Abstract
Context
The epidemiology of prosthetic joint infection (PJI) in a population based cohort has not been studied in the United States.
Objectives
To provide an accurate assessment of the true incidence, secular trends, clinical manifestations, microbiology and treatment outcomes of PJI in a population based cohort.
Design
Historical cohort study
Setting
Olmsted County, Minnesota, United States of America.
Participants
Residents who underwent total knee arthroplasty (TKA) or total hip arthroplasty (THA) between 1/ 1/ 1969 and 12/ 31/ 2007.
Methods
Incidence rates and trends in PJI were assessed using the Kaplan Meier method and log-rank test, as were treatment outcomes among PJI cases.
Results
7375 THA or TKA were implanted in residents of Olmsted County during the study period. Seventy five discrete joints in 70 individuals developed PJI, during a mean(+/− SD) follow up of 6.8 (+/− 6.1) years. The cumulative incidence of PJI was 0.5%, 0.8% and 1.4% after 1, 5 and 10 years following arthroplasty, respectively. Overall, the rate of survival free of clinical failure after treatment of PJI was 76.8 % ( 95% CI: 64.3 – 85.2) and 65.2 % ( 95% CI: 33.1 – 76.2) at 3 years and 5 years, respectively. The incidence and treatment outcomes did not significantly differ by decade of implantation, patient age at implantation, gender or joint location.
Conclusions
The incidence of PJI is relatively low in a population based cohort, and is a function of age of the prosthesis. Incidence trends and outcomes have not significantly changed over the past forty years.
BACKGROUND
About 1,000,000 prosthetic joints are implanted each year in the United States. The number is projected to increase to over 4 million by 2030 (1, 2). Prosthetic joint infection (PJI) is a rare but serious complication of total joint arthroplasty, and may result in permanent prosthesis removal or rarely loss of limb or life (3). The cost of medical and surgical management of each PJI is in excess of $ 50, 000(4, 5).
The epidemiology of PJI at the population level has not been studied in the United States. Data on the incidence, microbiology, risk factors and outcomes are derived from single-center cohorts or convenient cohorts that are susceptible to significant referral bias. More-over, most studies have been limited by methodological problems including lack of explicit case or risk factor definitions, incomplete case ascertainment, and incomplete follow up (6–12).
Olmsted County is located in south-eastern Minnesota, and its population consists largely of middle-class white individuals with characteristics similar to those of the general US non Hispanic white population (13). According to the 2000 census the population of Olmsted County was 124,277(14). Residents derive their health care services including joint arthroplasties and management of PJI largely from Mayo Clinic, Rochester, and the Olmsted Medical Center (Rochester). The Rochester Epidemiology Project (REP) allows the linkage of the medical records for local residents who encounter the health care system within the county, regardless of the provider. Medical histories, diagnoses, surgical interventions and other key information can thus be accurately obtained for incidence data and population based analytic studies. The Rochester Epidemiology Project, is uniquely positioned to study the natural history of diseases in a defined population (13). This study will thus serve to provide an accurate assessment of the true incidence, secular trends, clinical manifestations, microbiology and outcomes of PJI in a U.S. population based cohort, and serve as a source of comparison to previous hospital based studies.
METHODS
This is a historical population based cohort study of all Olmsted County residents who underwent total knee arthroplasty (TKA) or total hip arthroplasty (THA) between January 1, 1969 and December 31, 2007. The subgroup of total joint recipients who experienced infection of their prosthesis constituted cases (Table 1). Once the diagnoses of the study population and potential cases were confirmed and residency established, the complete (inpatient and outpatient) medical record were reviewed and pertinent information abstracted. For every identified case, the clinical presentation, specific microorganisms responsible for the PJI, the type of antimicrobial agent used, duration of treatment, as well as modality of surgical management was recorded. All cases were followed passively through their medical record from the date of diagnosis until death, occurrence of clinical failure or loss to follow up. All aspects of this study were reviewed and approved by the Institutional Review Boards at Mayo Clinic Rochester and Olmsted Medical Center Rochester.
Table 1.
