Abstract
Two lines of evidence indicate that there exists a reciprocal inhibitory relationship between opposed brain networks. First, most attention-demanding cognitive tasks activate a stereotypical set of brain areas, known as the task-positive network and simultaneously deactivate a different set of brain regions, commonly referred to as the task negative or default mode network. Second, functional connectivity analyses show that these same opposed networks are anti-correlated in the resting state. We hypothesize that these reciprocally inhibitory effects reflect two incompatible cognitive modes, each of which is directed towards understanding the external world. Thus, engaging one mode activates one set of regions and suppresses activity in the other. We test this hypothesis by identifying two types of problem-solving task which, on the basis of prior work, have been consistently associated with the task positive and task negative regions: tasks requiring social cognition, i.e., reasoning about the mental states of other persons, and tasks requiring physical cognition, i.e., reasoning about the causal/mechanical properties of inanimate objects. Social and mechanical reasoning tasks were presented to neurologically normal participants during fMRI. Each task type was presented using both text and video clips. Regardless of presentation modality, we observed clear evidence of reciprocal suppression: social tasks deactivated regions associated with mechanical reasoning and mechanical tasks deactivated regions associated with social reasoning. These findings are not explained by self-referential processes, task engagement, mental simulation, mental time travel or external vs. internal attention, all factors previously hypothesized to explain default mode network activity. Analyses of resting state data revealed a close match between the regions our tasks identified as reciprocally inhibitory and regions of maximal anti-correlation in the resting state. These results indicate the reciprocal inhibition is not attributable to constraints inherent in the tasks, but is neural in origin. Hence, there is a physiological constraint on our ability to simultaneously engage two distinct cognitive modes. Further work is needed to more precisely characterize these opposing cognitive domains.
Keywords: task-positive, task negative, default network, anti-correlated networks, fMRI, dual-process theory
1.1 Introduction
The last decade has witnessed rapidly growing interest in the brain’s task negative or default mode network (DMN1), both in health and disease (Buckner et al., 2008, Broyd et al., 2009, Andrews-Hanna, 2011). The DMN is a constellation of regions that includes areas in medial parietal/posterior cingulate, medial prefrontal, lateral inferior parietal and superior temporal cortices. This network exhibits activity decreases during performance of a wide variety of tasks (Shulman et al., 1997, Binder et al., 1999). Tasks that deactivate the DMN also activate a second common network, the task positive network (TPN)(Fransson, 2005, Fox et al., 2006), which includes areas in dorsal parietal and lateral prefrontal cortices. Spontaneous activity in these two networks is temporally anti-correlated in the resting state (Fox et al., 2005, Fox et al., 2009). Thus, mutual antagonism between the DMN and the TPN is regularly observed both in the resting state and during task performance (Greicius et al., 2003, Fox et al., 2005, Fransson, 2005, Golland et al., 2007, Tian et al., 2007, Fox et al., 2009, Uddin et al., 2009). While some methodological concerns about the validity of anti-correlations that involve regression of the mean signal have been noted (Murphy et al., 2009), observations that don’t use mean signal regression also identify anticorrelated regions (Chang and Glover, 2009, Fox et al., 2009, Chai et al., 2012). In addition, there is evidence that the hypothesized physiological inhibition is relevant to understanding performance in normal (Gordon et al., 2007) and diseased states (Lustig et al., 2003, Kennedy, 2006, Pomarol-Clotet et al., 2008, Fassbender et al., 2009). As a result, it has been suggested that the anti-correlation between networks may prove functionally more important than DMN activity itself (Fox et al., 2005, Broyd et al., 2009, Uddin et al., 2009). The goal of this investigation is to shed light on the cognitive significance of this tension between the TPN and DMN.
1.2 Cognitive significance of DMN vs TPN dichotomy
The relationship between the TPN and DMN has been likened to a two sided see-saw, such that activity in one network decreases below baseline as activity in the other increases above baseline (Meyer et al., 2012). This analogy is wholly consistent with findings from resting state functional connectivity analyses. However, evidence from task-based studies most clearly supports only one half of the full range of motion of the see-saw: activity in the TPN parametrically increases in activation in response to cognitive effort or task demand, while the DMN shows parametric decreases (McKiernan et al., 2003, Gordon et al., 2007, Mason et al., 2007). Demonstrations of the converse pattern, in which the DMN is activated and TPN deactivated, have been much more elusive. Accounting for this asymmetry represents a significant theoretical opportunity, as there are a number of competing accounts which critically depend on characterizing the processes which lead to activation of the DMN and deactivation of the TPN. We consider three broad hypotheses which might account for the relationship between the TPN and DMN: two that are frequently mentioned in the literature on the default network, and a third which derives from a distinct literature. The first hypothesis is that the relationship between the TPN and DMN reflects a tension between goal-directed cognition versus spontaneous cognition or mind-wandering. The second hypothesis is that this relationship reflects a tension between externally versus internally directed attention. The third hypothesis is that it reflects a tension between distinct cognitive modes associated with social and non-social domains. The motivation and evidence for these hypotheses will be briefly reviewed.
1.2.1 Goal directed vs. Spontaneous Cognition
The first hypothesis is directly related to the “task-positive” and “task negative” (or “default mode”) labels, which have come to be associated with the two networks (Raichle et al., 2001). These labels were suggested by early findings that a broad range of tasks activate the TPN and deactivate the DMN (Shulman et al., 1997). This hypothesis predicts that it should not be possible to identify goal-directed tasks that activate the DMN and/or deactivate the TPN. It is challenged by more recent findings that identify goal-directed tasks which activate the DMN above a resting baseline (Iacoboni, 2004, Sestieri et al., 2010, Spreng et al., 2010), including one task which demonstrates parametric increases in DMN activity associated with social working memory load (Meyer et al., 2012). Hence Spreng (Spreng, 2012) suggests that the labels ‘task positive’ and ‘task negative’ are “more likely the byproduct of the desire for rigorously controlled experimental designs (i.e., externally directed stimuli) than meaningful descriptors of functional brain networks.”
1.2.2 Internal vs. External attention
The second hypothesis appeals to attention to account for the tension between the TPN and DMN (Buckner and Carroll, 2007, Buckner et al., 2008, Broyd et al., 2009). These accounts build on the observation that a common feature of tasks that activate the TPN and deactivate the DMN is focused attention to the external environment. According to various accounts, focused attention may be in competition either with broad exploratory attention (Gilbert et al., 2007), or with a variety of types of internal attention, including attention to self (Gusnard, 2001, Fransson, 2006, Andrews-Hanna, 2011), conceptual association (Binder et al., 1999, Bar, 2009), episodic retrieval (Buckner and Carroll, 2007), and mental simulation or model building (Spreng et al., 2010). One study has presented evidence for both activation of DMN regions and deactivation of TPN regions (Spreng et al., 2010). On this basis, the authors argue for a variant of the internal versus external attention account. The TPN comprises regions which lie in two distinct networks, as defined by positive functional connectivity: the dorsal attention network (DAN) and the fronto-parietal control network (FPCN). On this basis, Spreng (Spreng, 2012) argues the TPN does not represent a unitary functional network, but rather reflects distinct cognitive factors which are confounded in many tasks. Hence, Spreng argues that the tension between the TPN and the DMN as a ‘false dichotomy.’ Instead, Spreng suggests that the true tension lies between the DAN and the DMN, and reflects “competition between exogenous and endogenous loci of information processing.” However, this account runs into three difficulties. First, findings from resting state functional connectivity indicate that the regions which are most anti-correlated with the DMN lie in both the FPCN and the DAN2. Since these analyses are neutral with regard to task, the value of characterizing the TPN as a network in tension with the DMN cannot be explained away by appeal to task confounds. Second, there have not been any demonstrations of goal-directed tasks which activate the DMN while deactivating the entire TPN. Spreng et al (Spreng et al., 2010) show deactivation of the DAN alongside activation of both the DMN and FPCN. However, that study does not identify specific deactivated regions, and therefore does not demonstrate alignment between task induced activations/deactivations and anti-correlated regions derived from functional connectivity. Third, the cognitive characterization of the tension as being due to internal versus external attention appears problematic for one class of tasks which have been consistently associated with the DMN. A large number of studies have implicated the DMN in social cognition (Amodio and Frith, 2006, Schilbach et al., 2008, Van Overwalle, 2009, Mars et al., 2012). Many of these tasks differ from other tasks which are classified as involving internally directed cognition in a significant respect: they require attention to external stimuli. For instance, Iacoboni (Iacoboni, 2004) finds activation of the default network above resting levels while participants watch videos of unfamiliar individuals engaged in social interactions. Social cognition tasks may essentially involve attention to ‘internal states’, however, these are often the ‘internal states’ of unfamiliar individuals, not of the participant.
1.2.3 Opposing domains
The third hypothesis, which we favor, is the ‘opposing domains hypothesis’. According to this hypothesis, the task positive and task negative networks reflect two incompatible cognitive modes, each of which can be directed towards understanding the external world. Instead of appealing to attention, this hypothesis is more closely allied to dual-process models of cognition, which hold that distinct cognitive modes can be engaged by externally directed tasks (Sloman, 1996, Evans, 2003, Kahneman, 2003). These modes are hypothesized to be associated with two broad cognitive domains, namely social information processing (reasoning about the minds of others) and non-social information processing (reasoning about physical objects). The opposing domains hypothesis is motivated by theoretical work in a distinct literature on the problem of consciousness (Nagel, 1974, Hill, 1997, Levine, 2000, Jack and Shallice, 2001, Robbins and Jack, 2006), however for current purposes we focus on considering this hypothesis as a candidate for explaining the tension between the TPN and DMN. A direct prediction of both this hypothesis and the internal vs. external attention hypothesis is that, in addition to tasks that activate the task positive network and deactivate the task negative network, it should also be possible to identify goal-directed tasks that activate the task negative network and deactivate the task positive network. In other words, it should be possible to identify goal directed tasks that push the see-saw to both extremes of its full range of motion. Further, since the definition of the TPN and DMN was initially established by reference to anti-correlated networks seen in resting connectivity (Fox et al., 2005), there should be a close correspondence between these anti-correlated networks and the regions shown to be in tension by the tasks. The key difference between these hypotheses is that the internal vs. external attention hypothesis predicts this pattern should be produced by external and internal tasks, even when the social processing demands of the tasks are similar. In contrast, the opposing domains hypothesis predicts that this pattern should be produced by social and non-social tasks, even when the attention demands of the tasks are similar. We know of no reports which test the first prediction, as existing reports confound internal focus with social processing (Sestieri et al., 2010, Spreng et al., 2010). The present study aims to test the second prediction.
