Abstract
Magnetic Resonance Navigation (MRN) relies on Magnetic Nanoparticles (MNPs) embedded in microcarriers or microrobots to allow the induction of a directional propelling force by 3-D magnetic gradients. These magnetic gradients are superposed on a sufficiently high homogeneous magnetic field (e.g. the Bo field of a MR scanner) to achieve maximum propelling force through magnetization saturation of the MNPs. As previously demonstrated by our group, such technique was successful at maintaining microcarriers along a planned trajectory in the blood vessels based on tracking information gathered using Magnetic Resonance Imaging (MRI) sequences from artifacts caused by the same MNPs. Besides propulsion and tracking, the same MNPs can be synthesized with characteristics that can allow for the diffusion of therapeutic cargo carried by these MR-navigable carriers through the Blood Brain Barrier (BBB) using localized hyperthermia without compromising the MRN capabilities. In the present study, localized hyperthermia induced by an alternating magnetic field (AC field) is investigated for the purpose of transient controlled disruption of the BBB and hence local delivery of therapeutic agents into the brain. Here, an external heating apparatus was used to impose a regional heat shock on the skull of a living mouse model. The effect of heat on the permeability of the BBB was assessed using histological observation and tissue staining by Evans blue dye. Results show direct correlation between hyperthermia and BBB leakage as well as its recovery from thermal damage. Therefore, in addition to on-command propulsion and remote tracking, the proposed navigable agents could be suitable for controlled opening of the BBB by hyperthermia and selective brain drug delivery.
I. Introduction
Brain tumours are extremely lethal and incredibly invasive and therefore, intervening with complex surgery is a top priority in most medical cases. Despite many efforts, drug delivery to the brain remains a challenge mainly because the Blood Brain-Barrier (BBB), which consists of endothelial cells that are tightly interconnected and cover all the interior of the cerebral vessel walls, is reputed to be insurmountable for most therapeutic molecules. In fact, nearly 98% of new drugs used in the Central Nervous System (CNS) to combat brain cancer and other chronic diseases cannot enter the brain following systemic administration [1]. On the other hand, systemic administration of toxic agents causes the active principles to distribute throughout all the organs. Therefore, while pathological regions are treated, they also promote side effects in healthy organs.
The extremely selective permeability of BBB and high cytotoxicity of anticancer drugs reinforce the importance of non-invasive targeted drug delivery for brain tumours and other chronic brain related disorders. Previously, our team has shown successful local delivery and tracking of therapeutic agents encapsulated in miniaturized magnetic carriers in the liver of a living animal by the gradient field of a modified Magnetic Resonance Imaging (MRI) scanner [2, 3]. The proposed carriers consist of therapeutic molecules and aggregates of Magnetic Nanoparticles (MNPs) with relatively high magnetization saturation embedded inside a biocompatible and biodegradable polymer, which serves as a transport mediator in the vasculature. This encapsulation also functions as a protective shield and prevents cells from further exposure to toxic drugs during the carriers’ commute to a target area.
Besides MR-propulsion and MR-tracking, the same MNPs can be synthesized to offer characteristics that will allow them to become magnetically excited by an alternating field (AC field) and to release their energy in the form of heat to their surrounding area. Inside brain microvasculature, such heat may thermally disrupt an intact BBB thereby creating a transient opening for the therapeutic agents to cross into the brain tissue. In fact, it has long been recognized that hyperthermia, otherwise known as elevation of body temperature, can lead to intense cellular stress and cause temporal disruption of the BBB [4–7] as well as death of cancer cells by enhancing cell sensitivity and vulnerability towards more established forms of cancer therapy, such as radiation and chemotherapy [8]. Coincidentally, a widely suggested method of hyperthermia is based on excitation of MNPs inside an AC field [9]. This technique makes use of the radiofrequency of electromagnetic waves to heat the MNPs and therefore it is just as effective in deep biological structures as it is at the surface.
To our knowledge, hyperthermic disruption of the BBB via induction of MNPs by an AC field for drug delivery to the brain has not yet been reported. Here, the feasibility of this technique, as well as its integration with our pioneered MRI-based propulsion and tracking technique for intervention of microcarriers and microrobots in the vasculature are discussed. Furthermore, results in this study suggest that there may be a direct relationship between the elevated brain tissue temperature and the extent of the penetration of the desired drug molecules across the BBB. This implies that by controlling the amount of heat and exposure time, we can adjust the BBB opening for various molecular dimensions. Finally in this study, the recovery of the BBB from thermal damage is examined and investigated. Although this is a preliminary study, it is in light of such technique that non-invasive local drug delivery to the region of the brain may become possible.