Definition of Terms
|
Statistical Analysis
Patient characteristics, clinical manifestations, microbiology, antimicrobial treatment, and surgical treatment modalities were summarized using descriptive statistics, including counts and percentages or means and standard deviations as appropriate. All patients who underwent an arthroplasty were followed prospectively until their last medical visit or first occurrence of PJI. The incidence of PJI was computed and expressed in two ways: 1) as the rate, per 1000 person joint-years, and 2) as the cumulative incidence (%) of PJI using the Kaplan-Meier method (one minus the “survival” estimate). With both sets of estimates, the rates of PJI were reported at 1, 2, 5 and 10 years following joint implantation for the overall cohort as well as by decade of implantation, patient age, gender and joint location (hip vs. knee). For these factors, Kaplan-Meier plots are shown to visually compare survival rates over time across categories, which were also formally compared with a log-rank test. Among the subgroup with subsequent PJI, the rate of survival free from clinical failure was also assessed using similar Kaplan-Meier methods as those described above. All analyses were carried out using the SAS statistical software package (version 9.2, SAS Institute, Cary, NC). A P value less than 0.05 was considered statistically significant.
RESULTS
Patient Selection
A total of 302 potentially eligible cases of PJI were identified from the Surgical Index of the Rochester Epidemiology Project. One hundred and eighty six were excluded after chart review, as the discharge diagnosis of PJI did not meet our case definition. The discharge diagnosis was not supported by any surgical findings, microbiology or histopathology reports. The status of two patients could not be verified because their medical records were not available for review. Of the 114 confirmed occurrences of PJI, 19 did not meet residency requirements (16 had not resided in Olmsted County for at least 365 days before the index arthroplasty, and 3 were residents only by virtue of being institutionalized). Nineteen Olmsted residents, diagnosed with PJI in the county, were excluded because the index arthroplasty was performed elsewhere. One patient had arthroplasty before 1969.
Incidence
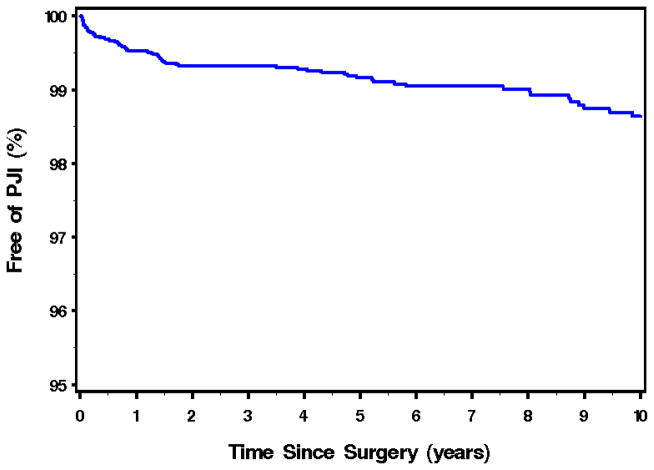
A total of 7375 total hip or knee arthroplasties were implanted in residents of Olmsted County during the study period. Excluding n=8 without any follow-up information available, the analysis cohort consisted of 7367 arthroplasties from 5456 unique subjects. Seventy-five discrete joints in 70 individuals developed infection, during a mean (± SD) follow up of 6.8 (± 6.1) years. Figure 1 illustrates the overall survival of total hip or knee arthroplasties free of infection for all residents over the study period. The overall unadjusted incidence rate (IR) of PJI was 1.50 per 1000 person joint years [95% CI: 1.18–1.87]. The highest IR was within the first year following arthroplasty (4.9/1000 person joint years [95% CI: 3.3–6.8]), with a decline to 3.5/1000 person joint years [95% CI: 2.6–4.7]; 2.0/1000 person joint years [95% CI: 1.5–2.6], and 1.6/1000 person joint years [95% CI: 1.3 – 2.1] at 2, 5 and 10 years respectively. The cumulative incidence of PJI expressed as a percentage of total knees and hips implanted, was 0.5% ( 95% CI: 0.3–0.7), 0.7% (95% CI: 0.5–0.9), 0.8% (95% CI: 0.6–1.1) and 1.4% (95% CI: 1.0–1.8) at 1,2, 5 and 10 years after implantation respectively. From univariate analysis, gender, age at implantation, joint location and year of implantation were not significantly associated with developing a PJI. Results of these analyses are summarised in Table 2 below.
