Abstract
The initiation and progression of pancreatic ductal adenocarcinoma (PDAC) is governed by a series of genetic and epigenetic changes, but it is still unknown whether these alterations are required for the maintenance of primary and metastatic PDAC. We show here that the c-Myc oncogene is upregulated throughout the entire process of neoplastic progression in human PDAC and in genetically engineered mice that express mutant Kras. To experimentally address whether c-Myc is essential for the growth and survival of cancer cells, we developed a novel mouse model that allows a temporally and spatially controlled expression of this oncogene in pancreatic progenitors and derived lineages of the exocrine pancreas. Unlike previous reports, upregulation of c-Myc was sufficient to induce the formation of adenocarcinomas after a short latency without additional genetic manipulation of cell survival pathways. Deficiency in Cdkn2a increased the rate of metastasis but had no effect on tumor latency or c-Myc-mediated cancer maintenance. Despite a macroscopically complete regression of primary, metastatic, and transplantable tumors following the ablation of c-Myc, some cancer cells remained dormant. A significant number of these residual neoplastic cells expressed cancer stem cell markers, and re-expression of exogenous c-Myc in these cells led to rapid cancer recurrence. Collectively, the results of this study suggest that c-Myc plays a significant role in the progression and maintenance of PDAC, but besides targeting this oncogene or its downstream effectors, additional therapeutic strategies are necessary to eradicate residual cancer cells to prevent disease recurrence.
Keywords: Pancreatic Cancer, Pancreatic Ductal Adenocarcinoma, Oncogenes, c-Myc, Disease Progression, Metastasis, Cancer Cell Dormancy, Cancer-initiating Cells, Genetically-engineered Mice, Cre recombinase, Tetracycline-controlled transactivation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and one of the most lethal human malignancies. Molecular studies have identified a number of genetic and epigenetic changes that affect the functionality of tumor susceptibility genes that, based on their frequent occurrence at particular stages of carcinogenesis, are suggested to play a role in the initiation and promotion of PDAC (1, 2). Among those genes, KRAS, CDKN2a, and TP53 have been shown to play a pivotal role in PDAC formation and progression (3, 4). Besides these frequently mutated loci, there are oncogenes that do not carry mutations but their deregulated expression contributes significantly to the pathogenic process. Specifically, increased expression of c-MYC has been reported in a significant subset of primary PDACs and derived cell lines (5, 6). Beside transcriptional upregulation, c-MYC is amplified in a subset of PDACs, and this gene was listed among the core signaling pathways that are genetically altered in pancreatic cancers (7). A recent study by Ying and colleagues (8) suggested that c-Myc is an essential mediator of Kras-induced changes in the metabolism of pancreatic cancer cells.
Although the stage at which c-MYC is upregulated during pancreatic carcinogenesis is unknown, experimental evidence in animal models suggests that an elevated expression of this oncogene contributes to neoplastic transformation in cells of the exocrine and endocrine pancreas. While expression of c-Myc under the elastase (Ela) promoter is sufficient to induce acinar-type tumors (9), the upregulation of this oncogene seems to have discrete biological effects on endocrine cells. A slight elevation in c-Myc expression in β-cells led to islet hyperplasia (10). A more pronounced upregulation of this oncogene, however, caused cell death, and only when apoptosis was inhibited did c-Myc facilitate the formation of insulinomas (11). The cureent paradigm that upregulation of c-Myc alone might be insufficient to cause cancer in the pancreas was supported by Lewis and coworkers (12), who used a retroviral-based gene transfer into Ela-TVA transgenics to ectopically express c-Myc. Unlike the Ela-Myc model, the upregulation of this oncogene using the retroviral approach did not lead to the initiation of tumors with features of the exocrine pancreas, but this experimental strategy did trigger the development of endocrine tumors in conjunction with a functional inhibition of Cdkn2a.
Collectively, the previous studies in transgenic mouse models support the notion that c-Myc preferentially transforms endocrine cells and leads to neoplasms that resemble human insulinomas. These neuroendocrine tumors, however, are less common, and it is evident that none of these current models are suitable to study the biological significance of c-Myc in PDAC initiation and disease maintenance. Here, we describe the development of a new pancreatic cancer model that allows a temporally and spatially controlled expression of c-Myc in pancreatic progenitors and derived lineages of the exocrine pancreas. Unlike any of the previous models, upregulation of c-Myc alone leads to a rapid initiation of ductal precursor lesions and formation of adenocarcinomas that have the propensity of metastasizing to the liver. The ligand-mediated downregulation of c-Myc expression in ductal precursor lesions as well as primary and metastatic pancreatic cancers resulted in cell death in a p19Arf/p53-independent manner. Nonetheless, a few cancer cells remain dormant and contribute to a swift disease recurrence upon re-expression of c-Myc. The results of this study suggest that c-Myc plays a significant role in the initiation and maintenance of pancreatic cancer, but a complete eradication of dormant cancer cells is a necessity to prevent cancer recurrence.
Materials and Methods
Mouse models and in vivo bioluminescence imaging
The generation and genotyping of the TetO-Myc strain as well as the CAG-βgeo-tTA and TeO-Luc transgenic lines were described previously (13, 14). The CAG-GFP reporter strain was generated by Kawamoto et al. (15). Pdx1-Cre transgenics and the Cdkn2a knockout strain (3, 16) were obtained from the NCI repository. TetO-H2B/GFP transgenic mice (17) were purchased from the Jackson Laboratory. The administration of doxycycline (Dox) and the use of the IVIS200 (Caliper Life Sciences, Alameda, CA) for in vivo bioluminescence imaging was described previously (14).