II. Background Information
A. Blood-Brain Barrier (BBB)
All capillaries in the mammalian body including humans are composed of endothelial cells. In the circumventricular organs, most of the capillaries are fenestrated to allow for rapid exchange of molecules such as the therapeutic agents between blood vessels and surrounding tissue. In the rest of the brain however, very complex inter-endothelial tight junctions interconnect the endothelial cells. The tight junctions seal the cell interspace and form a diffusion barrier that markedly controls the flow of molecules across the epithelium. In addition to the tight junctions, pericytes with smooth muscle-like properties constitute the BBB [10]. Only small electrically neutral lipid-soluble compounds with low molecular mass of less than 400–500 Daltons (Da), or those small electrically neutral lipid-soluble compounds with low air-water partition coefficients and an average cross-sectional area of 50 Å2, are able to diffuse passively through the BBB [11–13]. As mentioned before, this restrains admission of a considerable portion of pharmaceutical agents into the brain. For instance, currently many large drug molecules such as peptides, recombinant proteins, monoclonal antibodies, antisense and non-viral gene medicines are ineffective for the brain [14].
One of the main functions of the BBB is to keep the neurotransmitters and agents that act in the CNS separate from the peripheral tissues and blood, so that similar agents can be used in the two systems without ‘cross-talk’ [15]. Also, because of the BBB’s large surface area (180 cm2 per gram brain [13]) and the short diffusion distance between neurons and capillaries (8–20 μm [16]), the extent to which a molecule enters the brain is determined only by the permeability characteristics of the BBB and that has a predominant role in regulating the brain microenvironment. That is why circumventing the BBB is a priority for any drug delivery mechanism to the region of the brain. Successful crossing of this barrier will have a profound effect on the treatment of many brain related disorders and therefore hyperthermia is being investigated for that purpose.
B. Hyperthermia
In modern oncology, hyperthermia generally refers to heating of organs or tissues in various ways to temperatures between 40°C and 45°C, at which point it causes moderate and reversible cellular inactivation [8]. In this regard, induction of MNPs by an AC field has been investigated for elevation of tissue temperature on a variety of fronts. Chief among those is the fact that the MNPs can act as very small heat sources once placed in an AC field, regardless of their depth inside the biological entity. On the contrary, other techniques such as RF, microwave and High Intensity Focused Ultrasound (HIFU), are not able to accurately target desired deep-seated tissues.
During local hyperthermia to temperatures near 42°C in the region of the brain (3T = 5°C), morphological changes of individual endothelial cells in the monolayer lining of the micro-vessels begin to cause the tight junctions between adjacent endothelial cells to loosen, therefore allowing transport of large molecules through intercellular pathway [17]. Fortunately, the BBB seems to have the capability to restore functionality after brief hyperthermic disruption [18]. The rate of this restoration however, depends on the amount of heat and the exposure time.
C. Magnetic Nanoparticles (MNPs)
Among various methods of hyperthermia, whole body [5], microwave [6] and radiofrequency hyperthermia [7] are most commonly used to disrupt the BBB. In these techniques an entire region of the brain including neurons, astrocytes, vessel wall cells, and other glial cells are equally heated. In fact, this may be the reason for many undesirable acute side effects with hyperthermic disruption of the BBB by these techniques. Conversely, in the proposed hyperthermic disruption of the BBB by induction of MNPs inside an AC field, heat is exclusively dissipated to the ambient vessel wall cells by thermal conduction. Consequently, only the monolayer lining of the vessel walls and the endothelial cells are directly affected by the thermal stress.
The Specific Absorption Rate (SAR) or heat generated by the MNPs is mainly caused by three major mechanisms; hysteresis loss, Néel, and Brownian relaxations. Particles’ physical properties as well as magnitude and frequency of the applied AC field determine the relative strength of each of these mechanisms. SAR is proportional to the time rate of change of temperature of a magnetic material and is given by the following formula [19]:
| (1) |
In (1), c is the specific heat capacity of the sample (J·l−1·K−1), m is the mass of the magnetic particles (kg), Vs is the total volume (m3) and expressed in °K·s−1, is the temperature increment which is experimentally derived from the linear regression of the initial data points obtained from the time varying temperature curve. In the steady state, the difference in temperature ΔT is given by (2) where C is the concentration of the MNPs (mass of the particles per tissue volume) and λ represents the heat conductivity of a tissue volume with a radius R [20].
| (2) |
From (2) it is evident that higher concentration of MNPs per unit volume of the tissue leads to higher ΔT. In reality, for most therapeutic applications, the relatively poor energy transfer efficiency of the MNPs, i.e. poor SAR, introduces a great obstacle that hinders full functionality or demands large administration of the MNPs at the biological target location leading to an increase in possible side effects. Therefore, scientists have long been searching for MNPs with the highest possible SAR.