Figure 1. Kaplan Meir Plot of Overall Survival of Total Hip or Knee Arthroplasty free of Prosthetic Joint Infection for Residents of Olmsted County, 1969–2007*.
*Number of Total Joints = 7367
Table 2.
Cumulative Incidence of Prosthetic Joint Infection at Two and Five years following Arthroplasty, by Age, Gender, Joint Location and Year of Primary Arthroplasty
| Group | 2-year Cumulative Incidence %(95% CI) | 5 year Cumulative Incidence %(95% CI) |
|---|---|---|
| Overall | 0.67 (0.50 – 0.90) | 0.84 (0.64 – 1.11) |
| Age(years) | ||
| <60 | 0.55 (0.26 – 1.18) | 0.79 (0.41 – 1.57) |
| 60–69 | 0.50 (0.26 – 0.97) | 0.77 (0.43 – 1.37) |
| 70–79 | 0.83 (0.52 – 1.33) | 0.96 (0.62 – 1.53) |
| 80+ | 0.74 (0.41 – 1.39) | 0.74 (0.41 – 1.73) |
| Gender | ||
| Female | 0.67 (0.46 – 0.97) | 0.74 (0.52 – 1.08) |
| Male | 0.68 (0.42 – 1.12) | 1.02 (0.66 – 1.59) |
| Joint Location | ||
| Hip | 0.52 (0.33 – 0.83) | 0.65 (0.42 – 1.04) |
| Knee | 0.83 (0.57 – 1.21) | 1.03 (0.72 – 1.47) |
| Year of implantation | ||
| 1969 – 1989 | 0.32 (0.14 – 0.72) | 0.39 (0.18 – 0.83) |
| 1990 – 1999 | 0.99 (0.62 – 1.57) | 1.18 (0.77 – 1.82) |
| 2000+ | 0.69 (0.45 – 1.07) | 0.96 (0.61 – 1.50) |
Characteristics of Patients with PJI
Of the 70 subjects with new PJI, 43 (61.4%) were females and 27 (38.6%) males. Age at the time of infection ranged from 41.6 to 95.0 years, with an average (± SD) of 72.6 (± 12.2) years. Among PJI subjects in whom race was known, 41 (93.2%) of 44 were Caucasian. Sixty percent of PJI episodes occurred within 2 years of primary joint implantation (71% within 2 years of index arthroplasty). Forty three (57%) of the 75 were knee infections and 32 (43%) hips. The most common underling joint diseases were degenerative joint disease (n=41, 55%), fracture (n=16, 21%) and rheumatoid arthritis (n=12, 16%). A majority of the affected joints (n=47, 63%) were preceded by an invasive procedure prior to the index arthroplasty. Fifteen (20%) had undergone at least one revision surgery. The clinical manifestations of PJI in this cohort is summarized in Table 3.
Table 3.
Clinical Features of 75 Episodes of Prosthetic Joint Infection*
| Symptom/Sign | Present (%) | Absent (%) |
|---|---|---|
| Pain/Tenderness | 70 (94.6) | 4 (5.4) |
| Swelling/Effusion | 35 (48.6) | 37 (51.4) |
| Redness/Erythema | 28 (38.9) | 44 (61.1) |
| Differential warmth | 25 (34.2) | 48 (65.8) |
| Fever | 27 (36.5) | 47 (63.5) |
| Purulent drainage/Sinus tract | 29 (39.7) | 44 (60.3) |
% expressed as proportion of the total. When the total is <75, it implies data missing or undocumented in the chart.
Microbiology
The most common micro-organisms causing PJI were Staphylococci, accounting for 39 (52.0%) infections. Staphylococcus aureus was isolated in 21 (28.0%) episodes, only 2 of which were methicillin resistant. Eighteen (24.0%) infections were attributable to Coagulase negative staphylococci, 3 (4.0%) of which were further identified as Staphylococcus lugdunensis. Eight (10.7%) patients experienced polymicrobial infection, and 6 (8.0%) persons had a culture negative PJI. Only one non bacterial infection occurred, in a patient with Coccidiodes imitis knee infection (Table 4). Peri-operative blood cultures were positive for the offending microorganism in 14 out of 56 (25.0%) episodes. MSSA bacteremia was the most common, occurring in 10 (58.8%) of 17 episodes, followed by Streptococcus bacteremia, in 3 (30%) of 10.