Histologic analysis and immunostaining
Detailed protocols for the preparation of histological sections and for immunostaining can be found elsewhere (18). Antibodies against CK19 and Pdx1 were obtained from the Iowa Hybridoma Bank, and the c-Myc antibody (Y69) was purchased from Epitomics. A list of all other primary and secondary antibodies and staining conditions will be provided upon request. TUNEL staining was carried out using the in situ cell death detection kit (Roche Applied Sciences, Indianapolis, IN). Stained slides were examined with an Axio Imager microscope (Carl Zeiss) or a LSM5 PASCAL confocal microscope.
Orthotopic transplantation pancreatic tumors
Freshly isolated pancreatic cancer tissues from transgenic mice were washed in 1x PBS and cut into small pieces of 1–3 mm in diameter. One or two small pieces from a tumor were transplanted orthotopically into normal pancreatic tissues of 8-week-old NCrnu/nu mice. The rate of engraftment using this method as determined by in vivo imaging was 100%.
Flow cytometric analysis of GFP-labeled cancer cells
Areas with small residual tumor masses that contained GFP-positive cells were isolated under a fluorescent stereoscope and processed for enzymatic dissociation as described (19). GFP-positive cells were collected using FACS, and stained with a diluted cocktail of antibodies. These included biotinylated or fluorochrome-conjugated rat anti-mouse CD31, CD45, CD24, CD44 (BD Biosciences), CD133 (Miltenyi Biotec), Sca-1 (eBioscience) and a streptavidin protein tagged with a fluorochrome (BD Biosciences). After a 20-minute incubation on ice, cells were washed and resuspended for flow cytometry.
Results
Overexpression of c-Myc is a very early event during pancreatic carcinogenesis
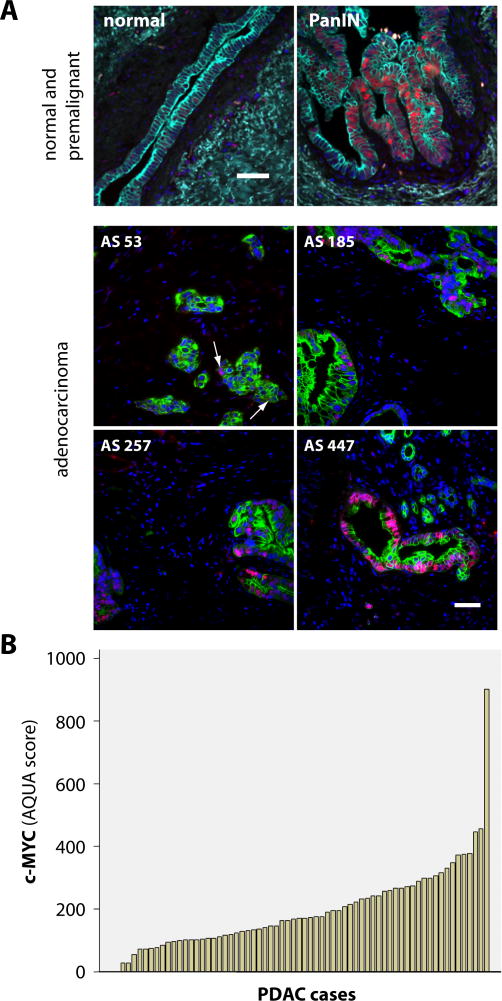
In an initial experiment, we determined the expression levels of the c-MYC protein in eight human pancreatic cancer cell lines including two lines (BXPC-3 and Hs766T) that were reported to carry wildtype Kras. In comparison to normal pancreatic ductal cells, all cancer cell lines exhibited elevated levels of c-MYC (Suppl. Fig. S1). To define the stage at which c-MYC is first upregulated during pancreatic carcinogenesis, we performed immunofluorescence staining on pancreatic tissues that were collected from patients of the UNMC rapid autopsy program. Nuclear localized c-MYC was not detected in normal cytokeratin (CK) 19-positive ductal cells, but it was clearly present in 8 out of 13 (62%) specimens with pancreatic intraepithelial neoplasia (PanINs, stages 1–3) which are the earliest known ductal precursor lesions (Fig. 1A, upper panel). Next, we performed a quantitative image analysis to determine the levels of immunofluorescence staining of c-MYC in 65 primary PDACs (Fig. 1A, lower panel). While there was no detectable expression of c-MYC in normal ductal cells (not shown), 25 of 65 pancreatic cancer specimens (38%) exhibited an AQUA score of greater than 200 (Fig. 1B), which is well above any background fluorescence and corresponds to samples where the majority of cancer cells stained positive for c-MYC.
Fig. 1. c-MYC is upregulated in ducal precursor lesions as well as pancreatic ductal adenocarcinomas (PDACs) in humans.
A. Immunofluorescence staining of c-MYC (red, nuclear) and CK19 (green) in the normal pancreas, pancreatic intraepithelial neoplasia, PanIN (upper panel), and selected pancreatic ductal adenocarcinomas (four lower panels). The AQUA score (AS) in the lower panel is indicative for the intensity of the c-MYC staining pattern; bars represent 50 μm. B. Waterfall plot of AQUA scores (automated quantitative image analysis) of 65 individual PDAC cases
In addition to human pancreatic cancer specimens, we analyzed the expression of c-Myc in genetically engineered mice that constitutively express mutant Kras in the exocrine pancreas. While c-Myc was highly overexpressed in a Kras-induced tumor, there was also a noticeable increase in the levels of this oncogene in a tumor-free, 10-month-old animal (Suppl. Fig. S2A). To assess whether c-Myc is upregulated in ductal precursor lesions, we stained this protein in pancreatic tissues of mice that express mutant Kras in a p53 haploinsufficient background. We observed that high-grade PanINs as well as PDACs exhibited a very strong nuclear staining in all tissue specimens (Suppl. Fig. S1B).
Collectively, the results from this inaugural study on the expression pattern of c-MYC during pancreatic tumorigenesis suggest that the upregulation of this oncogene is a very early event in pancreatic carcinogenesis in humans and Kras-induced mouse models for PDAC. The fact that c-Myc is still overexpressed in a significant subset of advanced PDACs supports the notion that this oncogene might also be required for the proliferation and survival of malignant cancer cells.