In contrast to other forms of magnetization, superparamagnetism can prevent formation of nanoparticle clusters in the biological entity [9]. That is why ultra-small magnetite or superparamagnetic iron oxide (magnetite: Fe3O4) nanoparticles have been given special attention for hyperthermia. These particles are commercially available and their physical properties are vastly studied. In addition, magnetite nanoparticles have shown great biocompatibility, biodegradability and low toxicity [21]. The SAR value of these particles varies with respect to particle diameter and magnetic properties of the AC field. Our previous studies with superparamagnetic magnetite nanoparticles have shown promising results with regards to hyperthermia [22]. Table 1 summarizes some of the key parameters required to elevate the temperature of the brain tissue from 37°C to 42°C using commercially available particles. These parameters are induced from numerous in-vitro experiments and simulations presented in [23]. In the same table, MNPs with much higher SAR but not yet commercially available are also reported.
Table 1.
Parameters required to elevate tissue temperature 5°C by hyperthermia of magnetite
The main difficulty of localized hyperthermic disruption of the BBB by induction of the MNPs is transportation of the MNPs and therapeutic agents through the vasculature to a desired area of the brain. Several criteria have been foreseen for such transportation. First, the carrier must have the ability to geometrically fit into the target microvasculature. Second, the carrier must allow for maximum MNPs concentration at the target area to reach sufficient thermal levels. It is also very important to consider factors such as immunological reactions, excessive toxicity, premature degradation and fast excretion of the carrier by blood enzymes, or unexpected capture by non-targeted tissues that may affect the carrier behavior. Compositions such as polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), and various environment-sensitive hydrogels are some of the well-known biomaterials for this purpose [25].
D. MRI-Based Drug Delivery System
Magnetic nanoparticles experience a thrust force when exposed to a gradient field. As seen in (4), this magnetic force, F⃗magnetic (N) is directly proportional to the volume of the MNPs, Vferro (m3) and their magnetization properties, M⃗ (A·m−1) as well as the gradient of the magnetic field, ∇B⃗ (T) [26].
| (4) |
Magnetic Drug Targeting (MDT) technique uses this to concentrate therapeutic drugs adsorbed, entrapped or covalently linked to aggregates of MNPs at a superficial target location following a local intravenous injection [27]. The main difficulty for this technique however, is that it lacks the ability to target deep tissues [9]. Therefore, instead of a conventional approach most often based on an external magnet, an improved alternative method based on the three dimensional gradient magnetic field of the MRI was proposed by our team [28]. In this technique, due to the large main magnetic field of the MRI, aggregates of the MNPs become magnetically saturated once inside the relatively high homogeneous field of a clinical MRI scanner and therefore, relatively small shifts in the gradient field can steer them towards a target anywhere in the tissue. Furthermore, the aggregates of the MNPs create a magnetic distortion on the images acquired by MRI sequences. Therefore, the same MRI platform is able to track the aggregates in real-time and confirm their presence at the target.
In order to maneuver in the microvasculature, and to avoid fast degradation of the MNPs in the blood circulation system, as well as preventing them from dissociation, which greatly limits the thrust force and distortion for tracking purposes, the MNPs along with the therapeutic agents must be encapsulated in biocompatible micrometer size carriers. Previously, our team was able to synthesize such complex microcarriers and to use them to target a specific region of the liver of a living rabbit [3] prior to the successful release of the therapeutic agent. The vascular structure of the liver however is very different from that of the brain. Therefore, the proposed micro-entities may require appropriate modifications before attempting MRN towards the brain.
III. Method
Various studies evaluated the required amount of thermal exposure and duration for temporal disruption of the BBB based on imposition of thermal stress on the tight junctions [29, 30]. According to the findings of these studies, the ideal temperature for transient disruption of the BBB falls in the range of 42°C–44°C for a period of 30 minutes.