1.3 Experimental design
To test our hypothesis, we designed tasks which were specific in terms of which domain they recruited from, but not in terms of the processes they recruited from a given domain. The motivation for this approach follows from two considerations. First, there already exist carefully controlled studies which have shown that social and physical reasoning are associated with distinct brain areas (Mitchell, 2002, Martin and Weisberg, 2003). However, these studies did not find evidence of activation and deactivation relative to baseline consistent with the opposing domains hypothesis. We hypothesize that this pattern was not observed because the tasks only required processing of surface features (e.g., patterns of movement, or semantic associations between two words). They did not require participants to represent the mental states of identifiable conspecifics, nor encourage participants to apply principles of physics in order to understand mechanical processes. Two recent reviews of work in social cognitive neuroscience argue that the desire to produce rigorously controlled studies has encouraged the use of task designs that are too artificial to shed light on many important social cognitive processes (Zaki and Ochsner, 2012, Schilbach et al., in press). Rather than designing our tasks to distinguish distinct process involved in social or mechanical reasoning, we used ecologically valid tasks designed to engage rich mechanical and mental state representations. For example, our social texts were modified from a prior study of false belief by adding additional emotional and moral content, producing narratives similar to soap opera (Appendix B).. Second, some studies have demonstrated co-activation of the DMN and regions in the TPN (Spreng et al., 2010, Meyer et al., 2012). According to the opposing domains hypothesis, this could occur because these studies involved cognitive components associated with both of the two distinct cognitive modes. Hence, a key goal of this study was to identify tasks which predominantly recruit processes associated with just one of these cognitive modes.
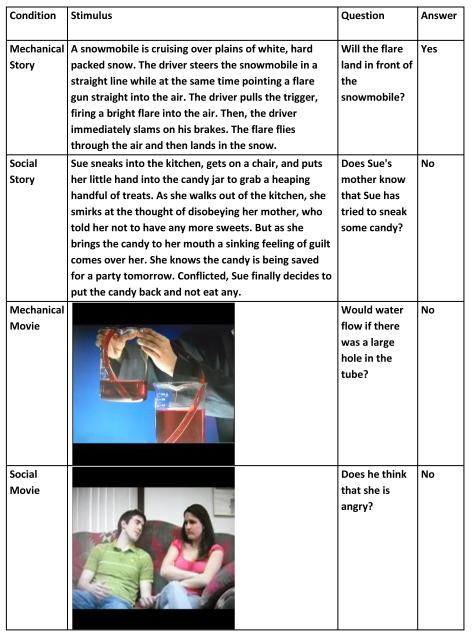
The experimental tasks followed a 2x2 factorial design with cognitive domain and perceptual modality as crossed factors (Figure 1). On each trial, after reading the text or watching the video clip for 20s, a simple yes/no question was presented as text and participants were given 7s to answer by pressing a key (Figure 2). The social videos depicted conversations between two individuals who often misunderstood each other. The questions concerned one actor’s belief about the emotional state of the other actor. The social texts were adapted from a prior study (Saxe and Powell, 2006) and described scenarios in which at least one protagonist had a false belief. The questions tested understanding of this false belief. The mechanical videos were clips excerpted from the Video Encyclopedia of Physics (Education Group & Associates., 1995). The questions were counterfactuals that tested understanding of the illustrated physical principle. The mechanical texts described puzzles similar to and adapted from examples found in popular scientific puzzle books. The questions asked participants to predict what would happen next. To disambiguate the opposing domains hypothesis from the external vs. internal attention hypothesis, we made the social texts significantly longer than the mechanical texts, while holding reading difficulty constant.
Figure 1.
Schematic illustration of task conditions
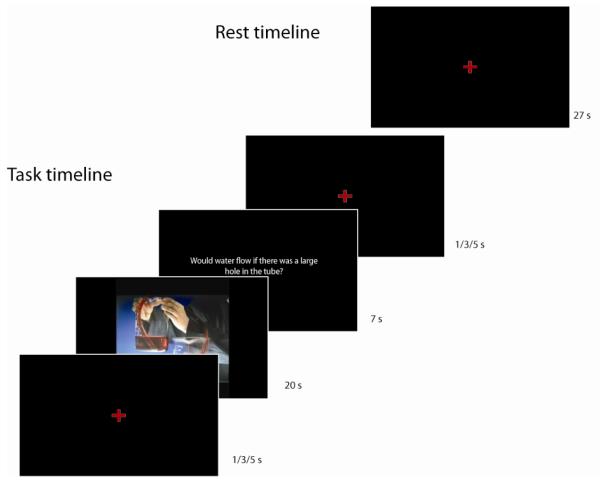
Figure 2.
Timeline for tasks and resting trials
2. Materials and Methods
2.1 Social and mechanical reasoning study
2.1.1 Participants
Forty-five student volunteers were paid $50 to participate. The majority (43/45) of participants were recruited from a separate behavioral study of individual differences in social and non-social reasoning. All participants were drawn from the undergraduate population of Case Western Reserve University. All participants were fluent English speakers and reported no history of neurological or psychiatric disorders. All participants had normal or corrected to normal vision and normal hearing. The mean age of the participants was 20.5 years (range 19-23 yr), with 24 female participants. Informed consent was obtained in accordance with guidelines provided by the institutional review board of Case Western Reserve University Hospitals.
2.1.2 Magnetic Resonance Imaging
Scans were collected using a 4T Bruker-Siemens hybrid MR scanner. Participants underwent an MP-RAGE high resolution anatomical scan, T2 weighted anatomical scan, and five functional task runs (300 volumes each). Functional runs used an echoplanar imaging sequence with 38 contiguous 3.8mm slices, 3.8 × 3.8 mm in-plane resolution, TE = 20 ms, flip angle = 90°, TR = 2.00 s. Participants practiced a training version of the task during their MPRage.
2.1.3 Stimulus presentation
Stimuli were presented using E-prime2.0 software. Images were projected onto a screen attached to the head coil using an Avotech projector and were viewed by subjects through a mirror. Sound was presented though integrated Avotech headphones. Participants responded to stimuli using an Avotech MR compatible serial response system by pressing one of two buttons using either the index or middle finger of the right hand.
2.1.4 Design
We used a two factor crossed design to examine the cortical regions recruited during social and scientific reasoning, using problems presented in two different modalities: text, and video with audio soundtrack. Each 10 minute scanner run consisted of 16 trials (4 presentations of each of the 4 conditions) and 4 rest periods, all 27 seconds in length. The order of stimuli and rest periods within each scanner run was determined randomly for each participant. Variable fixation periods of 1, 3, or 5 second duration separated each question or rest period from the next. On each trial, participants had 20 seconds to either read a text passage or watch a video. A short yes/no textual comprehension question was then presented on screen and participants were given 7 seconds to respond. All text passages and videos were unique and were not repeated. A brief description of the five conditions (including rest) follows:
2.1.5 Social Text Condition
The social reasoning texts were adapted from (Saxe and Powell, 2006) and elaborated to add additional emotional and moral context to the existing false belief content. Questions concerned the beliefs or attitudes of one of the protagonists. Texts had a Flesch Reading Ease score of 77.8 and Flesch Grade Level of 6.0. Average word counts were as follows: story text: 86.2 question text: 9.5 total: 95.7. All items are listed in appendix B.
2.1.6 Social Video Condition
The social reasoning videos were generated by Angela Ciccia, Dept. of Psychological Sciences, Case Western Reserve University, using student actors. Each video comprised an emotionally laden verbal exchange between two individuals, one male and one female, which typically involved a degree of misunderstanding. There were no direct linguistic cues used to demonstrate the emotion/construct of interest thereby requiring the participant to rely on extralinguistic and emotional cues to answer the emotional state question. Actors were filmed against one of two plain backgrounds to limit distraction. All questions focused on whether one protagonist understood the attitude or emotional states of the other protagonist.
2.1.7 Mechanical Text Condition
The mechanical reasoning texts were adapted from public domain science puzzles. Persons often featured in the descriptions. Texts had a Flesch Reading Ease score of 77.0 and Flesch Grade Level of 6.0. All questions asked whether or not a specific event would happen next. Average word counts were as follows: story: 75.3 question: 9.6 total: 84.9. All items are listed in appendix B.
2.1.8 Mechanical Video Condition
The mechanical reasoning videos consisted of clips taken, with permission, from the Video Encyclopedia of Physics. Each clip demonstrated a mechanical principle or phenomenon. The phenomenon was also described in a voiceover audio track. Many clips featured actors. Questions were designed to test understanding of the mechanical principle explained, with most questions phrased as counterfactuals.
2.1.9 Rest Condition
The rest condition involved the passive viewing of a red fixation cross centered on a black background.
2.1.10 Imaging analysis (task data)
A scanner specific atlas target was created from the MP-RAGE images of 25 young adults (Buckner et al., 2004). The resulting atlas represented Talairach space according to the SN method(Lancaster et al., 1995). After calculation of parameters for realignment within each BOLD run, and for coregistration of each BOLD run with the atlas aligned T1 & T2 structural images, BOLD stacks were resampled directly from the raw data into a 3mm cubic voxel atlas space. Each BOLD stack was then spatially smoothed with a Gaussian 3D filter with FWHM of 2 voxels (6mm).
Data for each subject were entered into a general linear model in which baseline and linear trend were estimated alongside a single uniform assumed response associated with each condition. Voxel activity was averaged and activity in each given condition was subtracted from baseline or another condition using the Washington University of Saint Louis software application fidl. The computerized anatomical reconstruction and editing toolkit, Caret, (Washington University in Saint Louis software) was used for visualization.
2.2. Resting state functional connectivity
Resting state data was retrieved from the public database NITRC on February 15, 2010. Two data sets were used: Beijing_Zang (Zang, Y.F.; n = 198 [76M/122F]; ages: 18-26; TR = 2; # slices = 33; # timepoints = 225) and Cambridge_Buckner (Buckner, R.L.; n = 198 [75M/123F]; ages: 18-30; TR = 3; # slices = 47; # timepoints = 119). The total combined number of subjects was 396 (245 female), aged 18-30 (mean age 21.1). The data was aligned to the same atlas space as the task data (711-2B), and smoothed to 6mm FWHM. Analysis methods were identical to those previously reported(Fox et al., 2005, Fox et al., 2009), with two exceptions: (i) no global (whole brain) regressor was used (i.e. only movement, white matter and ventricle regressors were used), unless otherwise specified. (ii) All statistical contrasts used a random effects method (i.e. one Fisher-z transformed correlation image per subject was entered into a single sample t-test), and the resulting statistical images were corrected for multiple comparisons.
3 Results
3.1 Behavior
Mean accuracy for the four conditions was as follows: Social movies 85.3%; Mechanical movies 71.7%, Social texts 80.4%, Mechanical texts 65.7%. fMRI analyses that control for task difficulty are reported below the primary analyses.
