS by neurons during neuroexcitation is taken up largely by the AQP4-containing astrocytes. Reuptake of K+ following neuroexcitation results in osmotic water influx into AQP4-expressing astrocytes and consequent ECS shrinkage, which maintains the electrochemical driving force for K+ reuptake. Reduced astrocyte water permeability in AQP4 deficiency would reduce ECS contraction and hence slow K+ reuptake. This hypothesis is attractive because it relates the neuroexcitation phenotypes directly to AQP4 water transport.
AQP4 and neuroinflammation
We recently discovered a novel role of AQP4 in neuroinflammation following studies of experimental autoimmune encephalomyelitis (EAE), an extensively used model of neuroinflammatory demyelinating diseases such as multiple sclerosis. EAE is mediated primarily by myelin-specific Th1 or Th17 cells. The motivation for this work is the central involvement of astrocytes in neuroinflammation and evidence, as discussed in the next section, that AQP4 is the target antigen in the neuroinflammatory autoimmune disease neuromyelitis optica (NMO). In an initial phenotype study, we found that compared with wild type mice, AQP4 knockout mice showed remarkably attenuated EAE following active immunization with myelin oligodendrocyte glycoprotein (MOG) peptide, with reduced motor dysfunction, brain inflammation and myelin loss72. In a follow-on study, potential mechanisms for the protective effect of AQP4 deficiency were investigated, including AQP4-dependent leukocyte and microglia cell function, immune cell entry in the CNS, intrinsic neuroinflammation, and humoral immune response40. As we found with active-immunization EAE, neuroinflammation was greatly reduced in AQP4 knockout mice in adoptive-transfer EAE, which involved injection of MOG-sensitized T-lymphocytes in naïve mice. A series of negative studies ruled out AQP4-dependent differences in immune cell function and CNS entry, microglial function and humoral immune responses: (a) AQP4 was absent in immune cells, including activated T-lymphocytes; (b) CNS migration of fluorescently labeled, MOG-sensitized T-lymphocytes was comparable in wildtype and AQP4 knockout mice; (c) microglia did not express AQP4; and (d) serum anti-AQP4 antibodies were absent in EAE. Remarkably, however, intracerebral injection of lipopolysaccharide produced much greater neuroinflammation in wildtype than in AQP4 knockout mice, indicating an intrinsic pro-neuroinflammatory role of AQP4. In analyzing possible cellular mechanisms, we found that the secretion of the major cytokines TNF-alpha and IL-6 was reduced in astrocyte cultures from AQP4 knockout mice. Further, adenovirus-mediated expression of AQP4, or of a different aquaporin, AQP1, increased cytokine secretion in astrocyte and non-astrocyte cell cultures, supporting the involvement of aquaporin water permeability in cytokine secretion.
These findings implicated a novel intrinsic pro-inflammatory role of AQP4, which we propose at the cellular level involves AQP4-dependent differences in astrocyte water permeability and consequent cell swelling and cytokine release (Figure 3C). AQP4-dependent neuroinflammation is likely further exaggerated by a positive-feedback cycle of secretion of pro-inflammatory cytokines and local cytotoxic brain swelling, which, as discussed above, is also AQP4-dependent.
AQP4 and neuromyelitis optica
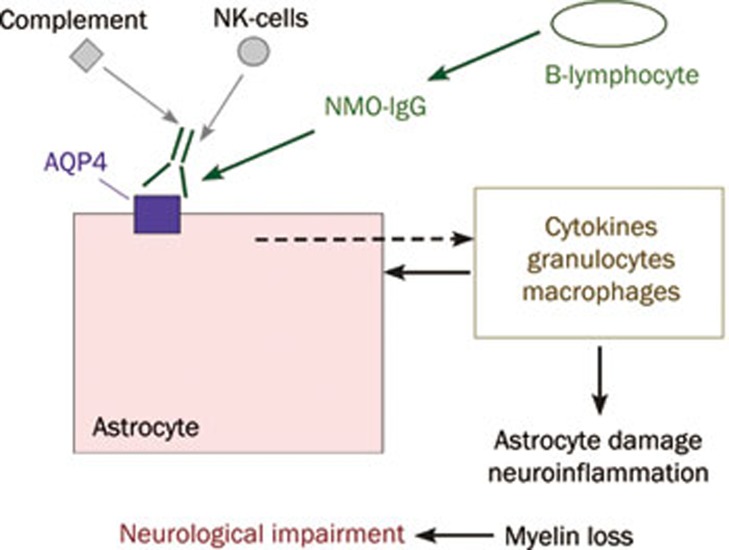