A. BBB Staining
Staining of the BBB is a traditional method for evaluating BBB leakage. Evans blue, an exogenous dye tracer is used here to assess the integrity of the BBB following a hyperthermic disruption. The dye molecules are able to easily diffuse through the fenestrated endothelial cells of all capillaries except those of the brain due to a functional BBB. However, once the BBB is compromised, Evans blue enters the brain and it fluoresces with excitation peaks at 470 and 540 nm and an emission peak at 680 nm [31]. Histological staining techniques can therefore reflect the extent of BBB leakage by studying the intensity of Evans blue dye in the brain.
B. Experimental Procedure
In the primary steps of this study, distribution of heat from an external heating device on the brain of living mice as well as feasibility studies in regards to hyperthermic disruption of the BBB were assessed. For this purpose, a two-phase in-vivo experiment was executed:
1) Phase i
Five mice were separately anesthetised by intravenous injection of 40mg/kg body weight of pentobarbital. Quickly after, each animal was positioned on a stereotaxic frame and the head of the animal was secured in place. Then, by removing the skin, the surface of the skull of the animal was exposed. At this point, four small holes (~1mm in diameter) were drilled into the skull at precise locations shown in Fig. 1a. Next, fibre optic thermocouples were placed inside the holes. An external heating device was used to focally elevate the temperature of a small region of the brain near Bregma (see Fig. 1c.) at a 40° angle for duration of 30 minutes. While the thermocouples recorded changes in temperature at specific distances (1, 2, 3, 5mm respectively) away from the heating point (see Fig. 1b.), a rectal thermometer monitored the internal body temperature of the animal. Since the body temperature drops rapidly during anaesthetic state, each animal was placed on a thermal pad. In addition, a heating lamp was placed 5 cm above the skull to keep the brain temperature at 37°C during the experiment. As a consequence, the body temperature was always kept at 36.5°C to 37°C.
Fig. 1.
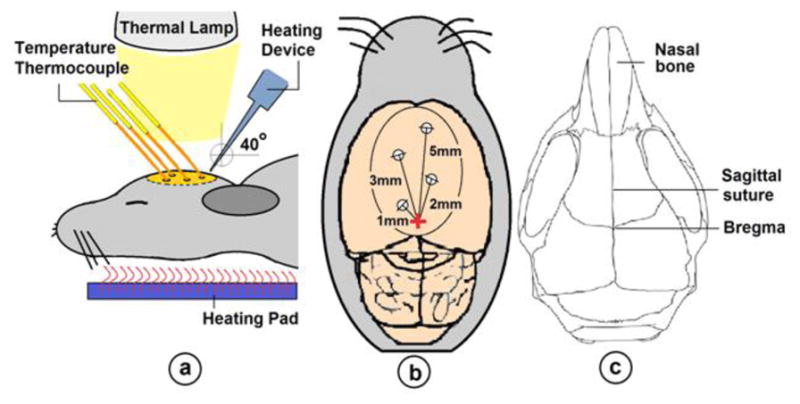
a) Experimental schematics for Part i. b) The dorsal view of the brain. The cross in the middle represents the position of the heating point. c) Heating was done over the skull near Bregma.
The purpose of this experiment was to examine the thermal distribution in the brain tissue. This resulted in a thermal map of the tissue represented in Section IV. It is important to mention that, the environment in the experimental suite was kept thermally neutral during all experiments.
2) Phase ii
The purpose of this part of the experiment was to examine the feasibility of hyperthermic disruption of the BBB as well as its recovery period from thermal damage using Evans blue staining technique. Here, nine mice, each 6–8 weeks of age, were randomly divided into three identical groups. In contrast to the previous part, no holes were drilled into the exposed surface of the skull of these animals. In order to correlate temperature patterns with stains left from the Evans blue dye, the heating parameters for all groups were kept the same as was described in the first part of this experiment.
a) Group I
All mice in this group were intravenously injected with 40mg/kg of body weight of pentobarbital and 4ml/kg body weight 2% Evans blue dye. As in Part i, quickly after anaesthesia each animal was positioned on a stereotaxic frame where a thermal pad and the lamp kept the body temperature steady at near 37°C. Just as before, the heating device was also placed at a 40° angle near and above Bregma and the exposure time was set to 30 minutes. All animals in this group were sacrificed one hour after injection of the dye. The animals’ brains were extracted and immersed in isopentane and kept on dry ice for further analysis.
b) Group II
To examine BBB’s ability to recover following hyperthermic disruption, all mice belonging to this group were prepared the same way as done in Group I except that the dye was injected 2 hours after 30 minutes of thermal treatment had ended. Exactly one hour after injection of the dye, the animals were sacrificed and their brains were removed, immersed in isopentane and kept on dry ice for further study.
c) Group III
All mice in this group served as controls and were injected with the Evans blue and before they were sacrificed. There was no staining of the dye found on the brain tissue of the mice from this group.
