Abstract
Chlamydia trachomatis, although commonly asymptomatic in women, can result in chronic sequelae, such as pelvic inflammatory disease, ectopic pregnancy and infertility. However, a clear relationship has not been determined between specific serovars and the ability to lead to upper genital tract infection or infertility. Thus, in order to investigate differences in pathogenicity, C3H/HeN mice were infected in the ovarian bursa with the C. trachomatis strains D (UW-3/Cx), F (N.I.1), F (IC-Cal-3) and E (Bour). Differences both in the amount of vaginal shedding as well as subsequent fertility rates were observed between D (UW-3/Cx) and F (N.I.1) compared to F (IC-Cal-3) and E (Bour). Approximately 50% of the mice infected with the D (UW-3/Cx) and F (N.I.1) strains had vaginal shedding for up to 3–4 weeks after infection and fertility rates of less than 25%. Furthermore, mice inoculated with D (UW-3/Cx) and F (N.I.1) showed infertility even in the absence of medroxy progesterone acetate (MPA) treatment. In contrast, both MPA and non-MPA treated mice infected with F (IC-Cal-3) or E (Bour) did not show vaginal shedding and had fertility rates between 45–88%. Mutations in the CT135 open reading frame have been associated with virulence. However, no nucleotide differences were found among the four isolates for CT135. This murine model of infection with C. trachomatis may help with the understanding of disease pathology in humans and ultimately vaccine development.
Keywords: Chlamydia, infertility, murine, pathogenicity
1. Introduction
Chlamydia trachomatis is a leading cause of pelvic inflammatory disease (PID), ectopic pregnancy and infertility among women [1–4]. There are approximately 100 million confirmed cases of Chlamydia each year, making it the most common bacterial sexually transmitted disease in the world [3, 4]. The exact percentage of cases that ultimately ascend to the upper genital tract causing tubal pathology and infertility is not well known. One study showed that the rate of PID among women that previously tested positive for Chlamydia was 5.6% [5], yet most women with PID or tubal infertility report no previous symptoms [6]. In addition to the host factors it is not understood if all or only certain C. trachomatis serovars, or specific strains, have the potential to induce upper genital tract pathology.
Using mouse toxicity assays and immunofluorescence analysis, the human C. trachomatis isolates were grouped into 15 serovars belonging to three complexes: B (B, Ba, E, D, L1, and L2 serovars), C (C, J, H, I, A, K, and L3 serovars) and the intermediate G and F serovar complex [7, 8]. DNA sequencing of the major outer membrane protein (MOMP) from the human serovars demonstrated that this protein has four variable domains (VD) and five constant domains (CD) [9]. Analysis of the amino acid sequence of the VD was found to correlate with the classification based on the mouse toxicity and immunofluorescence assays [10]. Since then, the assumption was made that the virulence of C. trachomatis isolates was dependent, at least for the most part, on MOMP.
Based on these assumptions several epidemiological studies were performed trying to correlate the prevalence and virulence of genital infections with specific serovars [11, 12]. From these studies it was established that the most common genital serovars, consisting of up to 75% of all clinical cases, are D, E, and F [13–15]. Studies have shown conflicting results in identifying associations between clinical symptoms and the infecting serovar. For example, infections with serovar F have been shown to be more likely in women reporting abdominal pain and PID [11, 16]. However, another study showed that serovar F presented with fewer clinical signs in both men and women [12]. Additionally, Dean et al. found that infections with serovar E were commonly asymptomatic while serovar D was associated with ascending infections [12].
Animal experiments have also addressed these issues. Tuffrey et al. first attempted to induce upper genital pathology in the mouse with the human serovar F [17]. They showed that an intrabursal, but not an intravaginal infection, with C. trachomatis F (N.I.1), in CBA/H animals pre-treated with medroxy progesterone acetate (MPA), resulted in salpingitis and infertility; in addition, intrabursal inoculation resulted in salpingitis in CBA and C3H/He-mg mice, but not in BALB/c [17, 18]. Ito et al. pretreated outbred CF-1 mice with MPA, inoculated the animals intravaginally with 4.8×107 to 9.5×107 IFU of serovar D, E, F, G, H, I, or K and collected vaginal cultures [19]. Based on the median duration of vaginal shedding they concluded that the infection was significantly longer with serovars D and E. In a separate experiment they infected CF-1 mice, pretreated with MPA, with serovars D and H and collected the genital tract at 13 and 27 days post infection [20]. Six out of eight mice infected with serovar D had positive uterine cultures while only one out of eight animals infected with serovar H had positive uterine cultures. The ovaries and oviducts were negative in all animals.
Due to the limitations of the current Chlamydia genetic systems, several investigators have used DNA sequencing in an attempt to identify virulence factors in this pathogen. For example, Sturdevant et al. [21] infected innate immunity-deficient C3H/HeJ female mice intravaginally with C. trachomatis serovar D (UW-3/Cx), that had undergone multiple in vitro passages and observed early and late clearance phenotypes. Strains of each serovar D phenotype were then used to infect naïve mice. Following infection, the late-clearance strains were shown to be more virulent than the early-clearance strains. Genome sequencing of the isolates demonstrated mutations in the CT135 gene suggesting that it may be a virulence factor.
Studies to further investigate the differential virulence of human C. trachomatis serovars in mice are crucial for elucidating the pathogenesis of these infections and development of an effective vaccine. Here, for the first time, we performed a comparative study between the C. trachomatis strains D (UW-3/Cx), E (Bour), F (IC-Cal-3) and F (N.I.1). Our results show that strains D (UW-3/Cx) and F (N.I.1) can induce infertility in C3H/HeN mice following an intrabursal infection both in the presence and absence of MPA; further, these strains differ in their ability to infect the upper and lower genital tract compared to strains F (IC-Cal-3) and E (Bour).
2. Materials and Methods
2.1 C. trachomatis stocks
The C. trachomatis serovar D (strain UW-3/Cx), F (strain IC-Cal-3) and E (strain Bour) were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and the C. trachomatis serovar F (strain N.I.1) was obtained from Dr. Tuffrey (Imperial College School of Medicine; London, UK). This strain (F N.I.1), originally thought to be serovar E, was isolated from the cervix of a non-gonococcal urethritis contact who was negative for Neisseria gonorrhoeae but positive for Gardnerella vaginalis, anaerobes and Mycoplasma hominis [17, 22]. All strains were grown in HeLa-229 cells as previously described [23]. Purified elementary bodies (EB) were stored at −70°C in 0.2 M sucrose, 20 mM sodium phosphate (pH 7.4), and 5 mM glutamic acid (SPG) [24].