3.2 fMRI
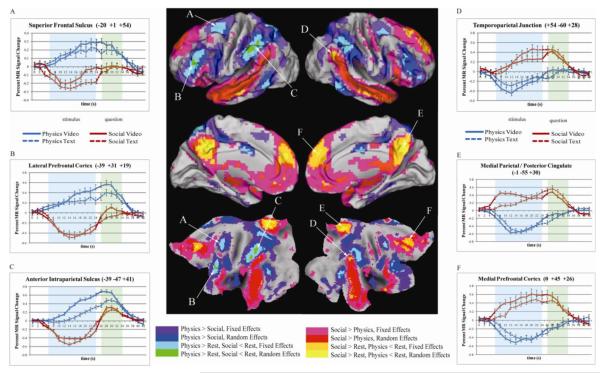
The fMRI data were analyzed using the method of strict cognitive conjunction: We report regions as sensitive to domain only when the contrast between social and mechanical tasks was statistically significant independently within each modality (text and video)(Friston et al., 2005, Nichols et al., 2005). Regions more associated with the social tasks (Figure 3, warm colors) overlapped substantially with the DMN. These regions in medial prefrontal, medial parietal/posterior cingulate, lateral parietal and superior temporal cortices have also been identified in prior studies of social cognition (Amodio and Frith, 2006), emotion identification (Phan, 2002), and autobiographical memory (Buckner and Carroll, 2007). Regions more associated with mechanical reasoning (Figure 3, cool colors) overlapped substantially with the TPN, including regions in both the DAN and the FPCN. These regions in dorso-lateral parietal and lateral prefrontal cortex have been identified in studies of abstract reasoning (Vincent et al., 2008), action observation and execution (Van Overwalle and Baetens, 2009), visual attention and working memory (Corbetta and Shulman, 2002, Owen et al., 2005, Fox et al., 2006). These findings are also consistent with prior studies that used closely controlled stimuli to contrast social and physical reasoning (Mitchell, 2002, Martin and Weisberg, 2003), except that the areas identified by contrasting our tasks were far more extensive. Fixed and random effects analyses were used to obtain upper and lower estimates of the extents of cortex sensitive to cognitive domain. Even using the statistically highly conservative (Friston et al., 2005) method of strict cognitive conjunction (Nichols et al., 2005), in our sample of 45 participants, we found 54% of the cortical surface to be sensitive to domain (fixed effects result). Generalizing to the population, 21% of the cortical surface was sensitive to domain (random effects result). Peak coordinates from the random effects analysis can be found in appendix A.
Figure 3.
Brain areas sensitive to cognitive domain and antagonistic areas. Colored areas pass multiple comparison correction in both video and text conditions independently. Warm colors (pink, red, orange, yellow) activate more for social than mechanical reasoning. Cool colors (purple, blue, cyan, green) activate more for mechanical than social reasoning. Bright colors (orange, yellow, cyan, green) identify antagonistic areas, which are significantly above rest for both tasks in one domain, and significantly below rest for both tasks in the other domain. Contrasts in each domain are cumulative, i.e. mechanical areas in blue have passed the contrast for purple (Physics>Social, fixed), areas in cyan have passed for purple and blue, and areas in green have passed for purple, blue and cyan. Graphs A-F show timecourses from antagonistic areas, without correction for hemodynamic lag. Blue and green shading indicate time points associated with stimulus and response periods, respectively. Error bars show standard error of mean across participants.
These observations serve to validate the tasks used by demonstrating that they preferentially recruit the hypothesized networks. As might be predicted, our ecologically valid and engaging tasks produced a far more extensive differentiation of brain regions than prior studies which either aimed to identify specific processes and/or which employed closely matched stimuli. However, direct contrasts between tasks cannot address the critical question at issue, of whether these tasks activated brain regions associated with one network above baseline, while deactivating regions associated with the other network.
3.3 Reciprocal suppression
To provide evidence for activation and deactivation, it is necessary to compare brain activity with a well-defined resting baseline. Our study combined two techniques to ensure a robust estimate of the resting baseline: brief variable delay periods between trials (1, 3 or 5 sec), and the introduction of a fifth trial type, equal in frequency and duration (27 sec) to the four experimental trial types, but consisting entirely of resting fixation. As a result, 28% of fMRI time was spent in resting fixation. We estimated activity relative to rest in two ways. First, we used the same assumed hemodynamic response function to model the task conditions and the resting fixation condition. We then identified regions that demonstrated a consistent pattern of activation and deactivation illustrative of reciprocal inhibition. That is, we identified voxels where each of the four contrasts (mechanical text – rest), (mechanical video – rest), (rest – social text), (rest – social video) were significantly positive, and where all four were significantly negative (alpha for each contrast p<0.05 two tailed, multiple comparison corrected, alpha for conjunction p<6.25×10−6). We found such regions (‘antagonistic brain areas’) throughout both the social reasoning and the mechanical reasoning networks (bright colors in Figure 3). The second analysis addressed an important methodological concern with the first analysis: The baseline may not have been properly estimated if the assumed hemodynamic response function failed to capture all task related activity. Hence, we generated a second set of models in which we made no assumptions about the hemodynamic response. This model estimated activity associated with each of the four experimental trial types on a frame-by-frame basis. In this model, both the variable delays between tasks and the resting fixation trials contributed to the baseline estimate. The timecourses in antagonistic areas derived from this model are shown in Figure 3(A-F). They start at and return to baseline levels, and clearly demonstrate deviations above and below the baseline. This demonstrates that the antagonistic brain areas exhibit genuine deviations from the resting baseline.
3.4 Correspondence with resting state anti-correlations
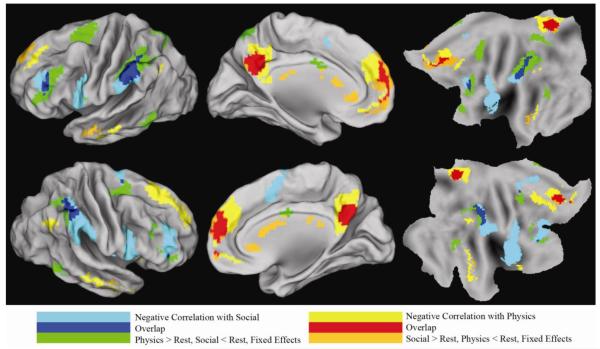
The dichotomy between TPN and DMN regions was originally based on the observation that activity in these networks is negatively correlated at rest, when cognition is unconstrained by any task (Fox et al., 2005). If the opposing domains hypothesis correctly characterizes the cognitive tension between these networks, then there should be a close correspondence between our task-based antagonistic regions and anti-correlated regions. To examine this hypothesis, we performed a functional connectivity analysis on resting state data from a separate group of 396 participants, who were not exposed to our tasks. First, we used the methods of Fox et al (Fox et al., 2009), which involves removal, by regression, of the signal averaged over the whole brain. This analysis revealed extended areas of negative correlation that broadly corresponded to the regions revealed by the strict conjunction contrast between social and mechanical tasks. However, the results obtained with this method might overestimate the degree to which brain areas are truly antagonistic. It has been shown that global signal regression can produce negative correlations between regions believed to lack inhibitory connections (Murphy et al., 2009). Therefore, we performed a second, more conservative, analysis omitting global signal regression (Chang and Glover, 2009, Chai et al., 2012). This highly conservative approach greatly reduced the extent of the observed negative correlations, revealing only the most anti-correlated areas. The correspondence between these maximally anti-correlated areas and the antagonistic areas was remarkable (Figure 4). Areas anti-correlated with the mechanical network had a whole brain spatial correlation of r=0.92 with regions activated by the social tasks and deactivated by the mechanical tasks. Areas anti-correlated with the social network had a whole brain spatial correlation of r=0.91 with regions activated by the mechanical tasks and deactivated by the social tasks. Hence, the antagonistic areas identified using social and mechanical reasoning tasks corresponded almost perfectly with the brain areas demonstrating the most robust RS-fcMRI anti-correlations.
Figure 4.
Correspondence between antagonistic areas derived using task induced deviations from rest and anti-correlations networks derived from resting functional connectivity (without regressing whole brain signal). All areas corrected for multiple comparisons. Resting connectivity data from a separate group of subjects was used to derive regions anti-correlated with social antagonistic areas, and separately with mechanical antagonistic areas. The overlap of these anti-correlated areas with antagonistic areas is shown. Core brain areas involved in social reasoning tend to suppress core regions involved in mechanical reasoning, and vice-versa, even during unconstrained thought in the absence of a task.
3.4 Additional analyses
A number of additional analyses were conducted to rule out potential confounds, detailed below.
3.4.1 Visual attention demands
Our first concern was that activation of parietal and frontal areas associated with visual attention might be driven by differences in visual attention demands. Visual attention demands are influenced by a variety of factors (e.g., eye-movements, covert attention, object tracking, contrast, crowding), which precludes quantification of the global visual demands involved in viewing complex visual stimuli such as the videos. On the other hand, the visual demands of text stimuli are independent of content. The social and mechanical texts were matched in terms of overall reading difficulty, font and other visual properties; however the length of the texts varied. The number of words therefore served as a direct index of the visual demands associated with each text stimulus. We examined the effect of text length by performing a median split on each of the two text conditions. We then compared the shorter texts with the longer texts (mean difference = 9 words). This contrast did not produce any differences that passed whole brain correction, however lowering the threshold and looking at regions of interest revealed more activity for longer texts in areas associated with mechanical cognition. Figure 5 illustrates the overall effect of text length in the social and mechanical networks (defined by random effects strict conjunction). Social regions were consistently less active for longer texts in both conditions. Mechanical regions were more active for longer texts in the mechanical reasoning condition. We had anticipated this association when designing the stimuli. Therefore we made the visual demands greater in the social condition by making the social texts longer, on average, than the mechanical texts (mean difference = 11 words). Therefore, the visual demands in the text conditions drove activity in the opposite direction to the effect of domain. Since areas reported here as preferring one domain to another independently passed multiple comparison correction for the text stimuli in addition to the video stimuli, we can rule out the possibility that the preference of regions in the dorsal attention network for mechanical cognition is due to differences in visual attention demands.
Figure 5.
Influence of text length and task difficulty on average activity in the social and mechanical networks (defined by random effects strict conjunction). Voxelwise analyses failed to identify any significant differences in individual regions due to these potential confounds (see section 3.4).