C. Data Analysis
Extracted brains were embedded in Optimal Cutting Compound where 50-micron coronal slices were made at −20°C in a cryostat. Results are shown in the next Section.
IV. Results
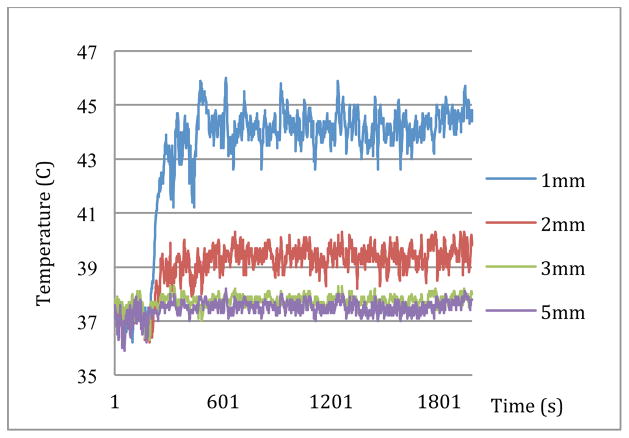
Results from the first part of the experiment are shown in Fig. 2. In this figure, recorded temperatures from 1mm and 2mm distances away from the heating device quickly raised once heating started and rapidly plateaued at approximately 44.3°C and 39.4°C respectively. Also, at the distances of 3mm and 5mm away from the heating device, temperatures reached 37.8°C and 37.6°C respectively.
Fig. 2.
Elevation of temperature in the brain tissue of a mouse by an external heating element
In phase ii, Evans blue dye was expected to have distributed throughout the entire body except the brain where they are forbidden entry prior to the hyperthermic disruption of the BBB. Following hyperthermia, the extracted brains from the animals of Group I revealed that hyperthermia could indeed disrupt the BBB and allow entry of a large and heavy molecule such as Evans blue into the brain tissue. Fig. 3 illustrates what the extracted brain from a mouse in this group resembles. As it is seen in this figure, the integrity of the BBB at where heat was applied was compromised leading to staining of that region of the brain.
Fig. 3.
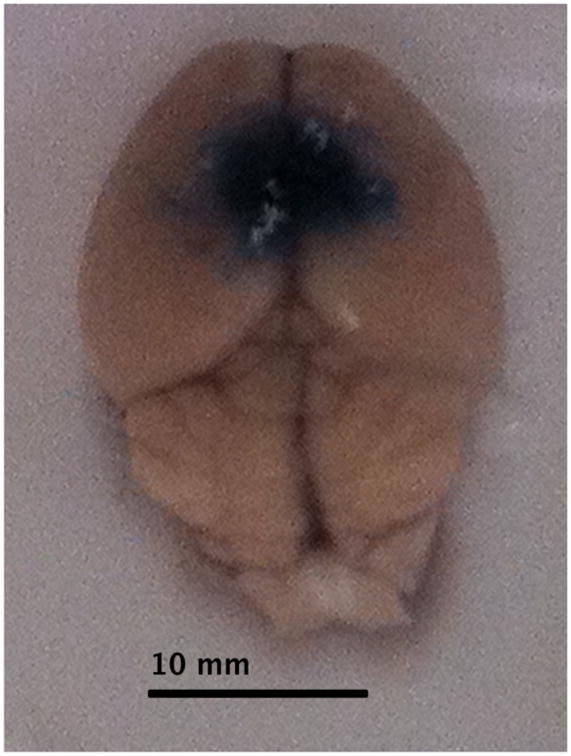
Appearance of the Evans blue dye near and around the heating point (Group I Animal #1)
A correlation between findings depicted in Fig. 2 and the sketch shown in Fig. 1b, lead to a thermal mapping of the brain in Fig. 4. The temperature curves presented in Fig. 2 indicate conduction of heat in the brain tissue regardless of the method heat has been produced. As mentioned before, MNPs are also able to create such thermal energy by relaxation processes. Thus, in the presence of micro-robots near the Bregma, distribution of heat generated by excitation of the embedded MNPs inside an AC field would be similar to Fig. 2. That is why the MNPs are considered the key component of the proposed micro-robots.
Fig. 4.
