Abstract
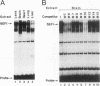
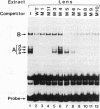
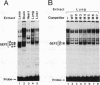
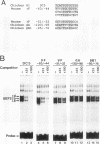
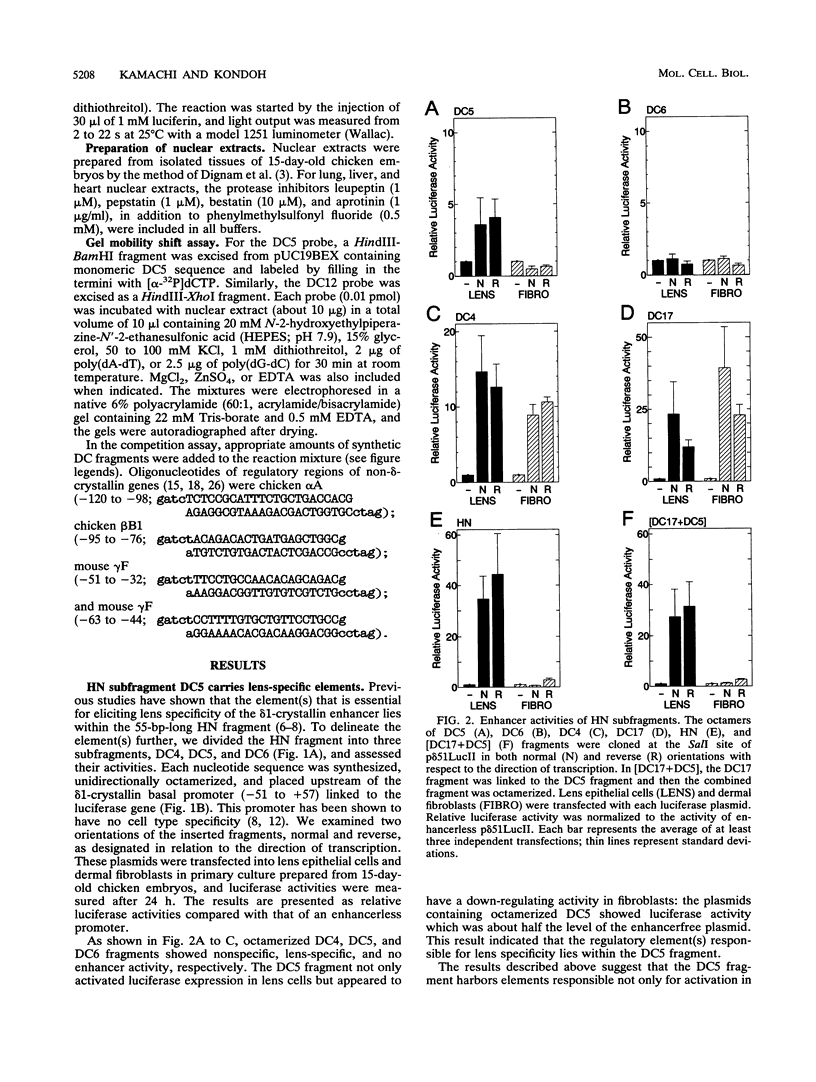
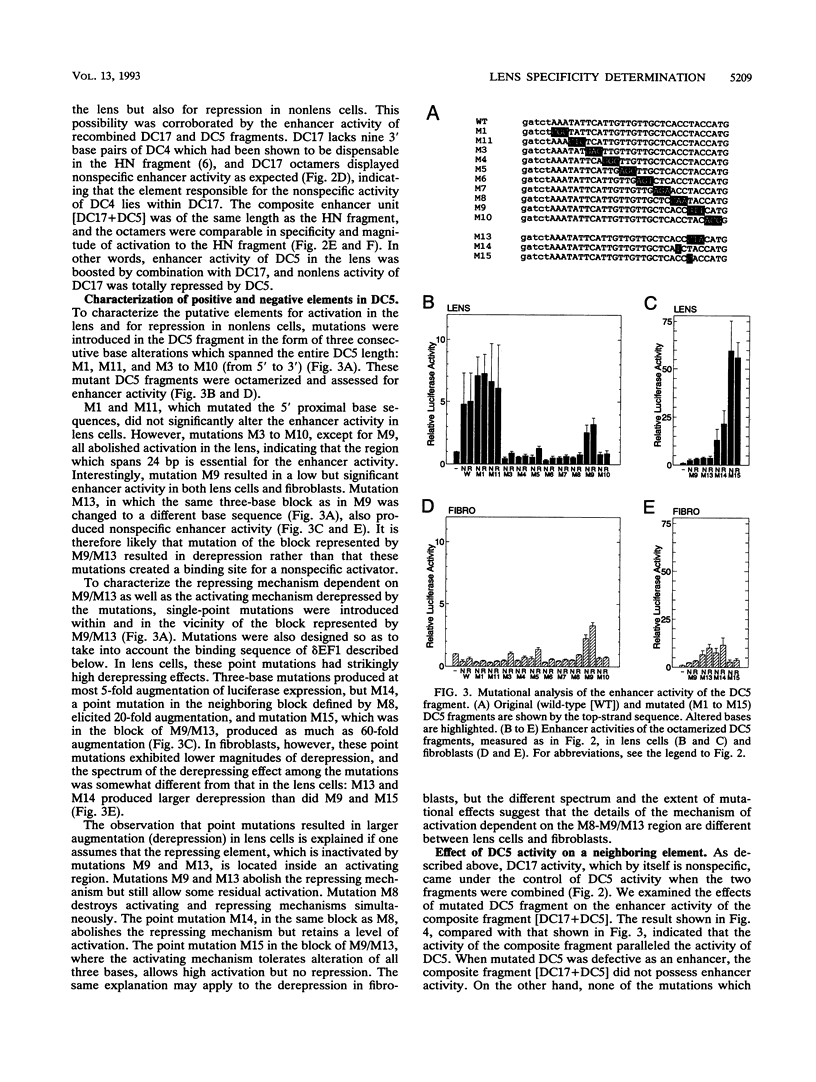
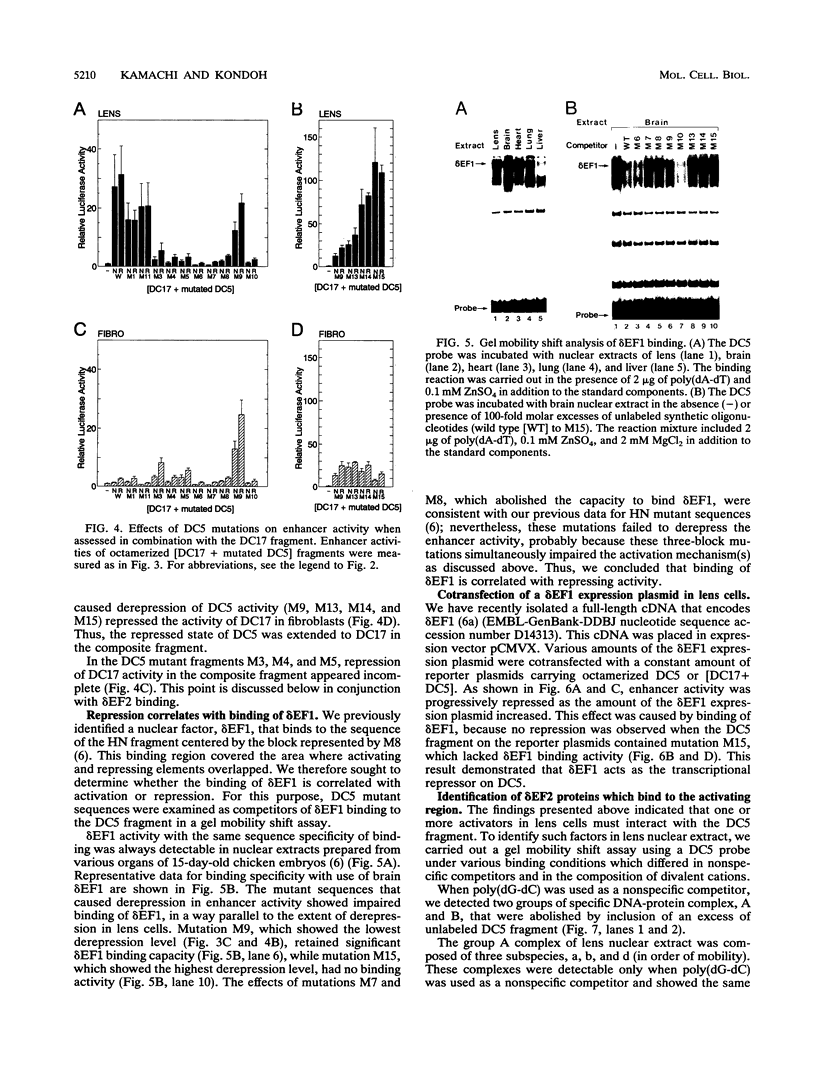
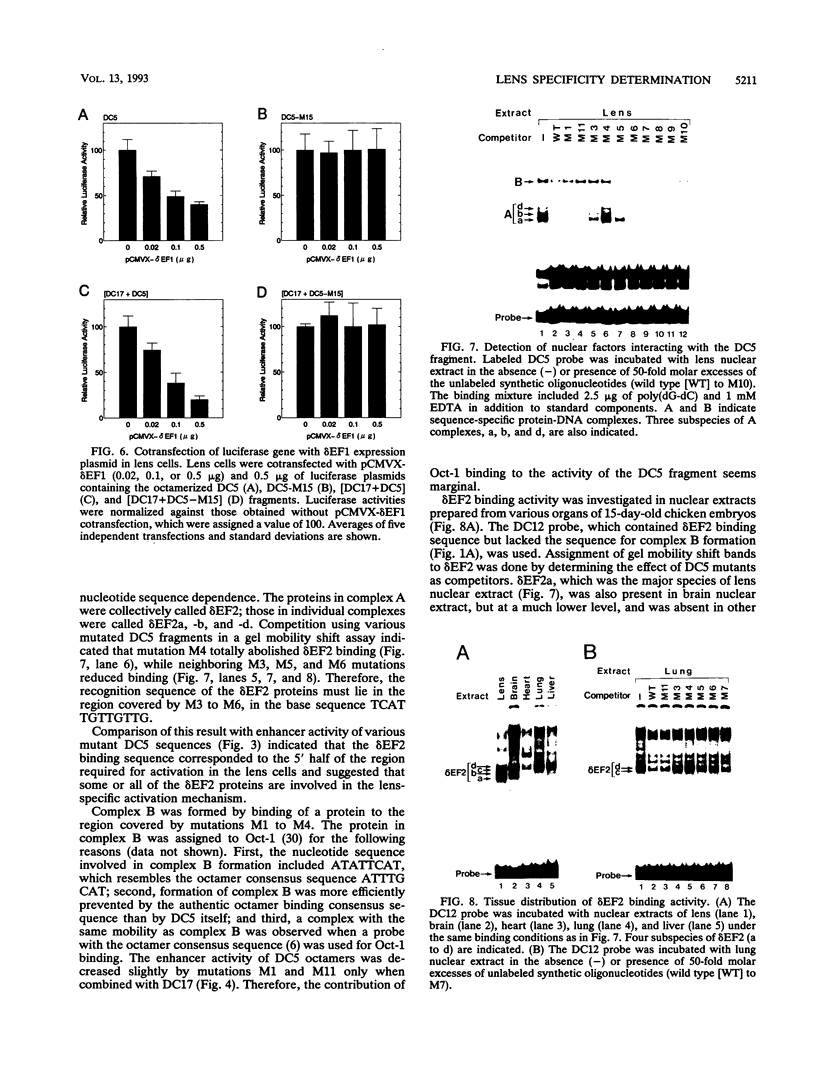
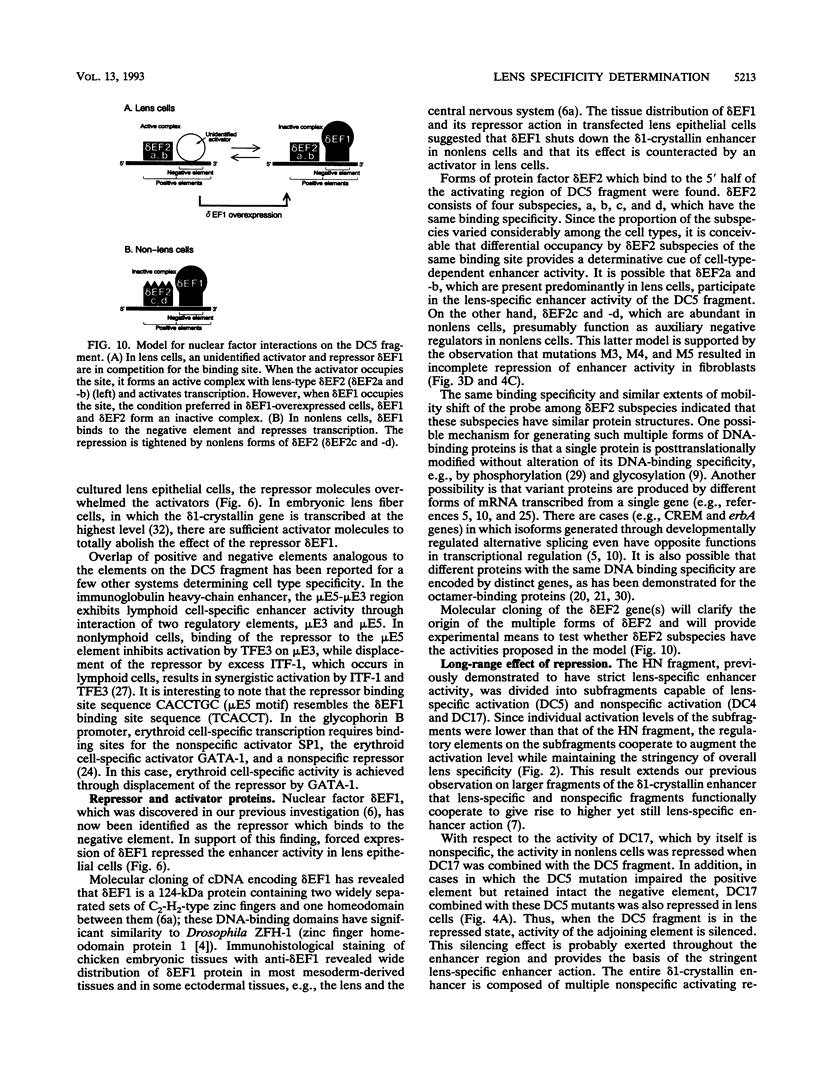
Lens-specific expression of the delta 1-crystallin gene is governed by an enhancer in the third intron, and the 30-bp-long DC5 fragment was found to be responsible for eliciting the lens-specific activity. Mutational analysis of the DC5 fragment identified two contiguous, interdependent positive elements and a negative element which overlaps the 3'-located positive element. Previously identified ubiquitous factors delta EF1 bound to the negative element and repressed the enhancer activity in nonlens cells. Mutation and cotransfection analyses indicated the existence of an activator which counteracts the action of delta EF1 in lens cells, probably through binding site competition. We also found a group of nuclear factors, collectively called delta EF2, which