3.4.2 Action observation and execution
A second potential confound relates to brain areas in lateral intra-parietal and inferior pre-central sulcus that are associated with action observation and execution (Van Overwalle and Baetens, 2009). These areas were more active in the mechanical than the social conditions. This could not be due to differences in action execution, as the response requirements were identical for social and mechanical conditions. Therefore, we audited the conditions for differences in action observation. In the social videos, two people were on screen throughout, continually engaged in speech acts, gestures and emotional expressions. In the physics videos, people or body parts were only occasionally visible. A count was made of the number of physical actions involving the limbs (i.e. not including speaking and facial expressions) and the length of time they occupied. No significant differences in the number or time spent performing actions were found between the social and mechanical videos (number: social 2.4 mechanical 2.1 t(37)=0.8, n.s.; time: social 5.9s mechanical 6.1s t(33)= 1.1, n.s.). For the texts, we counted the number of verbs that described actions. There were significantly more action verbs in the social than the mechanical texts (all non-auxiliary verbs: social texts 15.5 mechanical texts 10.4 t(36)=6.9, p<0.001; verbs describing a physical action involving the limbs performed by a person: social texts 9.4 mechanical texts 3.1 t(38)=8.0, p<0.001). Thus, by any measure, the number of actions in the social conditions was greater than or equal to the number of actions in the mechanical conditions. Therefore, demands associated with action observation drove activity in the opposite direction to the effect of domain, and cannot account for the consistent preference of regions associated with action observation for mechanical reasoning.
3.4.3 Task Difficulty
A third potential confound relates to task difficulty. Prior studies have found that more difficult tasks are associated with greater activation of dorsal attention network and fronto-parietal control network, and with greater deactivation of the default network (McKiernan et al., 2003, Mason et al., 2007). Task difficulty cannot account for the pattern seen in antagonistic areas, since rest is clearly less difficult than any of the four tasks and hence an explanation of activity in terms of task difficulty would require that all four conditions are either above or below resting level. However, task difficulty might account for other regions identified in the direct contrast between social and mechanical tasks. The best proxy measure for task difficulty is mean task accuracy. Mean accuracy was higher for the social conditions (mean=82%) than for the mechanical conditions (mean= 68%), consistent with the possibility that task difficulty might be confounding the contrast between domains. First, we performed a median split on the items in each condition. The median split produced conditions with a mean difference of 26% in accuracy, greater than the 14% mean difference in accuracy between the overall social and mechanical conditions. We then performed a voxelwise contrast comparing difficult questions to less difficult questions i.e. (more difficult social + more difficult mechanical) - (less difficult social + less difficult mechanical). When this analysis was done using the same method of strict conjunction employed for the main analyses, no areas passed multiple comparison correction. Hence, task difficult by itself cannot account for any of the regions identified in the contrast between domains. Might it have contributed to some regions? To examine this, we reversed the confound by contrasting the easier half of the mechanical conditions (mean = 82%) with the harder half of the social conditions (mean = 70%), as shown in figure 5. In social regions, the difference between conditions remained the same. In mechanical regions, we saw a significantly greater difference between conditions. Since an even greater differentiation was found between mechanical and social conditions when the contrast involved mechanical conditions that were easier than the social conditions, task difficulty can be ruled out as a confound.
3.4.4 Working Memory and Episodic memory
A fourth pair of potential confounds relates to mnemonic demands. A preference for mechanical cognition was observed in parts of the middle frontal gyrus associated with working memory (Owen et al., 2005). Conversely, a preference for social cognition was observed in medial parietal/posterior cingulate and medial prefrontal areas associated with episodic retrieval (Buckner and Carroll, 2007). This raises the concern that differences in episodic and working memory demands might account for the observed difference between domains. These demands are not easy to quantify, however three considerations suggest the differences in activity could not be accounted for by low-level differences in mnemonic demands. First, there was no episodic retrieval or future imagining demands associated with the tasks. Each stimulus in each condition was self-contained, making no reference to other stimuli, or to private or to public events that participants might be able to recall. Second, with regard to working memory demands, both the social and the mechanical conditions contained numerous details that needed to be held in mind in order to make sense of the narrative. Further, given that the social texts were an average of 11 words longer than the physics texts, the working memory demands would be expected to be higher in the social condition. Third, activity in the regions in question (middle frontal gyrus, medial parietal and medial prefrontal) were activated well above baseline for tasks in one domain, and suppressed well below baseline levels during tasks from the other domain (Figure 3, A-C). If the contrast between domains were driven by differences in episodic and/or working memory demands, then these demands would have to be greater than those associated with resting fixation for one set of tasks, and less than those associated with resting fixation in the other set of tasks. The existence of differences in mnemonic demands that fits this pattern does not appear plausible. Therefore, it is unlikely that mnemonic demands are driving the differences between domains.
3.4.5 Response related confounds
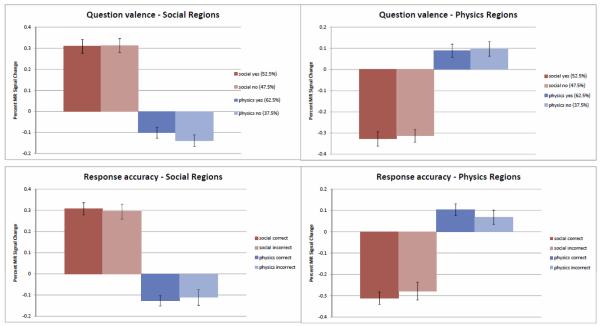
A fifth pair of potential confounds concerned the valence of correct response (i.e. whether the correct response was yes or no), and accuracy (whether response given was correct or not). These were examined using contrasts that were statistically equivalent to the tests used in the main analysis. For instance, to examine the effect of valence of correct response, the contrast (social movie correct response yes + mechanical movie correct response yes) − (social movie correct response no + mechanical movie correct response no) was combined using strict conjunction with the identical contrast for text conditions. Neither potential confound yielded any brain regions which passed multiple comparison correction. In addition, we examined the influence of both factors on averaged activity across the entire social network and the entire mechanical network (as identified using random effects strict conjunction). These can be seen in figure 6. It is clear from both these analyses that neither of the potential confounds examined could account for the pattern of results obtained.
Figure 6.
Influence of Question valence (correct answer yes or no) and response accuracy on average activity in the social and mechanical networks (defined by random effects strict conjunction). Voxelwise analyses failed to identify any significant differences in individual regions due to these potential confounds (see section 3.4).
4.1 Discussion
We identified social and mechanical reasoning tasks which produced a pattern of reciprocal activation and suppression in two brain networks. Each of the four tasks produced activation in one network, and deactivation in the other network. Hence, each task recruited processes that were excitatory with respect to one network, and suppressive with respect to the other. These antagonistic areas corresponded very closely to regions of maximal anti-correlation generated using resting connectivity, indicating their correspondence with previously described TPN and DMN networks.
Might these findings also be explained by other hypotheses? A number of researchers have characterized the DMN as a network specialized for internal attention (attention to self and/or internal states), which is opposed by a network specialized for external attention (Gusnard, 2001, Goldberg et al., 2006, Buckner and Carroll, 2007). While there may be competition between internal and external attention, this cannot account for the current findings. All of the tasks used here were externally focused: Each demanded perceptual attention and involved people and objects unfamiliar to the participants. There were no explicit demands to engage in hypothesized forms of internal attention, such as episodic memory, future thinking or self-referential cognition. In addition, the perceptual attention demands were higher for the social than the mechanical texts, which should produce the pattern opposite to that observed. While explicit task demands cannot account for our findings, it might be argued there are implicit demands to engage in internal attention that differentiate the tasks (Buckner and Carroll, 2007). If present, such implicit demands might account for regions revealed by the contrast between domains (but see additional analyses which cover some potential confounds). However, we do not know of any well-articulated notion of ‘internal’ or ‘self-referential’ cognition that might account for the differences relative to rest observed in antagonistic areas. The motivation for associating the default mode network with internal or self-referential cognition derives from the idea that this network is maximally activated when participants are at rest, a state in which the participant is effectively deprived of any external stimulus and hence must internally generate their thoughts. However, in our data, the antagonistic areas in the DMN were considerably more active when participants were attending to external stimuli, than at rest. Hence, the pertinent distinction which distinguishes which network is activated, and which network is deactivated, is between two types of externally directed attention: one in which the focus is on social interaction and the mental states of others, and another in which the focus is on inanimate objects and the physical principles that determine their mechanical interactions.
The spatial location of the antagonistic areas also argues against the external vs. internal attention hypothesis as an account of the tension between the TPN and DMN. First, the antagonistic regions in the TPN are distinct from regions that are most clearly associated with visual attention, i.e., frontal and parietal regions containing retinotopic maps (Jack et al., 2007). Second, the antagonistic regions in the DMN are distinct from regions involved in episodic memory (Spaniol et al., 2009), and the region in DMPFC is clearly dorsal to the region involved in self-oriented cognition (Mitchell et al., 2006). A recent meta-analysis of 107 neuroimaging studies found a preference for other-over self-related judgments in each of the social antagonistic areas identified here (dMPFC, MPC, rTPJ) (Denny et al., 2012). Finally, areas of maximal anti-correlation, the phenomenon which originally defined the TPN vs. DMN dichotomy, corresponded very closely with the antagonistic areas identified here, and did not correspond well with areas that would be predicted by the internal vs. external attention hypothesis. This provides compelling evidence for the opposing domains hypothesis over the internal vs. external attention hypothesis. Nonetheless, our findings do not rule out the possibility that there is a tension between external and internal attention. Figure 7A illustrates regions that were more active during rest than any of the tasks used here, and hence which may be associated with internal attention and/or spontaneous cognition. Further work may identify distinct processes that excite and inhibit other parts of those networks. On the other hand, it does appear that the contrast between the cognitive modes our tasks recruit accounts for much of the default network, illustrated in Figure 7B.
Figure 7.
Comparison of default-mode regions associated with internal attention and/or spontaneous cognition, and default-mode regions associated with the division between social vs mechanical reasoning. The default-mode network is shown in green. (A) Regions that are more active during rest than any of the four tasks used here are shown in blue. Overlap between default-mode network and consistently deactivated areas shown in light green and blue. (B) Regions preferring Social to mechanical reasoning are shown in red. Overlap with default-mode network shown in yellow/light green.
Numerous studies have demonstrated that DMN regions are involved in a broad range of social, emotional and moral cognitive processes. A number of comprehensive reviews and meta-analyses cover this work (Amodio and Frith, 2006, Schilbach et al., 2008, Van Overwalle, 2009, Mars et al., 2012). Many of the best regarded of these studies employed rigorously controlled experimental designs aimed at isolating specific social functions. Yet, with regard to the tension between the DMN and TPN, these studies may have missed the wood for the trees. Our study was designed to be specific in a different way: by recruiting processes from just one domain. Thus, for example, our social tasks involved a range of socio-emotional processes, including theory of mind, emotion recognition and moral cognition, yet we strove to minimize the degree to which these tasks involved the sorts of analytic reasoning, mental manipulation, and/or deliberately controlled attention that are often demanded by carefully controlled behavioral designs. Ultimately, the proof of our success lies in the data. We were able to show clear activation of DMN regions accompanied by clear deactivation of TPN regions, and vice-versa. This suggests that, while the tasks used here no doubt recruited a broad range of processes, they nonetheless successfully separated out cognitive processes preferentially associated with the DMN and TPN. In contrast, prior investigations appear to have engaged processes associated with both cognitive modes, and hence recruited regions in the TPN alongside the DMN (Spreng et al., 2010, Meyer et al., 2012).