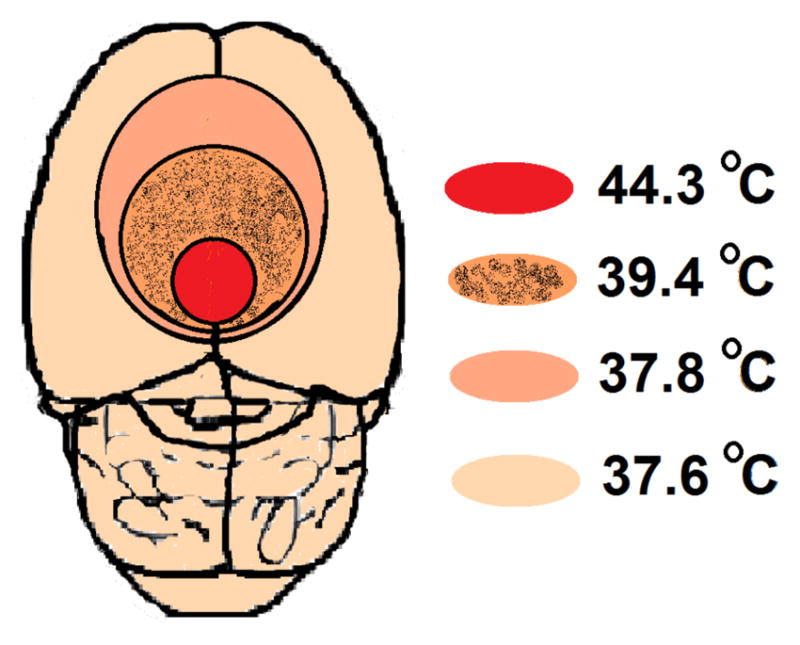
Brain Thermal Mapping
Results from the histological examination of the extracted brains are tabulated and presented in Table 2. As seen, all animals from the first group except one (#3) were affected by hyperthermia where Evans blue left a visible stain on the brain tissue around Bregma. It is believed that technical problems caused the anomaly for animal #3. Animals from the second group that received a 2-hour recovery period, showed substantial lower leakage area compared to the animals in Group I. The location of the leakage of the BBB and visible stains were also very close to the heating device where according to Fig. 2 must have reached to temperatures higher than 40°C. Therefore, although the BBB seem to have partially or in case of Animal #1 fully recovered from hyperthermic disruption, the recovery period may have not been sufficient for complete rejuvenation of the BBB. This requires further investigation. No evidence of BBB leakage for Group III could be found.
Table 2.
Histological examination of the BBB leakage of Evans blue dye
| Phase II | Animal # | Heating point position away from Bregma (mm) | Diameter of BBB leakage of Evans blue (mm) |
|---|---|---|---|
|
|
|||
| Group I Hyperthermic Disruption | 1 | 1.6 | 4.22 |
| 2 | 2.5 | 2.46 | |
| 3 | 1.66 | 0 | |
| Group II Recovery from Hyperthermia | 1 | 2.3 | 0 |
| 2 | 2.4 | 0.62 | |
| 3 | 2.2 | 1.98 | |
| Group III Control | 1 | N/A | 0 |
| 2 | N/A | 0 | |
| 3 | N/A | 0 | |
V. Discussion
Overcoming the BBB is an important field of current research that seeks a technique to reach the inside of the brain. To achieve brain localized drug delivery and increased efficacy, this project suggests that the therapeutic agents must be administered to the brain by means no more invasive than an intravenous injection of microcarriers or microrobots consisting of MNPs and therapeutic agents capable of remote propulsion and tracking compatible with MRN, and on-command actuation in the brain. The results of the preliminary experiments presented in this paper indicate that temperatures of 38°C and higher for an exposure time of 30 minutes are required for effective hyperthermic disruption of the BBB for crossing of large and heavy drug molecules. This crossing however is governed by the change of thermal energy or ΔT, which according to (2), is directly depended to the value of SAR. Therefore, controlling SAR leads to controlling the level of BBB leakage. For hyperthermia by induction of MNPs inside an AC field, as intended in this project, the SAR mainly depends on the field frequency and amplitude. Varying these parameters therefore, results in adjusting the BBB leakage to our favour. Thus, we have shown that this technique not only can be highly localized, it also provides advanced control over the opening of the BBB into the brain.