Development of genetically engineered mice that permit a temporally and spatially controlled expression of c-Myc in the exocrine pancreas
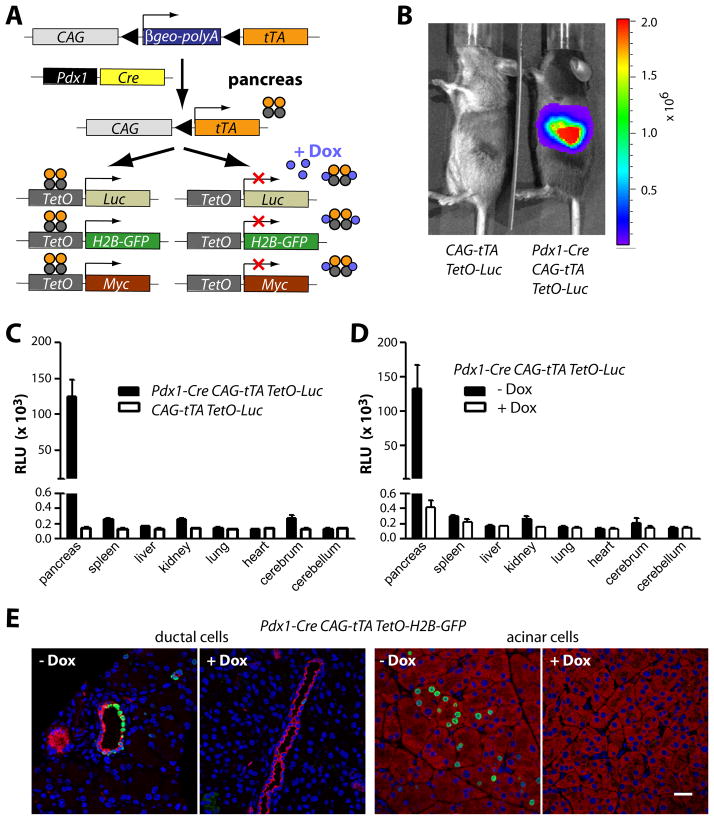
To study the role of c-Myc during pancreatic cancer initiation and progression, we developed a novel animal model that allows a ligand-controlled expression of c-Myc in progenitors and derived cell lineages in the pancreas of adult animals. The genetically engineered model is based on the combination of the Cre/loxP and the tetracycline (tet) responsive systems (Fig. 2A) and requires three transgenes: Pdx1-Cre, CAG-βgeo-tTA, and TetO-Myc. Cre recombinase under the regulation of the Pdx1 promoter initiates a constitutive expression of the tetracycline-controlled transactivator (tTA) in pancreatic progenitors and their descendants. The pancreas-specific expression of the tTA subsequently induces the activation of TetO-driven responder transgenes encoding c-Myc as well as the GFP and luciferase reporters. The expression of these responder genes can be controlled in a temporal manner though administration of doxycycline (Dox). Using bioluminescence imaging, we confirmed that expression of the luciferase reporter is restricted to the pancreas (Fig. 2B, 2C), and the level of transgene expression can be effectively controlled through administration of Dox (Fig. 2D). The inclusion of a TetO-H2B-GFP reporter revealed that the expression of GFP was confined to approximately 40% of pancreatic ductal cells and less than 10% of acinar cells under nonselective conditions (i.e., without the TetO-Myc transgene) (Fig. 2E).
Fig. 2. A novel genetically engineered model that permits a temporally and spatially controlled expression of oncogenes in the exocrine pancreas.
A. genetically engineered model on the basis of three transgenes, Pdx1-Cre, CAG-tTA, and TetO-driven responder genes B. In vivo bioluminescence imaging of a Pdx1-Cre/CAG-βgeo-tTA/TetO-Luc transgenic mouse and a littermate control without Pdx1-Cre. C. Conventional luciferase assay in selected tissues of the mice shown in panel B; D. luciferase assay in selected tissues of triple transgenic mice with and without administration of doxycycline (Dox); E. Immunofluorescence staining of H2B/GFP (green) and CK19 (red, ductal cells) or amylase (red, acinar cells) in triple transgenic mice with and without administration of Dox; bar represents 20 μm.
Expression of c-Myc causes formation of pancreatic adenocarcinoma with sporadic metastasis to the liver
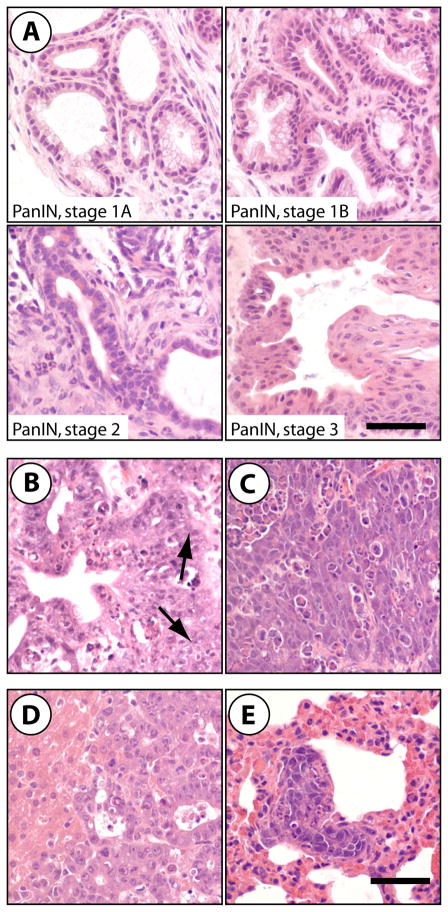
Despite normal appearance at birth, mice that conditionally express c-Myc in the pancreas developed pancreatic tumors as early as 14 days post partum. Mice presented in a moribund state were sacrificed for pathological examination (on average around 33 days of age), and all Pdx1-Cre CAG-tTA TetO-Myc triple transgenic mice developed pancreatic neoplasms in less than 5 months. In contrast, none of the littermate control mice lacking the Pdx1-Cre transgene developed cancer. The histopathological examination revealed that 43% of all triple transgenic mice developed only ductal lesions that include PanINs (Fig. 3A) and invasive PDACs with sporadic metastasis to the liver (Fig. 3B, 3D). A smaller subset (11%) exhibited exclusively poorly differentiated adenocarcinomas (Fig. 3C) that were capable of metastasizing to the liver, diaphragm, and lung (Fig. 3E). The remaining forty-six percent of animals had both ductal lesions and poorly differentiated carcinomas, with a few animals carrying moderately differentiated lesions that had an acinar-like appearance (not shown).