The mechanism by which the TPN and DMN suppress each other remains to be determined. Anatomical studies suggest that there are no direct inhibitory links between these brain networks, hence it is likely that the suppression is mediated by other regions. Since the effect of domain was consistent across two modalities of presentation (text and video), and the perceptual demands were similar for the social and physical tasks within each modality, it appears that the mechanism is distinct from mechanisms of perceptual attention. One possibility, suggested by Spreng (Spreng, 2012), is that regions in the FPCN mediate the relationship between the TPN and DMN. This is consistent with the observation that some regions in the FPCN were activated during both social and non-social tasks in the current investigation.
Why are DMN and TPN regions co-activated in some studies? It appears that, while there is a tendency for one network to be suppressed when the other is active, this tendency can be overridden by additional task demands e.g. where information from the DMN needs to be communicated to FPCN regions involved in planning (Spreng et al., 2010), or where information held in the DMN needs to be manipulated to perform a complex working memory task (Meyer et al., 2012). A further unanticipated clue concerning the relationship between the networks derives from the time course of BOLD activity seen in opposing areas. Notably, there is a clear pattern of activation and deactivation which persists throughout the stimulus presentation period (Figure 3, A-F). However, this pattern is disrupted during the question/response period. During this time period, we observe a pattern which is closer to what has typically been observed i.e. the TPN is not deactivated (Figure 3, A-C), even for conditions which activate the DMN (Figure 3, D-F). Hence it would appear that the decision making and response related processing which occurs during this period is sufficient to drive up activity in the TPN even when the task involves social-emotional processing. The co-activation of DMN and TPN regions during this period may reflect the communication of information represented in the DMN to areas involved in decision making and response. Mutual suppression is seen when the only task required of participants is to use perceptual information to build a representation. This suggests that the information processing resources which build representations used in social and mechanical reasoning are (at least in part) mutually incompatible3. Hence these findings suggest a limit to our capacity to form concurrent representations that span these domains. This is consistent with prior work which shows that entraining participants in an analytic cognitive set, by giving them mathematical problems, suppresses empathetic response (as measured by charitable donations) when a picture and story of a distressed individual is subsequently presented (Small et al., 2007). These findings also suggest a mechanism that may account for syndromes in which dysfunction in one domain is accompanied by cognitive strength in the other. Most notably, individuals with autism perform better than IQ matched controls on scientific reasoning (Baroncohen et al., 1986), spatial fluid intelligence tasks (Dawson et al., 2007, Hayashi et al., 2008), and some attention tasks (Baron-Cohen, 1998, O’Riordan et al., 2001). A number of individuals with autism also show extra-ordinary visual creativity. Fronto-temporal dementia is associated with declining social function, and some patients also display an increase in visual creativity as the disease progresses (Miller and Hou, 2004). The converse pattern appears in Williams syndrome, which is characterized by high levels of empathy but impaired visual-spatial ability (Brown et al., 2003). Further work is needed to determine how variability in human performance relates to the present biological observations.
These findings indicate that cognitive context, i.e., the primary cognitive mode that is engaged by a task, has a surprisingly powerful effect on the recruitment of brain areas. A number of brain areas showed patterns of activation that do not fit easily with widely held notions of their cognitive function. For instance, much of the ‘dorsal attention system’ (Corbetta and Shulman, 2002, Fox et al., 2006) was suppressed below resting levels during the social tasks, although these tasks clearly involved greater perceptual demands than resting fixation. Similarly, lateral frontal areas associated with working memory (Owen et al., 2005) showed radically different patterns of deactivation and activation in the social and mechanical reasoning tasks, although all the tasks required participants to maintain numerous details in working memory. Several researchers have drawn a close analogy between folk psychology and folk physics (Lewis, 1972, Gopnik, 1996, Saxe, 2005) because they both involve similar high-level psychological processes such as abstraction, inference, model building, prediction, and the postulation of unobservable processes or states. However, the present observations are difficult to reconcile with a view of functional organization which is driven by these apparent similarities: The degree to which different brain areas are recruited cannot be explained simply on the basis of the psychological processes that are required to perform a given task. These findings suggest a model of functional organization in which cognitive domain also plays a major role in determining how brain areas are recruited. It appears that there are fundamental differences in the information processing resources that are recruited, depending on the primary cognitive mode engaged by the task context. A similar finding has been demonstrated by studies using the Wason selection task, which show that the computational resources recruited depend on whether the task is framed as a social or mechanical problem (Cosmides, 1989, Fiddick et al., 2000). This and related work (e.g. on the representativeness heuristic (Kahneman and Tversky, 1972)) has helped spur dual-process theories of cognition which distinguish between System 1 and System 2 reasoning systems (Sloman, 1996, Evans, 2003, Kahneman, 2003). System 1 has been variously characterized as ‘intuitive’, ‘emotion-driven’ and ‘experiential’ whereas System 2 has been characterized as ‘controlled’, ‘rule-based’, ‘rational’ and ‘analytic’. We know of two lines of work which link cognitive neuroscience to this classical form of dual process theory: one which looks at logical reasoning (Goel and Dolan, 2003), the other moral judgments (Greene et al., 2004). Both identify areas in the DMN and TPN associated with System 1 and System 2 reasoning respectively. Hence, the link between dual-process theories of cognition and the DMN vs. TPN dichotomy appears worthy of further investigation.
A novel type of dual processing account has recently been proposed to account for disparities in performance on explicit and implicit measures of theory of mind. According to one version of this account, implicit measures reflect the operation of an inflexible and automatic implicit system, whereas explicit measures reflect the operation of a more flexible but cognitively demanding explicit system (Apperly and Butterfill, 2009). We reject this account for a variety of reasons. First, it has been shown that the information processing reflected by implicit measures of theory of mind is not wholly automatic because it is prone to dual-task interference (Schneider et al., 2012).
Concordant with this, the pattern of reciprocal inhibition between the TPN and DMN provides compelling evidence that the DMN is involved in controlled processing - if it were wholly automatic and its processing can proceed in parallel, then deactivation of the DMN would not be such a reliable function of non-social cognitive load (McKiernan et al., 2003, Gordon et al., 2007, Mason et al., 2007). Closing the loop, recent evidence shows that DMN activity associated with mentalizing is diminished by a concurrent dual task (Spunt and Lieberman, in press). Third, it does not appear accurate to describe the mentalizing system of the DMN as implicit in the sense of being unconscious, since this system is implicated in more cognitive representational aspects of emotion processing (Kober et al., 2008, Shamay-Tsoory et al., 2009, Zaki and Ochsner, 2011) and is reliably engaged during introspection, i.e. when we consciously represent our internal states (Schilbach et al., 2012).
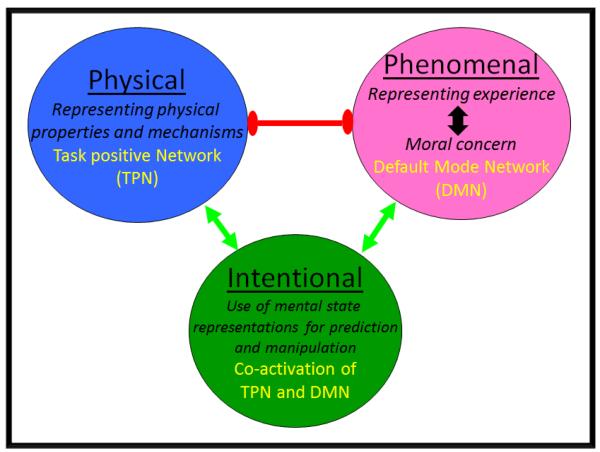
Rather than postulate two distinct systems whose operation is reflected by implicit and explicit measures of theory of mind, our theory postulates two distinct cognitive modes for mentalizing which both recruit the DMN but differ in terms of how they recruit the TPN (Figure 8). Implicit measures are thought to reflect the adoption of the Phenomenal stance, the ‘default mode’ of social cognition which is emotionally engaged and focused on the representation of experiential states. This stance is hypothesized to engage the DMN and deactivate the TPN. Explicit measures are thought to recruit a different mode, the Intentional stance, an emotionally disengaged form of social cognition in which processes associated with the TPN are recruited in order to manipulate and transform representations of internal mental states (held in the DMN) so that they can guide response. This is consistent with evidence suggesting that explicit theory of mind measures depend on additional non-social cognitive processes (Bloom and German, 2000), specifically executive functions such as inhibitory control (Carlson and Moses, 2001). The Intentional stance is thought to be engaged not just by explicit theory of mind tasks and tasks requiring mental manipulation of social representations (Meyer et al., 2012), but also in naturalistic settings when individuals are engaged in competitive or ‘manipulative’ (anti-) social interactions. This is consistent with meta-analytic findings which show tasks involving deception reliably recruit regions in the TPN associated with executive functions (Christ et al., 2009).
Figure 8.
Three cognitive stances, their relationships to each other, and the brain networks involved. Bidirectional arrows indicate mutual compatibility; barbell indicates mutual antagonism. The Intentional stance is a distinct cognitive mode in which processes associated with the task positive network operate on representations stored in the default network. Nonetheless, there remains a fundamental tension between cognitive modes involved in the representation of experiential mental states (the Phenomenal stance) and the representation of physical mechanisms (the Physical stance). The opposing domains hypothesis holds that this tension represents the cognitive basis of the reciprocal inhibition between default and task positive networks.
Why is there an antagonism between brain areas involved in social and mechanical reasoning? This may seem surprising, given that the instantiation of these functions in different brain regions might facilitate their occurring simultaneously without interference. One possibility is that incompatible heuristics are most effective for predicting the behavior of persons versus inanimate objects. It would be no less foolish to suppose that a person will continue in motion in a straight line unless acted upon by an external force, than it would be to suppose that a pool ball will alter its course because it wants to go into the pocket. Experimental evidence demonstrates that we have an automatic tendency to track the mental states of others, and that this can interfere with performance on an unrelated task (Samson et al., 2010). Hence, turning off the brain areas for the opposing domain may lessen the application of inappropriate cognitive strategies. However, our view is slightly different. We do not see the principle function of the cognitive mode which suppresses the TPN as the prediction of behavior, but as generating a distinctly social form of interpersonal understanding (‘intersubjectivity’). According to our model (Figure 8) and supporting behavioral work (Jack and Robbins, 2012) there is a tight linkage between thinking about experiential mental states and moral concern. Consistent with this, moral cognition produces an activation pattern that closely fits the default network (Harrison et al., 2008). Our hypothesis is that the inhibition between domains is driven by the need to differentiate members of our moral circle from objects suitable for manipulation. In terms of effective strategies, it would be a mistake to limit the actions one is willing to perform on an inanimate object out of a sense of compassion. In contrast, it is often advisable to demonstrate sensitivity to an in-group member’s feelings when interacting with them. Hence, our view is that the antagonism between domains reflects a powerful human tendency to differentiate between conscious persons and inanimate objects in both our attitudes and modes of interaction.