To eliminate the Mycoplasma from strain F (N.I.1), C3H/HeN (H-2k) female mice were treated twice with 2.5 mg MPA subcutaneously, 5 days before challenge and 2 days after challenge [25]. Mice were then inoculated intravaginally and in the left ovarian bursa with 105 IFU of F (N.I.1). Vaginal swabs were cultured at 7-day intervals for a period of 6 weeks following the challenge, and the presence of Chlamydia was confirmed by inoculation onto HeLa-229 cells [26]. Cultures from week 3 post-inoculation, the last positive timepoint, were amplified by passages in HeLa-229 cells without antibiotics, and the absence of Mycoplasma was confirmed by PCR using 16s rRNA primers: 5′-GGG AGC AAA CAG GAT TAG ATA CCC T and 5′-TGC ACC ATC TGT CAC TCT GTT ACC CTC [27]. A Mycoplasma-free stock was then used to inoculate mice as described below.
Genomic DNA from C. trachomatis D (UW-3/Cx), E (Bour), F (N.I.1), and F (IC-Cal-3) was extracted using the Wizard genomic DNA Purification Kit (Promega; Madison, WI). The MOMP gene (CT 681) was amplified without the leading sequence with Pfu Turbo DNA Polymerase (Agilent Technologies; Santa Clara, CA) using the primer set 5′ACGCCCATGGCACTGCCTGTGGGGAATCCTGCT and reverse primer: 5′ AGCGGTCGACTTAGAAGCGGAATTGTGCATTTACG. The CT135 gene was amplified from EB stocks and day 14 genital tissue culture from each C. trachomatis strain in two fragments using the primer sets 5′TGAGCTAAAGATCGTGATGGTC with 5′CTCATCATCACAGTATCGCAAG and 5′ CTGCCTTCGCCCGTTTGAG with 5′CTATACCACAAATCCGCCGC [21].
2.2 Infection of mice with C. trachomatis
Ten to 11 week-old female C3H/HeN (H-2(k)) mice were purchased from Charles River Laboratories (Wilmington, MA) and were housed at the University of California, Irvine, Vivarium. The animal protocols were approved by the UCI Animal Care and Use Committee. All experiments were repeated.
To enhance the susceptibility to C. trachomatis groups of mice were treated subcutaneously 4 days before the challenge with either 1 mg/mouse of MPA (Greenstone Ltd, Peapack, NJ), when infected with strains D (UW-3/Cx), F (IC-Cal-3) or F (N.1.1), or with 2 mg/mouse of MPA before infection with strain E (Bour) [18]. The same strains were used to infect groups of mice that were not treated with MPA. The vaginal cytology was checked before the challenge. Mice were inoculated in the left ovarian bursa with 103 or 105 C. trachomatis inclusion-forming units (IFU) per mouse, as previously described [26]. As a negative-control group, mice were inoculated in the left ovarian bursa with SPG.
2.3 Immunoassays
Blood was collected from the periorbital region; all immunoassays were performed with three pools of sera from each group. The Chlamydia-specific antibody titers in sera were determined by an enzyme-linked immunosorbent assay (ELISA) using total immunoglobulin G (IgG) (MP Biomedical; Solon, OH) as previously described; EBs from the homologous strain were used as the antigen [28].
2.4 Genital cultures
Vaginal swabs were cultured at 7-day intervals for a period of 6 weeks following the genital challenge, and samples were stained and counted as described [26].
2.5 Tissue cultures
2.6 Fertility Studies
At 6 weeks following the challenge, female mice were housed with a proven breeder male mouse for a maximum of 18 days and then repeated if necessary [26]. Fertility is defined as at least one embryo in each uterine horn.
2.7 Statistical analyses
The Mann-Whitney U test, Fisher’s exact test, and the Student’s t-test were used for statistical analysis using the program SigmaStat version 3.5.
3. Results
3.1 Immune response following infection with C. trachomatis
Table 1 shows the results of the humoral immune responses in sera from D28 post infection. The mice infected with 103 or 105 IFU of either D (UW-3/Cx) or F (N.I.1) had moderate IgG antibody titers (GMT and range). For example, the mice infected with 103 IFU of D (UW-3/Cx) had a titer of 800 (200–1,600), compared to 3,200 (3,200-3,200) in the 105 IFU group. The mice infected with 103 IFU of F (N.I.1) had a titer of 1,600 (800–3,200) compared to 8,063 (1,600–25,600) in the 105 IFU group. The mice infected with 103 or 105 IFU of F (IC-Cal-3) or E (Bour) had titers ≤400 (below the limit of detection (BLD) – 800)
Table 1.
Humoral immune response in serum from D28 after intrabursal inoculation
| Serovar | MPA (mg/mouse) | IFU/mouse | Chlamydia trachomatis-specific ELISA antibody titer Serum IgGa |
|---|---|---|---|
|
|
|
||
| D (UW-3/Cx) | 1 | 103 | 800 (200–1,600) |
| F (N.I.1) | 1 | 103 | 1,600 (800–3,200) |
| F (IC-Cal-3) | 1 | 103 | 400 (BLD-400) |
| E (Bour) | 2 | 103 | BLD |
|
|
|
||
| D (UW-3/Cx) | 0 | 105 | 3,200 (3,200-3,200) |
| F (N.I.1) | 0 | 105 | 8,063 (1,600–25,600) |
| F (IC-Cal-3 | 0 | 105 | 200 (BLD-200) |
| E (Bour) | 0 | 105 | 400 (BLD-800) |
Geometric mean of titers (range)
BLD: Below the limit of detection (<200)
3.2 Vaginal cultures for C. trachomatis
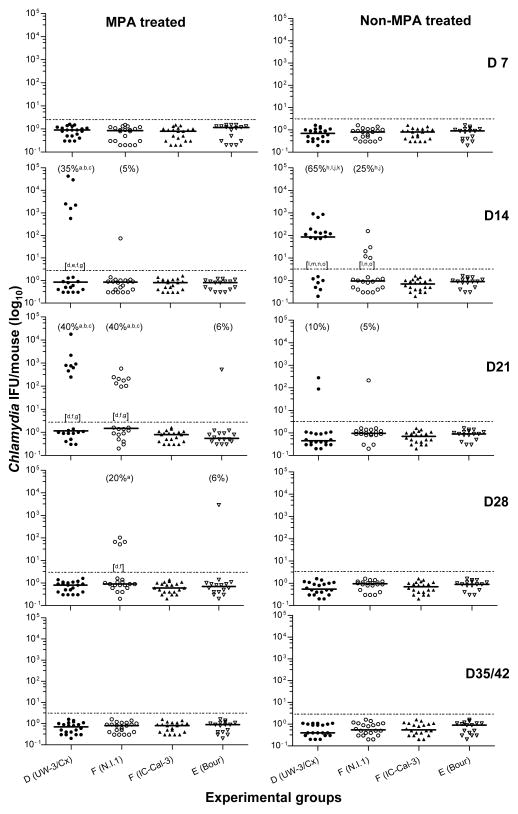
All mice treated with MPA were in diestrus at the time of challenge while mice not given MPA were not synchronized at the estrous cycle. Mice were inoculated intrabursally with the different C. trachomatis strains, and the resulting infection was followed by weekly vaginal cultures. As shown in Table 2, over the six-week period 45% (9/20) and 50% (10/20) of the MPA-treated mice infected with 103 IFU of D (UW-3/Cx) or F (N.I.1), respectively, had Chlamydia-positive vaginal cultures, significantly greater (P<0.05) than mice infected with E (Bour), (6%; 1/16) or F (IC-Cal-3), (0%; 0/20). The group infected with D (UW-3/Cx) in weeks 2 and 3, (35 and 40% respectively), along with the mice infected with F (N.I.1) in week 3, (40%), had significantly more animals shedding (P<0.05) than the animals infected with E (Bour), (6%), or F (IC-Cal-3), (0%) during this time (Fig. 1). Further, the mice infected with D (UW-3/Cx) in weeks 2 and 3, along with the mice infected with F (N.I.1) in week 3, shed significantly greater (P<0.05) number of IFU than the mice infected with F (IC-Cal-3) or E (Bour). Further, the D (UW-3/Cx) infected mice shed significantly greater (P<0.05) number of IFU than the F (N.I.1) infected mice in week 2 but significantly fewer (P<0.05) number of IFU in week 4.