Fig. 3. Expression of exogenous c-Myc in the exocrine pancreas causes ductal precursor lesions as well as adenocarcinomas that sporadically metastasize to the liver.
H&E-stained sections of pancreatic neoplasms of Pdx1-Cre/CAG-βgeo-tTA/TetO-Myctransgenic mice; A. PanIN lesions, stages 1A through stage 3; B. invasive pancreatic ductal adenocarcinoma (PDAC), arrows indicate invasive fronts; C. poorly differentiated pancreatic adenocarcinoma; D. liver metastasis from a primary PDAC; E. pulmonary metastasis; bars represent 50 μm.
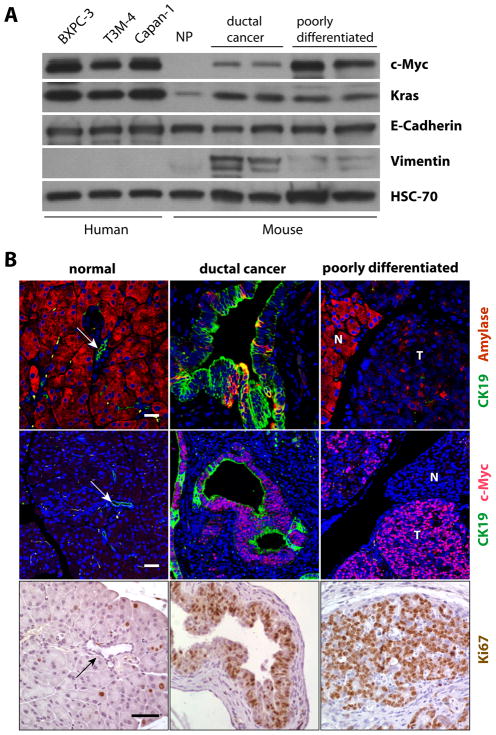
While c-Myc was undetectable in the normal pancreas of wildtype littermate controls by western blot analysis, this protein was clearly upregulated within ductal and poorly differentiated adenocarcinomas (Fig. 4A, Suppl. Fig. 3). The lower expression of c-Myc in ductal neoplasms compared to less differentiated tumors can partially be explained by the increased presence of vimentin-positive, cancer-associated fibroblasts. The western blot results also showed that the levels of exogenous c-Myc in pancreatic tumors of the newly generated mouse model do not greatly exceed the elevated expression of endogenous c-MYC in human pancreatic cancer cell lines (Fig. 4A) or Kras –associated tumors in mice (Suppl. Fig. 3).
Fig. 4. Upregulation of c-Myc results in highly proliferative pancreatic adenocarcinomas.
A. Western blot analysis to compare the levels of c-Myc expression between the two major types of adenocarcinomas (ductal and poorly differentiated) in transgenic mice and three selected human PDAC cell lines; NP, normal pancreas of a wildtype mouse. B. Immunofluorescence staining of amylase and CK19 (upper panel) or c-Myc and CK19 (middle panel) as well as immunohistochemical staining of Ki67 (lower panel) in the normal pancreas, ductal carcinomas, and poorly differentiated carcinomas in Pdx1-Cre/CAG-βgeo-tTA/TetO-Myc triple transgenic mice; N, normal; T, tumor; bars represents 20 μm in upper and middle panels and 50 μm in lower panels. The arrows in the images of the normal pancreas point to CK19-positive ducts.
All duct-type neoplastic lesions were positive for CK19, whereas the poorly differentiated adenocarcinomas exhibited very low expression of both CK19 and amylase, which typically is highly expressed in acinar cells (Fig. 4B). In addition to Sox9, some expression of Pdx1 was retained in ductal neoplasms, but these transcription factors were not detectable in the poorly differentiated subtype (Suppl. Fig. S4). All pancreatic adenocarcinomas expressed E-Cadherin, but Muc1, EGFR, and ErbB2 were largely restricted to the duct-type lesions (Suppl. Table 1). Regardless of the histopathologic subtype, all cancer tissues exhibited a widespread expression of Ki67 in addition to c-Myc, suggesting that these neoplastic lesions were highly proliferative (Fig. 4B). Similar to previous observations in islet cells, elevated levels of c-Myc can induce a pro-apoptotic response in untransformed normal epithelial cells of ducts and even in tumor cells (Suppl. Fig. S5A). The induction of apoptosis in response to oncogenic stress, however, was restricted to a smaller subset of cells expressing the oncogene. The c-Myc-induced proliferation rate was clearly higher than the number of apoptotic cells, which might explain the rapid onset of pancreatic tumorigenesis in this new cancer model.