bound to the 5'-located positive element. delta EF2a and -b were the major species in lens cells, whereas delta EF2c and -d predominated in nonlens cells. These delta EF2 proteins probably cooperate with factors bound to the 3'-located element in activation in lens cells and repression in nonlens cells. delta EF2 proteins also bound to a promoter sequence of the gamma F-crystallin gene, suggesting that delta EF2 proteins are involved in lens-specific regulation of various crystallin classes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini M. E., Lai Z. C., Rubin G. M. The Drosophila zfh-1 and zfh-2 genes encode novel proteins containing both zinc-finger and homeodomain motifs. Mech Dev. 1991 Jun;34(2-3):113–122. doi: 10.1016/0925-4773(91)90048-b. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Funahashi J., Kamachi Y., Goto K., Kondoh H. Identification of nuclear factor delta EF1 and its binding site essential for lens-specific activity of the delta 1-crystallin enhancer. Nucleic Acids Res. 1991 Jul 11;19(13):3543–3547. doi: 10.1093/nar/19.13.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K., Okada T. S., Kondoh H. Functional cooperation of lens-specific and nonspecific elements in the delta 1-crystallin enhancer. Mol Cell Biol. 1990 Mar;10(3):958–964. doi: 10.1128/mcb.10.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Goto K., Okada T. S., Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987 Oct;1(8):818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Katoh K., Takahashi Y., Fujisawa H., Yokoyama M., Kimura S., Katsuki M., Saito M., Nomura T., Hiramoto Y. Specific expression of the chicken delta-crystallin gene in the lens and the pyramidal neurons of the piriform cortex in transgenic mice. Dev Biol. 1987 Mar;120(1):177–185. doi: 10.1016/0012-1606(87)90116-3. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Yasuda K., Okada T. S. Tissue-specific expression of a cloned chick delta-crystallin gene in mouse cells. Nature. 1983 Feb 3;301(5899):440–442. doi: 10.1038/301440a0. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Liu Q. R., Tini M., Tsui L. C., Breitman M. L. Interaction of a lens cell transcription factor with the proximal domain of the mouse gamma F-crystallin promoter. Mol Cell Biol. 1991 Mar;11(3):1531–1537. doi: 10.1128/mcb.11.