Table 2.
Summary results of intrabursal inoculation
| Serovar | MPA (mg/mouse) | IFU/mouse | # of mice shed/Total # mice (%) | # of Fertilea mice/Total # mice (%) | Mean # embryos per uterine hornb
|
||
|---|---|---|---|---|---|---|---|
| Right | Leftc | Total | |||||
|
|
|
|
|
|
|
||
| D (UW-3/Cx) | 1 | 103 | 9/20 d,e,f (45) | 2/20 d,e,f (10) | 1.5 ± 2.7 g,h | 0.6 ± 1.4 g,i,j | 2.0 ± 3.3 g,h,j |
| F (N.I.1) | 1 | 103 | 10/20 d,e,f (50) | 5/20 d,f (25) | 4.4 ± 2.2 i | 0.8 ± 1.4 g,i,j | 5.1 ± 2.6 g |
| F (IC-Cal-3) | 1 | 103 | 0/20 | 9/20 d (45) | 2.1 ± 2.4 g | 2.2 ± 2.7 g | 4.3 ± 4.5 g |
| SPG | 1 | - | 0/26 | 24/26 (92) | 4.4 ± 1.8 | 4.3 ± 1.5 | 8.7 ± 2.0 |
| E (Bour) | 2 | 103 | 1/16 (6) | 11/16 (69) | 3.0 ± 2.6 | 2.9 ± 2.4 | 5.9 ± 4.3 |
| SPG | 2 | - | 0/16 | 12/16 (75) | 3.6 ± 2.8 | 2.8 ± 2.0 | 6.4 ± 4.2 |
|
|
|
|
|
|
|
||
| D (UW-3/Cx) | 0 | 105 | 14/20 k,l,m,n (70) | 8/20 k,l,m,n (40) | 3.5 ± 2.6 | 2.2 ± 2.1o,p | 5.6 ± 2.8 o,q |
| F (N.I.1) | 0 | 105 | 6/20 k,m,n (30) | 1/20 k,m,n (5) | 3.0 ± 2.9 | 0.2 ± 0.7 p,q,r | 3.2 ± 3.1 p,q,r |
| F (IC-Cal-3 | 0 | 105 | 0/20 | 16/20 (80) | 4.1 ± 2.4 | 3.4 ± 2.1 | 7.5 ± 4.0 |
| E (Bour) | 0 | 105 | 0/16 | 14/16 (88) | 3.8 ± 2.2 | 3.8 ± 1.8 | 7.6 ± 3.2 |
| SPG | 0 | - | 0/44 | 37/44 (84) | 3.8 ± 2.3 | 3.2 ± 2.1 | 7.0 ± 3.6 |
Fertility is defined as at least one embryo present in each uterine horn (bilateral)
Values are means ± standard deviations (16–44 animals per group)
Inoculation site
P<0.05 by the Fisher’s Exact test compared to the SPG MPA group
P<0.05 by the Fisher’s Exact test compared to the F (IC-Cal-3) MPA group
P<0.05 by the Fisher’s Exact test compared to the E (Bour) MPA group
P<0.05 by the Student’s t-test compared to the SPG MPA group
P<0.05 by the Student’s t-test compared to the F (N.I.1) MPA group
P<0.05 by the Student’s t-test compared to the F (IC-Cal-3) MPA group
P<0.05 by the Student’s t-test compared to the E (Bour) MPA group
P<0.05 by the Fisher’s Exact test compared to the SPG non-MPA group
P<0.05 by the Fisher’s Exact test compared to the F (N.I.1) non-MPA group
P<0.05 by the Fisher’s Exact test compared to the F (IC-Cal-3) non-MPA group
P<0.05 by the Fisher’s Exact test compared to the E (Bour) non-MPA group
P<0.05 by the Student’s t-test compared to the F (N.I.1) non-MPA group
P<0.05 by the Student’s t-test compared to the E (Bour) non-MPA group
P<0.05 by the Student’s t-test compared to the SPG non-MPA group
P<0.05 by the Student’s t-test compared to the F: IC-Cal-3 0 mg non-MPA group
Figure 1. Vaginal cultures of mice at days 7, 14, 21, 28, 35 and 42 after intrabursal infection with C. trachomatis.
The horizontal lines correspond to the median number of C. trachomatis IFU. Numbers in parenthesis represent percentage of mice with positive vaginal cultures and the statistical analyses. Letters in brackets correspond to statistical analyses of number of Chlamydia IFU.