Downregulation of c-Myc leads to cell death and pancreatic cancer regression
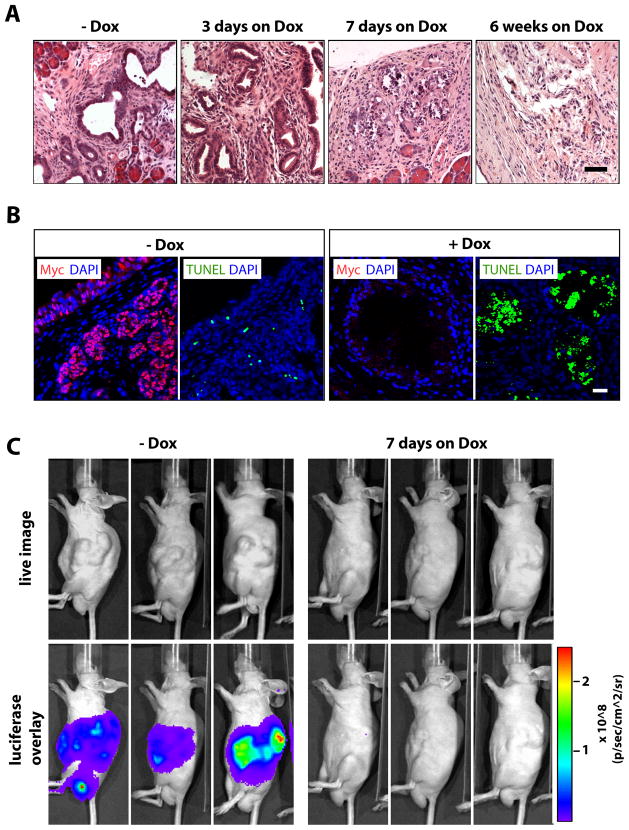
To determine whether the growth and survival of pancreatic cancer cells depended on the continuous presence of c-Myc, we treated 40 to 50-day-old Pdx1-Cre CAG-tTA TetO-Myc transgenic mice that developed palpable pancreatic neoplasms with Dox for a period of 3 days, 7 days, and 6 weeks. The histological examination of pancreatic tissues showed that a 7-day administration of Dox was sufficient to induce cancer regression (Fig. 5A). Interestingly, the selective ablation of pancreatic cancer cells did not result in a remodeling of the tumor associated stroma even after a prolonged treatment with Dox for 6 weeks. Using immunofluorescence staining, we confirmed that the cancer attrition process was a direct consequence of the Dox-controlled suppression of c-Myc expression and induction of cell death (Fig. 5B).
Fig. 5. Regression of primary pancreatic adenocarcinomas in response to the downregulation of c-Myc.
A. H&E-stained sections of pancreatic tissues from Pdx1-Cre/CAG-βgeo-tTA/TetO-Myc transgenic mice that were treated with Dox for 3 days, 7 days, or 6 weeks. B. TUNEL (green) and immunofluorescence staining of c-Myc (red) in pancreatic tissues from tumor-bearing mice prior to (−Dox) or after 7 days of treatment with Dox (+Dox), slides were counterstained with DAPI; bar represents 20 μm. C. Bioluminescence imaging on recipient mice that developed secondary pancreatic tumors following orthotopic transplantation of pancreatic cancer tissues that conditionally express c-Myc and luciferase. Expression of luciferase was examined in the same mice after 7 days of Dox treatment.
The initiation of pancreatic cancer in our model was multifocal and widespread, and animals quickly became moribund before they developed larger tumors. To study whether c-Myc was still required for the maintenance of cancers that arose in a more focal manner and grew more extensively in size, we orthotopically transplanted small pancreatic tumor tissues from diseased animals that also contained the TetO-Luc reporter transgene into wildtype recipients. These transplants quickly grew into larger tumors within 3 to 6 weeks. Using in vivo imaging (Fig. 5C), we monitored the growth of the secondary tumors and verified the suppression of the TetO-driven transgene expression following Dox administration. We observed a remarkable regression of the tumors after just 7 days of Dox treatment, and there was no recurrence of tumors while the animals were treated for one month.
The biochemical analysis of pancreatic cancers following c-Myc ablation revealed that the majority of tumors exhibited a significant decline in cancer cell proliferation and upregulation of p53 prior to tissue remodeling (Suppl. Fig. S6A and S6B). Sequencing of the p53 mRNA showed that Dox-treated cancers cells upregulated the transcript variant 1, which did not contain any somatic mutations. Surprisingly, after three days of Dox administration, tumors expressed less Bax protein, and activated Caspases-3, which plays a central role in the execution-phase of apoptosis, was virtually absent (Suppl. Fig. S5B and S6B). Instead, these neoplasms showed an upregulation of LC3, and Beclin, also known as autophagy-related gene (Atg) 6, was abundant in Dox-treated tumors in vivo and in purified cancer cells in culture (Suppl. Fig. S6C and S6D). In addition to LC3 and Beclin, we observed an upregulation of ATG5/12 in response to the downregulation of c-Myc, and inhibition of autophagy with chloroquine resulted in increased survival of Dox-treated cells (Suppl. Fig. S6E). Collectively, cancer cells showed an increase in the expression of several autophagy-associated proteins that may initially promote their survival but eventually may trigger type 2 (autophagic) cell death.