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Stevens W., Breitman M. L., Tsui L. C. Multiple regulatory elements of the murine gamma 2-crystallin promoter. Nucleic Acids Res. 1989 May 11;17(9):3563–3582. doi: 10.1093/nar/17.9.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Yasuda K. The cooperative interaction between two motifs of an enhancer element of the chicken alpha A-crystallin gene, alpha CE1 and alpha CE2, confers lens-specific expression. Nucleic Acids Res. 1992 Jul 25;20(14):3701–3712. doi: 10.1093/nar/20.14.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Rahuel C., Vinit M. A., Lemarchandel V., Cartron J. P., Roméo P. H. Erythroid-specific activity of the glycophorin B promoter requires GATA-1 mediated displacement of a repressor. EMBO J. 1992 Nov;11(11):4095–4102. doi: 10.1002/j.1460-2075.1992.tb05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C., Cohn L., Calame K. A dominant negative form of transcription activator mTFE3 created by differential splicing. Science. 1991 Oct 4;254(5028):94–97. doi: 10.1126/science.1840705. [DOI] [PubMed] [Google Scholar]
- Roth H. J., Das G. C., Piatigorsky J. Chicken beta B1-crystallin gene expression: presence of conserved functional polyomavirus enhancer-like and octamer binding-like promoter elements found in non-lens genes. Mol Cell Biol. 1991 Mar;11(3):1488–1499. doi: 10.1128/mcb.11.3.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruezinsky D., Beckmann H., Kadesch T. Modulation of the IgH enhancer's cell type specificity through a genetic switch. Genes Dev. 1991 Jan;5(1):29–37. doi: 10.1101/gad.5.1.29. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Hanaoka K., Hayasaka M., Katoh K., Kato Y., Okada T. S., Kondoh H. Embryonic stem cell-mediated transfer and correct regulation of the chicken delta-crystallin gene in developing mouse embryos. Development. 1988 Feb;102(2):259–269. doi: 10.1242/dev.102.2.259. [DOI] [PubMed] [Google Scholar]
- Thomas G., Zelenka P. S., Cuthbertson R. A., Norman B. L., Piatigorsky J. Differential expression of the two delta-crystallin/argininosuccinate lyase genes in lens, heart, and brain of chicken embryos. New Biol. 1990 Oct;2(10):903–914. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]