It must be mentioned that disturbance of the BBB at high levels may cause vasogenic edema and energy metabolic failure leading to subsequent structural brain damages. Evidently, the degree of pathophysiological changes in the vascular system of normal brain tissue is dependent on temperature and duration of heating. To minimize local hypo-perfusion and local brain cell death, thermal dosage as well as exposure period must be carefully selected and performed in a controlled environment.
VI. Conclusion
The main goal of modern pharmacology is the transport of drug molecules to specific targets while maintaining therapeutic efficacy. However, nearly 98% of drugs cannot enter the brain following systemic administration. Our group has previously pioneered a MRI-based drug delivery platform referred to as MRN that employs microcarriers or future microrobots capable of interventions in the vasculature. Here, our team is looking to equip these micro-entities with hyperthermic capabilities to disrupt the BBB and therefore be as effective in delivering therapeutic agents in the brain. This ability comes from the fact that these micro-entities rely on embedded MNPs that are excited once placed inside an AC field. This excitation leads to moderate elevation of temperature and thus transient disruption of the BBB. In light of this technique, local drug delivery for various disorders such as brain tumours, psychiatric, neurological and neurodegenerative disorders may also become feasible.
Acknowledgments
This study was possible, in part by the equipment financed by the Canadian Foundation for Innovation (CFI), Canada Research Chair (CRC) in Micro/Nanosystem, Canadian Institutes in Health Research (CIHR), the Fonds de Recherche en Santé du Québec’ (FRSQ), and the ‘Natural Sciences and Engineering Research Council of Canada’ (NSERC). Dr. Girouard is the holder of a new investigator award from the FRSQ and the ‘Heart and Stroke Foundation’ (HSFC).
Contributor Information
Seyed Nasrollah Tabatabaei, Ph.D. candidate with the Nanorobotics Laboratory, École Polytechnique de Montréal, QC, H3T 1J4, Canada.
Sonia Duchemin, Ph.D. candidate is a member of the Cerebrovascular Pharmacology Laboratory, Department of Pharmacology, Universite de Montreal, Pavillon Roger Gaudry, C.P. 6128, H3C 3J7, Montreal, QC, Canada.
Helene Girouard, Department of Pharmacology, Director of the Cerebrovascular Pharmacology Laboratory, Universite de Montreal, Pavillon Roger Gaudry, C.P. 6128, H3C 3J7, Montreal, QC, Canada
Sylvain Martel, Director of the Nanorobotics Laboratory, Department of Computer and Software Engineering, École Polytechnique de Montréal, QC, P.O. Box 6079 Station Centre-ville, H3C 3A7, Canada, (phone: +1 (514) 340-4711, ex. 5098; fax: +1 (514) 340-5280;).
References
- 1.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Molecular Interventions. 2003 Mar 1;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 2.Pouponneau P, Leroux JC, Martel S. Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials. 2009;30:6327–6332. doi: 10.1016/j.biomaterials.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Pouponneau P, Leroux J-C, Soulez G, Gaboury L, Martel S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2010.12.059. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Moriyama E, Salcman M, Broadwell RD. Blood-brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: I. Temperature and power measurements. Surgical Neurology. 1991;35:177–182. doi: 10.1016/0090-3019(91)90068-k. [DOI] [PubMed] [Google Scholar]
- 5.Sharma HS, Duncan JA, Johanson CE. Whole-body hyperthermia in the rat disrupts the blood-cerebrospinal fluid barrier and induces brain edema. In: Hoff JT, Keep RF, Xi G, Hua Y, editors. Brain Edema XIII. Vol. 96. Springer; Vienna: 2006. pp. 426–431. [DOI] [PubMed] [Google Scholar]
- 6.Lin JC, Yuan PMK, Jung DT. Enhancement of anticancer drug delivery to the brain by microwave induced hyperthermia. Bioelectrochemistry and Bioenergetics. 1998;47:259–264. [Google Scholar]
- 7.Masuda H, Hirata A, Kawai H, Wake K, Watanabe S, Arima T, Poulletier de Gannes F, Lagroye I, Veyret B. Local exposure of the rat cortex to radiofrequency electromagnetic fields increases local cerebral blood flow along with temperature. Journal of Applied Physiology. 2011;110:142–148. doi: 10.1152/japplphysiol.01035.2010. [DOI] [PubMed] [Google Scholar]
- 8.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. The Lancet Oncology. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. Journal of Physics D: Applied Physics. 2003;36:R167–R181. [Google Scholar]