3.2 Limitations
The most significant limitation of the current study is that our tasks were not designed to isolate specific cognitive processes involved in social (or mechanical) cognition. Hence, while the social and mechanical tasks used were successful in separating out processes associated with the DMN and TPN respectively, this study does not allow us to identify which of a number of the potential candidate processes associated with the social and mechanical tasks might be responsible for suppressing the opposing network. A second limitation is that our ecologically valid tasks did not afford an opportunity to parametrically vary processing load. Parametric variations in cognitive load would provide an additional method for identifying key processes responsible for deactivation of the TPN, as they have done in studies investigating deactivation of the DMN (McKiernan et al., 2003, Gordon et al., 2007, Mason et al., 2007). It is possible that future investigations may be able to pinpoint a single isolated cognitive process which leads to deactivation in the TPN. Alternatively, it may be that this deactivation only occurs when a range of different socio-emotional processes are simultaneously recruited. If the suppression of the TPN mirrors the pattern seen for the DMN, then it is likely that a family of different processes are capable of producing deactivation, and that the degree of suppression of the TPN increases with increasing recruitment of any of this family of processes.
A third limitation concerns the ability of the current study to accurately characterize the extension of the family of processes that deactivate the TPN. No single study can adequately address this issue, and it is possible that the characterization of the processes that suppress the TPN will be prone to the same process of revision which has marked the history of attempts to correctly characterize the family of processes which deactivate the DMN. All that we can definitely state on the basis of this investigation is that the social cognition tasks used here did reliably recruit processes which suppressed the TPN, even though attention was directed externally rather than internally. On the other hand, we note that all the tasks we know of which have been shown to recruit the DMN above resting levels have clearly involved social cognition (Iacoboni, 2004, Sestieri et al., 2010, Spreng et al., 2010, Meyer et al., 2012). Following the theoretical work which motivated this investigation (Robbins and Jack, 2006, Jack and Robbins, 2012), we hypothesize that any task that involves thinking about experiential mental states will both activate the DMN and cause suppression of the TPN.
Highlights.
Physical reasoning tasks activate the TPN and deactivate the DMN.
Social reasoning tasks deactivate the TPN and activate the DMN.
Activated/deactivated regions match areas of maximal anti-correlation.
Findings are not explained by task engagement, or internal vs. external attention.
TPN versus DMN dichotomy reflects opposing cognitive modes.
Acknowledgements
This work was supported by funding to AIJ from the NSF 0841313, the Leonard Krieger Fund and the University Hospitals Case Medical Center Spitz Brain Health fund; and by funding to AZS from NIH/NINDS 30NS048056. The authors would like to thank Hillel Chiel, Randy Buckner and Chris Frith for comments on earlier drafts.
Appendix A
Peak coordinates (foci) associated with random effects analyses. All z statistics are minima of the contrasts that are conjoined (i.e. minimum z from two contrasts for direct comparisons of social and mechanical, minimum of four contrasts for comparisons with rest). Spaces are reported in 711-2B space, a modified version of talairach.
| conjunction | X | Y | Z | z-stat | conjunction | X | Y | Z | z-stat |
|---|---|---|---|---|---|---|---|---|---|
| social > rest, mechanical < rest | −2 | −56 | 31 | 4.9 | mechanical > rest, social < rest | −52 | −39 | 42 | 3.7 |
| social > rest, mechanical < rest | −1 | 46 | 27 | 3.7 | mechanical > rest, social < rest | −37 | −46 | 39 | 3.6 |
| social > mechanical | 12 | −96 | 18 | 3.0 | mechanical > rest, social < rest | −39 | 32 | 19 | 3.4 |
| social > mechanical | 26 | −77 | −33 | 5.4 | mechanical > social | 39 | −78 | 26 | 3.1 |
| social > mechanical | −22 | −73 | −33 | 3.8 | mechanical > social | −24 | −69 | 41 | 4.8 |
| social > mechanical | −52 | −65 | 29 | 5.5 | mechanical > social | 33 | −69 | 42 | 3.2 |
| social > mechanical | −58 | −59 | 19 | 4.6 | mechanical > social | 17 | −67 | 48 | 5.9 |
| social > mechanical | 51 | −57 | 26 | 6.5 | mechanical > social | −12 | −66 | 53 | 6.1 |
| social > mechanical | 2 | −56 | 3 | 3.2 | mechanical > social | 35 | −63 | −37 | 4.9 |
| social > mechanical | 1 | −55 | 30 | 8.7 | mechanical > social | −52 | −59 | −6 | 7.0 |
| social > mechanical | 3 | −50 | −38 | 4.4 | mechanical > social | 57 | −55 | −10 | 5.7 |
| social > mechanical | −6 | −46 | 10 | 3.1 | mechanical > social | −19 | −49 | 47 | 3.3 |
| social > mechanical | 60 | −44 | 10 | 5.4 | mechanical > social | 37 | −46 | −52 | 3.1 |
| social > mechanical | 6 | −38 | 4 | 5.3 | mechanical > social | −30 | −44 | −7 | 4.0 |
| social > mechanical | −52 | −38 | 0 | 6.2 | mechanical > social | −45 | −44 | 45 | 7.0 |
| social > mechanical | −35 | −32 | 19 | 3.0 | mechanical > social | 40 | −43 | 46 | 7.3 |
| social > mechanical | −9 | −31 | −5 | 4.1 | mechanical > social | 30 | −42 | −7 | 5.1 |
| social > mechanical | 17 | −27 | −7 | 4.7 | mechanical > social | 54 | −37 | 43 | 7.5 |
| social > mechanical | 52 | −27 | −5 | 6.7 | mechanical > social | 14 | −36 | 42 | 3.8 |
| social > mechanical | −58 | −26 | −6 | 7.2 | mechanical > social | −60 | −34 | 37 | 6.1 |
| social > mechanical | 1 | −22 | 40 | 5.6 | mechanical > social | −13 | −33 | 39 | 4.3 |
| social > mechanical | −20 | −18 | −10 | 5.1 | mechanical > social | −23 | −15 | 51 | 3.8 |
| social > mechanical | 53 | −16 | −10 | 6.6 | mechanical > social | −37 | −10 | 4 | 5.5 |
| social > mechanical | 2 | −16 | 11 | 4.8 | mechanical > social | 40 | −7 | 4 | 4.6 |
| social > mechanical | 67 | −13 | −11 | 4.4 | mechanical > social | −2 | −3 | 32 | 5.6 |
| social > mechanical | 21 | −12 | −8 | 5.0 | mechanical > social | −23 | 0 | 55 | 5.9 |
| social > mechanical | −52 | −6 | −14 | 5.5 | mechanical > social | 23 | 0 | 54 | 4.2 |
| social > mechanical | 48 | 2 | −22 | 6.5 | mechanical > social | −48 | 1 | 27 | 5.8 |
| social > mechanical | −48 | 4 | −24 | 5.0 | mechanical > social | 48 | 2 | 21 | 5.7 |
| social > mechanical | 32 | 13 | −12 | 5.2 | mechanical > social | −29 | 13 | 7 | 3.5 |
| social > mechanical | −36 | 13 | −15 | 4.6 | mechanical > social | −5 | 20 | 48 | 3.0 |
| social > mechanical | 37 | 14 | −32 | 3.4 | mechanical > social | −42 | 31 | 23 | 6.6 |
| social > mechanical | 3 | 17 | 66 | 3.0 | mechanical > social | 42 | 35 | 21 | 6.5 |
| social > mechanical | 2 | 17 | −4 | 3.5 | mechanical > social | −45 | 38 | 13 | 5.5 |
| social > mechanical | 53 | 20 | 15 | 4.0 | mechanical > social | 41 | 47 | 7 | 3.3 |
| social > mechanical | 49 | 22 | 4 | 4.1 | |||||
| social > mechanical | −44 | 24 | −2 | 3.9 | |||||
| social > mechanical | 17 | 30 | 43 | 3.0 | |||||
| social > mechanical | 13 | 34 | 55 | 3.7 | |||||
| social > mechanical | 2 | 40 | −11 | 4.6 | |||||
| social > mechanical | −2 | 48 | 23 | 7.1 | |||||
| social > mechanical | 18 | 48 | 41 | 3.6 | |||||
| social > mechanical | 4 | 48 | 44 | 5.6 | |||||
| social > mechanical | −15 | 52 | 33 | 4.0 |
Appendix B: text stimuli
Physics Stories
A guitar is a musical instrument with five metal strings of varying thicknesses. Plucking the strings causes them to vibrate and produce a sound. Each string has a different pitch depending on its thickness, which affects the speed of the vibration. The lightest or thinnest string will vibrate the quickest, while the heaviest string will vibrate the slowest. To test the pitch, the lightest string is plucked. Will the lightest string that vibrates fastest produce the lowest pitch?
A block of solid lead is placed on a scale in the elevator of a building on campus. The elevator is taken to the top floor, and when the elevator doors open and the scale stops moving, the weight of the solid lead block is recorded. Now, the button for the basement is pressed, the doors of the elevator close, and the elevator moves downward. As it starts to move, the lead block’s weight changes.
According to the scale, does the weight of the lead block decrease?
On a hot, sunny day, two metal buckets sit in direct sunlight. The buckets are the same size, but one is dark black while the other bucket is painted shiny silver. In the morning, the temperatures are the same. As the sun heats up the buckets throughout the day, the black bucket heats up faster than the shiny bucket. That night, the air temperature drops and the buckets begin to cool at different rates.
Will the black bucket cool more quickly?
A snowmobile is cruising over plains of white, hard packed snow. The driver steers the snowmobile in a straight line while at the same time pointing a flare gun straight into the air. The driver pulls the trigger, firing a bright flare into the air. Then, the driver immediately slams on his brakes. The flare flies through the air and then lands in the snow.
Will the flare land in front of the snowmobile?
A rifle is being tested at a long, flat firing range. The gun is propped up on a table, with a few bullets sitting on the table next to the gun. After looking down the firing range to insure that nothing is in the way, the trigger is pulled, firing the gun perfectly straight. At the same instant that the trigger is pulled, a bullet rolls off the table and falls to the ground. The two bullets eventually hit the ground.
Will the bullets hit the ground at the same time?
A membrane separates a tank into two equal halves. The membrane allows water molecules to pass through, but not large molecules such as red dye. The tank is filled almost to the brim with water. Then concentrated red dye is poured into one of the sides until that side reaches the brim, changing its concentration. The dye causes water from the other side to pass through the membrane to the red side. The red side begins to overflow.
Will the clear side without dye empty completely?
To test the effects of buoyancy, a heavy rock has been placed into a boat that is floating in a small pool filled to the brim with water. The rock is then taken out of the boat, and the water level decreases. Finally, the rock is put into the water, and it sinks to the bottom of the pool. The water level increases.