a: P<0.05 by the Fisher’s exact test compared to the SPG MPA group,
b: P<0.05 by the Fisher’s exact test compared to the F (IC-Cal-3) MPA group
c: P<0.05 by the Fisher’s exact test compared to the E (Bour) MPA group
d: P<0.05 by the Mann Whitney test compared to the SPG MPA group
e: P<0.05 by the Mann Whitney test compared to the F (N.I.1) MPA group
f: P<0.05 by the Mann Whitney test compared to the F (IC-Cal-3) MPA group
g: P<0.05 by the Mann Whitney test compared to the E (Bour) MPA group
h: P<0.05 by the Fisher’s exact test compared to the SPG non-MPA group
i: P<0.05 by the Fisher’s exact test compared to the F (N.I.1) non-MPA group
j: P<0.05 by the Fisher’s exact test compared to the F (IC-Cal-3) non-MPA group
k: P<0.05 by the Fisher’s exact test compared to the E (Bour) non-MPA group
l: P<0.05 by the Mann Whitney test compared to the SPG non-MPA group
m: P<0.05 by the Mann Whitney test compared to the F (N.I.1) non-MPA group
n: P<0.05 by the Mann Whitney test compared to the F (IC-Cal-3) non-MPA group
o: P<0.05 by the Mann Whitney test compared to the E (Bour) non-MPA group
In the non-MPA treated groups infected with 105 IFU, over the 6-week period Chlamydia was recovered from 70% (14/20) of the mice infected with D (UW-3/Cx) and 30% (6/20) infected with F (N.I.1) (Table 2), significantly greater (P<0.05) compared to mice infected with E (Bour) or F (IC-Cal-3), that had no positive cultures (0/16 and 0/20, respectively). In addition, there were significantly more (P<0.05) mice with positive vaginal cultures overall with D (UW-3/Cx), 70%, than with F (N.I.1), 30%. Further, the animals infected with D (UW-3/Cx) had significantly more mice shedding (P<0.05) during week 2, (65%), than animals infected with F (N.I.1), (25%), E (Bour), (0%), or F (IC-Cal-3), (0%). There was significantly more mice shedding (P<0.05) over all 6 weeks, and significantly more mice shedding (P<0.05) in week 2 for animals infected with F (N.I.1) compared to mice infected with E (Bour) or F (IC-Cal-3). Finally, the mice infected with D (UW-3/Cx) or F (N.I.1) shed significantly greater (P<0.05) number of IFU in week 2 than mice infected with F (IC-Cal-3) or E (Bour), with mice infected with D (UW-3/Cx) having significantly greater (P<0.05) number of IFU than mice infected with F (N.I.1) (Fig. 1).
All MPA treated groups infected with 103 IFU had negative vaginal cultures starting at week 5 and the non-MPA treated groups infected with 105 IFU were negative by week 4. Both the MPA and non-MPA treated SPG inoculated negative-control group had no mice with positive cultures.
3.3 Genital Tissue Culture for C. trachomatis
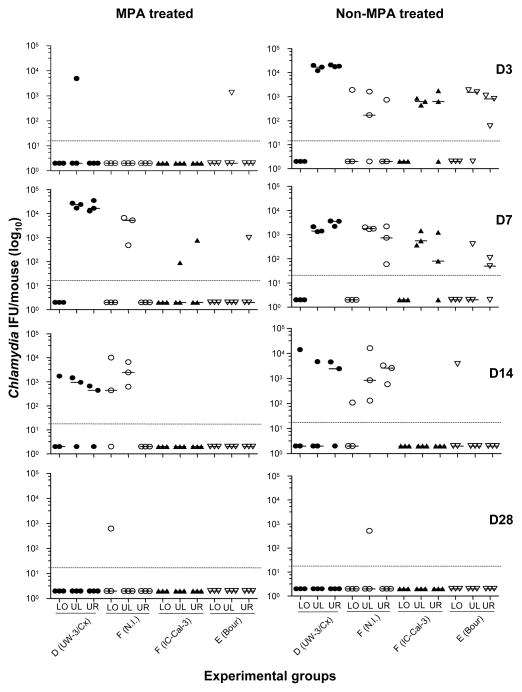
Mice either treated or not treated with MPA were inoculated in the left ovarian bursa. To assess the local spread of the infection, the tissue from the lower (cervix and vagina), upper right and upper left (uterus and ovary) genital tract was cultured for Chlamydia from three mice in each infection group for each time point. In the MPA-treated groups, over the six-week period, Chlamydia was recovered from 36% (13/36) of the tissue samples from mice infected with D (UW-3/Cx) and 25% (9/36) of F (N.I.1), significantly greater (P<0.05) compared to 6% (2/36) of the mice infected with F (IC-Cal-3) or with E (Bour) (Table 3). In addition, 50% (6/12) of the overall upper left genital tract tissue samples from D (UW-3/Cx) or F (N.I.1) were Chlamydia-positive, significantly greater (P<0.05) compared to 8% (1/12) positive tissues from the mice infected with F (IC-Cal-3) or E (Bour), while 42% (5/12) of the upper right genital tract tissue samples from the mice infected with D (UW-3/Cx) were positive, significantly greater (P<0.05) than the F (N.I.1) infected mice with no positive cultures (0/12). This corresponded to, at D7, 100% of the mice infected with either D (UW-3/Cx) or F (N.I.1) had positive upper left genital tissue cultures and 100% of the mice infected with D (UW-3/Cx) had positive upper right genital tissue cultures (Fig. 2). In addition, the number of Chlamydia IFU in the upper left genital tissues from the mice infected with either D (UW-3/Cx) or F (N.I.1) was significantly greater (P<0.05) than the F (IC-Cal-3) and E (Bour) infected groups. Further, one mouse infected with F (N.I.1) still had positive cultures from the lower genital tract at D28, while all other groups were negative. The total number of IFU (median; range) recovered from the mice infected with D (UW-3/Cx), (<20; <20–35,150) or F (N.I.1), (<20; <20–10,175) were significantly higher (P<0.05) compared with the mice infected with F (IC-Cal-3), (<20; <20–777) or E (Bour), (<20; <20–1,332). Mice inoculated with SPG were negative for all tissues at all time points.
Table 3.