Cdkn2a deficiency causes an increase in metastatic dissemination but has no effect on tumor latency or c-Myc-mediated cancer maintenance
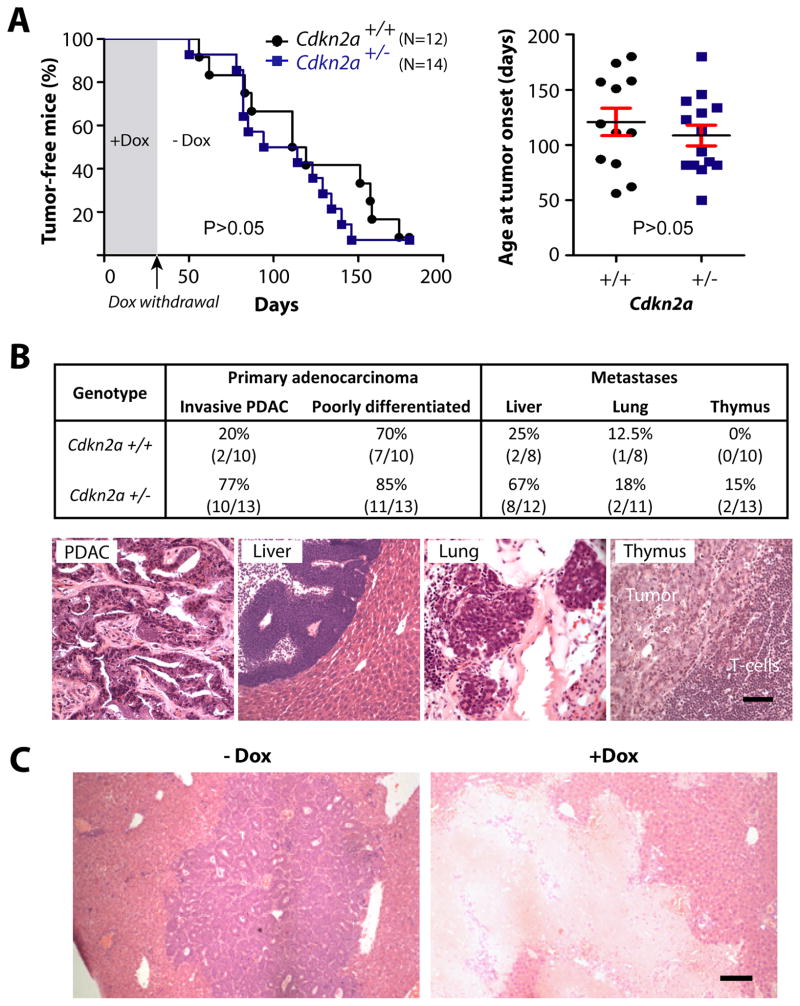
One of the most frequently altered genes in pancreatic cancers is Cdkn2a, which encodes p16Ink4a and p19Arf that play essential roles in the expression and functionality of cell cycle regulators including p53. Since c-Myc-induced adenocarcinomas in our model show a clear upregulation of p53 in response to the ablation of the oncogene, we addressed whether a loss of Cdkn2a might change the requirement for c-Myc in tumor maintenance. For this purpose, we introduced a conventional knockout allele of this locus into the conditional c-Myc expression model. To facilitate such a complex breeding scheme of multiple transgenes and a knockout allele, we kept the parental animals and the nursing offspring continuously on Dox to suppress the expression of c-Myc. The withdrawal of the ligand in adult mice at 4 weeks of age led to a rapid initiation of pancreatic cancer in all experimental cohorts (Fig. 6A), suggesting that cancer initiation in our model is not strictly dependent on the expression of c-Myc during early pancreatic development. More importantly, lack of one copy of the Cdkn2a locus did not significantly shorten the tumor latency; however, we noticed a remarkable increase in the occurrence of more aggressive primary PDACs that swiftly metastasized to the liver (Fig. 6B). Despite a significantly elevated rate of metastatic dissemination in response to the loss of just one copy of Cdkn2a, the growth and survival of these highly malignant, metastatic cells was still dependent on the expression of c-Myc. The downregulation of this oncogene led to a macroscopically complete remission of metastatic lesions (Fig. 6C). The biochemical analysis of pancreatic tumors revealed that, unlike mice that carried two functional copies of Cdkn2a, p53 was not upregulated following downregulation of c-Myc (Suppl. Fig. S7). While this suggests that there is a loss of heterozygosity of Cdkn2a during tumorigenesis, the results also indicate that neither p16Ink4a/p19Arf nor p53 play an essential role in the initiation of cell death in response to c-Myc ablation. However, regardless of the expression of p53, the downregulation of the oncogene led to a significant decline in active Caspase-3 and upregulation of LC3 prior to the induction of cell death.
Fig. 6. Haploinsufficiency in Cdkn2a (p19Arf/p16Ink4a) increases metastatic dissemination of pancreatic carcinomas expressing c-Myc.
A. Kaplan–Meier survival blot (left) and mean age of tumor onset (right, horizontal bars) in mice that conditionally express c-Myc in a Cdkn2a wildtype (Cdkn2a+/+) or haploinsufficient background (Cdkn2a+/−). Each marker represents the age of onset of the first palpable tumor per mouse (P value, logrank test). B. relative incidence and representative images of primary and metastatic pancreatic cancers that occurred repeatedly in mice conditionally expressing c-Myc in a heterozygous Cdkn2a knockout background. C. H&E-stained liver sections from Pdx1-Cre/CAG-βgeo-tTA/TetO-Myc/Cdkn2a+/− mice without Dox administration (−Dox) or following 5 weeks of Dox treatment (+Dox); bars represents 50 μm in panel B. and 100 μm in panel C.
Dormant cancer cells can facilitate tumor recurrence following re-expression of c-Myc
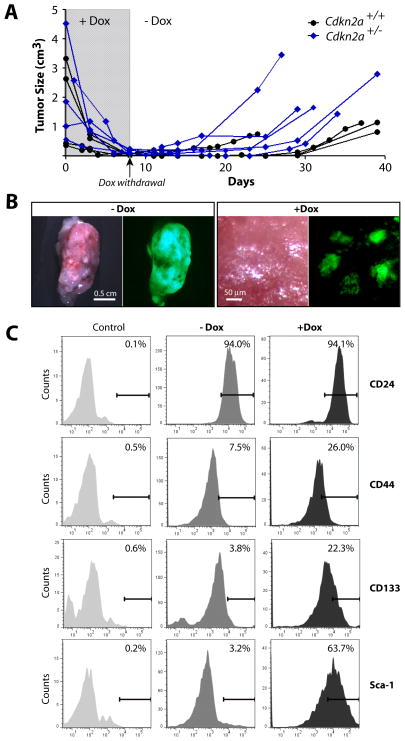
Since the tumor-associated stroma did not regress following the selective elimination of cancer cells, it was difficult to assess whether all neoplastic cells had undergone apoptosis. To examine whether some pancreatic cancer cells survived without exogenous c-Myc, we established a cohort of wildtype recipient mice that developed secondary lesions following the transplantation of small cancer tissues from c-Myc-expressing mice. In addition to the TetO-Luc transgene, we included a CAG-LSL-GFP reporter construct to permanently label individual cancer cells with GFP. Following the identification of tumors, we treated the recipients for 8 days with Dox until the tumors had regressed (Fig. 7A). The subsequent withdrawal of Dox from the drinking water led to a gradual reactivation of c-Myc within 3 to 6 days, and tumors swiftly reappeared in all animals regardless whether they were wildtype or haploinsufficient in Cdkn2a. Since a de novo transformation of normal pancreatic cells in this transplant model was unlikely, it was evident that a few cancer cells had survived without exogenous c-Myc, and these residual cells served as cancer-initiating cells upon re-expression of c-Myc.