Will the pool overflow?
There is a basket on a conveyor belt connecting two sides of a canyon. The basket carries materials from one side to the other. When the basket successfully reaches one of the sides it stops and the bottom of the basket opens, releasing its contents onto a platform. One day when the basket is in the process of moving across the canyon, the bottom of the basket breaks opens and its contents fall into the canyon.
Will the objects fall straight down when they are released?
A roller coaster uses electromagnets to accelerate to high speeds. It is designed so that it travels along a flat portion before heading straight up an incline. The car starts at rest and then accelerates, reaching top speed as it begins to climb the hill. As it climbs, gravity pulls on the car, causing it to slow down and eventually stop. It begins to fall back down the hill, gaining speed as it goes. The car reaches the bottom of the hill.
Will the car continue to accelerate?
Two cannonballs are brought to the top of a tower. Both are placed on a platform over the edge of the tower. When a button is pressed two things will occur simultaneously. A platform underneath one ball will drop, releasing the ball and allowing it to fall straight down. Also, a powerful spring behind the second ball will push it horizontally away from the tower and parallel to the ground. Eventually, both balls reach the ground.
Will the two balls hit the ground at the same time?
As a train approaches a bridge, it sounds its horn continuously to warn those ahead. The whistle’s pitch sounds relatively high and constant to an observer standing on the bridge as the train approaches. When the train goes under the bridge, the pitch changes to a lower pitch, and stays constant as it travels farther from the bridge. Then, the train starts to slow down as it nears its station, and the whistle’s pitch changes.
Does the pitch get higher as the train slows down?
A large rectangular block of wood sits on an ice rink and needs to be removed. A rope is tied around the block and workers pull the block of wood over the ice and off the rink. Due to the ice rink’s setup, the block can be pulled straight off the ice rink and over a concrete floor. While it takes a great amount of pulling to get the ice started moving, once in motion it is relatively easy to pull. Then the workers reach the concrete.
Will it be easier to drag the block over the concrete floor?
A cannonball and volleyball are brought to the top of the tallest building in the world. The balls are placed on a platform over the edge of the building. This platform is built in such a way that when a lever is pulled the balls are released at the exact same moment. The lever is released, and the cannonball and volleyball accelerate towards the ground. Due to air resistance, the volleyball eventually stops accelerating.
Will the two balls hit the ground with the same speed?
A communications satellite is moving in stable orbit around the Earth. The electrical panel on the satellite has a serious malfunction. The speed of the satellite begins to gradually decrease, eventually reaching half the original speed. A shuttle is sent up to repair the satellite, but before they can rendezvous the satellite begins to move out of its stable orbit due to its decreased speed.
Will the satellite fall to earth due to its decreased speed?
A concave mirror can be used to ignite a match with a hot light. A match is placed in the focal point of the mirror, so that any light that hits the mirror will be focused directly onto the match. A bright light is placed a few feet away from the mirror and turned on. Within a few moments the match bursts into flames. A new setup uses the same type of light and match, but uses a regular, flat mirror to reflect the light.
Will the regular mirror ignite the match?
A giant rock is put into a large bin and weighed. Then, a robotic arm picks up the rock and places it into a machine that smashes up the rock into smaller pieces so that the rock can be processed. After this machine breaks the rock into thousands of tiny pieces they are all collected and put back into the bin. This bin with all the tiny pieces of rock is weighed to ensure that no rock was lost in the process.
Will the rock weigh the same as before?
Scientists want to design a rocket to reach space with the greatest speed. They have created an experiment to test which of two possible designs will work best. Two identical rockets have been set to launch into outer space. One rocket is being launched in the same direction as Earth’s spin, while the other is being launched in the opposite direction of Earth’s spin. When they reach space, the two rockets are going different speeds.
Will the rocket launched in the same direction as Earth’s spin reach a higher speed?
Two identical rectangular magnets are resting near each other on a flat table. These magnets are identical in shape, size, and magnetic strength. One end of each of the magnets is blue and the other is red. The magnets are picked up and the blue ends are brought together. The closer that the blue ends are brought together, the stronger the magnets seem to repel away from each other. Then, the red ends are brought together.
Will the red ends of the magnets repel one another?
Two commercial jet airplanes are flying from Cleveland to Boston. The planes are identical in almost every way, except that one plane has clearance to fly faster. On their approach into the airport, each plane must make a turn in order to line up with a runway. The faster plane makes the turn at a considerably faster speed than the slower plane. One of the planes is able to turn more sharply.
Will the faster plane turn less sharply?
A paper cup is placed directly above a flame from a Bunsen burner that is turned on high. Within a few moments the cup bursts into flames. Then, an identical cup is filled with water and placed the same distance from the Bunsen burner’s flame. After a few moments, the water filled cup does not catch fire. A few minutes passes with the cup directly in the heat. The water temperature increases and it begins to boil away.
If the water boils away completely, will the cup burst into flames?
Social Stories
Justin is afraid to tell his father that he didn’t make the basketball team during tryouts. His father is proud of Justin’s older brother who is a basketball star. Justin wants to please his father, but he’s too clumsy to be good at basketball. Ashamed to tell his father the truth, he comes home late after school so his father thinks he was at practice. At the hardware store, Justin’s father happens to see the team coach and learns the truth. He quietly stares down at the ground.
Will Justin’s father come home feeling guilty?
James and Lauren plan on meeting for dinner to celebrate Lauren’s new job. James is determined to show up because he wants Lauren to feel that he supports her career. Last time he had to cancel to volunteer at Habitat for Humanity, but this time he promises to show up. During the day James’ dog gets hit by a car and needs to be rushed to the vet. He tries to call Lauren but her cell phone has run out of battery.
Will Lauren think that James cares about her career?
David worked all summer mowing lawns to save up for a new video game. He buys the game, but he has to mow the neighbor’s yard before he can play it. He carefully puts the game in the middle of his desk. David’s younger sister Hailey is bored without a summer job, so she sneaks into David’s room and plays the game all afternoon. She does not want David to know she played the game, but she mistakenly puts it on his dresser instead of the desk where he left it.
Does David trust his sister?
Sally invited Ann over to see the new doll that she got for her birthday. While they are playing in her bedroom, Sally’s mother comes in looking angry and asks her to step outside to talk. Before she leaves the room, Sally takes her new doll and puts it in her toy chest. Ann decides to play a trick on Sally while she is gone. She takes the doll out of the toy chest and hides it under the bed. She then goes back to innocently playing on Sally’s bed.
When Sally gets back to her room, will she look for the doll under her bed?
George is in a fishing competition. Last year he placed first because he cheated, pretending that he caught a fish that he actually purchased. This year a camera crew is following him to witness his catch. He nervously explains his techniques to the camera, praying to himself that he gets lucky and catches a fish. Suddenly, his pole bends over, and he screams with delight as he begins to reel in his line. In actuality, George’s lure is stuck on a small tire.
Does George care what other people think of him?
In preparation for her first high school dance, Sarah purchases a revealing red dress and high heels. To afford the dress, she steals money from her step-father’s wallet. She doesn’t feel guilty because it was his fault that her parents got divorced. When she gets home, Sarah puts her dress and shoes on the chair in her room. While she’s away, her older sister borrows the shoes to look good for the stepfather and then carelessly leaves the shoes in the basement.
Does Sarah’s sister think Sarah was wrong to steal?
Shannon spent all weekend studying for an exam, even though Saturday was her father’s birthday. Shannon decided that she must study, because if she did well she could get into medical school. The test went well, but Shannon feels guilty that she didn’t spend more time with her father. When Shannon gets her test back, she sees a ‘D’ written at the top of the paper, and in embarrassment quickly puts it in her bag. She didn’t notice that it is actually Jack’s test. Her real score is an ‘A’.
Does Shannon think that she received an A on the exam?
Pam and Mike plan on having dinner to celebrate their anniversary. They have been fighting recently because Mike admits to fantasizing about Pam’s twin sister. To make amends, he promises to make dinner reservations at Pam’s favorite restaurant. Pam looks forward to the date, but Mike, dreading the dinner, waits until the last minute to reserve a table. When he finally calls, the restaurant is booked. Relieved, Mike reserves a table at his favorite restaurant, where Pam’s sister works.
Does Pam think she is going to have fun at dinner?
Rebecca and Patrick plan on going to see their favorite band. When Rebecca calls to buy the tickets, they are all sold out. Worried that Patrick will hate her for not getting tickets, she calls him in tears to apologize. Patrick is not mad because he secretly bought the tickets last week, but he wants to keep it a surprise so he doesn’t tell her. Rebecca feels bad about not getting tickets, so she buys him lunch, which he gladly accepts.
Is Patrick concerned about Rebecca’s feelings?
Elise has a puppy that she thinks is the cutest dog ever. Elise’s mother does not let her bring her new puppy into her room because he chews on everything. Because of this Elise always keeps her door closed. One evening as Elise is rushing to the bathroom, the dog slips into her room unnoticed. That night there is a loud thunderstorm and Elise awakens to hear strange growling and scratching under her bed. Elise runs screaming to her mother’s bedroom.
Does Elise think that her dog has turned nasty?
Bill convinces Sue to purchase a season pass to an amusement park, but every time they have a chance to go it rains. Sue regrets getting the season passes, and complains to Bill all the time. In reality, she is relieved because she is afraid of roller coasters. This weekend the weather forecast predicts clear skies. Bill goes to sleep excited for the trip, while Sue dreads the upcoming good weather. While they sleep, the forecast changes to rain.
Will Bill and Sue wake up thinking they are going to the amusement park?
Chris has developed a bad habit of skipping class to play online video games. He used to like going to class to see his friends, but lately he has been depressed and would rather just laze around in his room. Today he looks at his calendar and notices a test next week but nothing important today, so he skips class again. He was not in class last week to hear the teacher announce that the test has been moved up a week.
Do Chris’s friends care if he misses the test?
Eric is an honors student, but he is having trouble keeping up with his challenging courses. He plans out his week so that he studies for exams, watches basketball with friends, and writes a history paper. He dreads writing the paper, so he puts it off to the last minute. He only checks out the library books for the project the day before the paper is due. While he’s away, his mother sees the books and returns them, assuming he is finished with the project because it is due tomorrow.
Does Eric’s mother think that her son will be thankful?
John and Mary are looking for their car keys. John is excited but nervous because he plans on proposing at dinner. Mary is stressed out because she just got fired at work but has not had the nerve to tell John. Both are looking for the keys frantically because they are already late for their dinner reservation. Losing her temper, Mary storms outside to see if John left the keys in the car. While Mary is outside, John finds the keys between the couch cushions.
Does John think Mary is upset about losing her job?