Results of genital tissue infection after intrabursal inoculation
| Serovar | MPA (mg/mouse) | IFU/mouse | Overall Median (Range) (+ Tissue/Total Culture)
|
Total % + Cultures (+Tissue/Total Culture) | ||
|---|---|---|---|---|---|---|
| Lower | Upper Left | Upper Right | ||||
|
|
|
|
|
|
||
| D (UW-3/Cx) | 1 | 103 | <20 (<20–1,739) (2/12) | 481a,b,c (<20–27,010) (6/12)e,f,g | <20a,d (<20–35,150) (5/12)e,h | 36e,f,g (13/36) |
| F (N.I.1) | 1 | 103 | <20 (<20–10,175) (3/12) | 241a,b,c (<20–6,623) (6/12)e,f,g | <20 - (0/12) | 25e,f,g (9/36) |
| F (IC-Cal-3) | 1 | 103 | <20 - (0/12) | <20 (<20–90) (1/12) | <20 (<20–777) (1/12) | 6 (2/36) |
| SPG | 1 | - | <20 (0/8) | <20 (0/8) | <20 (0/8) | 0/24 |
| E (Bour) | 2 | 103 | <20 - (0/12) | <20 (<20–1,332) (1/12) | <20 (<20–999) (1/12) | 6 (2/36) |
|
|
|
|
|
|
||
| D (UW-3/Cx) | 0 | 105 | <20 (<20–14,430) (1/12) | 1,369i (<20–19,980) (7/12)l | 3,016i,j,k (<20–21,090) (8/12)l,m,n | 44l (16/36) |
| F (N.I.1) | 0 | 105 | <20 (<20–1,924) (2/12) | 685i,k (<20–16,280) (9/12)l,n | 326i (<20–3,293) (7/12)l | 50l (18/36) |
| F (IC-Cal-3 | 0 | 105 | <20 - (0/12) | 185i (<20–1,443) (6/12)l | <20 (<20–1,776) (4/12) | 28l (10/36) |
| E (Bour) | 0 | 105 | <20 (<20–3,811) (1/12) | <20 (<20–1,850) (3/12) | <20i (<20–1,110) (5/12)l | 25l (9/36) |
| SPG | 0 | - | <20 (0/8) | <20 (0/8) | <20 (0/8) | 0/24 |
Lower = Cervix and Vagina/Upper = Uterus and Ovary
P<0.05 by the Mann Whitney test compared to the SPG MPA group
P<0.05 by the Mann Whitney test compared to the F (IC-Cal-3) MPA group
P<0.05 by the Mann Whitney test compared to the E (Bour) MPA group
P<0.05 by the Mann Whitney test compared to the F (N.I.1) MPA group
P<0.05 by the Fisher’s exact test compared to the SPG MPA group
P<0.05 by the Fisher’s exact test compared to the F (IC-Cal-3) MPA group
P<0.05 by the Fisher’s exact test compared to the E (Bour) MPA group
P<0.05 by the Fisher’s exact test compared to the F (N.I.1) MPA group
P<0.05 by the Mann Whitney test compared to the SPG non-MPA group
P<0.05 by the Mann Whitney test compared to the F (IC-Cal-3) non-MPA group
P<0.05 by the Mann Whitney test compared to the E (Bour) non-MPA group
P<0.05 by the Fisher’s exact test compared to the SPG non-MPA group
P<0.05 by the Fisher’s exact test compared to the F(IC-Cal-3) non-MPA group
P<0.05 by the Fisher’s exact test compared to the E (Bour) non-MPA group
Figure 2. Number of Chlamydia IFU in the lower (LO), upper left (UL) and upper right (UR) genital tissue at days 3, 7, 14 and 28 after intrabursal inoculation.
Data represent results of tree individual mice per group per time point.
In the non-MPA treated groups infected with 105 IFU, over the six-week period, Chlamydia was recovered from 44% (16/36) of the tissue samples from mice infected with D (UW-3/Cx) and 50% (18/36) with F (N.I.1), compared to 28% (10/36) of the mice infected with F (IC-Cal-3) or 25% (9/36) with E (Bour) (Table 3). However, these values were not significantly different (p>0.05). Also 58% (7/12) and 75% (9/12) of the upper left genital tissue samples from mice infected with D (UW-3/Cx) or F (N.I.1), respectively, were Chlamydia-positive; in addition, 67% (8/12) and 58% (7/12) of the upper right genital tissue samples from these mice were Chlamydia-positive, respectively. Yet, the upper left genital tissue from F (N.I.1) infected mice and the upper right genital tissue from D (UW-3/Cx) infected mice had significantly greater number of Chlamydia IFU than only the E (Bour) infected mice (P<0.05). The mice infected with F (IC-Cal-3) showed 50% (6/12) and 33% (4/12) positive cultures while mice infected with E (Bour) showed 25% (3/12) and 42% (5/12) positive cultures from the upper left and right genital tract tissue, respectively. Here, by D7 100% of the mice infected with either D (UW-3/Cx), F (N.I.1) or F (IC-Cal-3) showed positive cultures from the upper left and upper right genital tract tissues, which persisted to D14 for the animals infected with F (N.I.1). The total number of IFU recovered from the mice infected with D (UW-3/Cx), (<20; <20–21,090) or F (N.I.1), (30; <20–16,280) were significantly higher (P<0.05) compared with the mice infected with F (IC-Cal-3), (<20; <20–1,776) or E (Bour), (<20; <20–3,811). The SPG inoculated negative-control mice had no positive cultures at any time point.
3.4 Fertility Studies
Six weeks after the intrabursal infection, mice were mated to determine the effect of the chlamydial infection on fertility. As shown in Table 2, MPA-treated groups infected with 103 IFU of D (UW-3/Cx), (2/20; 10%), F (N.I.1), (5/20; 25%), or F (IC-Cal-3), (9/20; 45%), had significantly lower fertility rates (P<0.05) than the SPG negative-control group, 92% fertile (24/26). Also, the mice infected with D (UW-3/Cx) or F (N.I.1) had significantly lower fertility rates (P<0.05), 10% and 25%, respectively, than mice infected with E (Bour) with 69% fertility. Further, the mice infected with D (UW-3/Cx) had a significantly lower fertility rate (10%) (P<0.05) than the mice infected with F (IC-Cal-3) (45%).
The mean total number of embryos in the MPA-treated groups: 2.0 ± 3.3 for the mice infected with D (UW-3/Cx), 4.3 ± 4.5 for F (IC-Cal-3) and 5.1 ± 2.6 for F (N.I.1), were significantly less (P<0.05) than in SPG negative-control group, 8.7 ± 2.0 (Table 2). The mean total number of embryos in the mice infected with E (Bour), 5.9 ± 4.3, was not statistically different than the SPG negative-control group. The mean number of embryos in the left uterine horn for the mice infected with D (UW-3/Cx) was 0.6 ± 1.4, 2.2 ± 2.7 for F (IC-Cal-3), and 0.8 ± 1.4 for F (N.I.1), all significantly less (P<0.05) than the SPG negative-control group, 4.3 ± 1.5. The mice infected with D (UW-3/Cx) or F (IC-Cal-3) also had significantly less embryos (P<0.05) in the right uterine horn compared to the SPG group (1.5 ± 2.7 and 2.1 ± 2.4 versus 4.4 ± 1.8). The mice infected with D (UW-3/Cx) had significantly less (P<0.05) mean number of embryos than animals infected with E (Bour) or F (N.I.1), less embryos in the left uterine horn compared to mice infected with E (Bour) or F (IC-Cal-3), and less embryos in the right uterine horn compared to mice infected with F (N.I.1). Further, the mice infected with F (N.I.1) had significantly less (P<0.05) embryos in the left uterine horn compared to mice infected with E (Bour) or F (IC-Cal-3), but significantly more embryos (P<0.05) in the right uterine horn compared to mice infected with F (IC-Cal-3).