Table 4.
Microbiology of 75 Episodes of Prosthetic Joint Infection
| Microorganism/Class | Number | Percentage |
|---|---|---|
| MSSA | 19 | 25.3 |
| MRSA | 2 | 2.6 |
| Coagulase negative staphylococci | 18 | 24.0 |
| Streptococci | 13 | 17.3 |
| Enterococci | 3 | 4.0 |
| Gram Positive Bacilli | 2 | 2.7 |
| Gram Negative Bacilli | 1 | 1.3 |
| Anaerobes | 2 | 2.7 |
| Polymicrobial | 8 | 10.6 |
| Culture Negative | 6 | 8.0 |
| Fungi | 1 | 1.3 |
MSSA= Methicillin Susceptible Staphylococcus aureus
MRSA= Methicillin Resistant Staphylococcus aureus
Treatment
Over half of PJI episodes (41 out of 75, 54.7%) underwent debridement and retention of the prosthesis as the initial surgical management strategy. Sixteen (21.3%) episodes were treated by two-stage exchange surgery, while 5 (6.7%) had no surgical intervention. Other modalities of surgical management included resection arthroplasty (n=6), external arthrodesis (n=4), one stage surgery with oral antibiotic suppression (n=2), and amputation (n=1). All but 6 patients had adjunctive parenteral antibiotic therapy for a mean (± SD) duration of 27.8 (± 12.9) days. The most prescribed antibiotics were cephalosporins (n=36, 47.9%), vancomycin (n=18, 24.0%), and penicillins (n=13, 17.3%). For patients with retained prosthesis, long term oral antibiotic suppression was administered for a mean (± SD) duration of 26.2 (± 24.5) months during a maximum 5 year follow up period. Oral antibiotic choices were cephalosporins (n=19, 25.3%), penicillins (n=15, 20.0%), tetracyclines (n=10, 13.5%) and flouroquinolones (n=8, 10.7%).
Outcomes of patients with PJI
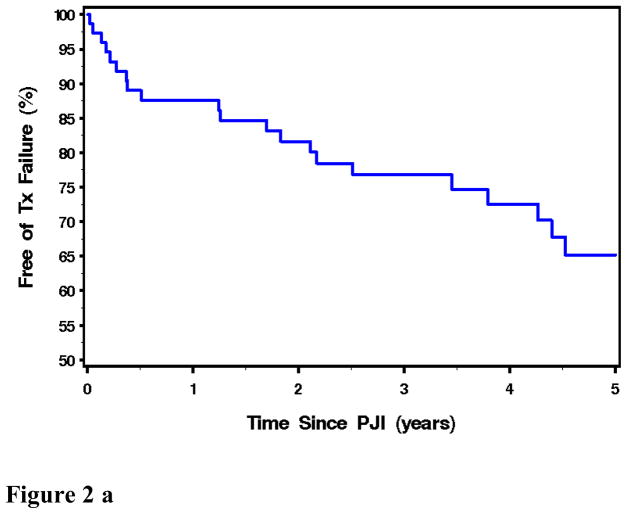
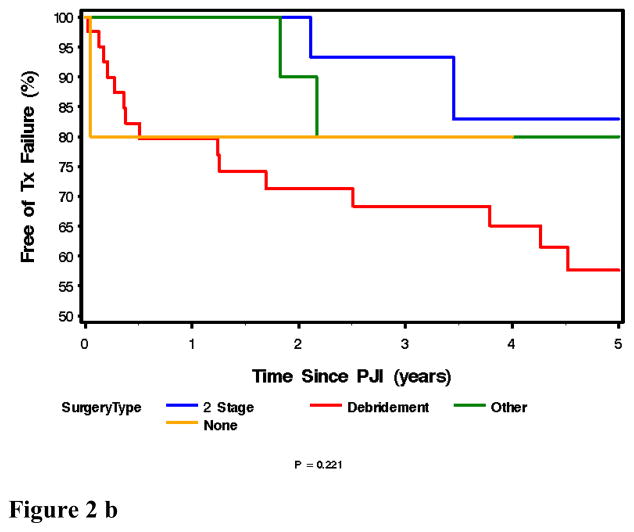
Seventy five episodes of PJI were passively followed for a mean (± SD) duration of 3.1 (± 1.8) years after diagnosis. Overall, the rate of survival free of clinical failure after treatment of PJI was 76.8% (95% CI: 64.3 – 85.1) and 65.2 % ( 95% CI: 33.1 – 76.2) at 3 years and 5 years, respectively (Figure 2a). There was not an overall difference in success rate across the various treatment types (p=0.22), although the rate of survival free of clinical failure was marginally (but not significantly) higher in the 2-stage exchange group when compared to only those treated with debridement/retention of prosthesis (p=0.069) (Figure 2b).