- 10.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4. McGraw-Hill; 2000. [Google Scholar]
- 11.Fischer H, Gottschlich R, Seelig A. Blood-Brain Barrier Permeation: Molecular Parameters Governing Passive Diffusion. Journal of Membrane Biology. 1998;165:201–211. doi: 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- 12.BÈduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM. Brain drug targeting. United Kingdom: Cambridge University Press; 2001. [Google Scholar]
- 14.Pardridge WM. Drug and gene targeting to the brain via blood-brain barrier receptor-mediated transport systems. International Congress Series. 2005;1277:49–62. [Google Scholar]
- 15.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 16.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel Organization and Structure in Experimental Brain Tumors: Microvessel Populations with Distinctive Structural and Functional Properties. Microvascular Research. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 17.Friedl Josef, Turner Ewa, Alexander HR. Augmentation of endothelial cell monolayer permeability by hyperthermia but not tumor necrosis factor: Evidence for disruption of vascular integrity via VE-cadherin down-regulation. International Journal of Oncology. 2003;23:611–616. [PubMed] [Google Scholar]
- 18.Jeliazkova-Mecheva VV, Hymer WC, Nicholas NC, Bobilya DJ. Brief heat shock affects the permeability and thermotolerance of an in vitro blood-brain barrier model of porcine brain microvascular endothelial cells. Microvascular Research. 2006;71:108–114. doi: 10.1016/j.mvr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Michael Lv, et al. Magnetically induced hyperthermia: size-dependent heating power of β-Fe2O3 nanoparticles. Journal of Physics: Condensed Matter. 2008;20:204133. doi: 10.1088/0953-8984/20/20/204133. [DOI] [PubMed] [Google Scholar]
- 20.Hergt R, Dutz S. Magnetic particle hyperthermia--biophysical limitations of a visionary tumour therapy. Journal of Magnetism and Magnetic Materials. 2007;311:187–192. [Google Scholar]
- 21.Suh WH, Suslick KS, Stucky GD, Suh YH. Nanotechnology, nanotoxicology, and neuroscience. Progress in Neurobiology. 2009;87:133–170. doi: 10.1016/j.pneurobio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabatabaei SN, Lapointe J, Martel S. Shrinkable Hydrogel-Based Magnetic Microrobots for Interventions in the Vascular Network. Advanced Robotics. 2011;25:1049–1067. [Google Scholar]
- 23.Tabatabaei SN. Master. Institute of Biomedical Engineering, University of Montreal; Montreal: 2010. Evaluation of hyperthermia using magnetic nanoparticles and alternating magnetic field. [Google Scholar]
- 24.Lee J-H, Jang Jt, Choi J-s, Moon SH, Noh S-h, Kim J-w, Kim J-G, Kim I-S, Park KI, Cheon J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nano. 2011;6:418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 25.Pillai O, Panchagnula R. Polymers in drug delivery. Current Opinion in Chemical Biology. 2001;5:447–451. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 26.Jiles D. Introduction to magnetism and magnetic materials. 1. New York: Chapman and Hall; 1990. [Google Scholar]
- 27.Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lübbe AS. Locoregional Cancer Treatment with Magnetic Drug Targeting. Cancer Research. 2000 Dec 1;60:6641–6648. [PubMed] [Google Scholar]
- 28.Martel S, Felfoul O, Mathieu JB, Chanu A, Tamaz S, Mohammadi M, Mankiewicz M, Tabatabaei N. MRI-based Medical Nanorobotic Platform for the Control of Magnetic Nanoparticles and Flagellated Bacteria for Target Interventions in Human Capillaries. The International Journal of Robotics Research. 2009 Sep 1;28:1169–1182. doi: 10.1177/0278364908104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Lin M. Microwave hyperthermia-induced blood-brain barrier alterations. Radiation Research. 1982;89:77–87. [PubMed] [Google Scholar]
- 30.Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161:926–939. doi: 10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hed J, Dahlgren C, Rundquist I. A simple fluorescence technique to stain the plasma membrane of human neutrophils. Histochemistry and Cell Biology. 1983;79:105–110. doi: 10.1007/BF00494347. [DOI] [PubMed] [Google Scholar]
- 32.Duguet E, Hardel L, Vasseur S. Cell Targeting and Magnetically Induced Hyperthermia. In: Volz S, editor. Thermal Nanosystems and Nanomaterials. Vol. 118. Springer; Berlin/Heidelberg: 2009. pp. 343–365. [Google Scholar]
- 33.Moghimi SM, Hunter AC, Murray JC. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacological Reviews. 2001 Jun 1;53:283–318. [PubMed] [Google Scholar]