For the non-MPA-treated infected groups, the mice infected with 105 IFU of D (UW-3/Cx) (8/20; 40%) or F (N.I.1) (1/20; 5%) had significantly lower fertility rates (P<0.05) than the SPG negative-control group, 84% fertile (37/44), and the mice infected with E (Bour) or F (IC-Cal-3) that had 88% (14/16) and 80% (16/20) fertility rates, respectively. In addition, the mice infected with F (N.I.1) had a significantly lower fertility rate (P<0.05) than animals infected with D (UW-3/Cx).
Only the mice infected with D (UW-3/Cx) or F (N.I.1) had significantly less mean total number of embryos (P<0.05) than the SPG negative-control group (5.6 ± 2.8 and 3.2 ± 3.1 compared to 7.0 ± 3.6). The mice infected with F (N.I.1) also had significantly fewer embryos (P<0.05) in the left uterine horn (0.2 ± 0.7) than the SPG negative-control group (3.2 ± 2.1) and significantly less mean embryos and embryos in the left uterine horn (P<0.05) than those infected with D (UW-3/Cx), E (Bour) or F (IC-Cal-3). The mice infected with D (UW-3/Cx) had significantly less embryos (P<0.05) in the left uterine horn, 2.2 ± 2.1, than the mice infected with E (Bour), 3.8 ± 1.8.
3.5 DNA Sequencing
The MOMP and CT135 genes were both sequenced from genomic DNA from the infecting stocks of D (UW-3/Cx), F (N.I.1), F (IC-Cal-3) and E (Bour). In addition, CT135 was sequenced from the D14 positive genital tissue samples from each strain. The MOMP gene from the C. trachomatis D (UW-3/Cx), F (IC-Cal-3) and E (Bour) were identical to their corresponding reference strain (GenBank Accession Numbers DQ064284, DQ064287, and DQ064286, respectively). The MOMP sequence from serovar F (N.I.1) was identical to the MOMP sequence from F (IC-Cal-3). Further, no nucleotide differences were found between the four strains for CT135; the gene sequence from the genomic DNA stock was identical to the sequence from the genital tissue tract sample for each strain.
4. Discussion
This study shows that some C. trachomatis human strains can induce infertility in C3H/HeN mice by an intrabursal infection, and that strains D (UW-3/Cx) and F (N.I.1) are more virulent than strains F (IC-Cal-3) and E (Bour). MPA-treated mice infected with 103 IFU of D (UW-3/Cx) or F (N.I.1) had significant vaginal shedding (~50%) and very low fertility rates (≤;25%). In addition, low fertility rates (≤40%) were observed in mice infected with 105 IFU of these strains in the absence of MPA treatment. In comparison, mice infected with F (IC-Cal-3) or E (Bour) exhibited moderately low fertility rates (45 and 69%, respectively) only with MPA treatment and did not show vaginal shedding either in the presence or absence of MPA treatment. This difference in virulence was also supported by the genital tract tissue cultures, where 100% of the MPA-treated mice infected with D (UW-3/Cx) or F (N.I.1) showed positive cultures throughout the time course, compared to few to no positive cultures from mice infected with F (IC-Cal-3) or E (Bour). In addition, only the mice inoculated with D (UW-3/Cx) or F (N.I.1) exhibited a Chlamydia-specific serum antibody response. Therefore, these results demonstrate differential pathogenicity between C. trachomatis strains.
Prior comparative studies with C. trachomatis strains in mice have been limited due to either the absence of upper genital tract pathology and infertility (intravaginal model) or the necessity of MPA for such pathology (intrabursal model) [19, 29–31]. In our study, infection with D (UW-3/Cx) resulted in high vaginal shedding as well as significant infertility. Studies utilizing the intravaginal inoculation model showed that among serovars D-K, serovar D was the most pathogenic as shown by infection of the uterine horn tissue, and serovars D and E had the longest duration of infection in both inbred and outbred mice strains [19, 29]. Further, serovar F, along with serovars G and K, have been shown to have a shorter duration of vaginal shedding than serovars D and E (B complex), but greater than serovars from the C complex (H, I, and J) [19]. In our study, infection with strain F (IC-Cal-3), in mice treated with MPA, had only a moderate effect on the fertility rate (45%). Thus, it was less pathogenic than D (UW-3/Cx) (10% fertility), but more virulent than E (Bour) (69% fertility). However, strain F (N.I.1) showed vaginal shedding and a severe reduction in fertility rates (25 and 5%) with and without MPA, respectively. Additionally, Tuffrey et al. showed that an intrabursal infection with F (N.I.1) in MPA pretreated C3H (CRC) mice resulted in salpingitis and infertility (31% fertility rate) compared to the control group of mice (93% fertility rate) [31].
In our study, both MPA and non-MPA-treated mice infected intrabursally with E (Bour) exhibited the highest levels of fertility (69 and 88%, respectively), and genital tissues from these mice had the lowest yields of IFU among all the strains studied, as well as a lack of an antibody response. Numerous studies involving serovar E have resulted in minimal upper genital pathology and no infertility after a vaginal infection [30, 32]. Darville et al. showed that an intravaginal infection with serovar E or C. muridarum caused equivalent destruction of the uterine horn epithelium but only C. muridarum resulted in pathology in the oviduct, while Ramsey et al. showed serovar E could moderately reduce fertility but only after a secondary inoculation [30, 32]. In addition, Miyarei et al. reported that strain E (Bour) completed in vitro conversion to EB in a longer period compared to the strains D (UW-3/Cx), E (UW-5/Cx), F (UW-6/Cx) and L2 (434/Bu) [33]. Interestingly, E (Bour), which was originally isolated from an adult with active trachoma, possesses genetic characteristics specific to genital strains [34]. F (IC-Cal-3) was also isolated from the eye but it was of a nine-day old baby and therefore, acquired from the genital tract of the mother.
Here we show for the first time induction of infertility with C. trachomatis human strains in the absence of MPA treatment. Previously, Tuffrey and Taylor-Robinson showed that treatment with MPA was necessary to induce vaginal infection in CBA mice with F (N.I.1) [18]. It has long been assumed that MPA works by halting the oestrous cycle and thus maintaining the target epithelial cells necessary for a vaginal infection [18]. Yet, MPA may induce a general immunosuppressive effect necessary for a more severe chlamydial genital infection that could lead to the development of infertility [35, 36]. In our study, we have shown that differential pathogenicity between strains remained with and without MPA; in fact, the pathogenicity of E (Bour) may be so low in mice, that it is unaltered by the presence of MPA, even at the higher dose (2 mg/mouse compared to 1 mg/mouse) given to these mice.