Julia and her best friend Ashley have been fighting because Julia has been flirting with Ashley’s boyfriend online. Julia regrets upsetting her friend and decides to give her an ice cream cake when she comes over for a study date the next evening. Julia buys the ice cream cake and puts it in the freezer. That night, the power goes out and the ice cream cake melts into a gooey mess. When Ashley comes over, Julia tells her she has a special treat for her, and goes to open the freezer.
Does Julia think that Ashley will be impressed?
The Smith family went camping for the first time all together. Mr. Smith loves the outdoors, and he used to go camping every weekend with his ex-wife. Jessica, his new wife, hates camping but wants to impress her new husband. Jessica quickly becomes grumpy and then starts an argument around the campfire, and everyone goes to bed in a sour mood. A distracted Mr. Smith forgets to secure the food cooler, and that night a bear breaks into their cooler and eats all of the food.
Does the bear want to ruin Mr. Smith’s new marriage?
Laura is preparing for a horse show, but has been distracted by a young man working at the stable. Every time he comes to clean out the horse stalls she blushes and runs away. This distraction has put her behind schedule, and the day of the competition she does not have time to braid her horse’s mane. She cries on the way to school knowing she will not be able to compete that afternoon. During the day, the stable boy braids the horse’s mane for Laura, hoping it will impress her.
Does Laura know how the stable boy feels?
Jenny has a horrible fight with her brother. He is always being cruel to her. She goes out to buy some ice cream to cheer herself up. Just as she gets home a friend calls and asks Jenny out to see a movie. Excited, she rushes out and carelessly leaves the ice cream on the table. Jenny’s brother feels guilty. He sees the ice cream and puts it in the freezer. While she is watching the movie, Jenny suddenly remembers that she has left the ice cream out and feels sad again.
Does Jenny think her brother is helping her out?
Sue sneaks into the kitchen, gets on a chair, and puts her little hand into the candy jar to grab a heaping handful of treats. As she walks out of the kitchen, she smirks at the thought of disobeying her mother, who told her not to have any more sweets. But as she brings the candy to her mouth a sinking feeling of guilt comes over her. She knows the candy being saved for a party tomorrow. Conflicted, Sue finally decides to put the candy back and not eat any.
Does Sue’s mother think all of the candy is in the jar?
Charlie is seen by his classmates as a nerd. He has been in love with Leah since fifth grade, but she is so popular he has never had the guts to tell her. He decides to hold a surprise birthday party for Leah. Charlie calls Leah’s friends to invite them, but forgets to invite the most popular girl at school, Chloe. Jealous that she can’t go, Chloe tells Leah about the party. Leah is touched that Charlie planned the party, so she decides to act surprised at the party anyways.
Will Charlie think that he has surprised Leah?
Footnotes
DMN: Default Mode Network (Raichle, 2001) - also known as the task negative network (Fox et al, 2005) - network including midline and inferior parietal regions commonly deactivated during demanding cognitive tasks
TPN: Task Positive Network (Fox et al 2005) - network including parietal and frontal areas which is anti-correlated with the DMN and which is commonly activated during demanding cognitive tasks. The TPN overlaps the DAN and FCPN.
DAN: Dorsal Attention Network (Fox et al, 2006) - network defined by positive resting state functional connectivity which includes regions involved in visual attention. Network includes much of intra-parietal and pre-central sulci.
FPCN: Fronto-parietal Control Network (Vincent at al, 2008) - network defined by positive resting state connectivity which includes regions involved in problem solving, working memory and executive functions. Much of the network lies directly anterior to the DAN in parietal and frontal cortices.
While the TPN was aligned with the dorsal attention network in Fox et al’s initial papers (Fox et al, 2005; Fox et al, 2006) the spatial characterization of the TPN in those analyses was constrained both by negative correlations with seeds in the DMN and by positive correlations with points generated by studies of visual attention. Later studies have more simply identified areas which are negatively correlated with DMN seeds (Chang & Glover, 2009; Fox et al, 2009, Chai et al, 2012). These regions do overlap the FPCN.
In contrast, we suggest the co-activation of these networks at the time of response suggests it is possible for processes associated with either network to operate on representations stored in the DMN and TPN (social and mechanical representations, respectively).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The Brain’s Default Network and Its Adaptive Role in Internal Mentation. Neuroscientist. 2011 doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperly IA, Butterfill SA. Do humans have two systems to track beliefs and belief-like states? Psychol Rev. 2009;116:953–970. doi: 10.1037/a0016923. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Superiority on the Embedded Figures Test in autism and in normal males: Evidence of an “innate talent”? Behavioral and Brain Sciences. 1998;21:408. [Google Scholar]
- Baroncohen S, Leslie AM, Frith U. Mechanical, Behavioral and Intentional Understanding of Picture Stories in Autistic-Children. Brit J Dev Psychol. 1986;4:113–125. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bloom P, German TP. Two reasons to abandon the false belief task as a test of theory of mind. Cognition. 2000;77:B25–31. doi: 10.1016/s0010-0277(00)00096-2. [DOI] [PubMed] [Google Scholar]
- Brown JH, Johnson MH, Paterson SJ, Gilmore R, Longhi E, Karmiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia. 2003;41:1037–1046. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner R, Carroll D. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Dev. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Van Essen DC, Watson JM, Brubaker LE, McDermott KB. The contributions of prefrontal cortex and executive control to deception: evidence from activation likelihood estimate meta-analyses. Cereb Cortex. 2009;19:1557–1566. doi: 10.1093/cercor/bhn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nature Reviews Neuroscience. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cosmides L. The logic of social exchange: has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition. 1989;31:187–276. doi: 10.1016/0010-0277(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Dawson M, Soulieres I, Gernsbacher MA, Mottron L. The level and nature of autistic intelligence. Psychol Sci. 2007;18:657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A Meta-analysis of Functional Neuroimaging Studies of Self- and Other Judgments Reveals a Spatial Gradient for Mentalizing in Medial Prefrontal Cortex. J Cogn Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Education Group & Associates . The Video encyclopedia of physics demonstrations preview videotape. The Education Group; Los Angeles, CA: 1995. [Google Scholar]
- Evans JST. In two minds: dual-process accounts of reasoning. Trends in Cognitive Sciences. 2003;7:454–459. doi: 10.1016/j.tics.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddick L, Cosmides L, Tooby J. No interpretation without representation: the role of domain-specific representations and inferences in the Wason selection task. Cognition. 2000;77:1–79. doi: 10.1016/s0010-0277(00)00085-8. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Friston K, Penny W, Glaser D. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan R. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Goldberg I, Harel M, Malach R. When the Brain Loses Its Self: Prefrontal Inactivation during Sensorimotor Processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Gopnik A. The scientist as child. Philos Sci. 1996;63:485–514. [Google Scholar]
- Gordon A, Smith R, Keramatian K, Luus B, Weinberg A, Smallwood J, Schooler J, Christoff K. Mind-wandering, awareness, and task performance: An fMRI study. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale. 2007;61:366–366. [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The Neural Bases of Cognitive Conflict and Control in Moral Judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yucel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci U S A. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Kato M, Igarashi K, Kashima H. Superior fluid intelligence in children with Asperger’s disorder. Brain Cogn. 2008;66:306–310. doi: 10.1016/j.bandc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Hill CS. Imaginability, conceivability, possibility and the mind-body problem. Philosophical Studies. 1997;87:61–85. [Google Scholar]
- Iacoboni M. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Jack AI, Patel GH, Astafiev SV, Snyder AZ, Akbudak E, Shulman GL, Corbetta M. Changing Human Visual Field Organization from Early Visual to Extra-Occipital Cortex. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AI, Robbins P. The Phenomenal Stance Revisited. Review of Philosophy and Psychology. 2012 [Google Scholar]
- Jack AI, Shallice T. Introspective physicalism as an approach to the science of consciousness. Cognition. 2001;79:161–196. doi: 10.1016/s0010-0277(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice - Mapping bounded rationality. Am Psychol. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Subjective Probability - Judgment of Representativeness. Cognitive Psychol. 1972;3:430–454. [Google Scholar]
- Kennedy DP. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett L, Joseph J, Blissmoreau E, Lindquist K, Wager T. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Human Brain Mapping. 1995;3:209–223. [Google Scholar]
- Levine J. Conceivability, identity, and the explanatory gap. From Anim Animat. 2000:3–12. [Google Scholar]
- Lewis D. Psychophysical and theoretical identifications. Australasian Journal of Philosophy. 1972;50:249–258. [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural Foundations for Understanding Social and Mechanical Concepts. Cognitive Neuropsychology. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Spunt RP, Berkman ET, Taylor SE, Lieberman MD. Evidence for social working memory from a parametric functional MRI study. Proc Natl Acad Sci U S A. 2012;109:1883–1888. doi: 10.1073/pnas.1121077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Hou CE. Portraits of artists: emergence of visual creativity in dementia. Arch Neurol. 2004;61:842–844. doi: 10.1001/archneur.61.6.842. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable Medial Prefrontal Contributions to Judgments of Similar and Dissimilar Others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel T. What Is It Like to Be a Bat. Philos Rev. 1974;83:435–450. [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological Medicine. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P, Jack AI. The phenomenal stance. Philosophical Studies. 2006;127:59–85. [Google Scholar]
- Samson D, Apperly IA, Braithwaite JJ, Andrews BJ, Scott SEB. Seeing it their way: Evidence for rapid and involuntary computation of what other people see. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1255–1266. doi: 10.1037/a0018729. [DOI] [PubMed] [Google Scholar]
- Saxe R. Against simulation: the argument from error. Trends Cogn Sci. 2005;9:174–179. doi: 10.1016/j.tics.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff S, Rotarskajagiela A, Fink G, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behavioral and Brain Sciences. doi: 10.1017/S0140525X12000660. in press. [DOI] [PubMed] [Google Scholar]
- Schneider D, Lam R, Bayliss AP, Dux PE. Cognitive Load Disrupts Implicit Theory-of-Mind Processing. Psychol Sci. 2012;23:842–847. doi: 10.1177/0956797612439070. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks .2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sloman SA. The empirical case for two systems of reasoning. Psychological Bulletin. 1996;119:3–22. [Google Scholar]
- Small DA, Loewenstein G, Slovic P. Sympathy and callousness: The impact of deliberative thought on donations to identifiable and statistical victims. Organizational Behavior and Human Decision Processes. 2007;102:143–153. [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spreng RN. The fallacy of a “task-negative” network. Front Psychol. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science. doi: 10.1177/0956797612450884. in press. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liu Y, Yu C, Wang K, Zhou Y, Song M, Li K. The relationship within and between the extrinsic and intrinsic systems indicated by resting state correlational patterns of sensory cortices. Neuroimage. 2007;36:684–690. doi: 10.1016/j.neuroimage.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. NeuroImage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. You, Me, and My Brain: Self and other representations in social cognitive neuroscience. In: Todorov A, et al., editors. Social neuroscience: Toward understanding the underpinnings of the social mind. Oxford University Press; New York: 2011. [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]