Figure 2.
Figure 2a, above: Kaplan Meir Plot of Overall Outcome Free of Treatment Failure for All Patients with Prosthetic Joint Infection. N= 75.
Figure 2b, above: Kaplan Meir Plot of Outcome Free of Treatment Failure for Patients with Prosthetic Joint Infection by Type of Initial Surgical Strategy. N= 75.
DISCUSSION
This is the first population based epidemiologic study of prosthetic joint infections in the United States. We began our case finding from March 10, 1969, when Dr. Coventry performed the first Food and Drug Administration (FDA)-approved total hip replacement surgery in the United States at Rochester Methodist Hospital, Rochester, Minnesota. All incident cases of PJI in Olmsted County from this date forth, were included, thus limiting sampling bias associated with case selection. We applied the same strict reference standard to all cases to minimize measurement bias. Every potential case was reviewed, referring to original microbiology, pathology and surgical reports, to confirm the diagnosis. We also applied strict residency status requirements that excluded patients who had not lived in Olmsted County for a year before arthroplasty and/or had their prostheses implanted elsewhere.
The limitations of this study are inherent to its retrospective nature. The details and accuracy of the clinical data collected is largely dependent on the written record and could not otherwise be verified by the investigators. The other limitation is the unexpectedly small sample size. This is a reflection of the true low incidence of PJI in this population, as well as our strict inclusion/exclusion criteria. The latter decision was taken a priori, to ensure that we had a “clean” population based cohort as possible even at the expense of potentially generating enough power to show statistically significant differences in incidence or outcomes between various sub groups of the study population. Findings of this study can be generalized to a predominantly middle class White U.S. population. The extent to which socio-economic factors and race/ethnicity affect outcomes in total joint arthroplasties is unknown. The major findings of our study are thus discussed in the context of the strengths and limitations outlined above.
Joint infection is a time dependent outcome following arthroplasty - the cumulative incidence increases with age of the implant. The cumulative incidence of PJI was 0.7% at 2 years and increased only slightly to 0.8% after 5 years of implantation. Our study confirms that the greatest hazard occurs in the first two years after primary or revision arthroplasty with about 60–70% of infections occurring during this period. This is in keeping with the widely held view that the mechanism of PJI is predominantly peri-operative contamination of the implant which manifests as early infection (within 3 months) or may be delayed for up to two years due to biofilm formation, with the micro-organisms assuming a dormant planktonic state under adverse conditions.
Our study highlights the limitations of estimating the incidence density for PJI (number of new infections per person joint years). Those estimates assume a constant risk over follow-up time, which is clearly not the case. We note how drastic the incidence rate changes depending on how much follow-up time you allow per person-joint (4.9 and 1.6 per 1000 person joint years at 1 and 10 years respectively).These results become difficult to explain and can be misleading.
The secular trends of PJI noted in this study may be considered paradoxical. Our study shows no statistically significant difference in incidence of PJI rates over four decades of implantation. The incidence of PJI is influenced by a number of factors. These include pre-operative morbidities such as obesity and diabetes mellitus, and intra-operative factors such as aseptic technique, surgical skills, and the operating room environment. We hypothesize that some of these factors acting in opposing directions, counterbalanced each other, resulting in no net statistically significant secular trend in the incidence of PJI. Our findings may also be an artefact of the small numbers.
We did not notice any trends that associate PJI with age of patient at time of prosthesis implantation, gender, or site of implant(hip vs. knee).