Comparative genomic studies have yet to elucidate a clear explanation for the differences in pathogenicity between strains of the same C. trachomatis serovar, both in women and in mice. Since serovars share high genomic identity (>99.6%), even between MOMP (>80%), the basis for serovar identification, a genetic determinant for pathogenicity probably it is going to be very difficult to identify. The current genetic systems in Chlamydia preclude the definitive identification of gene(s) involved in virulence and DNA sequencing, an inadequate surrogate, will probably require characterization of thousands of isolates before a tentative conclusion could be reached [37, 38]. As shown here, the MOMP sequence is identical between F (IC-Cal-3) and F (N.I.1) but these two strains differ significantly in virulence and therefore, we can exclude MOMP as the cause underlying the differences in pathogenicity. Yet, genotype variants in MOMP were found to occur more often in serovar F than in other serovars, and there was a strong correlation between women infected with these variants and the occurrence of PID [12]. Jeffrey et al. found several ORFs with polymorphisms between serovars from clinical isolates; however only genotypes from two loci (CT154 and CT326) correlated with rectal tissue tropism and only within serovar G (isolates 9301, 9768, 11222 and 11074), and not E (isolates 11023 and 150) or J (isolate 6276) [39]. Moreover, two serovar A ocular isolates (A23497 and A/HAR-13) were found to have distinct genotypes within 6 genes, 4 potentially affecting growth rate and 2 involved in iron transport [40]. Finally, after infecting C3H/HeJ mice intravaginally with serovar D (UW-3/Cx), Sturdevant et al. observed that some mice had a prolonged infection compared to others, correlating to the presence of a frameshift mutation in the CT135 gene [21]. Yet, our sequence analysis of CT135, from the original stock as well as from the day 14 upper left genital culture tissue, from the four C. trachomatis strains studied here, found no variation between them. Therefore, this gene may account for population differences in serovar D but does not explain clinical virulence between strains.
Few studies have shown that genetic variability within a population of a single serovar can affect the development and severity of infection. For example, Ramsey et al. showed that the Nigg strain of C. muridarium was more virulent in C3H/HeN mice, based on the ID50, plaque size and replication rate in vitro, than the Weiss strain [41]. Kari et al. also observed that differences in growth rate and plaque morphology between two strains of C. trachomatis ocular subtype A corresponded to their infectivity in Macaca fascicularis [40]. Slow growth rate was also observed in nonfusing inclusion mutants of C. trachomatis correlating to a reduction in clinical signs and symptoms in women [42, 43].
Hence, faster growing serovars, or strains, may be correlated to higher virulence, possibly due to an increased host immune response. Rank et al. observed that pathology between a wild-type and azithromycin-resistant strain of Chlamydia caviae in vivo corresponded to the ability to induce an inflammatory response [44]. Yet, slower growing strains may have the potential to result in long-term or chronic pathology such as PID and infertility. In fact, studies have shown that the chronic inflammation due to prolonged infection may lead to greater tissue damage and disease pathology [45, 46]. Interestingly, this idea was also shown by an evolutionarily model where human papillomavirus (HPV) transmission and infection dynamics were linked to sexual behavior in humans [47]. The authors hypothesize that HPV strains with a high risk of causing cancer evade the host’s immune system by producing fewer virions. This reduces the transmission rate but allows for a longer infection period, and thus show an evolutionarily advantage in monogamous long-term relationships. In summary, the dynamics of Chlamydia growth and infection, the host’s immune response, and perhaps sexual behavior, all must be considered when evaluating virulence.
Our results illustrate a reproducible model in which some C. trachomatis strains can result in shedding and tubal infertility in mice, even in the absence of MPA immunosuppression. This model is an important contribution to the field for it contains similar elements as the C. muridarium model, such as the presence of upper genital tract pathology, thus making it a very useful system for analyzing the relationship between lower and upper genital pathology that may be extrapolated to human disease [26, 48]. Testing in mice multiple strains of C. trachomatis isolated from patients with a variety of clinical presentations may help determine the specific genes involved in pathogenicity. In addition, establishing in mice a model with C. trachomatis that replicates the spectrum of the infections observed humans could aid in the development of effective therapeutic and preventive measures.
Acknowledgments
This work was supported by the Public Health Service grant AI 067888 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 2.Ness RB, Smith KJ, Chang CC, Schisterman EF, Bass DC. Prediction of pelvic inflammatory disease among young, single, sexually active women. Sex Transm Dis. 2006;33:137–142. doi: 10.1097/01.olq.0000187205.67390.d1. [DOI] [PubMed] [Google Scholar]
- 3.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 4.Chlamydia screening among sexually active young female enrollees of health plans--United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58:362–365. [PubMed] [Google Scholar]
- 5.Low N, Egger M, Sterne JA, Harbord RM, Ibrahim F, Lindblom B, Herrmann B. Incidence of severe reproductive tract complications associated with diagnosed genital chlamydial infection: The Uppsala Women’s Cohort Study. Sex Transm Infect. 2006;82:212–218. doi: 10.1136/sti.2005.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Land JA, Van Bergen JE, Morre SA, Postma MJ. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum Reprod Update. 2009;16:189–204. doi: 10.1093/humupd/dmp035. [DOI] [PubMed] [Google Scholar]
- 7.Wang SP, Grayston JT. Classification of Trachoma Virus Strains by Protection of Mice from Toxic Death. J Immunol. 1963;90:849–856. [PubMed] [Google Scholar]
- 8.Wang SP, Grayston J. Microimmunofluorescence serology of Chlamydia trachomatis. In: de la Maza LM, Peterson EM, editors. Medical Virology III. Elsevier Science Pub; New York: 1984. [Google Scholar]
- 9.Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitch WM, Peterson EM, de la Maza LM. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol Biol Evol. 1993;10:892–913. doi: 10.1093/oxfordjournals.molbev.a040048. [DOI] [PubMed] [Google Scholar]
- 11.Geisler WM, Suchland RJ, Whittington WL, Stamm WE. The relationship of serovar to clinical manifestations of urogenital Chlamydia trachomatis infection. Sex Transm Dis. 2003;30:160–165. doi: 10.1097/00007435-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Dean D, Oudens E, Bolan G, Padian N, Schachter J. Major outer membrane protein variants of Chlamydia trachomatis are associated with severe upper genital tract infections and histopathology in San Francisco. J Infect Dis. 1995;172:1013–1022. doi: 10.1093/infdis/172.4.1013. [DOI] [PubMed] [Google Scholar]