Fig. 7. Dormant cancer cells can facilitate tumor recurrence following re-expression of c-Myc.
A. Changes in the size of individual pancreatic cancers following orthotopic transplantation into wildtype recipient mice that were treated with Dox after tumors became palpable. Following near complete regression after 8 days of Dox treatment, all tumors, regardless whether they were Cdkn2a haploinsufficient, recurred after withdrawal of Dox. B. Expression of GFP in pancreatic cancer tissues of recipient animals prior to Dox treatment (left) and after 8 days of Dox administration; bars represent 0.5cm (left panel) and 100 μm (right panel). Note the presence of small residual clusters of GFP-positive cells in pancreatic tissues following cancer regression. C. Flow cytometric analysis of various stem cell markers in the bulk of GFP-positive tumor cells from secondary cancers (−Dox) and in cancer cells that remained dormant following the ablation of exogenous c-Myc.
The analysis of GFP-labeled cells in cancers and pancreatic tissues before and after tumor-bearing mice were treated with Dox confirmed that the vast majority of, but not all, cancer cells died following downregulation of c-Myc (Fig. 7B). A closer examination of histological sections revealed that the GFP-labeled residual tumor cells did not express c-Myc (exogenous or endogenous). They also did not proliferate, and they were not undergoing any form of cell death (Suppl. Fig. S8). The fact that residual cancer cells were still identifiable in mice that were continuously treated with Dox for 4 weeks clearly indicate that a small subset of cancer cells remained dormant without expression of c-Myc.
Next, we used fluorescence-activated cell sorting from enzymatically dissociated pancreatic tissues to isolate GFP-positive cancer cells from tumor-bearing recipient animals prior to and after Dox treatment. Since the residual cells were capable of forming tumors upon reactivation of c-Myc, we used flow cytometry to determine whether a higher subset of these dormant cells also exhibit expression of previously reported pancreatic cancer stem cell makers (CD24, CD44, CD133, and Sca-1). CD45 and CD31-positive hematopoietic and endothelial cells were excluded from this assay. Nearly all cells from large tumors and residual cancer cells of both cohorts expressed CD24, but there was a noticeable increase in the number of CD44 and CD133-positive cells (Fig. 7C). Interestingly, Sca1+ cells could rarely be detected in large tumors, but the majority of dormant cancer cells were positive for this particular stem cell marker. Based on these observations, it is likely that the regression of tumors following ablation of c-Myc may enrich for residual cancer cells that display cell surface markers associated with pancreatic cancer stem cells.
Since it has been suggested previously that activating point mutations in Kras can prevent the death of mammary gland tumor cells following downregulation of c-Myc (20), we sequenced the exons of the Kras gene in isolated residual pancreatic cancer cells from three Dox-treated mice. We also included six recurring tumors following re-expression of c-Myc (i.e. the cellular descendants of residual cancer cells) and one untreated control in this study. None of these tumor cell samples carried mutations in codons 12, 13, and 61 of Kras (not shown), and therefore a gain-of-function of Kras does not appear to be the underlying mechanism for the survival of a small subset of residual cancer cells following ablation of c-Myc.
Discussion
c-Myc induces pancreatic cancer in a cell context-dependent manner without additional genetic manipulation of cell survival pathways
We have demonstrated that expression of exogenous c-Myc in Pdx1-positive pancreatic progenitors and derived cell lineages of the exocrine pancreas is entirely sufficient to initiate the development of premalignant lesions that swiftly progress into invasive and metastatic adenocarcinomas. c-Myc does not appear to preferentially transform endocrine cells, and unlike previously suggested (11, 12), there is no need to introduce other mutations to initiate tumorigenesis. c-Myc is abundant in preneoplastic and invasive pancreatic cancer cells, and we therefore conclude that the ability of c-Myc to cause neoplastic transformation in the pancreas is dependent on the cellular context. Our results also show that similar to islet cells, expression of c-Myc in the ductal epithelium triggers an oncogenic stress response that induces cell death. However, c-Myc promoted extensive cell proliferation and only a subset of these cells underwent apoptosis. This might account for the rapid onset of tumorigenesis without the need of manipulating additional cell survival pathways through expression of Bcl-xL or inhibition of p53 as reported previously (11, 12). Not only does c-Myc cause a different magnitude of cellular stress response in pancreatic progenitors or derived exocrine cells compared to islet cells, it also acts differently from other oncogenes such as mutant Kras where deficiency in p19Arf or p53 promotes malignant progression (21, 22). c-Myc-induced pancreatic tumors in our new model retain a regulatable expression of p53, and the fact that Cdkn2a deficiency did not accelerate the onset of tumorigenesis might indicate that the p19Arf/p53 axis does not play a key role in preventing c-Myc-induced neoplastic transformation. Similar to suprabasal keratinocytes in the skin (23), the target cells for neoplastic transformation in our PDAC model may have an inherent resistance towards c-Myc-induced apoptosis. This notion might be supported by the fact that normal pancreatic progenitors express elevated levels of c-Myc during embryogenesis where this protein is essential for the growth and differentiation of the exocrine pancreas (24, 25).