Staphylococci have been consistently isolated as the predominant pathogen in PJI. The major difference between this population based cohort and previous studies however, is the very high bacteremia (59%) associated with Staphylococcus aureus joint infections. Haematogenous seeding of total joint prosthesis is not unusual, especially with S. aureus, but rates this high have not been previously reported. There are several plausible explanations of this phenomenon. We postulate that our patients tend to present to their health care providers earlier, with joint pain and systemic symptoms (fever and other constitutional symptoms), and are less likely to have consumed antibiotics prior to evaluation. The health care providers are therefore more likely to sample their blood for microbiologic cultures compared to the usual chronic PJI host.
Overall, 3 out of every 4 infected prosthetic joints were free of clinical failure, three years after diagnosis and treatment. Two stage exchange surgery is associated with a more favorable 3 year outcome (>93%) compared to debridement and prosthesis retention (68%). There was no significant difference in success rates over the four decades.
CONCLUSIONS
The incidence of Prosthetic Joint Infection (PJI) is time dependent, with the greatest hazard within two years of arthroplasty. The 5 year cumulative incidence of PJI in this population is less than 1%, and has not significantly changed over the past 40 years. Staphylococci are the predominant pathogens, but Streptococci also play an important role in this population. Staphylococcus aureus PJI is often associated with bacteremia. Two stage exchange surgery has better outcomes free of clinical failure compared to debridement with prosthesis retention. The findings of our study highlight important similarities and major differences between the epidemiology of PJI in a population based cohort versus a hospital based sample.
Acknowledgments
Funding/Support This study was made possible by funding received by the Rochester Epidemiology Project (Grant #R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases) and the Small Grants Program, Division of Infectious Diseases, Department of Internal Medicine, Mayo Clinic, Rochester. This publication was also made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at: http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from: http://nihroadmap.nih.gov
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No relevant Conflict of interest of any of the authors.
References
- 1.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of Primary and Revision Total Hip and Knee Arthroplasty in the United States from 1990 through 2002. Journal of Bone and Joint Surgery, America. 2005;87:1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. Journal of Bone and Joint Surgery, America. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: Case-Control Study. Clinical Infectious Diseases. 1998;27:1247–1253. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 4.Herbert CK, Williams RE, Levy RS, Barrack RL. Cost of treating an infected total knee replacement. Clinical Orthopedics. 1996:140–5. doi: 10.1097/00003086-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Sculpo TP. The economic impact of infected total joint arthroplasty. Instr Course Lecture. 1993;42:349–51. [PubMed] [Google Scholar]
- 6.Wilson MG, Kelley K, Thornhill T. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in 67 cases. J Bone Joint Surg Am. 1990;72(6):878–883. [PubMed] [Google Scholar]
- 7.Fitzgerald RH, Jr, Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA, II, Coventry MB. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847–55. [PubMed] [Google Scholar]
- 8.Insall J, Scott WN, Ranawat CS. The total condylar knee prostheses. A report of two hundred and twenty cases. J Bone Joint Surg Am. 1979;61:173–80. [PubMed] [Google Scholar]
- 9.Petty W, Bryan RS, Coventry MB, Peterson LF. Infection after total knee arthroplasty. Orthop Clin North Am. 1989;20:201–10. [PubMed] [Google Scholar]
- 10.Wymenga AB, van Horn JR, Theeuwes A, Muytjens HL, Slooff TJ. Peri-operative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2651 hip operations. Acta Orthop Scan. 1992;63:665–71. doi: 10.1080/17453679209169732. [DOI] [PubMed] [Google Scholar]
- 11.Rand JA, Fitzgerald RH., Jr Diagnosis and management of the infected total knee arthroplasty. Orthop Clin North Am. 1989;20:201–10. [PubMed] [Google Scholar]
- 12.Bengston S, Knutson K. The infected knee arthroplasty. A 6-year follow up of 357 cases. Acta Orthop Scand. 1991;62:301–11. doi: 10.3109/17453679108994458. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.US Census Bureau. [Accessed December 13,2008];Olmsted County QuickFacts. Available at http://quickfacts.census.gov/qfd/states/27/27109.html.