NMO is a neuroinflammatory demyelinating disease that, unlike multiple sclerosis, primarily affects optic nerve and spinal cord, leading to blindness, paralysis and death73. A defining feature of NMO is the presence of serum autoantibodies directed against extracellular epitopes on AQP474. Recent data suggest that most, if not all NMO patients are seropositive for AQP4 autoantibodies (NMO-IgG), which recognize 3-dimensional epitopes on the extracellular surface of AQP475. There is emerging evidence for a pathogenic role of NMO-IgG in NMO, as administration of human NMO-IgG to naïve mice or to rats with pre-existing neuroinflammation produces NMO-like pathology76, 77, 78, 79. The most compelling evidence to date implicating a pathogenic role of NMO-IgG is the appearance of characteristic NMO lesions, with neuroinflammation, loss of glial fibrillary acidic protein (GFAP) and AQP4 immunoreactivity, demyelination and perivascular complement deposition, following direct intracerebral injection of NMO-IgG in mice79. As diagrammed in Figure 4, it is thought that NMO-IgG binding to AQP4 in astrocytes initiates an inflammatory cascade involving recruitment of leukocytes (granulocytes, macrophages, NK cells, lymphocytes), cytokine release, and complement and NK cell-mediated astrocyte damage80. The consequent neuroinflammation and myelin loss produce neurological deficits. A glutamate excitotoxicity mechanism for NMO pathogenesis has also been proposed based on NMO-IgG-induced internalization of glutamate transporters in transfected cells81; however, the relevance of glutamate transporter internalization to astrocytes in the CNS remains unproven. The initiating events in NMO-IgG production and CNS penetration remain unknown, as do the reasons why NMO-IgG produces much greater pathology in spinal and optic nerve compared to brain, with no significant pathology in peripheral AQP4-expressing organs.
Figure 4.
Proposed mechanism of NMO disease pathogenesis. NMO-IgG binding to AQP4 on astrocytes causes complement- and NK-mediated cell injury, resulting in leukocyte recruitment and cytokine release.
There has been recent interest in determining whether NMO-IgG targets the M1 vs M23 isoforms of AQP4, and OAP vs non-OAP associated AQP4. One report that analyzed NMO serum specimens concluded that OAPs were the exclusive target of NMO-IgG24. However, this conclusion cannot be correct because the clinical assay for serum anti-AQP4 autoantibody uses M1 AQP482, and we10 and others76, 80 reported strong binding of some NMO autoantibodies to cells expressing only M1 AQP4. The paper of Nicchia24 was also flawed in that they reported OAPs sized smaller than the diffraction limit of light, which was not possible. We recently examined the issue of NMO binding specificity utilizing a two-color fluorescence ratio imaging assay of AQP4-expressing cells stained with NMO patient serum or a recombinant monoclonal NMO autoantibody (NMO-rAb), together with a C-terminus anti-AQP4 antibody83. NMO-rAb titrations showed single site binding with dissociation constants down to 44 nmol/L. Different NMO-rAbs and NMO patient sera showed wide variation in NMO-IgG binding to M1 vs M23 AQP4. We found that differences in binding affinity rather than stoichiometry accounted for M23>M1 binding specificity. Binding and OAP measurements in cells expressing M23 AQP4 mutants with OAP-disrupting mutations indicated that the differential binding of NMO-IgG to M1 vs M23 was due to OAP assembly rather than to differences in the M1 vs M23 N-termini. Measurements using purified Fab fragments derived from NMO-rAbs suggested that a structural change in the AQP4 epitope upon array assembly, and not bivalent NMO-IgG binding, accounts for the greater binding affinity to OAPs.
Current NMO therapies are directed toward reducing the inflammatory response (immunosuppression) and the NMO-IgG load (B-cell depletion and plasmapheresis). A complement targeted monoclonal antibody therapy is in clinical trials. Our laboratory has focused on the development of a novel therapeutic approach involving blocking of the binding of pathogenic NMO antibodies to AQP4, which is believed to be the initiating event in NMO pathogenesis. We are currently developing both a monoclonal antibody approach involving tight-binding, non-pathogenic, engineered recombinant NMO antibodies ('aquaporumabs'), as well as drug-like small-molecule blockers identified by high-throughput screening.