- 13.Morre SA, Rozendaal L, Van Valkengoed IG, Boeke AJ, Van Voorst Vader PC, Schirm J, De Blok S, Van Den Hoek JA, Van Doornum GJ, Meijer CJ, Van Den Brule AJ. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: an association with clinical manifestations? J Clin Microbiol. 2000;38:2292–2296. doi: 10.1128/jcm.38.6.2292-2296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suchland RJ, Eckert LO, Hawes SE, Stamm WE. Longitudinal assessment of infecting serovars of Chlamydia trachomatis in Seattle public health clinics: 1988–1996. Sex Transm Dis. 2003;30:357–361. doi: 10.1097/00007435-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Lan J, Melgers I, Meijer CJ, Walboomers JM, Roosendaal R, Burger C, Bleker OP, Van Den Brule AJ. Prevalence and serovar distribution of asymptomatic cervical Chlamydia trachomatis infections as determined by highly sensitive PCR. J Clin Microbiol. 1995;33:3194–3197. doi: 10.1128/jcm.33.12.3194-3197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millman K, Black CM, Stamm WE, Jones RB, Hook EW, 3rd, Martin DH, Bolan G, Tavare S, Dean D. Population-based genetic epidemiologic analysis of Chlamydia trachomatis serotypes and lack of association between ompA polymorphisms and clinical phenotypes. Microbes Infect. 2006;8:604–611. doi: 10.1016/j.micinf.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 18.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Lett. 1981;12:111–115. [Google Scholar]
- 19.Ito JI, Jr, Lyons JM, Airo-Brown LP. Variation in virulence among oculogenital serovars of Chlamydia trachomatis in experimental genital tract infection. Infect Immun. 1990;58:2021–2023. doi: 10.1128/iai.58.6.2021-2023.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons JM, Morre SA, Airo-Brown LP, Pena AS, Ito JI. Acquired homotypic and heterotypic immunity against oculogenital Chlamydia trachomatis serovars following female genital tract infection in mice. BMC Infect Dis. 2005;5:105. doi: 10.1186/1471-2334-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun. 2010;78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–616. [PMC free article] [PubMed] [Google Scholar]
- 23.Pal S, Sarcon AK, de la Maza LM. C3H male mice with severe combined immunodeficiency cannot clear a urethral infection with a human serovar of Chlamydia trachomatis. Infect Immun. 2009;77:5602–5607. doi: 10.1128/IAI.00766-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furr PM, Taylor-Robinson D. Factors influencing the ability of different mycoplasmas to colonize the genital tract of hormone-treated female mice. Int J Exp Pathol. 1993;74:97–101. [PMC free article] [PubMed] [Google Scholar]
- 26.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossewaarde JM, De Vries A, Bestebroer T, Angulo AF. Application of a Mycoplasma group-specific PCR for monitoring decontamination of Mycoplasma-infected Chlamydia sp. strains. Appl Environ Microbiol. 1996;62:328–331. doi: 10.1128/aem.62.2.328-331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons JM, Morre SA, Airo-Brown LP, Pena AS, Ito JI. Comparison of multiple genital tract infections with Chlamydia trachomatis in different strains of female mice. J Microbiol Immunol Infect. 2005;38:383–393. [PubMed] [Google Scholar]
- 30.Ramsey KH, Dewolfe JL, Salyer RD. Disease outcome subsequent to primary and secondary urogenital infection with murine or human biovars of Chlamydia trachomatis. Infect Immun. 2000;68:7186–7189. doi: 10.1128/iai.68.12.7186-7189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuffrey M, Falder P, Gale J, Quinn R, Taylor-Robinson D. Infertility in mice infected genitally with a human strain of Chlamydia trachomatis. J Reprod Fertil. 1986;78:251–260. doi: 10.1530/jrf.0.0780251. [DOI] [PubMed] [Google Scholar]
- 32.Darville T, Andrews CW, Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyairi I, Mahdi OS, Ouellette SP, Belland RJ, Byrne GI. Different growth rates of Chlamydia trachomatis biovars reflect pathotype. J Infect Dis. 2006;194:350–357. doi: 10.1086/505432. [DOI] [PubMed] [Google Scholar]
- 34.Carlson JH, Hughes S, Hogan D, Cieplak G, Sturdevant DE, Mcclarty G, Caldwell HD, Belland RJ. Polymorphisms in the Chlamydia trachomatis cytotoxin locus associated with ocular and genital isolates. Infect Immun. 2004;72:7063–7072. doi: 10.1128/IAI.72.12.7063-7072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill JA, Barbieri RL, Anderson DJ. Immunosuppressive effects of danazol in vitro. Fertil Steril. 1987;48:414–418. [PubMed] [Google Scholar]
- 36.Pal S, Hui W, Peterson EM, de la Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 37.Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of Chlamydia psittaci using recombinant DNA introduced by electroporation. Proc Natl Acad Sci U S A. 2009;106:292–297. doi: 10.1073/pnas.0806768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeffrey BM, Suchland RJ, Quinn KL, Davidson JR, Stamm WE, Rockey DD. Genome sequencing of recent clinical Chlamydia trachomatis strains identifies loci associated with tissue tropism and regions of apparent recombination. Infect Immun. 2010;78:2544–2553. doi: 10.1128/IAI.01324-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kari L, Whitmire WM, Carlson JH, Crane DD, Reveneau N, Nelson DE, Mabey DC, Bailey RL, Holland MJ, Mcclarty G, Caldwell HD. Pathogenic diversity among Chlamydia trachomatis ocular strains in nonhuman primates is affected by subtle genomic variations. J Infect Dis. 2008;197:449–456. doi: 10.1086/525285. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey KH, Sigar IM, Schripsema JH, Denman CJ, Bowlin AK, Myers GA, Rank RG. Strain and virulence diversity in the mouse pathogen Chlamydia muridarum. Infect Immun. 2009;77:3284–3293. doi: 10.1128/IAI.00147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisler WM, Suchland RJ, Rockey DD, Stamm WE. Epidemiology and clinical manifestations of unique Chlamydia trachomatis isolates that occupy nonfusogenic inclusions. J Infect Dis. 2001;184:879–884. doi: 10.1086/323340. [DOI] [PubMed] [Google Scholar]
- 43.Xia M, Suchland RJ, Bumgarner RE, Peng T, Rockey DD, Stamm WE. Chlamydia trachomatis variant with nonfusing inclusions: growth dynamic and host-cell transcriptional response. J Infect Dis. 2005;192:1229–1236. doi: 10.1086/444394. [DOI] [PubMed] [Google Scholar]
- 44.Rank RG, Bowlin AK, Tormanen KI, Wang Y, Maurelli AT. Effect of inflammatory response on in vivo competition between two chlamydial variants in the guinea pig model of inclusion conjunctivitis. Infect Immun. 2011;80:612–619. doi: 10.1128/IAI.06054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 46.Campbell LA, Patton DL, Moore DE, Cappuccio AL, Mueller BA, Wang SP. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil Steril. 1993;59:45–50. [PubMed] [Google Scholar]
- 47.Orlando PA, Gatenby RA, Giuliano AR, Brown JS. Evolutionary ecology of Human Papillomavirus: trade-offs, coexistence, and origins of high-risk and low-risk types. J Infect Dis. 2012;205:272–279. doi: 10.1093/infdis/jir717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]