Ablation of c-Myc results in an upregulation of autophagy-related proteins and regression of primary and metastatic pancreatic cancer
The downregulation of c-Myc in established neoplasms resulted in a macroscopically complete regression of tumors. Prior to the induction of cancer cell death in response to the ablation of the transforming oncogene, adenocarcinomas exhibited a noticeable upregulation of p53. The introduction of a conventional Cdkn2a knockout allele into the conditional c-Myc expression model promoted a more rapid metastatic dissemination of cancer cells similar to other models (4, 26). Although p53 was no longer upregulated in response to the ablation of c-Myc, which is indicative for the loss of heterozygosity of Cdkn2a, the survival of cancer cells within primary and metastatic lesions was still dependent on the transforming oncogene, suggesting that p19Arf and p53 do not play essential roles in the initiation of cancer cell death. This conclusion might be applicable to pancreatic cancer cells that are addicted to other oncogenes. For example, it has been reported recently that the survival of cancer cells in mice that carry a knockout of p53 depends on the continued expression of oncogenic Kras (8, 27). Regardless of the presence of Cdkn2a or p53, we observed in our model a decline in the expression of regulators of apoptosis (i.e. type 1 cell death) in response to c-Myc ablation prior to tissue remodeling. In particular, cleaved Caspase-3, a crucial mediator of apoptosis, was no longer expressed. At the same time, these cells exhibited a noticeable upregulation of autophagy-related proteins that may initially promote the survival of cancer cells but ultimately contribute to their demise. Collectively, these results indicate that the downregulation of c-Myc may initiate additional cell death mechanisms other than apoptosis such as type 2 (autophagic) cell death (28), which may have implications for the treatment of pancreatic cancers.
Residual cancer cells can initiate disease recurrence upon re-expression of c-Myc
While it has been demonstrated that other malignant cell types are addicted to c-Myc [for references see review by Jonkers and Berns (29)], our model for reversible metastatic pancreatic cancer exhibits some distinct characteristics. Following cancer regression upon downregulation of c-Myc, the reactivation of the transforming oncogene led to swift recurrence of pancreatic cancer. Unlike osteogenic sarcomas and certain skin lesions where all neoplastic cells seemed to be addicted to c-Myc and terminally differentiate upon its ablation (23, 30), pancreatic cancer appears to be more similar to hepatocellular carcinoma where a sustained repression of c-Myc is a necessity to prevent cancer recurrence (31). In contrast to the liver cancer model, however, the ablation of c-Myc led to rapid cell death, and there was no evidence that cancer cells reverted into normal cells. In fact, the substantial attrition of the bulk of the tumor led to an enrichment of dormant cells that exhibited markers for pancreatic cancer-initiating cells in humans such as CD44 and CD133 (32, 33). Interestingly, a significant subset of genetically labeled, residual cancer cells was Sca-1-positive. Although it has not previously been shown that Sca-1 is associated with pancreatic cancer stem cells, recent work reported that this cell surface marker is expressed in terminal duct and centroacinar cells that play a role in tissue homeostasis in the normal pancreas (34). Only a small subset of adenocarcinoma cells is capable of surviving the ablation of c-Myc, and the underlying mechanisms for this biological phenomenon remain to be elucidated. We show in this report that the survival of stromal cells does not generally depend on paracrine interactions with carcinoma cells. Besides examining cell intrinsic mechanisms, it will also be interesting to assess in future studies whether the persistent stroma provides a suitable microenvironment that facilitates cancer cell dormancy and disease recurrence.
Supplementary Material
Acknowledgments
Financial support: PHS grants R21 CA155175 (K.-U.W), U54 CA163120 (K.-U.W., S.K.B., M.A.H.), R01 CA112537 (M.H.), and CA118740 (H.R.) from the National Cancer Institute; the S.G. Komen for the Cure Promise Grant KG091116 (H.R.); and the Nebraska Cancer and Smoking Disease Research Program NE DHHS LB506 2011-36 (K.-U.W.).
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–5. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–9. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 6.Mahlamaki EH, Barlund M, Tanner M, et al. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–8. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci U S A. 1991;88:93–7. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DJ, Junttila MR, Pouyet L, et al. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell. 2008;14:447–57. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–34. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 12.Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003;17:3127–38. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Triplett AA, Harms DW, et al. Temporally and spatially controlled expression of transgenes in embryonic and adult tissues. Transgenic Res. 2010;19:499–509. doi: 10.1007/s11248-009-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto S, Niwa H, Tashiro F, et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470:263–8. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 16.Serrano M, Lee H, Chin L, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 17.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner KU, Krempler A, Triplett AA, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24:5510–20. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–86. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Bardeesy N, Aguirre AJ, Chu GC, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–51. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell. 1999;3:565–77. doi: 10.1016/s1097-2765(00)80350-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakhai H, Siveke JT, Mendoza-Torres L, Schmid RM. Conditional inactivation of Myc impairs development of the exocrine pancreas. Development. 2008;135:3191–6. doi: 10.1242/dev.017137. [DOI] [PubMed] [Google Scholar]
- 25.Bonal C, Thorel F, Ait-Lounis A, et al. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–19. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Finch A, Prescott J, Shchors K, et al. Bcl-xL gain of function and p19 ARF loss of function cooperate oncogenically with Myc in vivo by distinct mechanisms. Cancer Cell. 2006;10:113–20. doi: 10.1016/j.ccr.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012 doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonkers J, Berns A. Oncogene addiction: sometimes a temporary slavery. Cancer Cell. 2004;6:535–8. doi: 10.1016/j.ccr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Jain M, Arvanitis C, Chu K, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–4. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 31.Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 33.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Rovira M, Scott SG, Liss AS, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.