Prospects for AQP4-based therapies
In addition to the possibility of NMO-IgG blocker therapy for NMO discussed just above, the involvement of AQP4 in brain water balance, astrocyte migration, neuroexcitation and neuroinflammation suggest the therapeutic potential of AQP4 modulators. Notwithstanding the challenges in drug delivery to the central nervous system and their multiplicity of actions, AQP4 inhibitors have potential utility in reducing cytotoxic brain swelling, seizure intensity, glial scar formation, and neuroinflammation; enhancers of AQP4 expression have potential utility in reducing vasogenic brain swelling. AQP4 modulators may thus offer new therapeutic options for stroke, tumor, infection, hydrocephalus, epilepsy, neuroinflammatory conditions and traumatic brain and spinal cord injury. However, the discovery of potent and selective small-molecule AQP4 inhibitors is a major challenge, as no confirmed small-molecule AQP4 inhibitors have been identified to date and identification of inhibitors of AQP4 by conventional screening methods has so far been unsuccessful. Further, AQP4 inhibitor therapy for brain swelling will require care because cytotoxic and vasogenic edema often coexist in varying proportions during the evolution of a CNS insult. For example, AQP4 inhibition may be beneficial early in the course of spinal cord trauma, but deleterious later on. Because of the involvement of AQP4 in neurosensory phenomena, transient visual, auditory and olfactory impairment are possible with AQP4 inhibition. In epilepsy therapy, while AQP4 inhibition reduces seizure duration and hence severity, it appears to increase seizure susceptibility. Of the various theoretical applications of AQP4-based therapies, the indications with greatest potential for success are likely NMO-IgG/AQP4 blocker therapy for NMO, and AQP4 inhibition therapy for acute cytotoxic cerebral edema in ischemic stroke.
References
- Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–500. [PubMed] [Google Scholar]
- Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A. 1994;91:13052–6. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995;92:4328–31. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108:2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, et al. The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci U S A. 1996;93:10908–12. doi: 10.1073/pnas.93.20.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ma T, Verkman AS. cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. Evidence for distinct transcriptional units. J Biol Chem. 1995;270:22907–13. doi: 10.1074/jbc.270.39.22907. [DOI] [PubMed] [Google Scholar]
- Moe SE, Sorbo JG, Sogaard R, Zeuthen T, Petter Ottersen O, Holen T. New isoforms of rat Aquaporin-4. Genomics. 2008;91:367–77. doi: 10.1016/j.ygeno.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Rossi A, Crane JM, Verkman AS.Aquaporin-4 Mz isoform: brain expression, supramolecular assembly and neuromyelitis optica antibody binding Glia 2011. doi: 10.1002/glia.21177 [DOI] [PMC free article] [PubMed]
- Crane JM, Bennett JL, Verkman AS. Live cell analysis of aquaporin-4 m1/m23 interactions and regulated orthogonal array assembly in glial cells. J Biol Chem. 2009;284:35850–60. doi: 10.1074/jbc.M109.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely JD, Christensen BM, Nielsen S, Agre P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry (Mosc) 1999;38:11156–63. doi: 10.1021/bi990941s. [DOI] [PubMed] [Google Scholar]
- Yang B, van Hoek AN, Verkman AS. Very high single channel water permeability of aquaporin-4 in baculovirus-infected insect cells and liposomes reconstituted with purified aquaporin-4. Biochemistry (Mosc) 1997;36:7625–32. doi: 10.1021/bi970231r. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–6. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, et al. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci U S A. 2009;106:7437–42. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hub JS, Grubmuller H, de Groot BL. Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations. Handb Exp Pharmacol. 2009;190:57–76. doi: 10.1007/978-3-540-79885-9_3. [DOI] [PubMed] [Google Scholar]
- Walz T, Fujiyoshi Y, Engel A. The AQP structure and functional implications. Handb Exp Pharmacol. 2009;190:31–56. doi: 10.1007/978-3-540-79885-9_2. [DOI] [PubMed] [Google Scholar]
- Landis DM, Reese TS. Arrays of particles in freeze-fractured astrocytic membranes. J Cell Biol. 1974;60:316–20. doi: 10.1083/jcb.60.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Staehelin LA, Ellisman MH. Rectangular arrays of particles on freeze-cleaved plasma membranes are not gap junctions. Exp Cell Res. 1974;86:187–90. doi: 10.1016/0014-4827(74)90670-3. [DOI] [PubMed] [Google Scholar]
- Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–80. [PubMed] [Google Scholar]
- Verbavatz JM, Ma T, Gobin R, Verkman AS. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci. 1997;110:2855–60. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A. 1998;95:11981–6. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–39. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, Brown D, et al. Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am J Physiol Renal Physiol. 2004;287:F501–11. doi: 10.1152/ajprenal.00439.2003. [DOI] [PubMed] [Google Scholar]
- Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, et al. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia. 2009;57:1363–73. doi: 10.1002/glia.20855. [DOI] [PubMed] [Google Scholar]
- Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci U S A. 2003;100:13609–14. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Verkman AS. Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys J. 2008;94:702–13. doi: 10.1529/biophysj.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Van Hoek AN, Skach WR, Verkman AS. Aquaporin-4 dynamics in orthogonal arrays in live cells visualized by quantum dot single particle tracking. Mol Biol Cell. 2008;19:3369–78. doi: 10.1091/mbc.E08-03-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Verkman AS. Determinants of aquaporin-4 assembly in orthogonal arrays revealed by live-cell single-molecule fluorescence imaging. J Cell Sci. 2009;122:813–21. doi: 10.1242/jcs.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Verkman AS. Reversible, temperature-dependent supramolecular assembly of aquaporin-4 orthogonal arrays in live cell membranes. Biophys J. 2009;97:3010–8. doi: 10.1016/j.bpj.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M, Crane JM, Verkman AS. Aquaporin-4 (AQP4) associations and array dynamics probed by photobleaching and single-molecule analysis of green fluorescent protein-AQP4 chimeras. J Biol Chem. 2010;285:8163–70. doi: 10.1074/jbc.M109.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–62. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–63. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–3. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, et al. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161:764–72. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kong H, Hua X, Xiao M, Ding J, Hu G. Altered blood-brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport. 2008;19:1–5. doi: 10.1097/WNR.0b013e3282f2b4eb. [DOI] [PubMed] [Google Scholar]
- Li Z, Gao L, Liu Q, Cao C, Sun XL, Ding JH, et al. Aquaporin-4 knockout regulated cocaine-induced behavior and neurochemical changes in mice. Neurosci Lett. 2006;403:294–8. doi: 10.1016/j.neulet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Liu L, Lu Y, Kong H, Li L, Marshall C, Xiao M, et al. Aquaporin-4 deficiency exacerbates brain oxidative damage and memory deficits induced by long-term ovarian hormone deprivation and D-galactose injection Int J Neuropsychopharmacol 2011. DOI: 10.1017/S1461145711000022 [DOI] [PubMed]
- Wu N, Lu XQ, Yan HT, Su RB, Wang JF, Liu Y, et al. Aquaporin 4 deficiency modulates morphine pharmacological actions. Neurosci Lett. 2008;448:221–5. doi: 10.1016/j.neulet.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Nicchia GP, Frigeri A, Liuzzi GM, Svelto M. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. FASEB J. 2003;17:1508–10. doi: 10.1096/fj.02-1183fje. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011;25:1556–66. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–8. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Badaut J, Ashwal S, Adami A, Tone B, Recker R, Spagnoli D, et al. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab. 2011;31:819–31. doi: 10.1038/jcbfm.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem. 2005;280:13906–12. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;283:15280–6. doi: 10.1074/jbc.M801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108:2563–8. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005;95:254–62. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- Tait MJ, Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience. 2010;167:60–7. doi: 10.1016/j.neuroscience.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527–37. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain. 2008;131:1087–98. doi: 10.1093/brain/awn014. [DOI] [PubMed] [Google Scholar]
- Kimura A, Hsu M, Seldin M, Verkman AS, Scharfman HE, Binder DK. Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann Neurol. 2010;67:794–801. doi: 10.1002/ana.22023. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–16. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins — new players in cancer biology. J Mol Med. 2008;86:523–9. doi: 10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–4. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loitto VM, Karlsson T, Magnusson KE. Water flux in cell motility: expanding the mechanisms of membrane protrusion. Cell Motil Cytoskeleton. 2009;66:237–47. doi: 10.1002/cm.20357. [DOI] [PubMed] [Google Scholar]
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–9. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Patil RV, Verkman AS. Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin-4 water channels. Invest Ophthalmol Vis Sci. 2002;43:573–9. [PubMed] [Google Scholar]
- Li J, Verkman AS. Impaired hearing in mice lacking aquaporin-4 water channels. J Biol Chem. 2001;276:31233–7. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Lu DC, Zhang H, Zador Z, Verkman AS. Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J. 2008;22:3216–23. doi: 10.1096/fj.07-104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–6. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2:825–7. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;24:8049–56. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Hrabetova S, Nicholson C, Manley GT. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J Neurosci. 2008;28:5460–4. doi: 10.1523/JNEUROSCI.0257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador Z, Magzoub M, Jin S, Manley GT, Papadopoulos MC, Verkman AS. Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain. FASEB J. 2008;22:870–9. doi: 10.1096/fj.07-9468com. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning. Biophys J. 2010;99:1284–91. doi: 10.1016/j.bpj.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100:13615–20. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–13. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Zhang H, Verkman AS. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J Biol Chem. 2007;282:21866–72. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol Cell Neurosci. 2008;37:1–10. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang H, Verkman AS. Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci. 2009;10:94. doi: 10.1186/1471-2202-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One. 2010;5:e10455. doi: 10.1371/journal.pone.0010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–29. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–43. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386:623–7. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–61. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- Hinson SR, Roemer SF, Lucchinetti CF, Fryer JP, Kryzer TJ, Chamberlain JL, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205:2473–81. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–24. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]